3. Results and Discussion
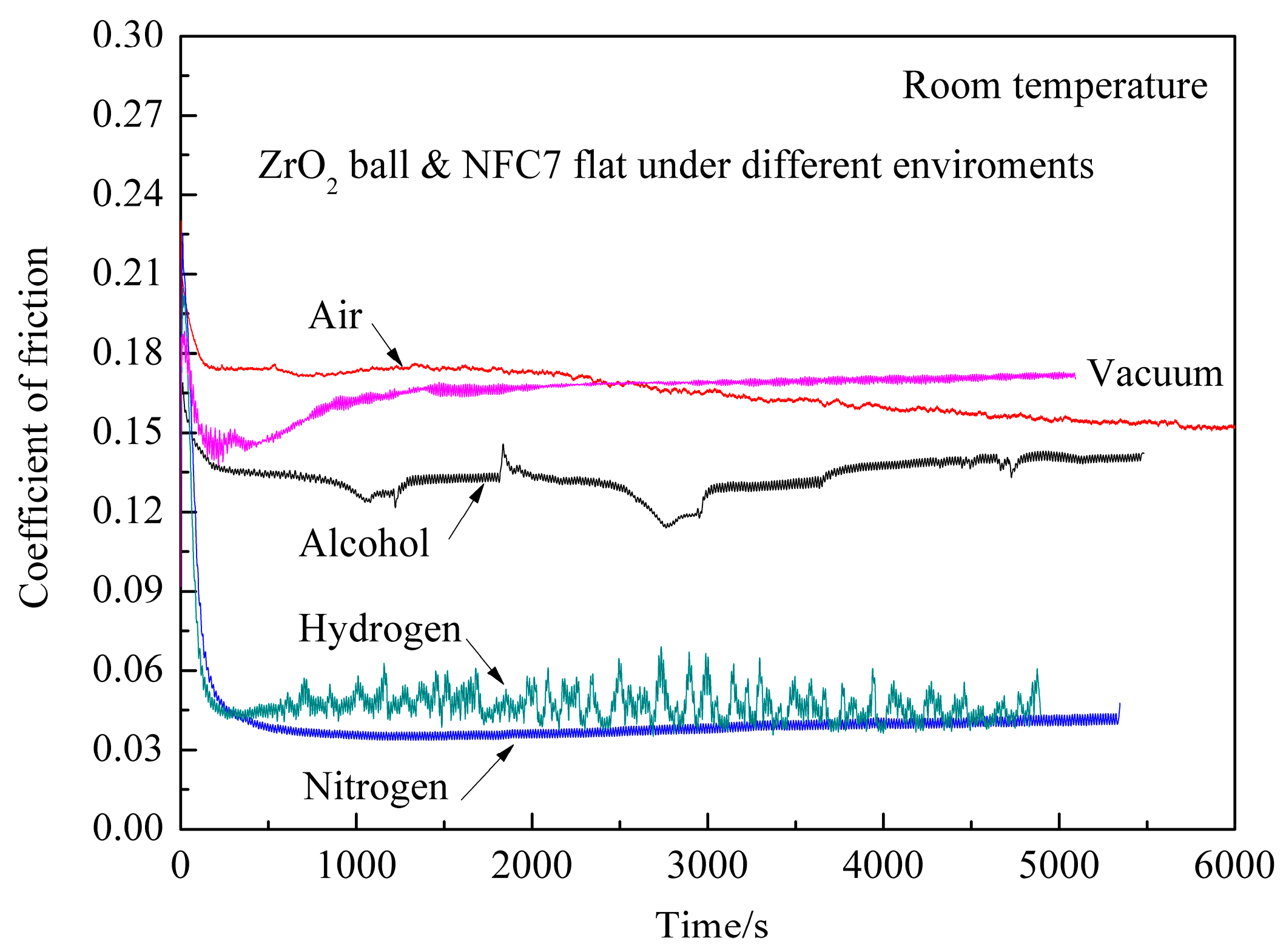
Figure 2 shows CoF of ZrO
2/DLC films with sliding time under various environments at room temperature. In ambient air, CoF is 0.08 at the initial stage, then increases rapidly to 0.2, and finally decreases to 0.16 with the increase of sliding time. It is interesting to find that with the increase of sliding time CoF is relatively stable, which is different from the typical CoF curve of DLC films in ambient air [
15]. This means that ZrO
2 as the friction mate material for DLC films is beneficial to the antifriction behavior of DLC films in ambient air. In dry nitrogen, CoF is 0.13 at the initial stage, then increases to 0.22 rapidly, and finally decreases to around 0.04 in subsequent tribotest. In dry hydrogen, CoF is 0.11 at the initial stage, then increases to 0.20 abruptly, and finally decreases. Average CoF is about 0.05 in hydrogen. In vacuum, CoF is 0.05 at the initial stage, then increases rapidly to 0.19, decreases to 0.14, and finally increases to around 0.17, which fluctuates typically in vacuum [
16]. The low and stable CoF indicates that this friction pair is applicable used to the vacuum environment. In alcohol environment, CoF is 0.15 at the initial stage, and then CoF fluctuates during the tribotest. It is concluded from the above experimental results that ZrO
2/DLC films exhibit excellent antifriction and stability behaviors under different environments at room temperature.
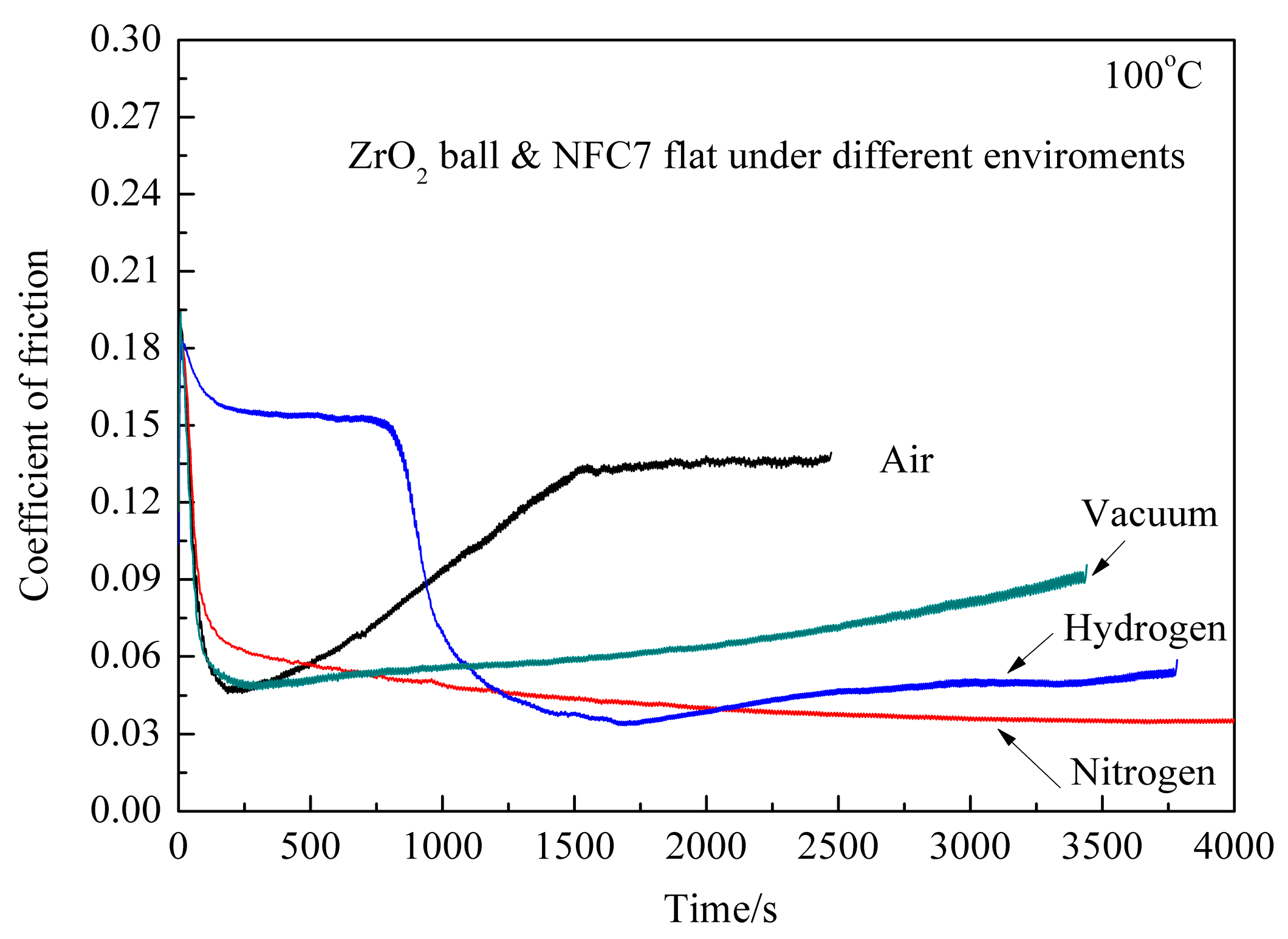
Figure 3 shows CoF of ZrO
2/DLC films with sliding time under different environments at the temperature of 100 °C. It is found from the experimental results that CoF is low at initial stage, then increases to the maximum value, decreases, and finally increase again with the increase of sliding time. In ambient air, CoF is 0.15 at the initial stage, then increases to 0.19, and finally CoF decreases to the minimum value of 0.04, which exhibits ultralow friction. Moreover, the CoF is lower than that at room temperature. It is interesting to find that the friction pair exhibits ultralow friction in ambient air. This also means that ZrO
2 is excellent friction mate material for DLC films in ambient air. In dry nitrogen, CoF is 0.11 at the initial stage, then increases to 0.18, and finally decreases slowly along with the sliding time. Average CoF is 0.04. In dry hydrogen, CoF is 0.09 at the initial stage, then increases to 0.19, and decreases to 0.03, and this ultralow friction value is maintained. The whole sliding process is divided into four stages: the running-in process, the low friction stage, the ultralow friction stage and at stable stage. The first fall of CoF from 0.19 to 0.15 happens at around 200 s, whereas the second dramatic decrease from 0.15 to about 0.03 happens at around 700 s, entering ultralow friction regime. The ultralow friction state in the present test is stable at about 500 s. Comparing with the tribotest results at room temperature, the stability of CoF is significantly improved. In vacuum, CoF is 0.10 at the initial stage, then increases to 0.19, decreases to 0.05, and finally increases slightly to 0.08. The low and stable CoF indicates that the friction pair exhibits excellent antifriction behaviors in vacuum environment at the temperature of 100 °C. It is concluded that the maximum and minimum values of CoF are both lower than those at room temperature, and the stability of the antifriction behavior is improved at the temperature of 100 °C.
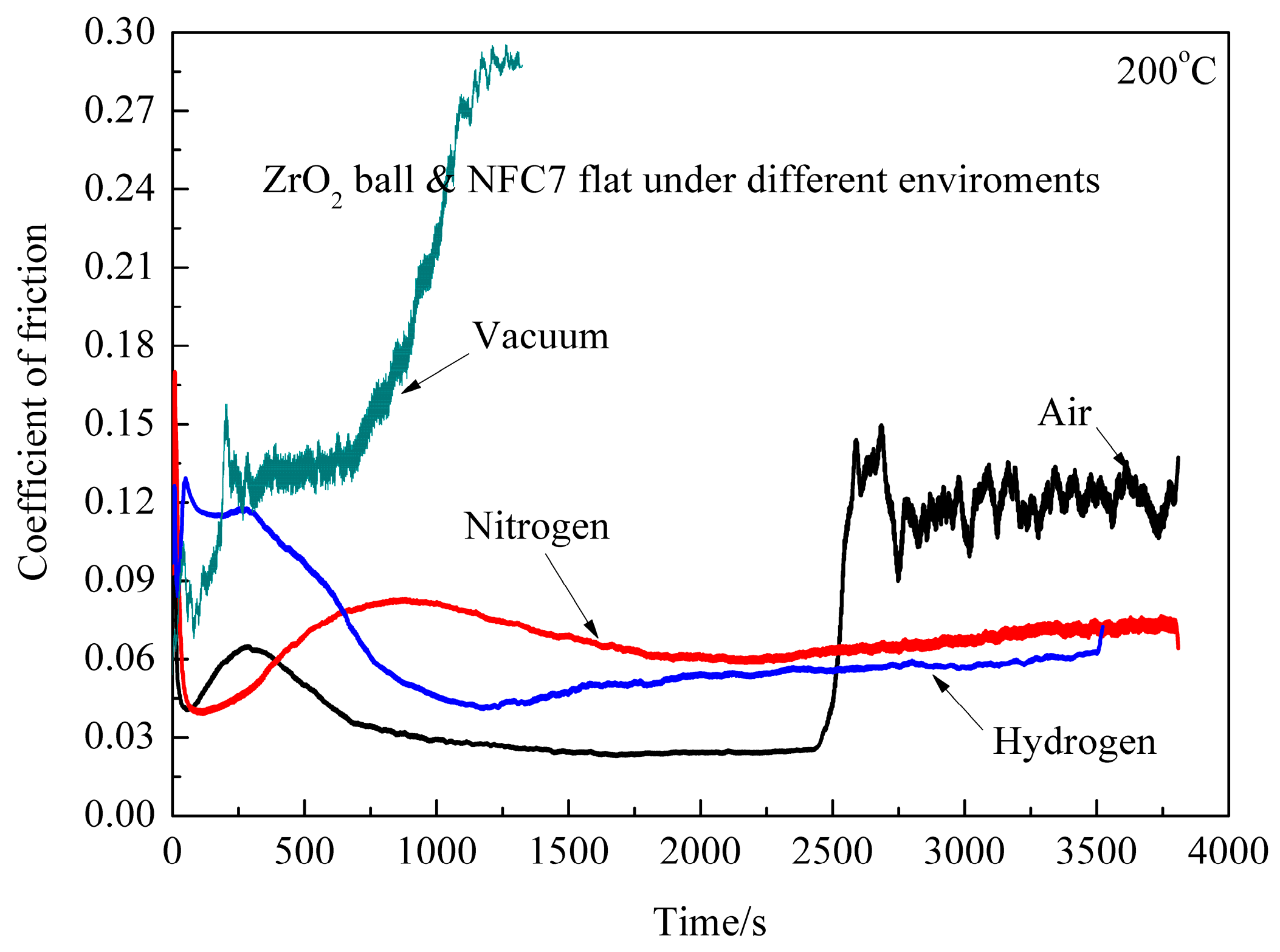
Figure 4 shows CoF of ZrO
2/DLC films with sliding time under different environments at the temperature of 200 °C. It is found that the CoF curve is different from that at room temperature and the temperature of 100 °C. In ambient air, CoF is 0.11 at the initial stage, then decreases to 0.06, and finally reaches CoF 0.02, which exhibits ultralow friction. It is shown that ZrO
2/DLC films exhibit ultralow friction in ambient air. In dry nitrogen, CoF is 0.09 at the initial stage, then increases rapidly to 0.17, decreases to 0.04 abruptly, and increases slowly along with the sliding time. Average CoF is about 0.07. In dry hydrogen, CoF is 0.09 at the initial stage, then increases to 0.12, decreases to 0.05, and finally increases to around 0.06 along with the sliding time. The whole sliding process can also be divided into four stages, although it is not obvious. In vacuum, CoF is 0.09 at the initial stage, then increases to 0.3, which indicates that DLC films were failed. It is concluded that the friction pair exhibits excellent antifriction behavior in ambient air and hydrogen environment at the temperature of 200 °C.
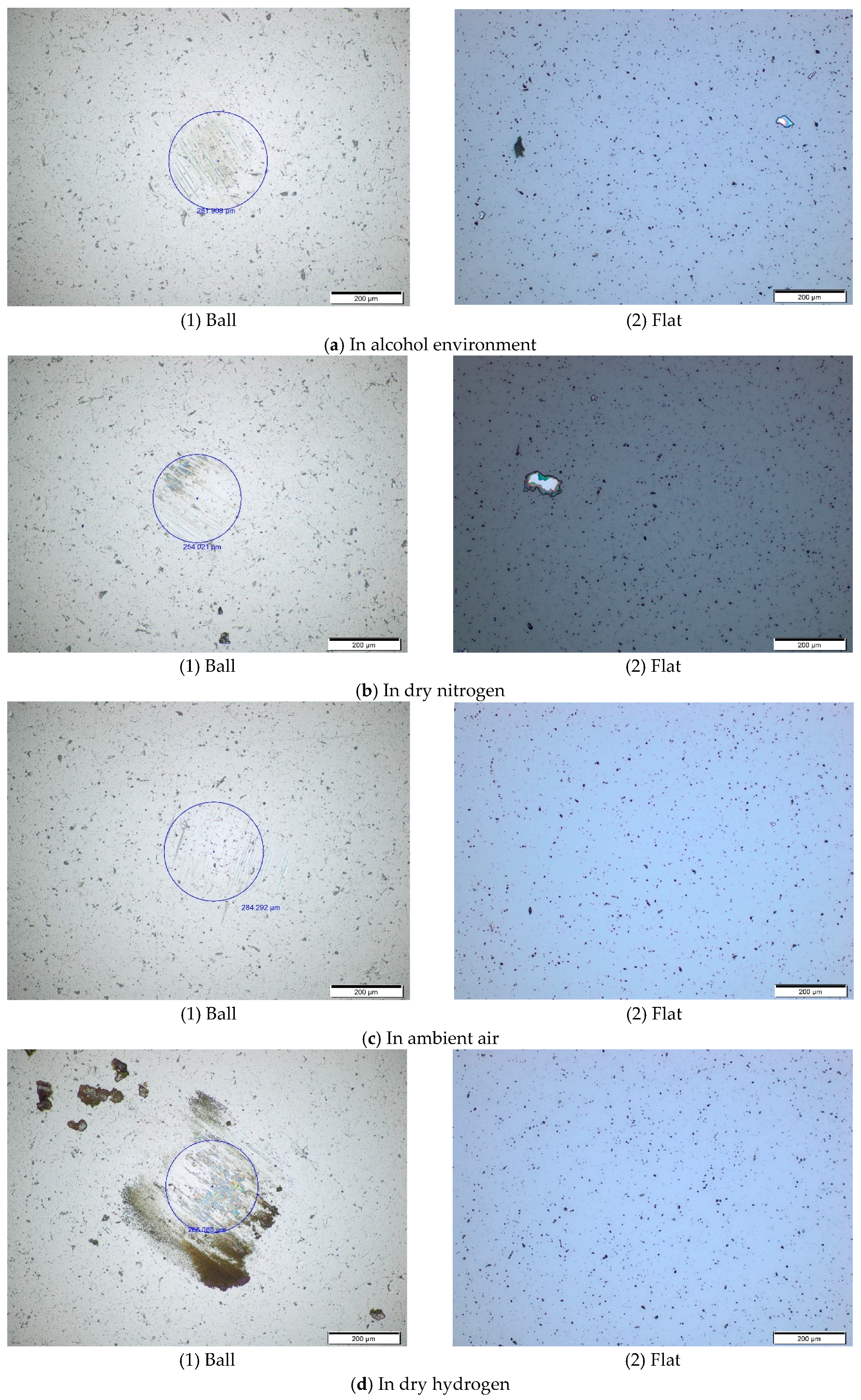
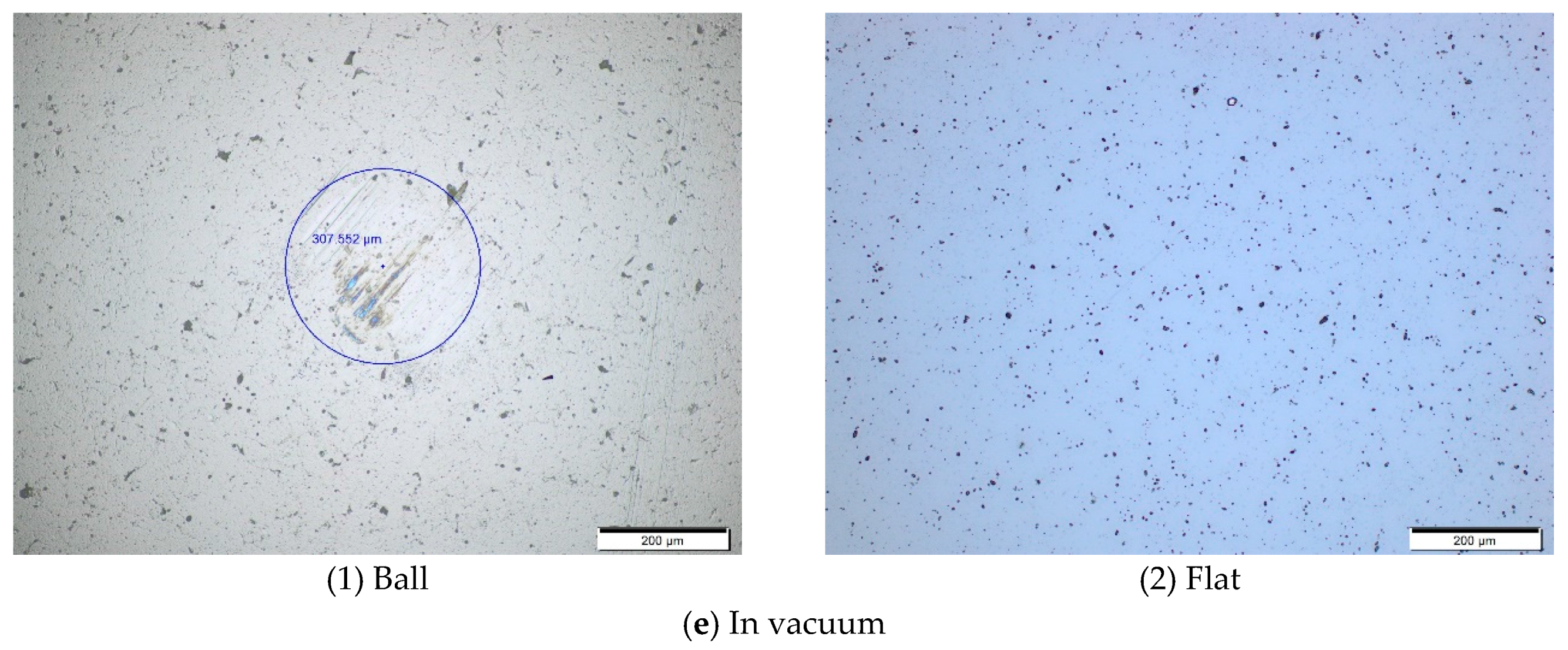
Figure 5,
Figure 6 and
Figure 7 show the worn surface topography of ball and flat under different temperatures and environments. It is found that there is no visible wear scar on flat from room temperature to 200 °C, whereas a wear scar with circle shape as well as transferred films and wear debris within or around the wear scar are found on the surface of ball. Therefore, we only provide the figures of ball, as shown in
Figure 6 and
Figure 7. For the tribotests at room temperature, there are obvious and deep plow grooves on the ball surface in alcohol environment, as shown in
Figure 5a. In dry nitrogen, there are not only plow grooves but also the color transferred from the films onto the worn surface of ball, as shown in
Figure 5b. In ambient air, there are only shallow plow grooves on the worn surface of the ball, as shown in
Figure 5c. In dry hydrogen, there are not only shallow plow grooves, but also large amounts of the color transferred from the films around the center of the wear scar, and even black wear debris around the wear scar on the ball surface, as shown in
Figure 5d. In vacuum, there are also shallow plow grooves and color transferred from the films within the wear scar, as shown in
Figure 5e.
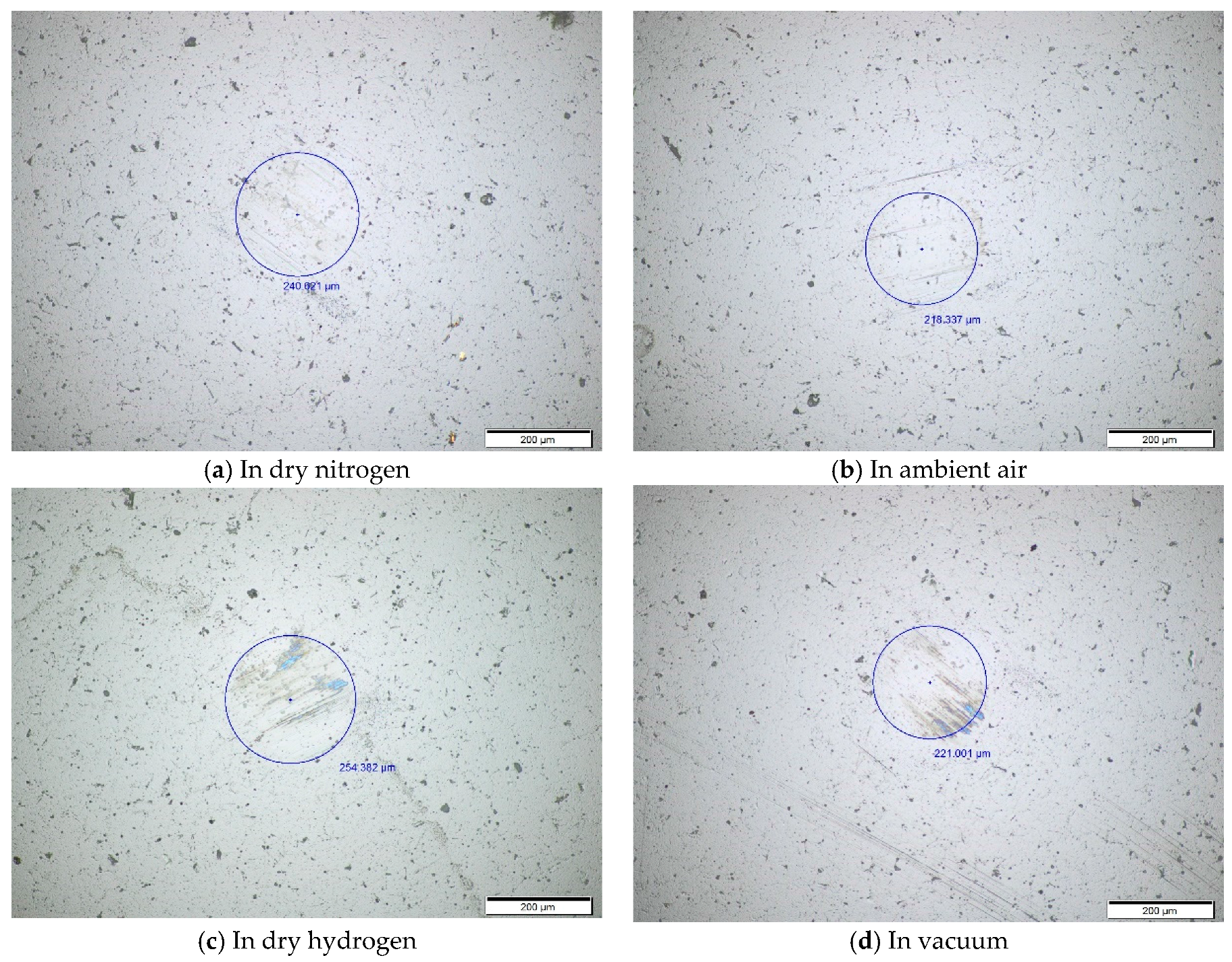
The worn surface topography of ball under different environments at the temperature of 100 °C is almost the same as that at room temperature. There are shallow plow grooves on the ball surface in ambient air and dry nitrogen, as shown in
Figure 6a,b. However, in dry hydrogen and vacuum, there are not only shallow plow grooves but also the color transferred from the films onto the worn surface of the ball, as shown in
Figure 6c,d.
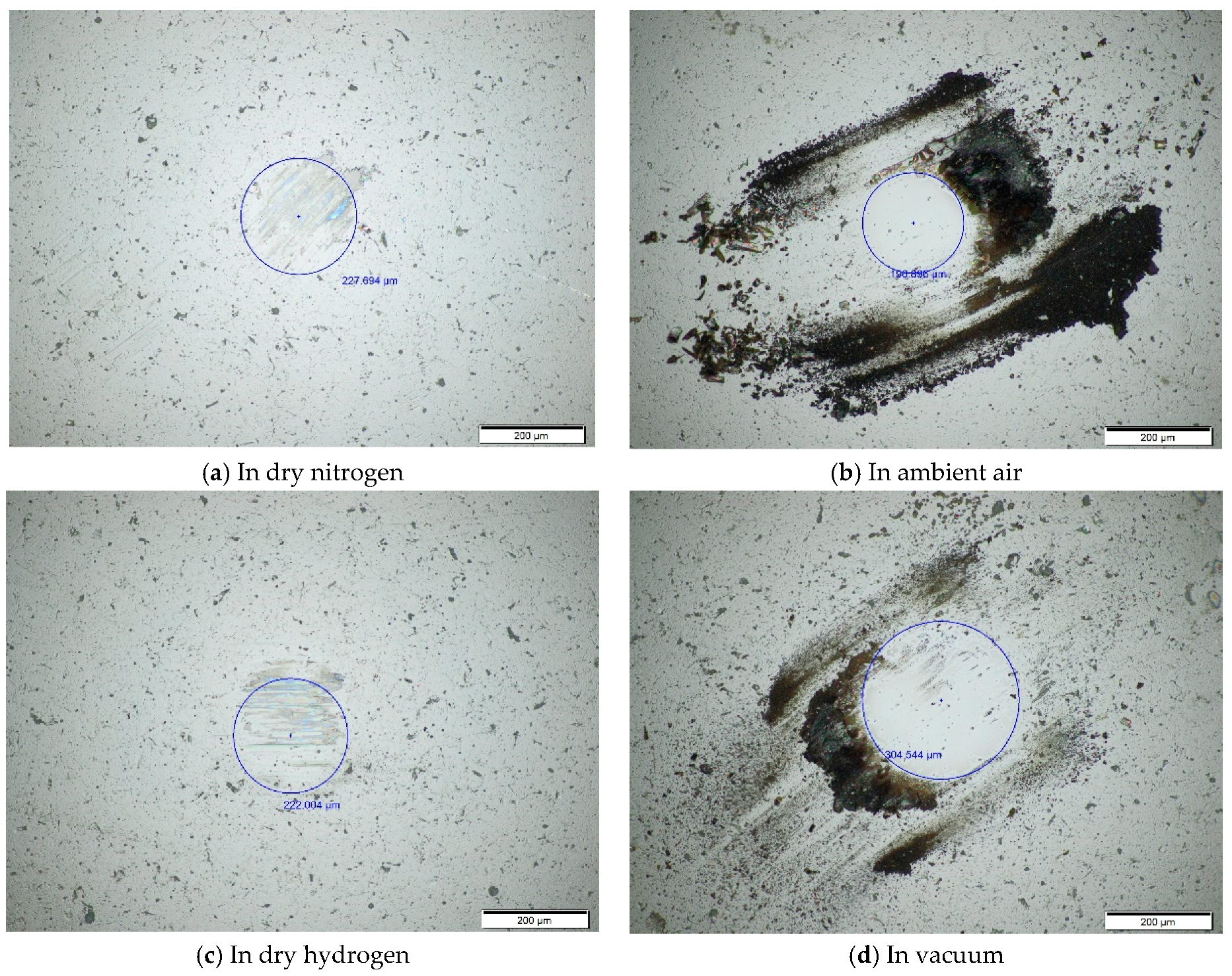
The worn surface topography of the ball at the temperature of 200 °C is slightly different from that at room temperature and 100 °C. In dry nitrogen, there are shallow grooves and the color transferred from the films onto the worn surface of the ball, as shown in
Figure 7a. In ambient air, the wear scar is very smooth and there is no plow groove, however, there is black wear debris outside the wear scar on the worn surface of the ball, as shown in
Figure 7b. In ambient air, CoF at the stable stage is as ultralow as 0.02, and then CoF increases to around 0.13. The results show that ZrO
2/DLC films exhibit ultralow friction behavior at the stable stage. It is speculated that ultralow friction of the friction pair is attributed to the transferred films, which is formed during sliding. However, the transferred films are worn quickly out in the subsequent tribotests. In dry hydrogen, there are not only shallow plow grooves but also the color transferred from the films onto the wear scar of the ball surface, as shown in
Figure 7c. In vacuum, there is black wear debris around the edge of the wear scar on the worn surface the ball, as shown in
Figure 7d.
Table 1 shows CoF of ZrO
2/DLC films and wear rate of ZrO
2 ball, respectively. Numbers represent the friction testing conditions of the testing temperature and environment gas, respectively.
Figure 8 shows CoF of ZrO
2/DLC films under different temperatures and environments. The blue circle in the curve represents CoF of the friction pair under different temperatures and environments. The dark yellow block in the curve represents the wear rate of the friction pair under different temperatures and environments. Numbers 1, 2 and 3 represent the temperatures of room temperature, 100 °C and 200 °C in ambient air, respectively. Numbers of 4, 5 and 6 represent the temperatures of room temperature, 100 °C and 200 °C in nitrogen environment, respectively. From room temperature to 200 °C, CoF decreases with the increase of temperatures in ambient air. CoF is almost the same with the increase of temperatures in dry nitrogen and hydrogen environment. CoF is as low as 0.08 at the temperature of 100 °C, and high at room temperature and the temperature of 200 °C in vacuum. The tendency of the wear rate is almost the same as that of CoF. It is found from the experimental results that the friction pair exhibits high wear-resistance behaviors, even though CoF increases slowly with the increase of the temperatures in nitrogen and hydrogen environment. The wear rate presented here is relatively low because there is no coating on the ball before the tribotests. It is found that CoF and the wear rate of ZrO
2/DLC films are ultralow and stable in dry hydrogen because the transferred films are produced on the worn surface on the ball side, especially at the temperature of 100 °C; therefore, it is necessary to measure the microstructure of the transferred films on the worn surface of the ball, analyze the tribochemical reaction and the formation of the transferred films during sliding and understand the possible ultralow friction mechanism of ZrO
2/DLC films.
For a good understanding of the temperatures and environments influencing the tribological process, the microstructure of the worn surface of the disc and ball is investigated before and after the tribotests by Raman spectroscopy. It is helpful to understand how ZrO
2/DLC films behave within the contact area. The tribochemistry reactions in this tribological system unavoidably take place under high temperature and friction heating conditions. Raman spectroscopy would add strong evidence to the tribochemistry mechanism of the friction pair.
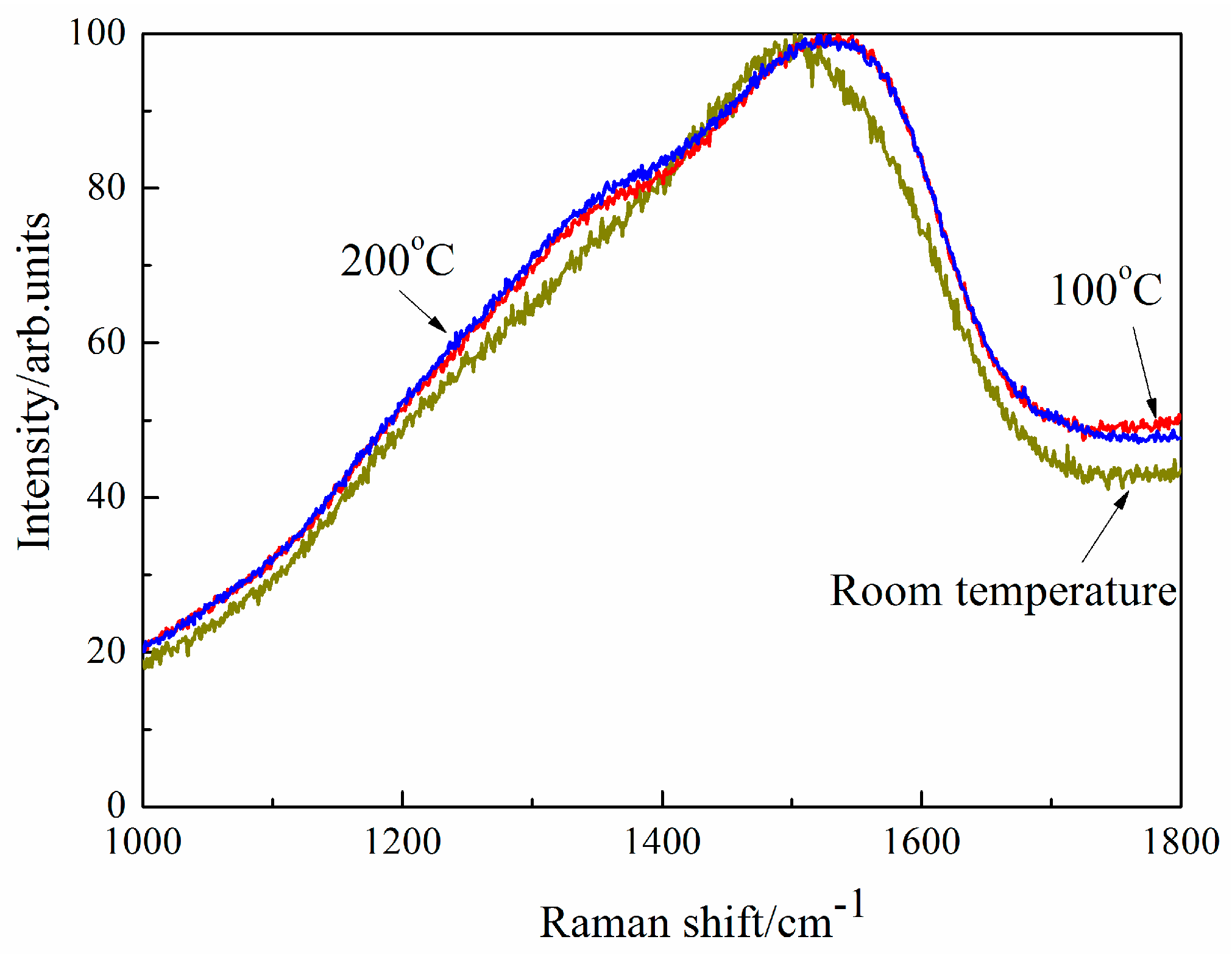
Figure 9 shows Raman spectra of DLC films on the flat under different temperatures. From room temperature to 200 °C, Raman spectroscopes of DLC films on the flat are almost the same original DLC films. There is a small difference in the band around 1507 cm
−1 at room temperature and the temperatures of 100 °C and this peak moves high wave shift slightly, and the peak of DLC films becomes obvious in the band around 1360 cm
−1 at the temperatures of 100 °C, which means there is slightly graphitization in DLC films. Raman spectroscopes of DLC films on the flat are almost the same at the temperatures of 100 °C and 200 °C.
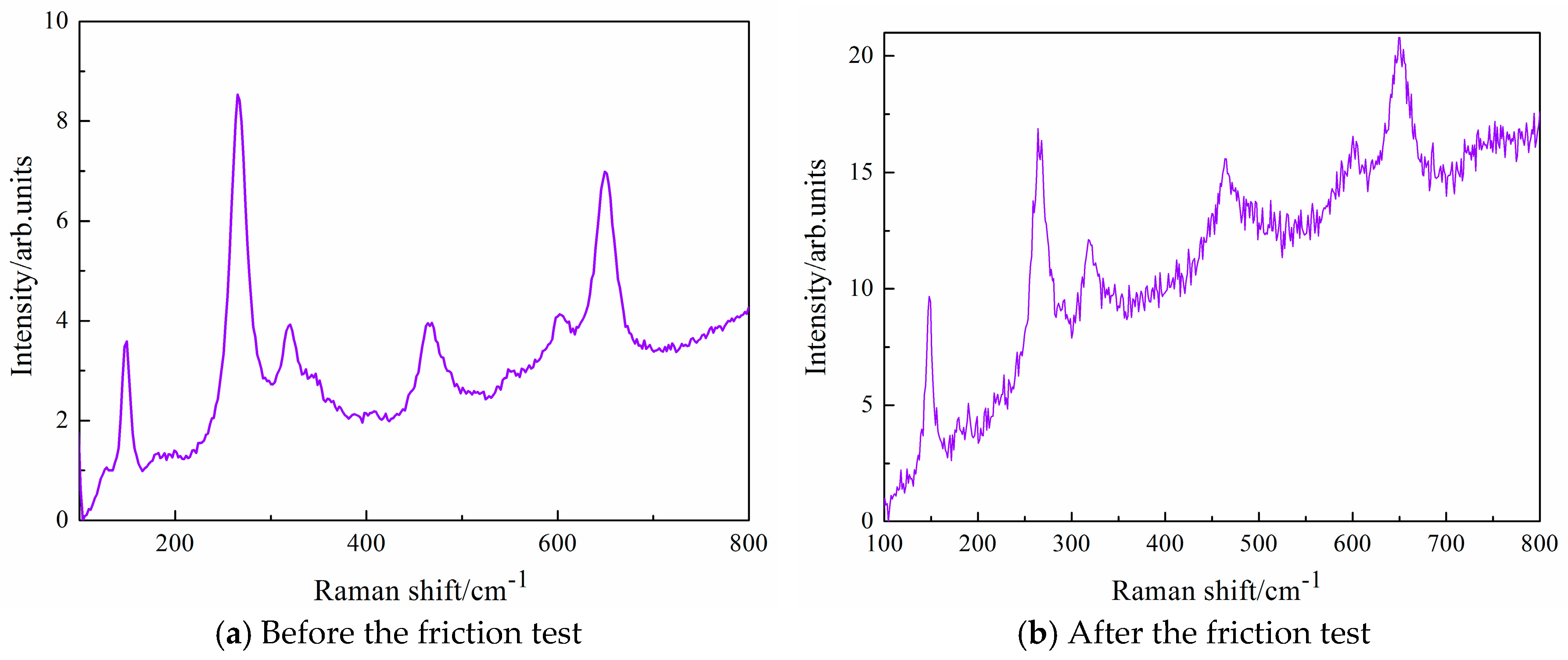
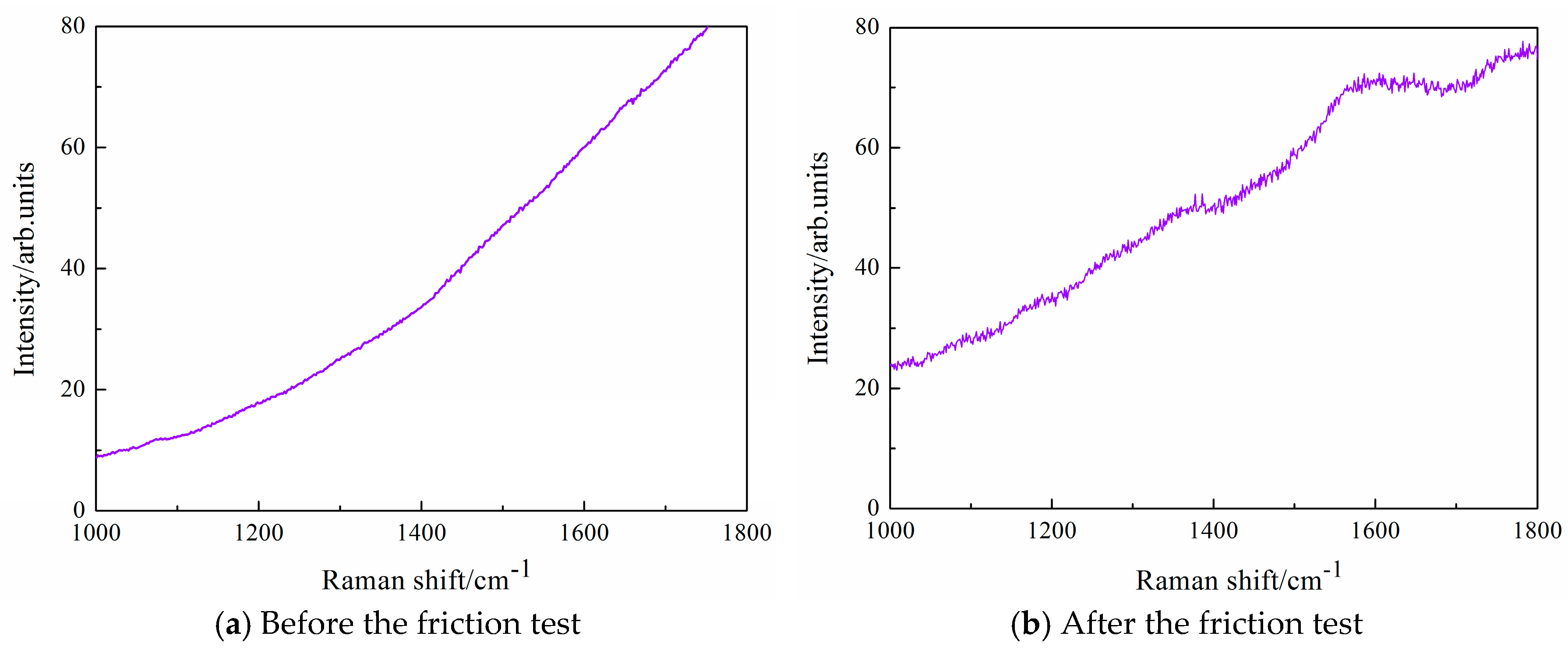
Figure 10 and
Figure 11 shows Raman spectroscopy of ZrO
2 ball in dry hydrogen at the temperatures of 100 °C. Raman spectroscopy of ZrO
2 ball is shown because this friction pair exhibits ultralow friction and high wear-resistance in dry hydrogen at the temperatures of 100 °C. Raman spectrum of ZrO
2 ball before the tribotest exhibits bands at 149 cm
−1, 265 cm
−1, 320 cm
−1, 468 cm
−1, 602 cm
−1 and 649 cm
−1, which are attributed to the tetragonal phase of ZrO
2 [
17]. The peak at 468 cm
−1 is the characteristic band of a cubic fluorite structure. There are bands at 148 cm
−1, 268 cm
−1, 318 cm
−1, 464 cm
−1, 600 cm
−1, 649 cm
−1, 1377 cm
−1 and 1579 cm
−1 for ZrO
2 ball after the friction test, as shown in
Figure 10 and
Figure 11. Raman spectrum of the worn surface of ZrO
2 ball contains five of the eight bands attributed to the tetragonal phase of zirconia, however, shift towards lower values (148 cm
−1, 268 cm
−1, 318 cm
−1, 464 cm
−1 and 600 cm
−1) comparing with the untested ZrO
2 ball. The peak at 464 cm
−1 is also the characteristic band of a cubic fluorite structure, whereas the peaks at 268 cm
−1 and 320 cm
−1 can be assigned to the tetragonal phase. It is found that there are two new obvious 1377 cm
−1 and 1579 cm
−1 bands, which are typical peaks of D and G bands of amorphous carbon films, respectively [
18]. This means that amorphous carbon films are formed on the contact surface of ball during sliding. The experimental results show that DLC films are transferred easily to ZrO
2 ball during the tribological process. DLC films slide against the transferred films that develop on the surface of the ceramic ball counterface, which results in the ultralow friction of ZrO
2/DLC films. The transfer films improve the tribological properties of the friction system.
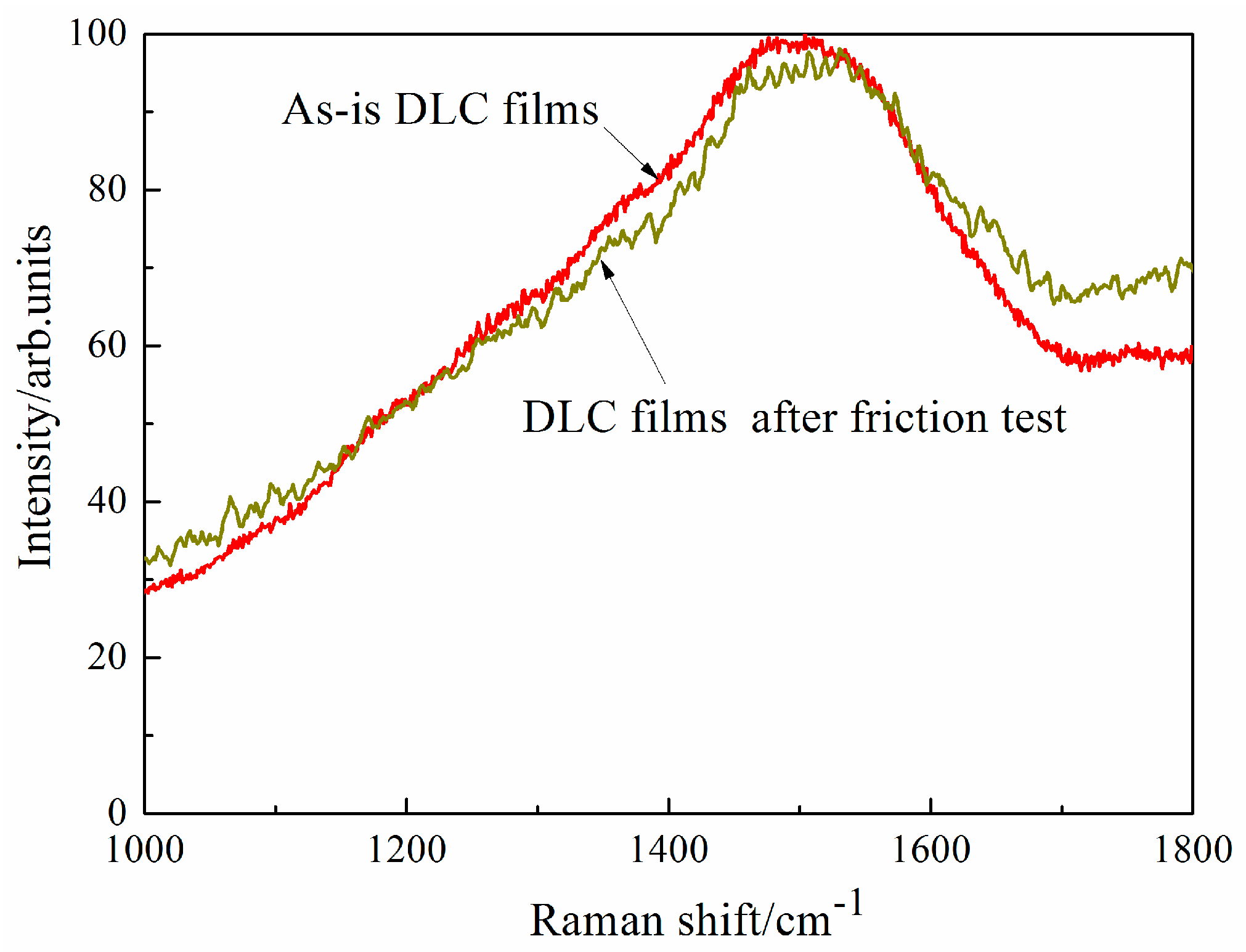
Figure 12 shows Raman spectroscopy of DLC films on flat before and after the tribotest test in dry hydrogen at 100 °C. There is no large different between as-is DLC films and DLC films after the friction test, however, the luminescence (in the range of 1600 cm
−1 and 1800 cm
−1) of DLC films after the friction test is higher than that of as-is DLC films, which indicates that there is more hydrogen in DLC films after the tribotest in hydrogen. As shown in
Figure 9, the same phenomena in DLC films is observed at 200 °C, which is helpful to improve the antifriction and anti-wear behaviors of ZrO
2/DLC films friction pair under various environments.
At room temperature, CoF is low and stable in the application of solid lubrication of DLC films. CoF is ultralow when the transferred films are formed on ball in dry nitrogen, hydrogen and even vacuum environment. CoF in dry nitrogen is lowest because the chemically passive surface cannot to be oxidized in inert gas environment. At 100 °C, there is the slight graphitization in DLC films (as shown in
Figure 9) under high friction heating and pressure simultaneously. DLC films are transferred to the contact surface of ball, and the friction interface of ball is partially covered by the transferred films. According to Raman spectroscopy analysis, the transferred films on the worn surface on ball are carbon-related materials, which result in low CoF compared with the tribotest in ambient air. At 200 °C, CoF is as ultralow as 0.02 in ambient air due to the tribochemistry reaction during sliding. However, CoF increases in dry nitrogen, hydrogen and vacuum at 200 °C. The experimental results show that the antifriction behavior of ZrO
2/DLC films is influenced significantly by the environment temperature.
From the tribotest results, it is found that the environment gas influences strongly the tribological properties of ZrO
2/DLC films. CoF is almost the same in dry nitrogen from room temperature to 200 °C. CoF is low and stable because the tribotests are performed in the inert environment and there are also the transferred films on the worn surface of ZrO
2 ball. However, CoF is unstable in vacuum in the absence of gas molecules. It is shown that the antifriction behavior of ZrO
2/DLC films is influenced by the environment gas. CoF decreases from 0.16 to 0.02 from room temperature to 200 °C in ambient air. However, the microstructure of DLC films is almost the same from room temperature to 200 °C according to Raman measurements (as shown in
Figure 9) and our previous work [
12]. It is surprising that there are almost no transferred films on the worn surface of ZrO
2 ball after the tribotests from room temperature to 200 °C. Therefore, there should be another possible factor influencing the antifriction behavior of ZrO
2/DLC films in ambient air. It is well known that hydrogen plays an important role in the shielding action in DLC films. CoF is almost the same as 0.05 in dry hydrogen from room temperature to 200 °C. It is also found that there are the transferred films on the worn surface of ZrO
2 ball in dry hydrogen.
Like the friction behavior, the wear resistance behavior follows the tendency. The lowest wear rate of ZrO
2 ball in ambient air at room temperature is 5.8% comparing to the wear rate of the same friction pair in vacuum at the temperature of 200 °C. The wear rate of ZrO
2 ball under different environments is very low except for the tribotests in vacuum at 200 °C. It is also found that there is no visible wear of DLC films on disc. The ultralow wear rate shows that the transferred films between the two contact surfaces are composed of low strength atomic carbon interlayer [
10].
Many factors such as temperature, environment and even tribochemistry reaction in the present case are involved. The synergism of the passive behaviors of DLC films and the transferred films on the worn surface of ZrO2 ball is beneficial to ultralow friction under different environments and temperatures. The di-hydrated carbon atoms on DLC films surfaces are expected to provide the shielding of chemical passivation. Such repulsive forces at sliding interface may act the weak van der Waals attractions. The shielding effect has positive implications for ultralow friction behavior. Overall, these experiments demonstrate that hydrogen has significant effects on the antifriction and anti-wear behaviors of the tribological system. The transferred films on ZrO2 ball are beneficial to low and stable friction in our work. However, we cannot explain why CoF is super low where there are no transferred films on the worn surface of ZrO2 ball at 200 °C and there are large amounts of black wear debris around the worn surface in ambient air.
It is concluded from the experimental results that Zr element in ZrO
2 ball is significantly beneficial to improve the antifriction and anti-abrasive behaviors of the friction pair and the formation of the transferred films on the worn surface of ZrO
2 ball under various environments during sliding. There is the tribochemical reaction producing a transferred layer that covers on the worn region of ZrO
2 ball because there are large amounts of black wear debris from the chemical reaction products. It is assumed that there exists the transferred films on ZrO
2 ball at the initial stage, but thin transferred films are easily worn out during sliding in ambient air. According to the experimental results and Raman analysis of the friction system, the transferred films play important roles in the antifriction and anti-abrasive behaviors of ZrO
2/DLC films. The transferred layer produced by the tribochemical reaction can achieve ultralow CoF, which is in accordance with the friction results of ceramic material in ambient air. The tribochemistry reactions are unavoidable under high temperature and friction heating conditions in ambient air. It is well known that Zr could be reacted with water molecular under high temperature [
17]. It is the hydrothermal metal oxidation for ZrO
2 under the environmental temperatures and frictional heating in ambient air [
19]. The possible oxidation reactions during sliding are listed as shown in Equation (3).
Zr is derived from the wear debris on ZrO
2 ball during the tribotests and it may react chemically with water molecular to form ZrO
2 and liberate hydrogen. Firstly, Zr is oxidized to ZrO
2 and ZrH
x, and then ZrH
x disappeared and ZrO
2 and hydrogen were produced finally. Equation (3) shows the chemical reaction products of ZrO
2 and hydrogen according to hydrothermal oxidation. The hydrothermal oxidation involves water molecular, high pressure and high temperature. The hydrothermal oxidation of the friction pair would be happening especially under high temperature during sliding. CoF of ZrO
2/DLC films is 0.16 because the hydrothermal oxidation could not occur at room temperature. The hydrothermal oxidation takes place in the accumulation of the friction heating, and then CoF decreases slightly with the increase of sliding time at room temperature. CoF at the initial stage is high, causing high flash temperature, and the hydrothermal oxidation liberates hydrogen, which is beneficial to ultralow friction. CoF becomes low and stable when the hydrothermal oxidation reaches the balance. At 200 °C, the hydrothermal oxidation is obviously promoted. The liberated hydrogen due to hydrothermal oxidation is introduced to enter and terminate the contact interface of ZrO
2 ball and DLC films, which achieves ultralow and stable friction (0.02) in ambient air at 200 °C. Therefore, it is shown for DLC films that it is important to select the appropriate friction mate materials and the tribological conditions to build a tribological system with ultralow friction [
20]. It is well known that Zr is a good dehydrogenation catalyst. Zr from the wear debris or ZrO
2 are used as the dehydrogenation catalyst in this tribological system during sliding. Therefore, it is reasonable to speculate that the wear debris of DLC films is catalyzed and dehydrogenated, and producing black materials that are disturbed around the worn surface of ZrO
2 ball in ambient air, as shown in
Figure 7. This black wear debris on the worn surface on ball leads to high CoF at the end of tribotests in the absence of the transferred films during siding. The friction heating promotes the tribochemistry reaction, especially under high temperature, which explains why CoF is low and stable at the stable stage, and then becomes high at the end of tribotests in ambient air. The products of the tribochemical reaction affect ultralow friction behaviors of this tribological system. Hydrogen is probably to diffuse to the contact surface of DLC films in hydrogen environment, achieving low and stable friction. The elimination of the possibility of strong covalent at sliding interfaces as well as the excellent shielding of carbon atoms by di-hydration are the reasons to explain ultralow friction.