Barriers and Chemistry in a Bottle: Mechanisms in Today’s Oxygen Barriers for Tomorrow’s Materials
Abstract
:1. Introduction
2. Background
2.1. Oxidative Degradation Mechanisms
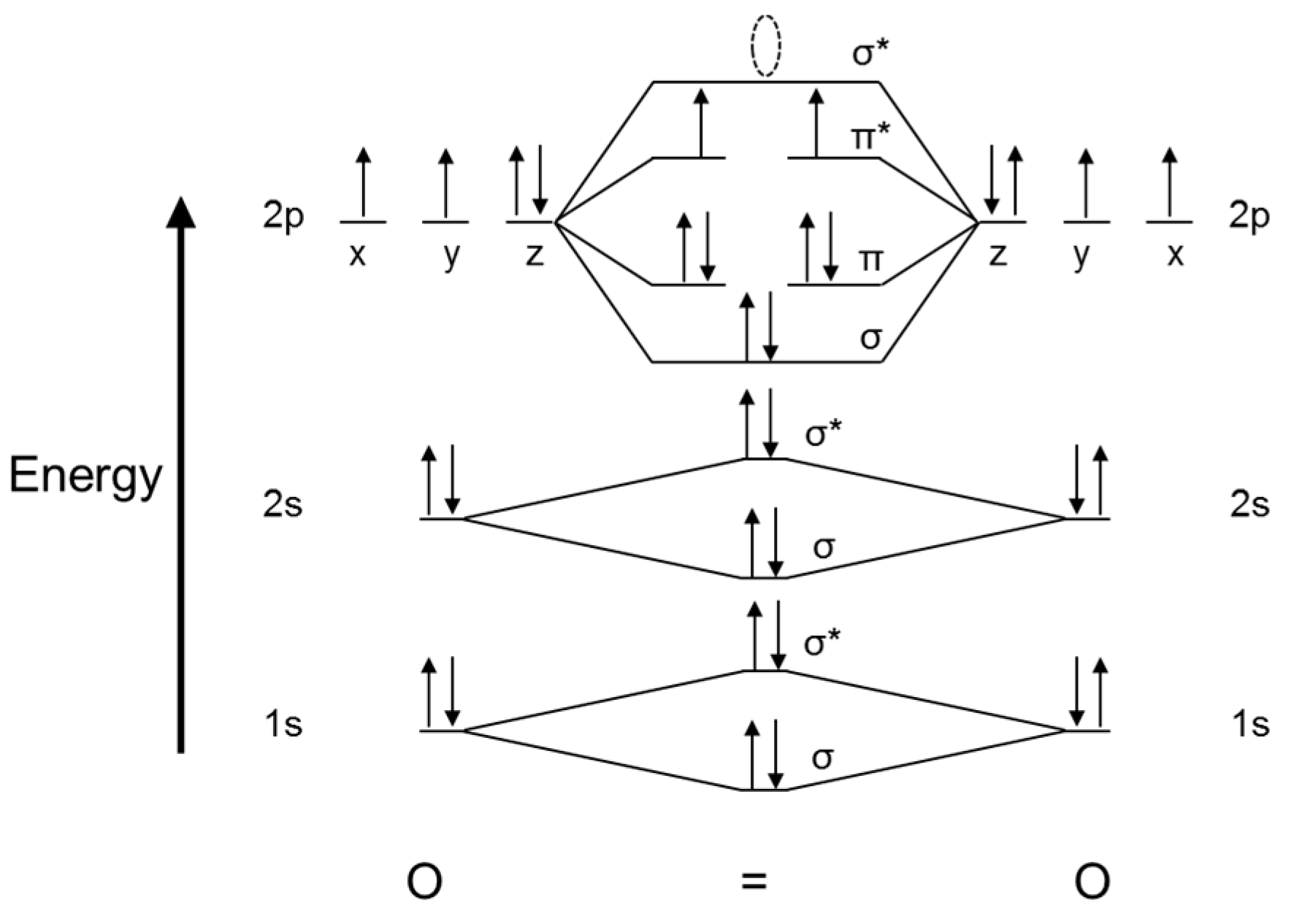
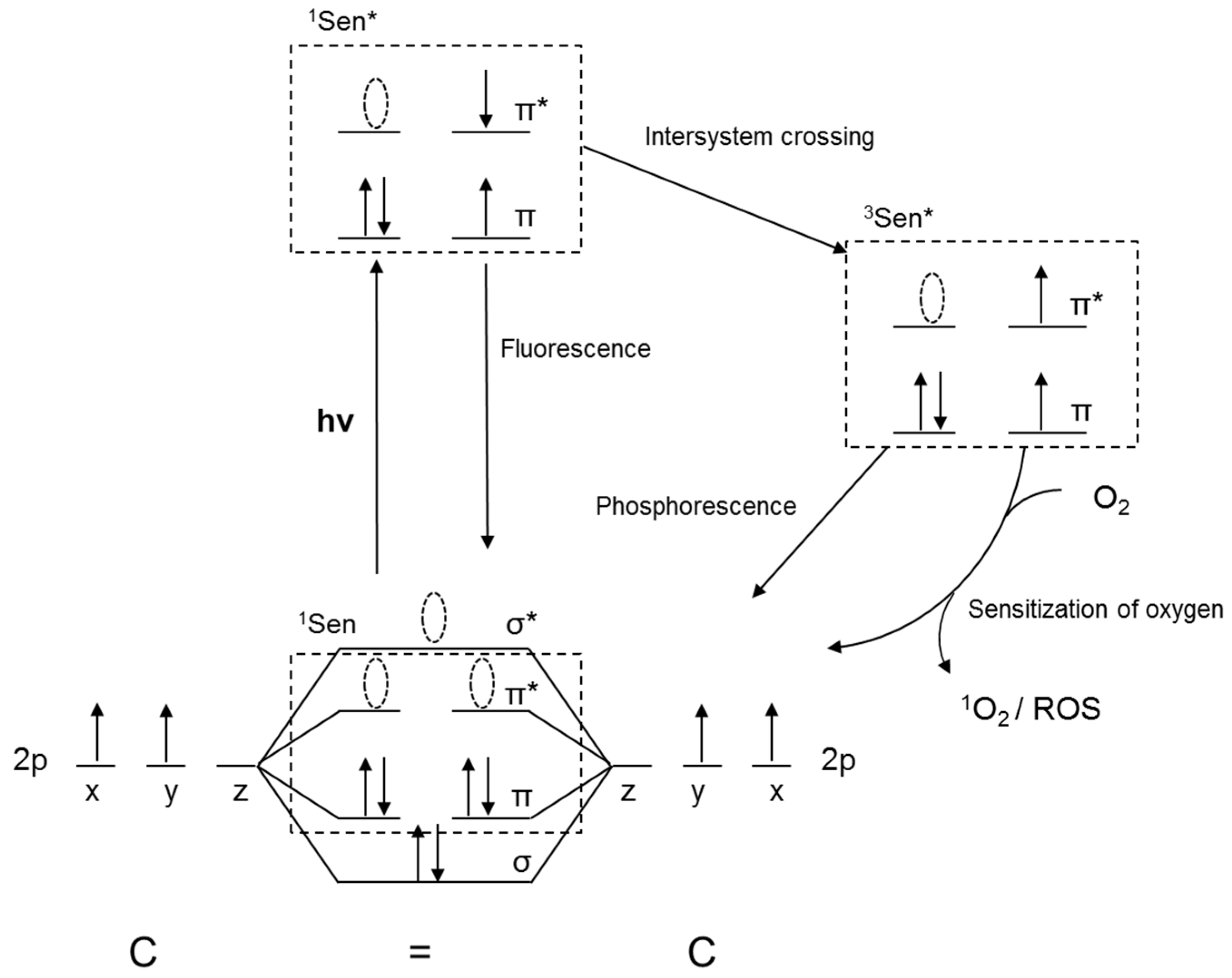
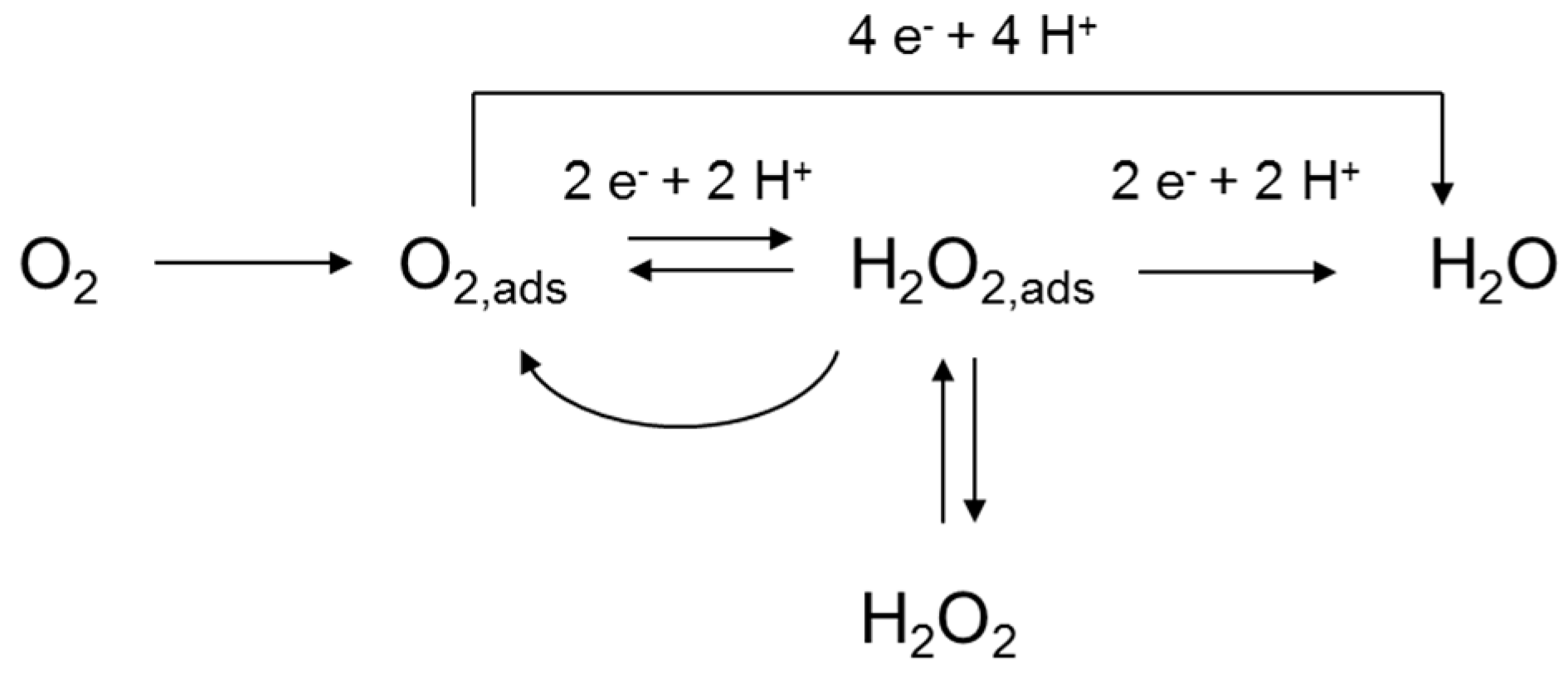
2.1.1. Chemistry of Oxygen
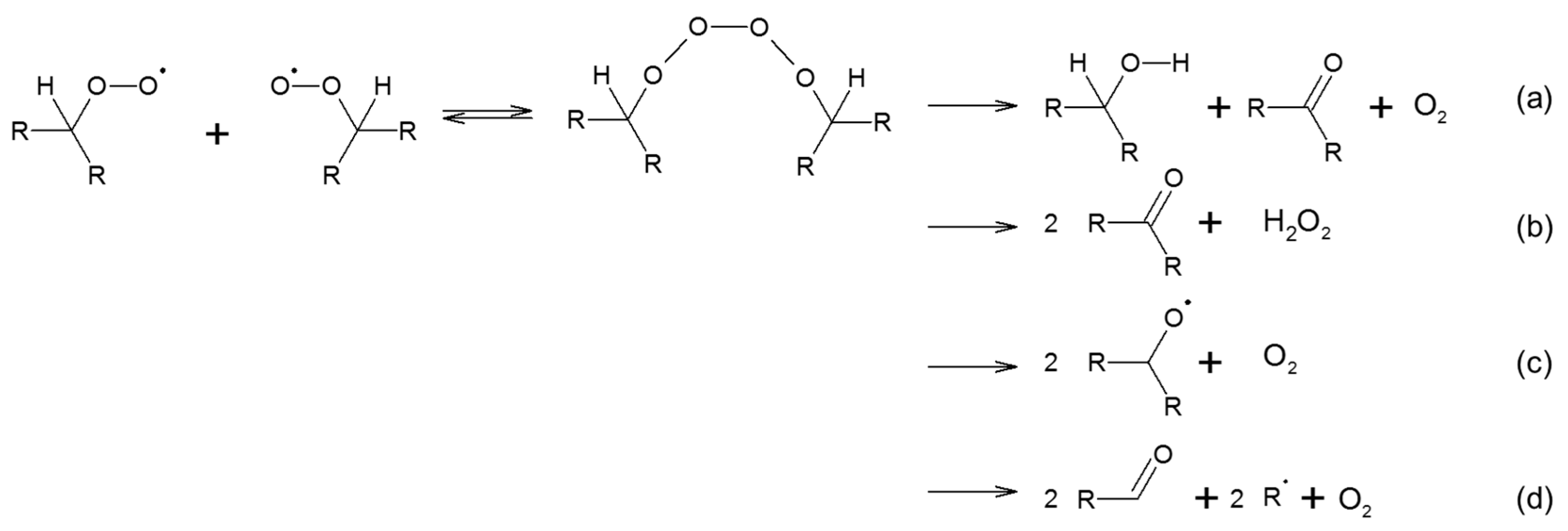
2.1.2. Oxidation of Unsaturated Compounds
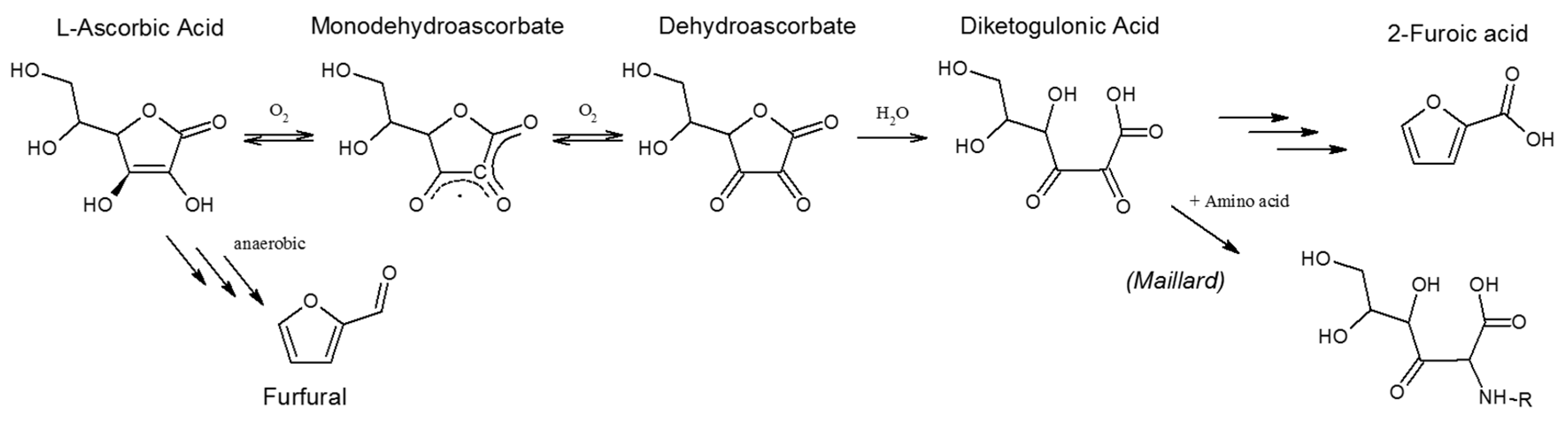
2.1.3. Oxidation of Alcoholic Compounds
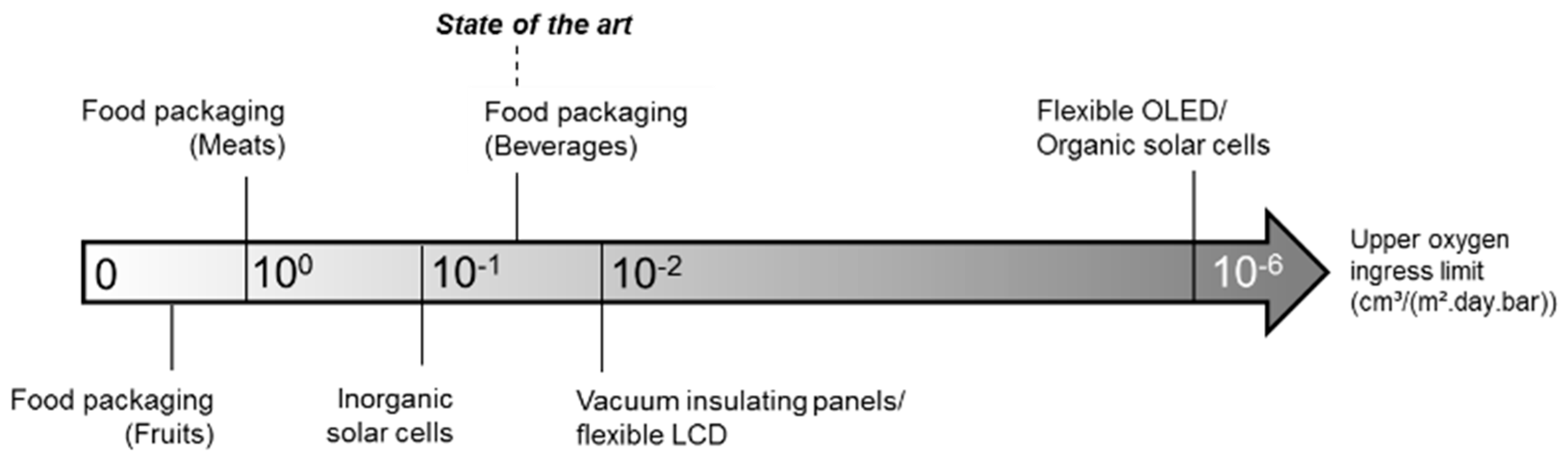
2.2. Measuring Oxygen in Packaging
- Oxygen transmission rate (OTR). The most common definition of OTR is the volume of O2 per package per day. The exact conditions need to be specified and preferably resemble the real storage environment of the packaging. Usually, the partial oxygen pressure is regulated to 0.21 atm, but temperature may vary between 20 °C and 25 °C and humidity between 0% and 100% RH [54,55].
- Permeability. This is usually defined in terms of Equation (3) [52].With P the permeability (most commonly expressed in units of cm3 mm m−2 day−1 atm−1). Herein, V is the volume of the gas permeating in time t (cm3 day−1), L is the thickness of the film or bottle wall (mm), A the surface area of the film over which permeation is measured (m2) and Δp the difference in partial pressure of the gas inside and outside the packaging (atm). The effect of temperature can then be quantified by a conventional Arrhenius equation, as shown in Equation (4) [52].With P the permeability at temperature T (K), P0 a pre-exponential factor (cm3 mm m−2 day−1 atm−1), Ep the activation energy for the permeability action (J mol−1) and R the ideal gas constant (J mol−1 K−1). The activation energy of oxygen in amorphous PET has been determined to be 37.7 × 103 J mol−1 [52].
- Barrier improvement factor (BIF). This is a unitless factor, usually used in a commercial setting, defined as the ratio between either OTR or permeability of the given barrier composition and a standard packaging [53].
2.2.1. Colorimetric Indicators
2.2.2. Potentiometric Detectors
2.2.3. Fluorescence Detectors
3. Passive Barriers
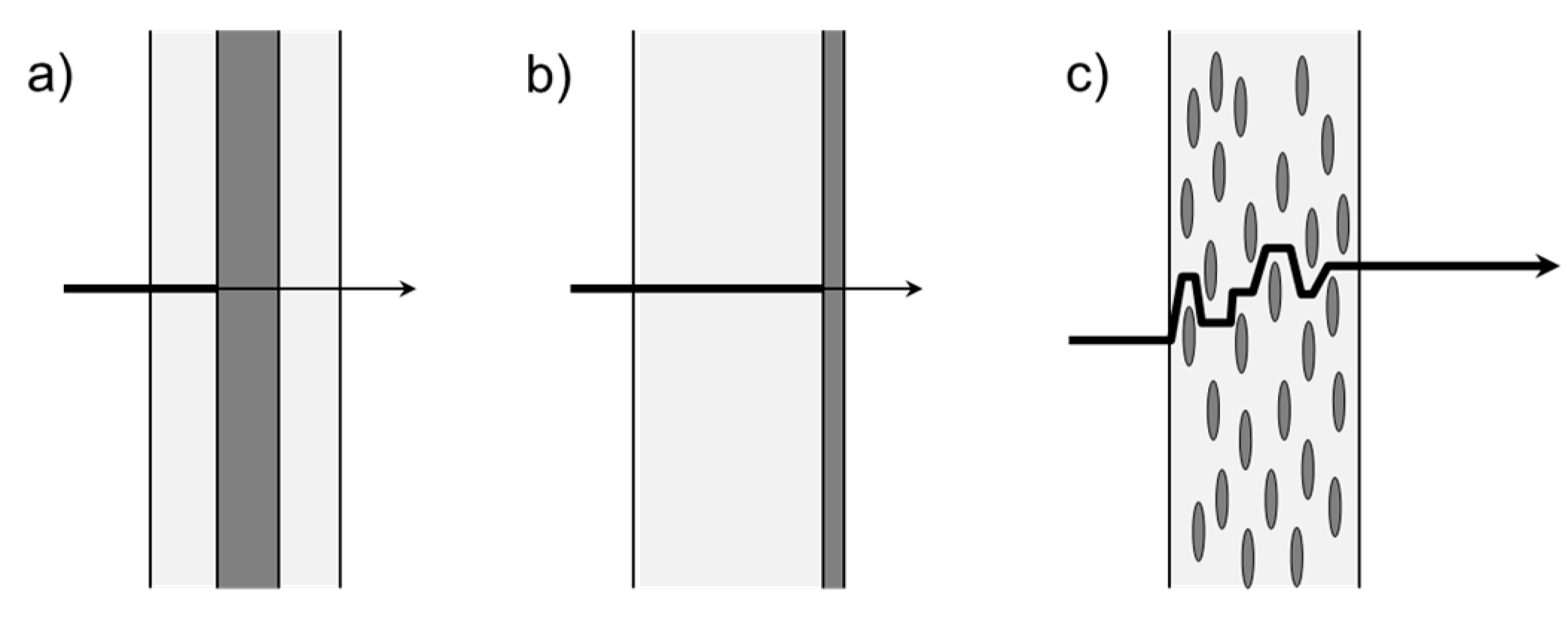
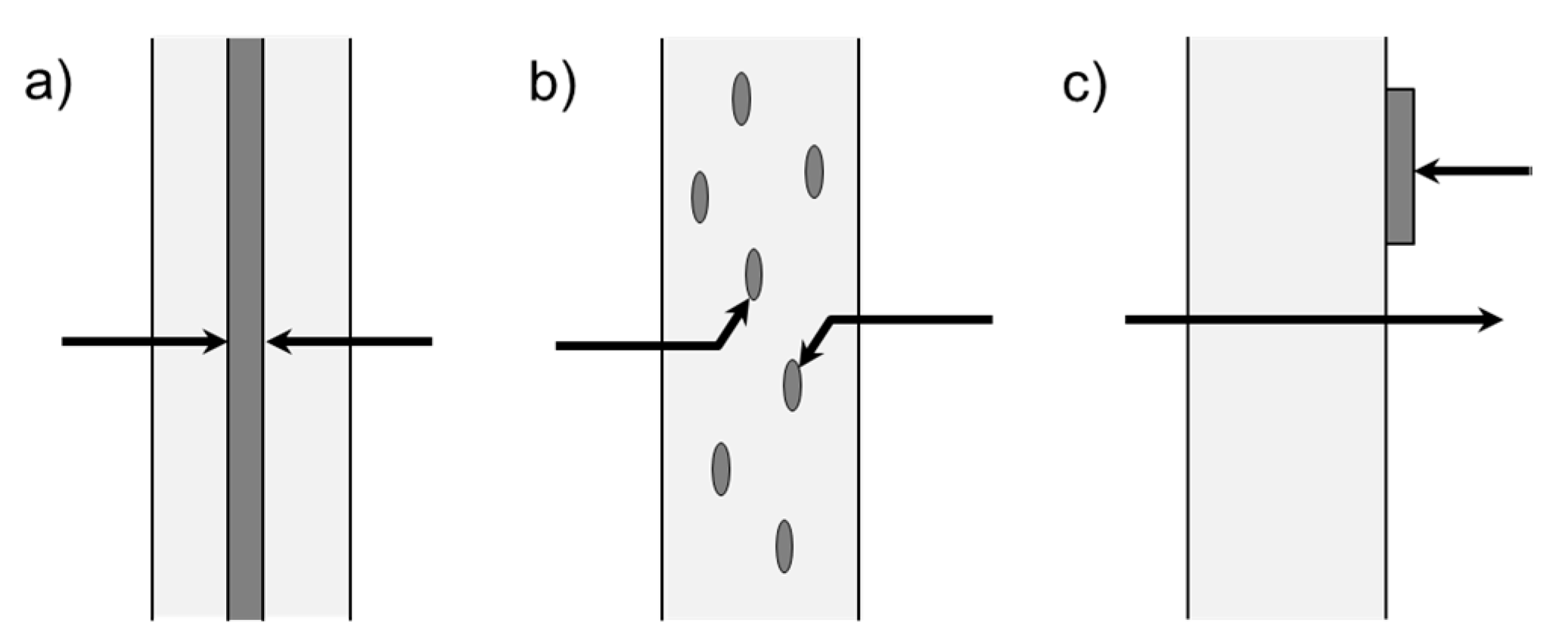
3.1. Multilayer
3.2. Coatings
3.3. Composites
4. Active Barriers
4.1. Antioxidants
4.2. Iron-Based
4.3. Pd/Pt-Based
4.4. Co-Based
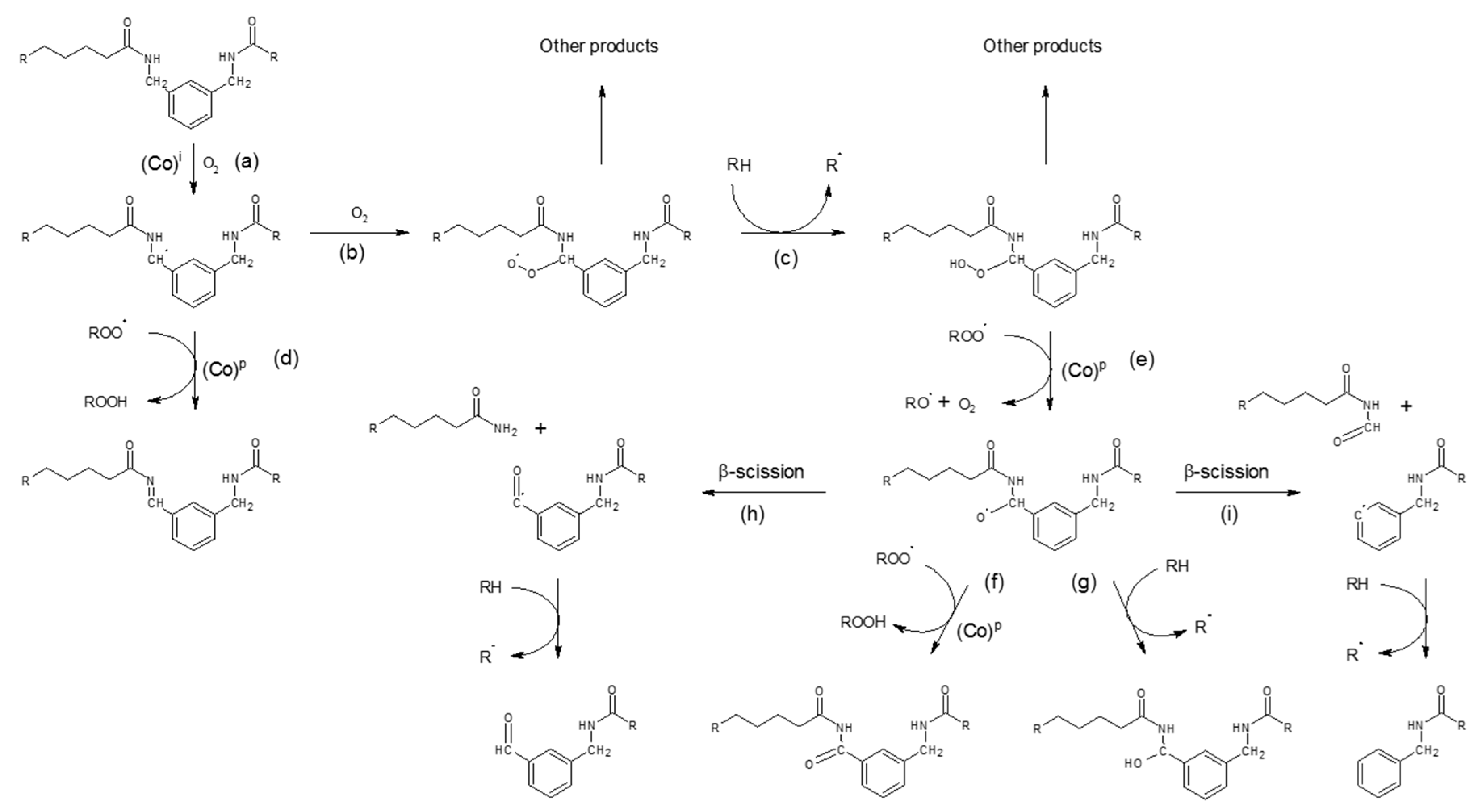
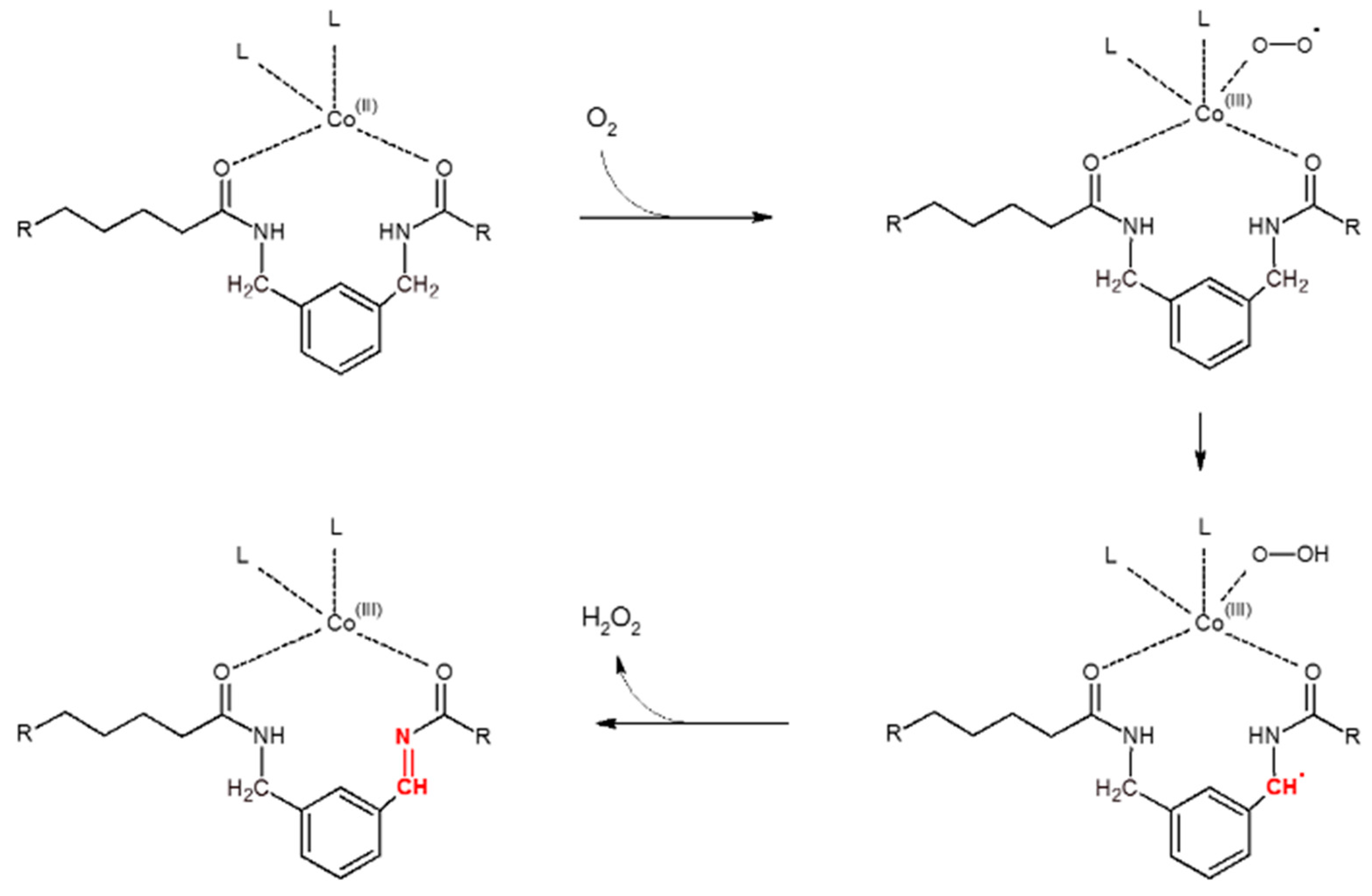
4.4.1. Polyamide Sacrificials
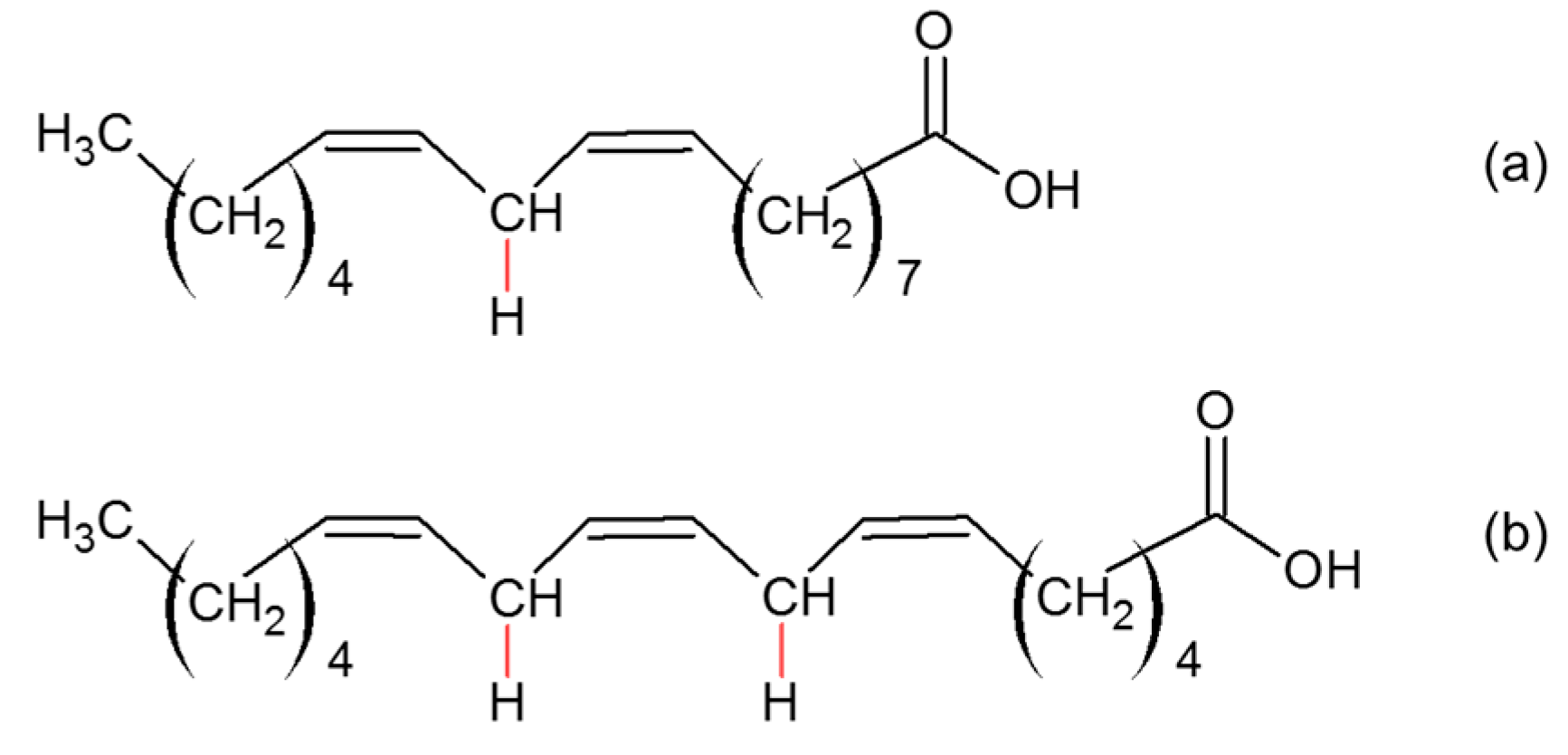
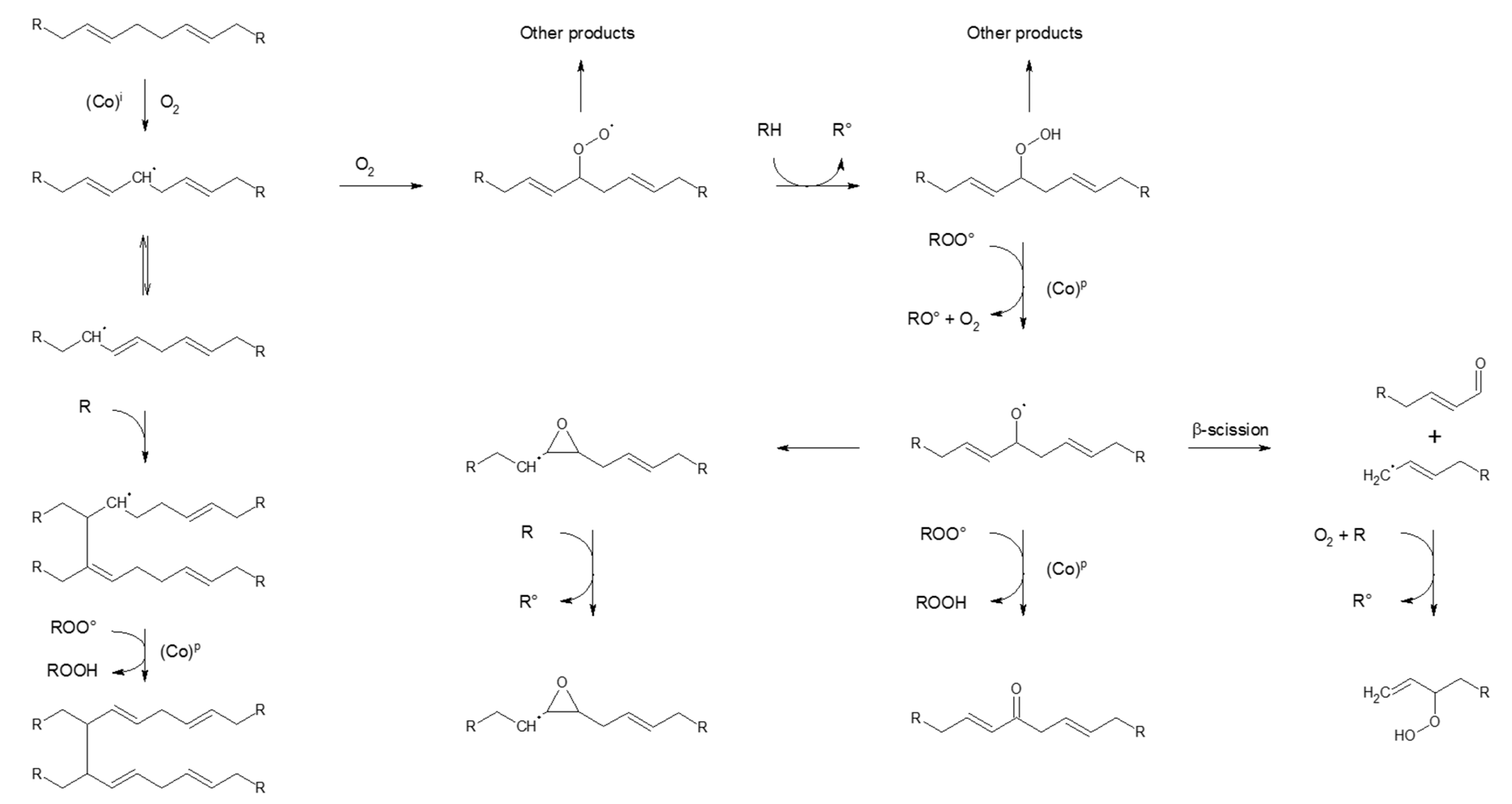
4.4.2. Unsaturated (Co-)Polymer Sacrificials
5. Prospectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amienyo, D.; Gujba, H.; Stichnothe, H.; Azapagic, A. Life cycle environmental impacts of carbonated soft drinks. Int. J. Life Cycle Assess. 2013, 18, 77–92. [Google Scholar] [CrossRef]
- Accorsi, R.; Cascini, A.; Ferrari, E.; Manzini, R.; Pareschi, A.; Versari, L. Life cycle assessment of an extra-virgin olive oil supply chain. In Proceedings of the 18th Summer School “Francesco Turco”—Industrial Mechanical Plants, Senigallia, Italy, 11–13 September 2013; pp. 172–178. [Google Scholar]
- Humbert, S.; Rossi, V.; Margni, M.; Jolliet, O.; Loerincik, Y. Life cycle assessment of two baby food packaging alternatives: Glass jars vs. plastic pots. Int. J. Life Cycle Assess. 2009, 14, 95–106. [Google Scholar] [CrossRef]
- Lange, J.; Wyser, Y. Recent innovations in barrier technologies for plastic packaging—A review. Packag. Technol. Sci. 2003, 16, 149–158. [Google Scholar] [CrossRef]
- Vermeiren, L.; Devlieghere, F.; van Beest, M.; de Kruijf, N.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Tian, F.; Decker, E.A.; Goddard, J.M. Controlling lipid oxidation of food by active packaging technologies. Food Funct. 2013, 4, 669. [Google Scholar] [CrossRef] [PubMed]
- Cichello, S.A. Oxygen absorbers in food preservation: A review. J. Food Sci. Technol. 2014, 52, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Ahvenainen, R. New approaches in improving the shelf life of minimally processed fruit and vegetables. Trends Food Sci. Technol. 1996, 7, 179–187. [Google Scholar] [CrossRef]
- Bessemans, N.; Verboven, P.; Verlinden, B.E.; Nicolaï, B.M. A novel type of dynamic controlled atmosphere storage based on the respiratory quotient (RQ-DCA). Postharvest Biol. Technol. 2016, 115, 91–102. [Google Scholar] [CrossRef]
- Gill, C.O. Extending the storage life of raw chilled meats. Meat Sci. 1996, 43, 99–109. [Google Scholar] [CrossRef]
- Simone, A. Inside Aseptic, 2nd ed.; GEA Procomac SpA: Sala Baganza, Italy, 2013. [Google Scholar]
- Miesbauer, O.; Kiese, S.; Carmi, Y.; Kücükpinar, E.; Noller, K. Development of novel opaque and transparent barrier films for VIP-encapsulation Part-II : Barrier film production for VIPs. In 11th International Vacuum Insulation Symposium; Empa: Dübendorf, Switzerland, 2013; pp. 19–20. [Google Scholar]
- Vasko, K.; Noller, K.; Mikula, M.; Amberg-Schwab, S.; Weber, U. Multilayer coatings for flexible high-barrier materials. Open Phys. 2009, 7, 371–378. [Google Scholar]
- Amberg-Schwab, S.; Weber, U.; Burger, A.; Nique, S.; Xalter, R. Development of passive and active barrier coatings on the basis of inorganic-organic polymers. Monatshefte fur Chemie 2006, 137, 657–666. [Google Scholar] [CrossRef]
- García-Torres, R.; Ponagandla, N.R.; Rouseff, R.L.; Goodrich-Schneider, R.M.; Reyes-De-Corcuera, J.I. Effects of dissolved oxygen in fruit juices and methods of removal. Compr. Rev. Food Sci. Food Saf. 2009, 8, 409–423. [Google Scholar] [CrossRef]
- Berlinet, B.C.; Brat, P.; Ducruet, V. Quality of Orange Juice in Barrier Packaging Material. Packag. Technol. Sci. 2008, 21, 279–286. [Google Scholar] [CrossRef]
- Van Dijck, S.; Dessaint, A.; Deckers, J. Heat Resistant and Biaxially Stretched Blow-Molded Plastic Container Having a Base Movable to Accomodate Internal Vacuum Forces and Issued from a Double-Blow Process. WIPO Patent 2015/177112, 29 November 2015. [Google Scholar]
- Dessaint, A.; Windelinckx, S. Hot-Fillable Plastic Container Having Vertical Pillars and Concave Deformable Sidewall Panels. Eur. Patent 2,698,320, 19 February 2014. [Google Scholar]
- Min, D.B.; Boff, J.M. Chemistry and Reaction of Singlet Oxygen in Foods. Compr. Rev. Food Sci. Food Saf. 2002, 1, 58–72. [Google Scholar] [CrossRef]
- DeRosa, M.C.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Jorgensen, M.; Norrman, K.; Krebs, F.C. Stability/degradation of polymer solar cells. Sol. Energy Mater. Sol. Cells 2008, 92, 686–714. [Google Scholar] [CrossRef]
- Park, S.J.; Gesquiere, A.J.; Yu, J.; Barbara, P.F. Charge Injection and Photooxidation of Single Conjugated Polymer Molecules. J. Am. Chem. Soc. 2004, 126, 4116–4117. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Goes, M.; Verhoeven, J.W.; Hofstraat, H.; Brunner, K. OLED and PLED devices employing electrogenerated, intramolecular charge-transfer fluorescence. ChemPhysChem 2003, 4, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1757. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Chemistry and Reactions of Reactive Oxygen Species in Foods. Crit. Rev. Food Sci. Nutr. 2006, 46, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Recamales, Á.F.; Sayago, A.; González, M.L.; Hernanz, D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 2006, 39, 220–229. [Google Scholar] [CrossRef]
- Mestdagh, F.; De Meulenaer, B.; De Clippeleer, J.; Devlieghere, F.; Huyghebaert, A. Protective influence of several packaging materials on light oxidation of milk. J. Dairy Sci. 2005, 88, 499–510. [Google Scholar] [CrossRef]
- Cladman, W.; Scheffer, S.; Goodrich, N.; Griffiths, M.W. Shelf-life of milk packaged in plastic containers with and without treatment to reduce light transmission. Int. Dairy J. 1998, 8, 629–636. [Google Scholar] [CrossRef]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, E. Mechanism of Catalysis By Metal Complexes in Autoxidation of an Olefin. Tetrahedron 1964, 20, 1819–1829. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef]
- Kehrer, J.P. The Haber–Weiss reaction and mechanisms of toxicity. Toxicology 2000, 149, 43–50. [Google Scholar] [CrossRef]
- Bouwman, E.; van Gorkum, R. A study of new manganese complexes as potential driers for alkyd paints. J. Coat. Technol. Res. 2007, 4, 491–503. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Comninellis, C.; Kapalka, A.; Malato, S.; Parsons, S.A.; Poulios, I.; Mantzavinos, D. Advanced oxidation processes for water treatment: Advances and trends for R&D. J. Chem. Technol. Biotechnol. 2008, 83, 769–776. [Google Scholar]
- Kuchel, L.; Brody, A.L.; Wicker, L. Oxygen and its reactions in beer. Packag. Technol. Sci. 2006, 19, 25–32. [Google Scholar] [CrossRef]
- Thomsen, M.K.; Kristensen, D.; Skibsted, L.H. Electron spin resonance spectroscopy for determination of the oxidative stability of food lipids. J. Am. Oil Chem. Soc. 2000, 77, 725–730. [Google Scholar] [CrossRef]
- Zufall, C.; Tyrell, T. The Influence of Heavy Metal Ions on Beer Flavour Stability. J. Inst. Brew. 2008, 114, 134–142. [Google Scholar] [CrossRef]
- Pérez-López, A.J.; Saura, D.; Lorente, J.; Carbonell-Barrachina, Á.A. Limonene, linalool, α-terpineol, and terpinen-4-ol as quality control parameters in mandarin juice processing. Eur. Food Res. Technol. 2006, 222, 281–285. [Google Scholar] [CrossRef]
- Lu, F.S.H.; Bruheim, I.; Jacobsen, C. Maillard reaction and lipid peroxidation contribute to non-enzymatic browning in krill-based products: A model study on proposed mechanisms. Eur. J. Lipid Sci. Technol. 2015, 117, 421–430. [Google Scholar] [CrossRef]
- Sun, H.; Lu, L.; Ge, C.; Tang, Y. Effect of Packaging Films on the Quality of Canola Oil under Photooxidation Conditions. Math. Probl. Eng. 2015, 2015, 764516. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Schmidbauer, S.; Hohenleutner, A.; König, B. Studies on the photodegradation of red, green and blue phosphorescent OLED emitters. Beilstein J. Org. Chem. 2013, 9, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Ziegenbalg, D.; Kreisel, G. OLEDs as prospective light sources for microstructured photoreactors. Photochem. Photobiol. Sci. 2014, 13, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Danilewicz, J.C. Interaction of sulfur dioxide, polyphenols, and oxygen in a wine-model system: Central role of iron and copper. Am. J. Enol. Vitic. 2007, 58, 53–60. [Google Scholar]
- Gómez-Plaza, E.; Cano-López, M. A review on micro-oxygenation of red wines: Claims, benefits and the underlying chemistry. Food Chem. 2011, 125, 1131–1140. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. Am. J. Enol. Vitic. 2006, 3, 306–313. [Google Scholar]
- Thellen, C.; Schirmer, S.; Ratto, J.A.; Finnigan, B.; Schmidt, D. Co-extrusion of multilayer poly(m-xylylene adipimide) nanocomposite films for high oxygen barrier packaging applications. J. Membr. Sci. 2009, 340, 45–51. [Google Scholar] [CrossRef]
- Van Bree, I.; De Meulenaer, B.; Samapundo, S.; Vermeulen, A.; Ragaert, P.; Maes, K.C.; De Baets, B.; Devlieghere, F. Predicting the headspace oxygen level due to oxygen permeation across multilayer polymer packaging materials: A practical software simulation tool. Innov. Food Sci. Emerg. Technol. 2010, 11, 511–519. [Google Scholar] [CrossRef]
- Tashiro, H.; Nakaya, M.; Hotta, A. Enhancement of the gas barrier property of polymers by DLC coating with organosilane interlayer. Diam. Relat. Mater. 2013, 35, 7–13. [Google Scholar] [CrossRef]
- Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor; ASTM D3985-05(2010)e1; ASTM International: West Conshohocken, PA, USA, 2010.
- Dhar, P.; Bhardwaj, U.; Kumar, A.; Katiyar, V. Poly (3-hydroxybutyrate)/cellulose nanocrystal films for food packaging applications: Barrier and migration studies. Polym. Eng. Sci. 2015, 55, 2388–2395. [Google Scholar] [CrossRef]
- Liu, R.Y.F.; Hu, Y.S.; Schiraldi, D.A.; Hiltner, A.; Baer, E. Crystallinity and oxygen transport properties of PET bottle walls. J. Appl. Polym. Sci. 2004, 94, 671–677. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. Effect of water on the oxygen barrier properties of poly(ethylene terephthalate) and polylactide films. J. Appl. Polym. Sci. 2004, 92, 1790–1803. [Google Scholar] [CrossRef]
- Knudsen, R.; Black, J.D. Polyamide-Polydiene Blends with Improved Oxygen Reactivity. U.S. Patent 8,409,680, 2 April 2013. [Google Scholar]
- Folkeson, E. Determining the Oxygen Transmission Rate of Carton Packages; Report; Lund University: Lund, Sweden, 2012. [Google Scholar]
- Yoshikawa, Y.; Nawata, T.; Goto, M.; Fujii, Y. Oxygen Indicator. U.S. Patent 4,169,811, 2 October 1979. [Google Scholar]
- Lee, S.K.; Sheridan, M.; Mills, A. Novel UV-activated colorimetric oxygen indicator. Chem. Mater. 2005, 17, 2744–2751. [Google Scholar] [CrossRef]
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar] [CrossRef] [PubMed]
- Blinka, T.A.; Bull, C.; Barmore, C.R.; Speer, D.V. Method of Detecting the Permeability of an Object to Oxygen. U.S. Patent 5,583,047, 10 December 1996. [Google Scholar]
- Baldini, F.; Bacci, M.; Cosi, F.; Del Bianco, A. Absorption-based optical-fibre oxygen sensor. Sens. Actuators B Chem. 1992, 7, 752–757. [Google Scholar] [CrossRef]
- Mills, A. Oxygen indicators and intelligent inks for packaging food. Chem. Soc. Rev. 2005, 34, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Veale, J.R. Apparatus and Method for Nondestructive Monitoring of Gases in Sealed Containers. U.S. Patent 6,639,678, 28 October 2003. [Google Scholar]
- Cocola, L.; Allermann, H.; Fedel, M.; Sønderby, S.; Tondello, G.; Bardenstein, A.; Poletto, L. Validation of an in-line non-destructive headspace oxygen sensor. Food Packag. Shelf Life 2016, 9, 38–44. [Google Scholar] [CrossRef]
- Lund, A.; Jacobsen, T.; Hansen, K.V.; Mogensen, M. Limitations of potentiometric oxygen sensors operating at low oxygen levels. Sens. Actuators B Chem. 2011, 160, 1159–1167. [Google Scholar] [CrossRef]
- Ramamoorthy, R.; Dutta, P.K.; Akbar, S.A. Oxygen sensors: Materials, methods, designs. J. Mater. Sci. 2003, 38, 4271–4282. [Google Scholar] [CrossRef]
- Garza, A.C.; Bavisotto, V.S. Apparatus for and Method of Determining Oxygen and Carbon Dioxide in Sealed Containers. U.S. Patent 3,849,070, 19 November 1974. [Google Scholar]
- Standard Test Method for Oxygen Transmission Rate Through Dry Packages Using a Coulometric Sensor; ASTM F1307-02; ASTM International: West Conshohocken, PA, USA, 2002.
- Pascall, M.A.; Fernandez, U.; Gavara, R.; Allafi, A. Mathematical modeling, non-destructive analysis and a gas chromatographic method for headspace oxygen measurement of modified atmosphere packaged soy bread. J. Food Eng. 2008, 86, 501–507. [Google Scholar] [CrossRef]
- Larsen, H.; Kohler, A.; Magnus, E.M. Ambient oxygen ingress rate method-an alternative method to Ox-Tran for measuring oxygen transmission rate of whole packages. Packag. Technol. Sci. 2000, 13, 233–241. [Google Scholar] [CrossRef]
- Ullsten, N.H.; Hedenqvist, M.S. A new test method based on head space analysis to determine permeability to oxygen and carbon dioxide of flexible packaging. Polym. Test. 2003, 22, 291–295. [Google Scholar] [CrossRef]
- Shahriari, M.R. Method and Composition for a Platinum Embedded Sol Gel Optical Chemical Sensor with Improved Sensitivity and Chemical Stability. U.S. Patent 2008/0199360, 21 August 2008. [Google Scholar]
- Klimant, I.; Krause, C. Oxygen Sensors Disposed on a Microtiter Plate. U.S. Patent 2004/0171094, 2 September 2004. [Google Scholar]
- Hatchett, D.W.; Bennett, B.L.; Devinder, P.S.S. Device and Method for Non-Invasive Oxygen Sensing of Sealed Packages. U.S. Patent 2007/0243618, 18 October 2007. [Google Scholar]
- Huber, C.; Nguyen, T.-A.; Krause, C.; Humele, H.; Stangelmayer, A. Oxygen Ingress Measurement into PET Bottles using Optical-Chemical Sensor Technology. Monatsschrift für Brauwissenschaft 2006, 59, 5–15. [Google Scholar]
- Bacigalupi, C.; Lemaistre, M.H.; Boutroy, N.; Bunel, C.; Peyron, S.; Guillard, V.; Chalier, P. Changes in nutritional and sensory properties of orange juice packed in PET bottles: An experimental and modelling approach. Food Chem. 2013, 141, 3827–3836. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, R.; Cazzola, D.; Cobror, S.; Oriani, L. Oxygen permeation in PET bottles with passive and active walls. Packag. Technol. Sci. 2008, 21, 405–415. [Google Scholar] [CrossRef]
- Suzuki, T.; Hirata, S.; Yazaki, J.; Miyazaki, S.; Nohara, S. Multi-Layer Blow Molded Container and Process for Preparation Thereof. U.S. Patent 4,079,850, 21 March 1978. [Google Scholar]
- Degroote, L. Rigid Plastic Container Having Gas-Barrier Properties and High Transparency. Eur. Patent 1,504,999, 9 February 2005. [Google Scholar]
- Collette, W.N.; Schmidt, S.L. Oxygen Scavenging Composition for Multilayer Preform and Container. WIPO Patent 96/18685, 20 June 1996. [Google Scholar]
- Collette, W.N.; Schmidt, S.L.; Krishnakumar, S.M. Multilayer Preform and Container with Polyethylene Naphthalate (PEN), and Method of Forming Same. U.S. Patent 5,976,653, 2 November 1999. [Google Scholar]
- Akkapeddi, M.K.; Socci, E.P.; Kraft, T.J.; Pratt, J.D. Delamination-Resistant, Barrier Polyamide Compositions for 3-layer PET Beverage Bottles. U.S. Patent 2005/0009976, 13 January 2005. [Google Scholar]
- Lagarón, J.M.; Cabedo, L.; Cava, D.; Feijoo, J.L.; Gavara, R.; Gimenez, E. Improving packaged food quality and safety. Part 2: Nanocomposites. Food Addit. Contam. 2005, 22, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Fasihi, M.; Abolghasemi, M.R. Oxygen barrier and mechanical properties of masterbatch-based PA6/nanoclay composite films. J. Appl. Polym. Sci. 2012, 125, E2–E8. [Google Scholar] [CrossRef]
- Hu, Y.S.; Prattipati, V.; Hiltner, A.; Baer, E.; Mehta, S. Improving transparency of stretched PET/MXD6 blends by modifying PET with isophthalate. Polymer 2005, 46, 5202–5210. [Google Scholar] [CrossRef]
- Grunlan, J.C.; Grigorian, A.; Hamilton, C.B.; Mehrabi, A.R. Effect of clay concentration on the oxygen permeability and optical properties of a modified poly(vinyl alcohol). J. Appl. Polym. Sci. 2004, 93, 1102–1109. [Google Scholar] [CrossRef]
- Zhang, X. Processing–structure–properties relationship of multilayer films. 1. Structure characterization. Polymer 2001, 42, 8179–8195. [Google Scholar] [CrossRef]
- Kim, Y.J.; Germonprez, R. Barrier Compositions and Film Made Therefrom Having Improved Optical and Barrier Properties. U.S. Patent 5,314,987, 24 May 1994. [Google Scholar]
- Lewis, J. Material challenge for flexible organic devices. Mater. Today 2006, 9, 38–45. [Google Scholar] [CrossRef]
- Lewis, J.S.; Weaver, M.S. Thin-Film Permeation-Barrier Technology for Flexible Organic Light-Emitting Devices. IEEE J. Sel. Top. Quantum Electron. 2004, 10, 45–57. [Google Scholar] [CrossRef]
- Burrows, P.E.; Graff, G.L.; Gross, M.E.; Martin, P.M.; Shi, M.K.; Hall, M.; Mast, E.; Bonham, C.; Bennett, W.; Sullivan, M.B. Ultra barrier flexible substrates for flat panel displays. Displays 2001, 22, 65–69. [Google Scholar] [CrossRef]
- Kushida, H.; Kosugi, A.; Nagayama, H. Saturated Polyester Bottle-Shaped Container with Hard Coating and Method of Fabricating the Same. U.S. Patent 4,569,869, 11 February 1986. [Google Scholar]
- Walker, F.H.; Pepe, F.R.; Dickenson, J.B. Packaging Materials Having Barrier Coatings Based on Water Epoxy Resin Copolymers. U.S. Patent 6,777,088, 17 August 2004. [Google Scholar]
- Lange, J.; Nicolas, B.; Galy, J.; Gerard, J.-F. Influence of structure and chemical composition on oxygen permeability of crosslinked epoxy-amine coatings. Polymer 2002, 43, 5985–5994. [Google Scholar] [CrossRef]
- Leterrier, Y. Durability of nanosized oxygen-barrier coatings on polymers. Prog. Mater. Sci. 2003, 48, 1–55. [Google Scholar] [CrossRef]
- Howells, D.G.; Henry, B.M.; Leterrier, Y.; Månson, J.A.E.; Madocks, J.; Assender, H.E. Mechanical properties of SiOx gas barrier coatings on polyester films. Surf. Coat. Technol. 2008, 202, 3529–3537. [Google Scholar] [CrossRef]
- Shirakura, A.; Nakaya, M.; Koga, Y.; Kodama, H.; Hasebe, T.; Suzuki, T. Diamond-like carbon films for PET bottles and medical applications. Thin Solid Films 2006, 494, 84–91. [Google Scholar] [CrossRef]
- Leterrier, Y.; Andersons, J.; Pitton, Y.; Månson, J.-A.E. Adhesion of silicon oxide layers on poly (ethylene terephthalate). II: Effect of coating thickness on adhesive and cohesive strengths. J. Polym. Sci. B Polym. Phys. 1997, 35, 1463–1472. [Google Scholar] [CrossRef]
- Greener, J.; Ng, K.C.; Vaeth, K.M.; Smith, T.M. Moisture Permeability Through Multilayered Barrier Films as Applied to Flexible OLED Display. J. Appl. Polym. Sci. 2007, 106, 3534–3542. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kodama, H.; Hasebe, T.; Shirakura, A.; Suzuki, T. Oxygen transmission of transparent diamond-like carbon films. Diam. Relat. Mater. 2005, 14, 1112–1115. [Google Scholar] [CrossRef]
- Inagaki, N.; Tasaka, S.; Nakajima, T. Preparation of oxygen gas barrier polypropylene films by deposition of SiOx films plasma-polymerized from mixture of tetramethoxysilane and oxygen. J. Appl. Polym. Sci. 2000, 78, 2389–2397. [Google Scholar] [CrossRef]
- Singh, B.; Bouchet, J.; Rochat, G.; Leterrier, Y.; Manson, J.A.E.; Fayet, P. Ultra-thin hybrid organic/inorganic gas barrier coatings on polymers. Surf. Coat. Technol. 2007, 201, 7107–7114. [Google Scholar] [CrossRef]
- Hu, Y.S.; Prattipati, V.; Mehta, S.; Schiraldi, D.A.; Hiltner, A.; Baer, E. Improving gas barrier of PET by blending with aromatic polyamides. Polymer 2005, 46, 2685–2698. [Google Scholar] [CrossRef]
- Pal, R. Permeation models for mixed matrix membranes. J. Colloid Interface Sci. 2008, 317, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Kang, D.; Nair, S. Rigorous calculations of permeation in mixed-matrix membranes: Evaluation of interfacial equilibrium effects and permeability-based models. J. Membr. Sci. 2013, 448, 160–169. [Google Scholar] [CrossRef]
- Vinh-Thang, H.; Kaliaguine, S. Predictive Models for Mixed-Matrix Membrane Performance: A Review. Chem. Rev. 2013, 113, 4980–5028. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.K. Biological and Bioenvironmental Heat and Mass Transport, 7th ed.; CRS Press: Boca Raton, FL, USA, 2002; ISBN 0-8247-0775-3. [Google Scholar]
- Krook, M.; Morgan, G.; Hedenqvist, M.S. Barrier and mechanical properties of injection molded montmorillonite/polyesteramide nanocomposites. Polym. Eng. Sci. 2005, 45, 135–141. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Morphology and barrier properties of nanobiocomposites of poly(3-hydroxybutyrate) and layered silicates. J. Appl. Polym. Sci. 2008, 108, 2787–2801. [Google Scholar] [CrossRef]
- Nielsen, L.E. Models for the Permeability of Filled Polymer Systems. J. Macromol. Sci. A Chem. 1967, 1, 929–942. [Google Scholar] [CrossRef]
- Gonzo, E.E.; Parentis, L.; Gottifredi, J.C. Estimating models for predicting effective permeability of mixed matrix membranes. 2006, 277, 46–54. [Google Scholar] [CrossRef]
- Doudou, B.B. Relationship between Draw Ratio and Strain-Induced Crystallinity in Uniaxially Hot-Drawn PET MXD6 Films. J. Plast. Film Sheeting 2005, 21, 233–251. [Google Scholar] [CrossRef]
- Callander, D.D.; Ferrari, G.; Giovannini, A.; Scrivani, M.T.; Ferrero, S. Dispersions of High Carboxyl Polyamides into Polyesters. U.S. Patent 2014/0107300, 17 April 2014. [Google Scholar]
- Miyabe, T.; Kato, T.; Mitadera, J. Polyester-Based Resin Composition, Method for Producing Same, and Molding Using Resin Composition. U.S. Patent 2015/0030793, 29 January 2015. [Google Scholar]
- Liu, Z.; Mehta, S.; Huang, X.; Schiraldi, D.A. Method to Make Single-Layer PET Bottles with High Barrier and Improved Clarity. U.S. Patent 7,919,159, 5 April 2011. [Google Scholar]
- Choi, W.J.; Kim, H.J.; Yoon, K.H.; Kwon, O.H.; Hwang, C.I. Preparation and barrier property of poly(ethylene terephthalate)/clay nanocomposite using clay-supported catalyst. J. Appl. Polym. Sci. 2006, 100, 4875–4879. [Google Scholar] [CrossRef]
- Kim, H.M.; Lee, J.K.; Lee, H.S. Transparent and high gas barrier films based on poly(vinyl alcohol)/graphene oxide composites. Thin Solid Films 2011, 519, 7766–7771. [Google Scholar] [CrossRef]
- Prud’homme, R.K.; O’Neil, C.D.; Ozbas, B.; Aksay, I.A.; Register, R.A.; Adamson, D.H. Functional Graphene-Polymer Nanocomposites for Gas Barrier Applications. U.S. Patent 2010/0096595, 22 April 2010. [Google Scholar]
- Gilmer, J.W.; Barbee, R.B.; Matayabas, J.C.J.; Lan, T. Polymer/Clay Nanocomposite Having Improved Gas Barrier Comprising a Clay Material with a Mixture of Two or More Organic Cations and a Process for Preparing Same. U.S. Patent 6,486,253, 22 November 2002. [Google Scholar]
- Knoll, R.; Mueller, C. Containers Having Improved Barrier and Mechanical Properties. U.S. Patent 6,841,211, 11 January 2005. [Google Scholar]
- Tammaji, K.S.; Palaniandavar, S.G.K.; Mohan, T.P.; Ramamoorthy, M.; Dillyraj, B.; Raja, M.S. Polyester Gas Barrier Resin and a Process Thereof. U.S. Patent 2008/0319117, 25 December 2008. [Google Scholar]
- Choudalakis, G.; Gotsis, A.D. Permeability of polymer/clay nanocomposites: A review. Eur. Polym. J. 2009, 45, 967–984. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z.; Karandrea, E.; Giliopoulos, D.; Papageorgiou, D.G.; Ladavos, A.; Katerinopoulou, A.; Achilias, D.S.; Triantafyllidis, K.S.; Bikiaris, D.N. Effect of clay structure and type of organomodifier on the thermal properties of poly(ethylene terephthalate) based nanocomposites. Thermochim. Acta 2014, 576, 84–96. [Google Scholar] [CrossRef]
- Silvestre, C.; Duraccio, D.; Cimmino, S. Food packaging based on polymer nanomaterials. Prog. Polym. Sci. 2011, 36, 1766–1782. [Google Scholar] [CrossRef]
- Lin, J.; Shenogin, S.; Nazarenko, S. Oxygen solubility and specific volume of rigid amorphous fraction in semicrystalline poly(ethylene terephthalate). Polymer 2002, 43, 4733–4743. [Google Scholar] [CrossRef]
- Priolo, M.A.; Gamboa, D.; Holder, K.M.; Grunlan, J.C. Super Gas Barrier of Transparent Polymer-Clay Multilayer Ultrathin Films. Nano Lett. 2010, 10, 4970–4974. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.C.; Carranza, S.; Bonnecaze, R.T.; Tung, K.K.; Freeman, B.D.; Paul, D.R. Modeling of oxygen scavenging for improved barrier behavior: Blend films. J. Membr. Sci. 2009, 329, 183–192. [Google Scholar] [CrossRef]
- Di Maio, L.; Scarfato, P.; Galdi, M.R.; Incarnato, L. Development and oxygen scavenging performance of three-layer active PET films for food packaging. J. Appl. Polym. Sci. 2015, 132, 1–10. [Google Scholar] [CrossRef]
- Simándi, L.I. Advances in Catalytic Activation of Dioxygen by Metal Complexes; Simandi, L.I., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; ISBN 0-306-47816-1. [Google Scholar]
- Johnson, D.R.; Tian, F.; Roman, M.J.; Decker, E.A.; Goddard, J.M. Development of Iron-Chelating Poly(ethylene terephthalate) Packaging for Inhibiting Lipid Oxidation in Oil-in-Water Emulsions. J. Agric. Food Chem. 2015, 63, 5055–5060. [Google Scholar] [CrossRef] [PubMed]
- López-Rubio, A.; Almenar, E.; Hernandez-Muñoz, P.; Lagarón, J.M.; Catalá, R.; Gavara, R. Overview of Active Polymer-Based Packaging Technologies for Food Applications. Food Rev. Int. 2004, 20, 357–387. [Google Scholar] [CrossRef]
- Morita, Y.; Komatsu, T.; Inoue, Y. Oxygen Scavenger Container Used for Cap. U.S. Patent 4,756,436, 12 July 1988. [Google Scholar]
- Yamada, S.; Sakuma, I.; Himeshima, Y.; Aoki, T.; Uemura, T.; Shirakura, A. Oxygen Scavenger. U.S. Patent 5,143,763, 1 September 1992. [Google Scholar]
- Frisk, P. Oxygen Scavenging Container. U.S. Patent 5,804,236, 8 September 1998. [Google Scholar]
- Baiano, A.; Marchitelli, V.; Tamagnone, P.; Del Nobile, M.A. Use of Active Packaging for Increasing Ascorbic Acid Retention in Food Beverages. J. Food Sci. 2006, 69, E502–E508. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Degradation of Ascorbic Acid in Aqueous Solution. J. Agric. Food Chem. 1998, 46, 5078–5082. [Google Scholar] [CrossRef]
- Van Bree, I.; Baetens, J.M.; Samapundo, S.; Devlieghere, F.; Laleman, R.; Vandekinderen, I.; Noseda, B.; Xhaferi, R.; De Baets, B.; De Meulenaer, B. Modelling the degradation kinetics of vitamin C in fruit juice in relation to the initial headspace oxygen concentration. Food Chem. 2012, 134, 207–214. [Google Scholar] [CrossRef]
- Li, J.; Chotiko, A.; Narcisse, D.A.; Sathivel, S. Evaluation of alpha-tocopherol stability in soluble dietary fiber based nanofiber. LWT Food Sci. Technol. 2016, 68, 485–490. [Google Scholar] [CrossRef]
- Bacigalupi, C.; Maurey, A.; Boutroy, N.; Peyron, S.; Avallone, S.; Chalier, P. Changes in nutritional value of a multi-vitamins fortified juice packed in glass and standard PET bottles. Food Control 2016, 60, 256–262. [Google Scholar] [CrossRef]
- Maldonado, J.A.; Bruins, R.B.; Yang, T.; Wright, A.; Dunne, C.P.; Karwe, M.V. Browning and Ascorbic Acid Degradation in Meals Ready-to-Eat Pear Rations in Accelerated Shelf Life. J. Food Process. Preserv. 2015, 39, 2035–2042. [Google Scholar] [CrossRef]
- Tikekar, R.V.; Anantheswaran, R.C.; Elias, R.J.; Laborde, L.F. Ultraviolet-induced oxidation of ascorbic acid in a model juice system: Identification of degradation products. J. Agric. Food Chem. 2011, 59, 8244–8248. [Google Scholar] [CrossRef] [PubMed]
- Grudic, V.; Blagojevic, N.; Vukasinovic-Pesic, V.; Brasanac, S. Kinetics of degradation of ascorbic acid by cyclic voltammetry method. Chem. Ind. Chem. Eng. Q. 2015, 21, 351–357. [Google Scholar] [CrossRef]
- Byun, Y.; Darby, D.; Cooksey, K.; Dawson, P.; Whiteside, S. Development of oxygen scavenging system containing a natural free radical scavenger and a transition metal. Food Chem. 2011, 124, 615–619. [Google Scholar] [CrossRef]
- Teumac, F.N.; Zenner, B.D.; Ross, B.A.; Dearduff, L.A.; Rassouli, M.R. Metal Catalyzed Ascorbate Compounds as Oxygen Scavengers. U.S. Patent 6,465,065, 15 October 2002. [Google Scholar]
- Koyama, M.; Oda, Y.; Yamada, M. Oxygen-Absorbing Resin Composition Containing Water-Absorbing Polymer, Olefin Resin and Oxygen Scavenger. U.S. Patent 5,274,024, 28 December 1993. [Google Scholar]
- Tung, D.; Sisson, E.A.; Leckonby, R.A. Oxygen-Scavenging Resin Compositions Having Low Haze. U.S. Patent 6,780,916, 24 August 2004. [Google Scholar]
- Busolo, M.A.; Lagaron, J.M. Oxygen scavenging polyolefin nanocomposite films containing an iron modified kaolinite of interest in active food packaging applications. Innov. Food Sci. Emerg. Technol. 2012, 16, 211–217. [Google Scholar] [CrossRef]
- Mu, H.; Gao, H.; Chen, H.; Tao, F.; Fang, X.; Ge, L. A nanosised oxygen scavenger: Preparation and antioxidant application to roasted sunflower seeds and walnuts. Food Chem. 2013, 136, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Foltynowicz, B.Z.; Kozak, W.; Fiedorow, R. Studies of Oxygen Uptake on O2 Scavengers Prepared from Different Iron-containing Parent Substances. Packag. Technol. Sci. 2002, 15, 75–81. [Google Scholar] [CrossRef]
- Al Ghatta, H. Oxygen-Scavenging Compositions and the Application Thereof in Packaging and Containers. U.S. Patent 2006/0208218, 21 September 2006. [Google Scholar]
- Chau, C.-C.; Incorvia, S.A.; Powers, T.H.; Solovyov, S.E. Laminated and Thermoformed Articles Containing Oxygen Scavenger. U.S. Patent 2010/0282633, 11 November 2010. [Google Scholar]
- Venkateshwaran, L.N.; Chokchi, D.J.; Chiang, W.L.; Tsai, B.C. Oxygen-Scavenging Compositions and Articles. U.S. Patent 5,744,056, 28 April 1998. [Google Scholar]
- Carmichael, A.; Overend, A.S.; Rule, M.; Valus, R.J.; Leeming, C. Composition for Scavenging Oxygen, Container, Package and Closure Containing Said Composition. WIPO Patent 2010/116192, 14 October 2010. [Google Scholar]
- Krikor, H.; Dessaint, A.; Verheyen, L. Seal Capable of Generating Molecular Hydrogen and Suitable for Closing a Container and for Scavenging Oxygen. Eur. Patent 2,404,753, 11 January 2012. [Google Scholar]
- Hermans, A. Packaging Method and Packaging Comprising a Closed Oxygen-Scavenging Container Containing an Oxygen-Sensitive Substance. Eur. Patent 2,604,128, 19 June 2014. [Google Scholar]
- Umegaki, T.; Yan, J.M.; Zhang, X.B.; Shioyama, H.; Kuriyama, N.; Xu, Q. Boron- and nitrogen-based chemical hydrogen storage materials. Int. J. Hydrogen Energy 2009, 34, 2303–2311. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P. A review: Hydrogen generation from borohydride hydrolysis reaction. J. Power Sources 2009, 187, 527–534. [Google Scholar] [CrossRef]
- Sha, Y. The Mechanisms of the Fuel Cell Oxygen Reduction Reaction on Pt and Other 8–11 Column Metal Surfaces. Ph.D. Thesis, California Institute of Technology, Pasadena, CA, USA, 2011. [Google Scholar]
- Jiang, S.P. Hydrogen Oxidation at the Nickel and Platinum Electrodes on Yttria-Tetragonal Zirconia Electrolyte. J. Electrochem. Soc. 1997, 144, 3777. [Google Scholar] [CrossRef]
- Wang, B. Recent development of non-platinum catalysts for oxygen reduction reaction. J. Power Sources 2005, 152, 1–15. [Google Scholar] [CrossRef]
- Mori, K.; Hara, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Hydroxyapatite-Supported Palladium Nanoclusters: A Highly Active Heterogeneous Catalyst for Selective Oxidation of Alcohols by Use of Molecular Oxygen. J. Am. Chem. Soc. 2004, 126, 10657–10666. [Google Scholar] [CrossRef] [PubMed]
- Akkapeddi, M.K. In-Situ Polymerized Polymer-Platinum Group Metal Nanoparticle Blends and Oxygen Scavenging Containers Made Therefrom. U.S. Patent 2014/0034641, 6 February 2014. [Google Scholar]
- Adzic, R.R.; Zhang, J.; Sasaki, K.; Vukmirovic, M.B.; Shao, M.; Wang, J.X.; Nilekar, A.U.; Mavrikakis, M.; Valerio, J.A.; Uribe, F. Platinum monolayer fuel cell electrocatalysts. Top. Catal. 2007, 46, 249–262. [Google Scholar] [CrossRef]
- Markovic, N.M.; Ross, P.N., Jr. Surface science studies of model fuel cell electrocatalysts. Surf. Sci. Rep. 2002, 45, 117–229. [Google Scholar] [CrossRef]
- Hicks, R.F.; Qi, H.; Young, M.L.; Lee, R.G. Structure sensitivity of methane oxidation over platinum and palladium. J. Catal. 1990, 122, 280–294. [Google Scholar] [CrossRef]
- Rule, M.; Valus, R.J.; Tattum, S.B. Scavenging Oxygen. U.S. Patent 2010/0028499, 4 February 2010. [Google Scholar]
- Cochran, M.A.; Folland, R.; Nicholas, J.W.; Robinson, M.E.R. Packaging. U.S. Patent 5,021,515, 4 June 1991. [Google Scholar]
- Ball, M.J. Oxidation Studies of a Novel Barrier Polymer System. Ph.D. Thesis, Aston University, Birmingham, UK, 1995. [Google Scholar]
- Patel, S.N. Thermal and Oxidative Degradation of an Aromatic Polyamide. Ph.D. Thesis, The University of Leeds, Leeds, UK, 1992. [Google Scholar]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, E.; Galfré, E.; Ruggeri, N. Use of Metal Complexes as Oxygen Absorber/Scavenger Elements for Packaging Applications. U.S. Patent 2012/0263974, 18 October 2012. [Google Scholar]
- Schmidt, S.L.; Collette, W.N.; Coleman, E.A.; Krishnakumar, S.M. Transparent Package with Aliphatic Polyketone Oxygen Scavenger. U.S. Patent 5,952,066, 14 September 1999. [Google Scholar]
- Cyr, M.J.; Clauberg, H.; Stewart, M.E.; Falling, S.N.; Rogers, M.E. Polyether Containing Polymers for Oxygen Scavenging. U.S. Patent 6,455,620, 24 September 2002. [Google Scholar]
- Bandi, S.; Mehta, S.; Schiraldi, D.A. The mechanism of color generation in poly(ethylene terephthalate)/polyamide blends. Polym. Degrad. Stab. 2005, 88, 341–348. [Google Scholar] [CrossRef]
- DeRoover, B.; Coppens, G.; Devaux, J.; Legras, R.; Momtaz, A. Contribution to poly(m-xylylene adipamide) characterization: Hydrolysis, condensation, and oxidation in the melt. J. Polym. Sci. A Polym. Chem. 1996, 34, 1039–1047. [Google Scholar] [CrossRef]
- Drbohlav, J.I.; Yuan, Z. Oxygen Scavenging Resin with Short Induction Period. U.S. Patent 8,647,728, 11 February 2014. [Google Scholar]
- Fava, F. Oxygen Scavenging Plastic Material. U.S. Patent 2013/0089686, 11 April 2013. [Google Scholar]
- Cai, G.; Ching, Y.; Yang, H. Oxygen Scavenging Compositions Suitable for Heat Triggering. U.S. Patent 6,610,215, 26 August 2003. [Google Scholar]
- Ve Speer, D.; Blinka, T.A.; Becraft, M.L. Methods and Compositions for Improved Initiation of Oxygen Scavenging. U.S. Patent 5,811,027, 22 September 1998. [Google Scholar]
- Speer, D.V.; Roberts, W.P.; Morgan, C.R. Methods and Compositions for Oxygen Scavenging. U.S. Patent 5,211,875, 27 June 1993. [Google Scholar]
- Behrendt, K.; Dauzvardis, M.J.; Hoch, R. Oxygen Scavenging Compositions, Articles Containing Same, and Methods of Their Use. U.S. Patent 2013/0285277, 31 October 2013. [Google Scholar]
- Li, S.; Dauzvardis, M.J. Oxygen Scavengers, Compositions Comprising the Scavengers, and Articles Made from the Compositions. U.S. Patent 2016/0108207, 21 April 2016. [Google Scholar]
- Deshpande, G.N. Thermoplastic Polymers Comprising Oxygen Scavenging Molecules. U.S. Patent 2014/0228524, 14 August 2014. [Google Scholar]
- Share, P.; Evans, R.H. Oxygen-Scavenging Composition and Articles Thereof. U.S. Patent 2014/0262917, 18 September 2014. [Google Scholar]
- Knudsen, R.; Murray, A. Polar Soluble Scavenging Compositions. U.S. Patent 2014/0145109, 29 May 2014. [Google Scholar]
- Stewart, M.E.; Armentrout, R.S. Oxygen-Scavenging Polymer Blends Suitable for Use in Packaging. U.S. Patent 7,985,456, 26 July 2011. [Google Scholar]
- Richaud, E.; Okamba Diogo, O.; Fayolle, B.; Verdu, J.; Guilment, J.; Fernagut, F. Review: Auto-oxidation of aliphatic polyamides. Polym. Degrad. Stab. 2013, 98, 1929–1939. [Google Scholar] [CrossRef]
- Coquillat, M.; Verdu, J.; Colin, X.; Audouin, L.; Nevière, R. Thermal oxidation of polybutadiene. Part 2: Mechanistic and kinetic schemes for additive-free non-crosslinked polybutadiene. Polym. Degrad. Stab. 2007, 92, 1334–1342. [Google Scholar] [CrossRef]
- Li, H. Kinetics and Mechanisms of the Oxidation Processes for Unsaturated-Hydrocarbon-Modified Scavengers. Ph.D. Thesis, The University of Toledo, Toledo, Spain, 2010. [Google Scholar]
- Rabek, J.F.; Lucki, J.; Ranby, B. Comparative studies of reactions of commercial polymers with molecular oxygen, singlet oxygen, atomic oxygen and ozone-I. Reactions with cis-1,4-polybutadiene. Eur. Polym. J. 1979, 15, 1089–1100. [Google Scholar] [CrossRef]
- De Paoli, M.A. The chemical effects of photo-oxidation on butadiene rubber. Eur. Polym. J. 1983, 19, 761–768. [Google Scholar] [CrossRef]
- Gijsman, P. Review on the thermo-oxidative degradation of polymers during processing and in service. E-Polymers 2008, 8, 1–34. [Google Scholar] [CrossRef]
- Cahill, P.J.; Chen, S.Y. Oxygen Scavenging Condensation Copolymers for Bottles and Packaging Articles. U.S. Patent 6,083,585, 4 July 2000. [Google Scholar]
- Hu, L.; Avakian, R.W. Oxygen Scavenging Terpolymers. Eur. Patent 2,443,169, 25 June 2015. [Google Scholar]
- Liu, Z. Oxygen Scavenging Compositions and Method of Preparation. U.S. Patent 2008/0171169, 17 July 2008. [Google Scholar]
- Ebner, C.L.; Berrier, A.L. Oxygen Scavenger Block Copolymers and Compositions. U.S. Patent 7,754,798, 13 July 2010. [Google Scholar]

| Barrier Polymer | O2 Permeability (cm3 mm m−2 day−1 atm−1) RH 50%, 23 °C | Source |
|---|---|---|
| Polystyrene (PS) | 100–150 | [4] |
| Polyethylene (PE) | 50–200 | [4] |
| Polypropylene (PP) | 50–100 | [4] |
| Poly(lactic acid) (PLA) | 10 | [86] |
| Poly(vinyl chloride) (PVC) | 2–8 | [4] |
| Poly(ethylene terephthalate) (PET) | 1–5 | [4] |
| Polyamide-6 (PA6) | 1,4 | [87] |
| Poly(ethylene naphthalate) (PEN) | 0.5 | [4] |
| poly(m-xylylene adipamide) (MXD6) | 0.05 | [88] |
| Poly(vinyl alcohol) (PVOH) | 0.02–1 | [89] |
| Ethylene vinyl alcohol (EVOH) | 0.04–0,4 | [86] |
| Poly(vinylidene chloride) (PVDC) | 0.01–0,3 | [4] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michiels, Y.; Puyvelde, P.V.; Sels, B. Barriers and Chemistry in a Bottle: Mechanisms in Today’s Oxygen Barriers for Tomorrow’s Materials. Appl. Sci. 2017, 7, 665. https://doi.org/10.3390/app7070665
Michiels Y, Puyvelde PV, Sels B. Barriers and Chemistry in a Bottle: Mechanisms in Today’s Oxygen Barriers for Tomorrow’s Materials. Applied Sciences. 2017; 7(7):665. https://doi.org/10.3390/app7070665
Chicago/Turabian StyleMichiels, Youri, Peter Van Puyvelde, and Bert Sels. 2017. "Barriers and Chemistry in a Bottle: Mechanisms in Today’s Oxygen Barriers for Tomorrow’s Materials" Applied Sciences 7, no. 7: 665. https://doi.org/10.3390/app7070665
APA StyleMichiels, Y., Puyvelde, P. V., & Sels, B. (2017). Barriers and Chemistry in a Bottle: Mechanisms in Today’s Oxygen Barriers for Tomorrow’s Materials. Applied Sciences, 7(7), 665. https://doi.org/10.3390/app7070665




