Reel-to-Reel Atmospheric Pressure Dielectric Barrier Discharge (DBD) Plasma Treatment of Polypropylene Films
Abstract
:1. Introduction
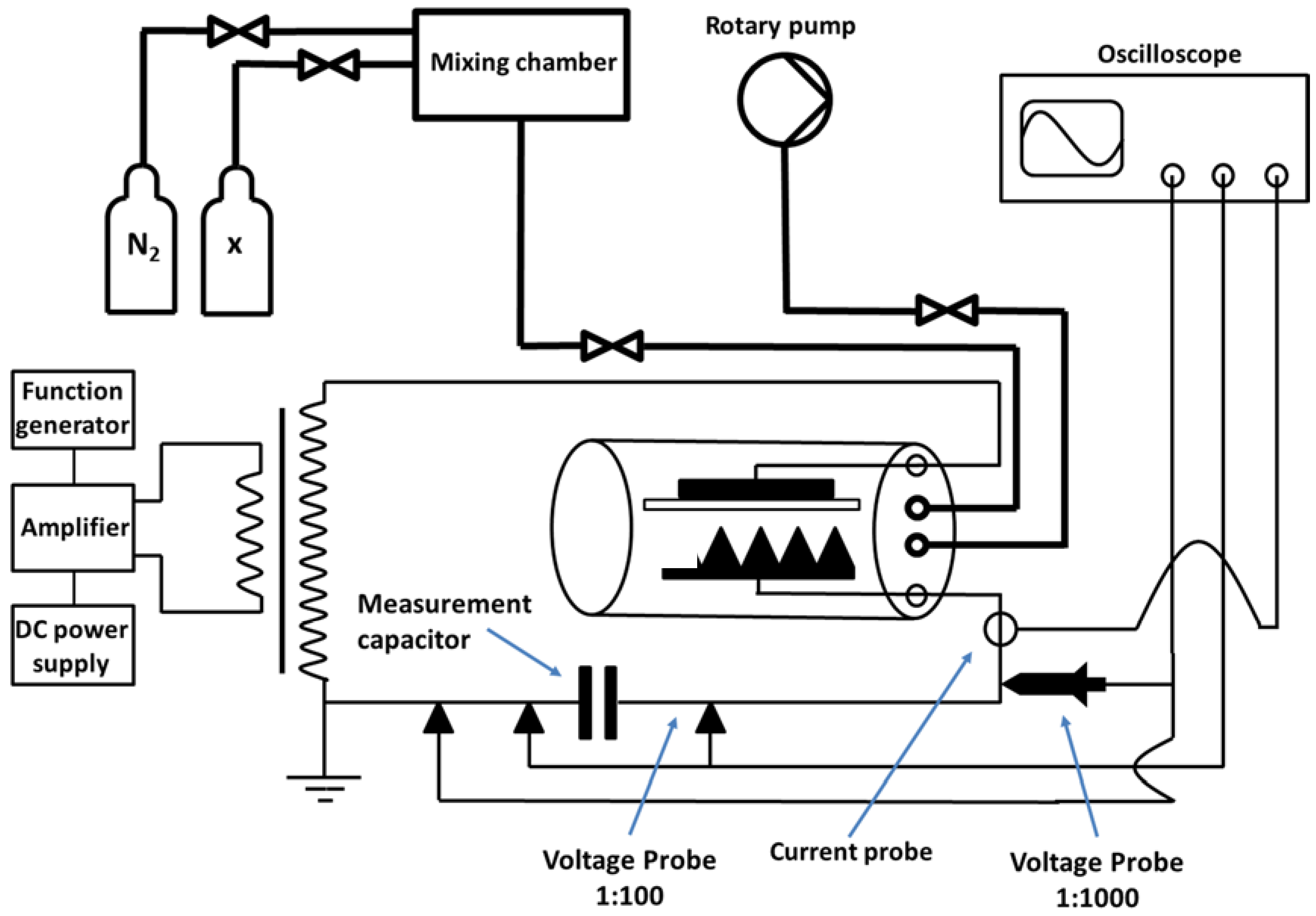
2. Experimental Section
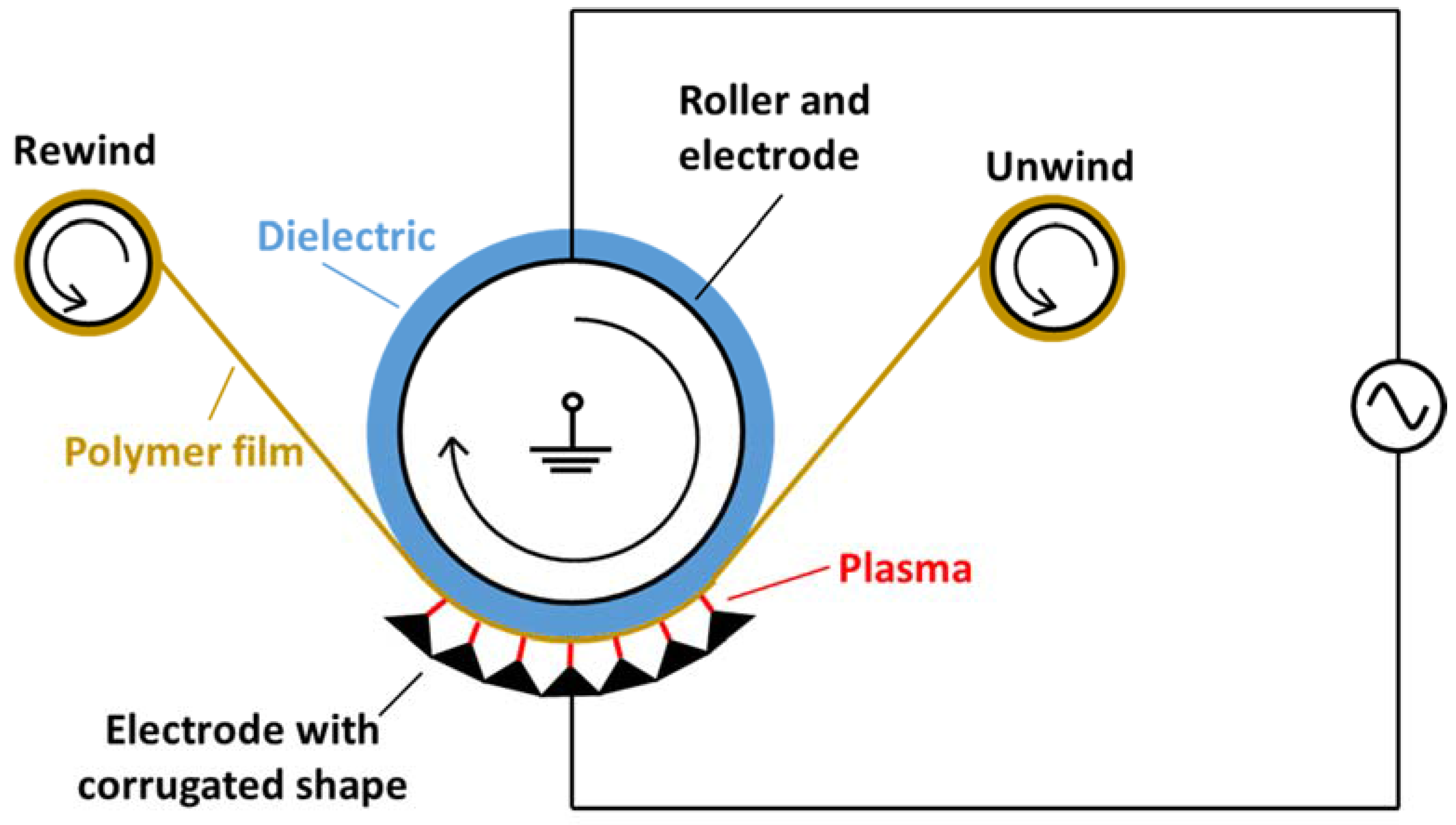
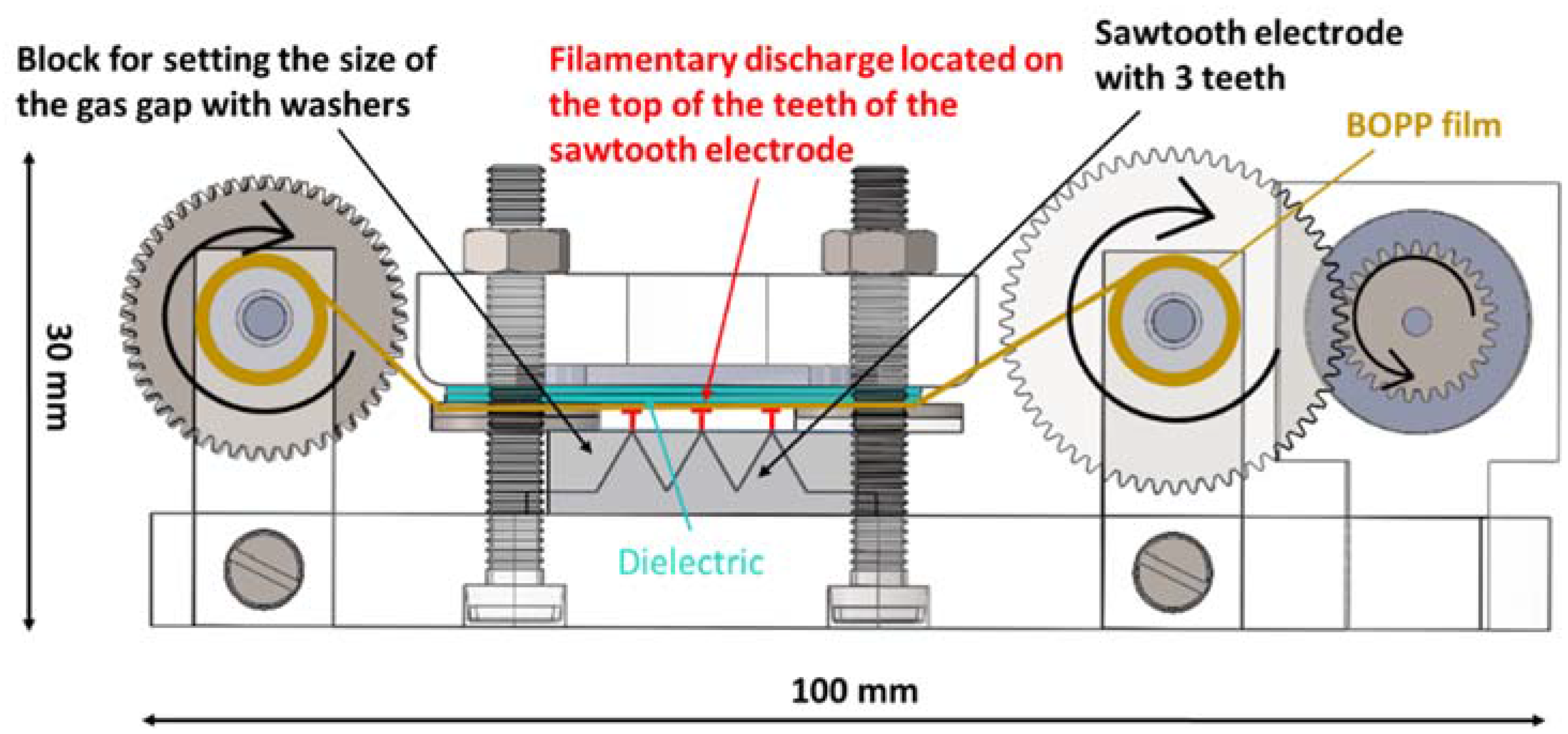
2.1. Design of Process Chamber
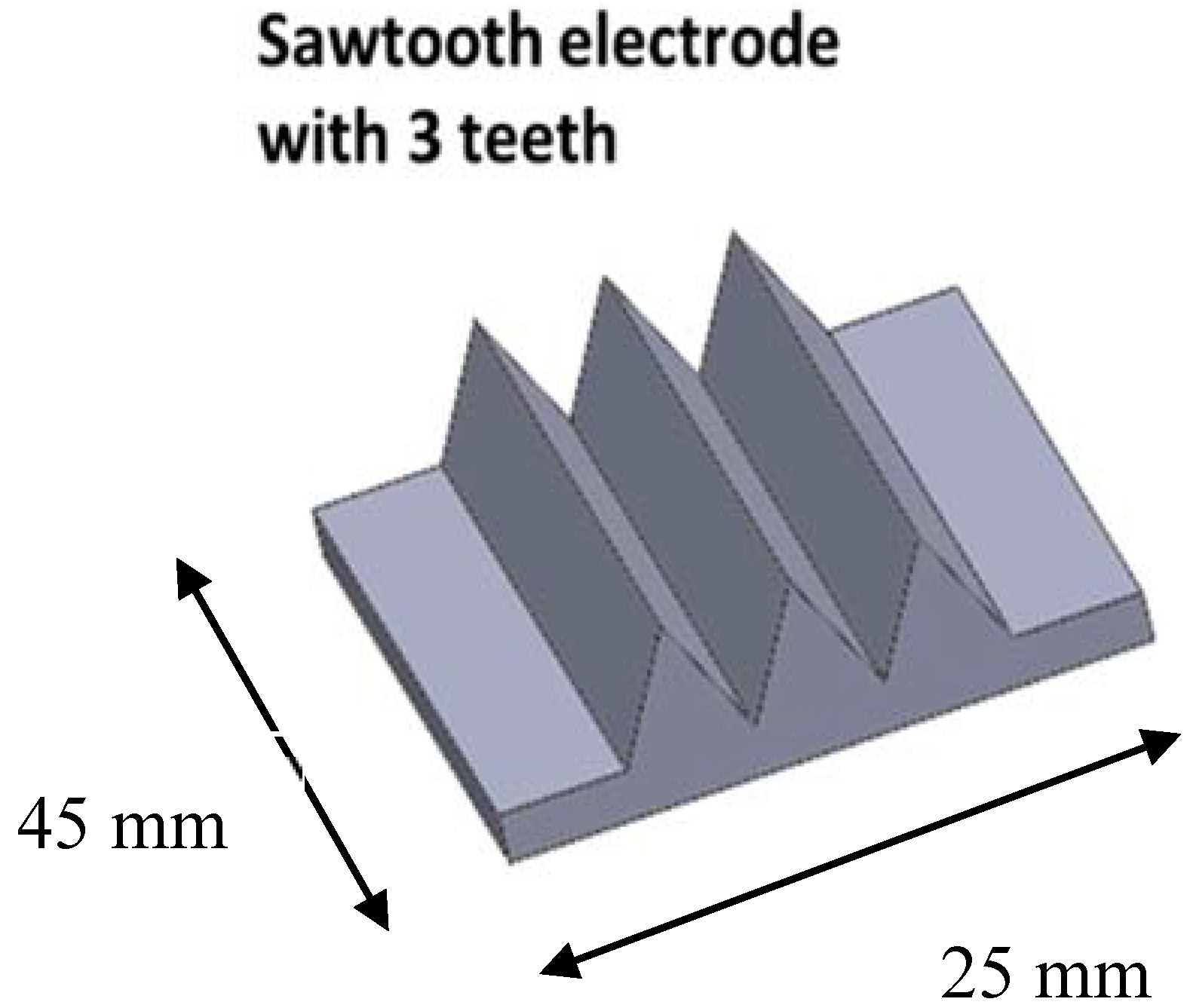
2.2. Design of the DBD Reactor
2.3. Power Supply Unit
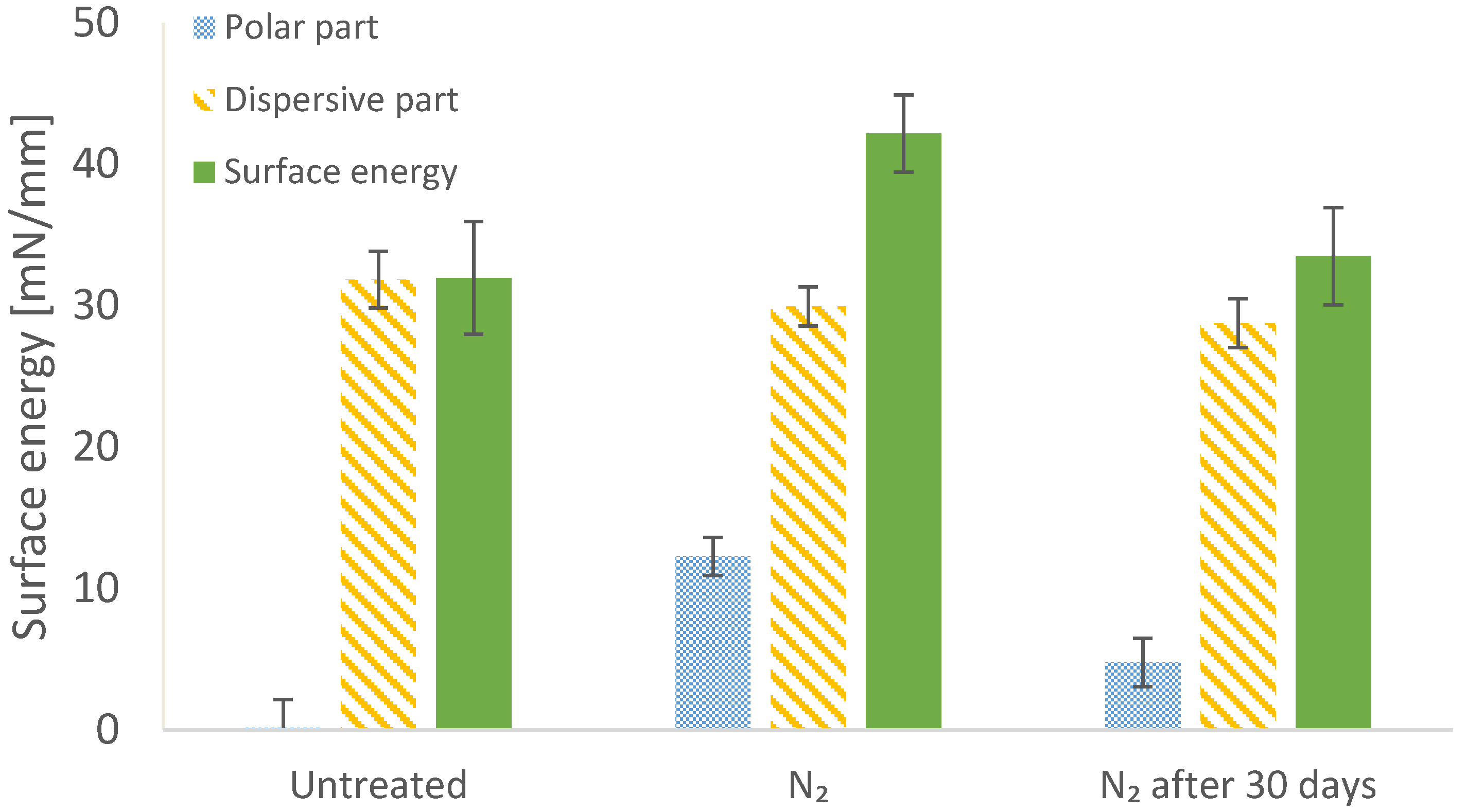
2.4. Treatment of BOPP Film in Nitrogen
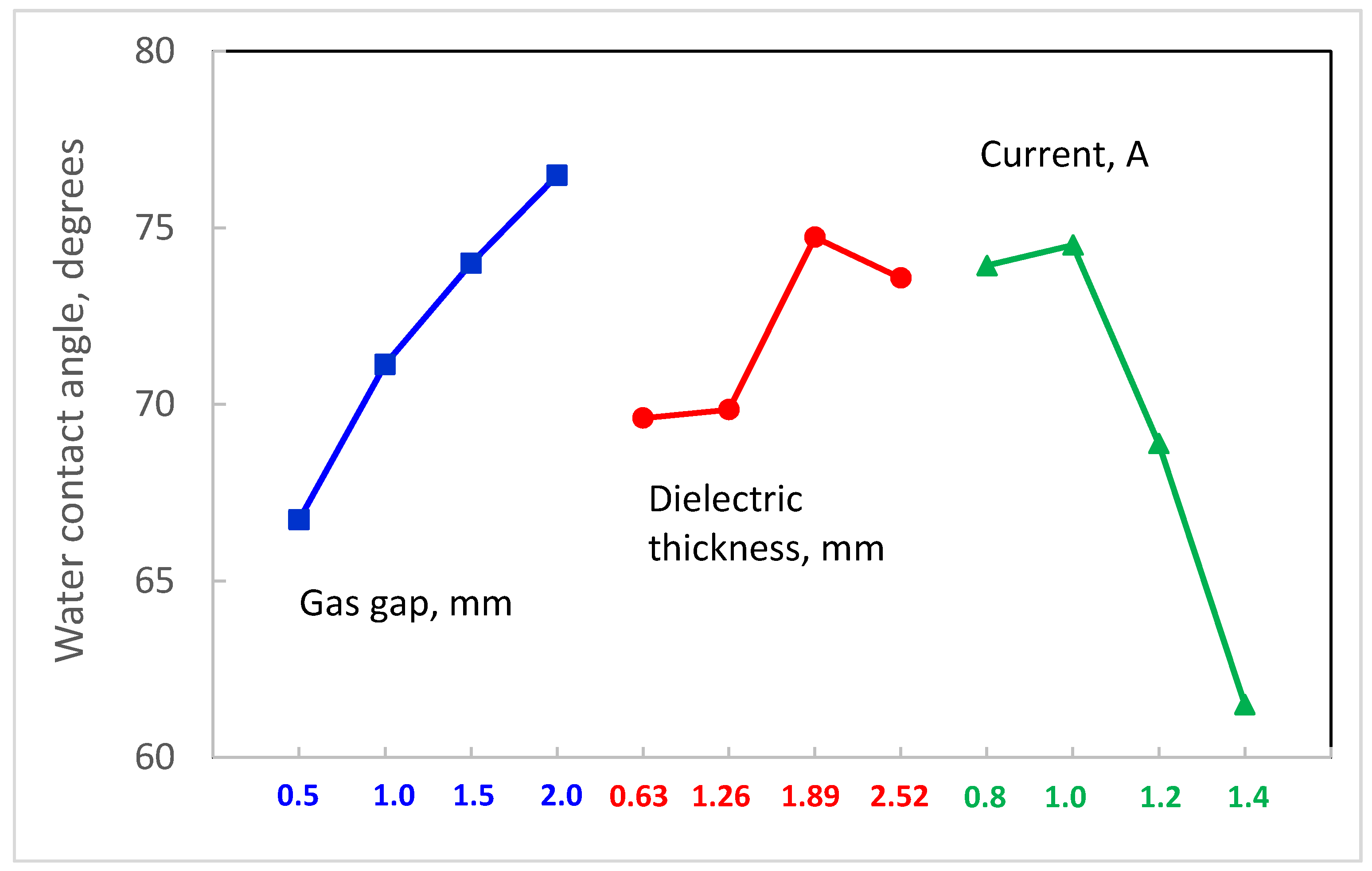
- the thickness of the dielectric covering the upper electrode (four levels were tested: 0.63 mm, 1.26 mm, 1.89 mm, 2.52 mm)
- the size of the gas gap measured between the upper electrode and the top of the teeth on the sawtooth electrode (0.5 mm, 1.0 mm, 1.5 mm, 2.0 mm)
- the DC power supply current setting (0.8 A, 1.0 A, 1.2 A, 1.4 A)
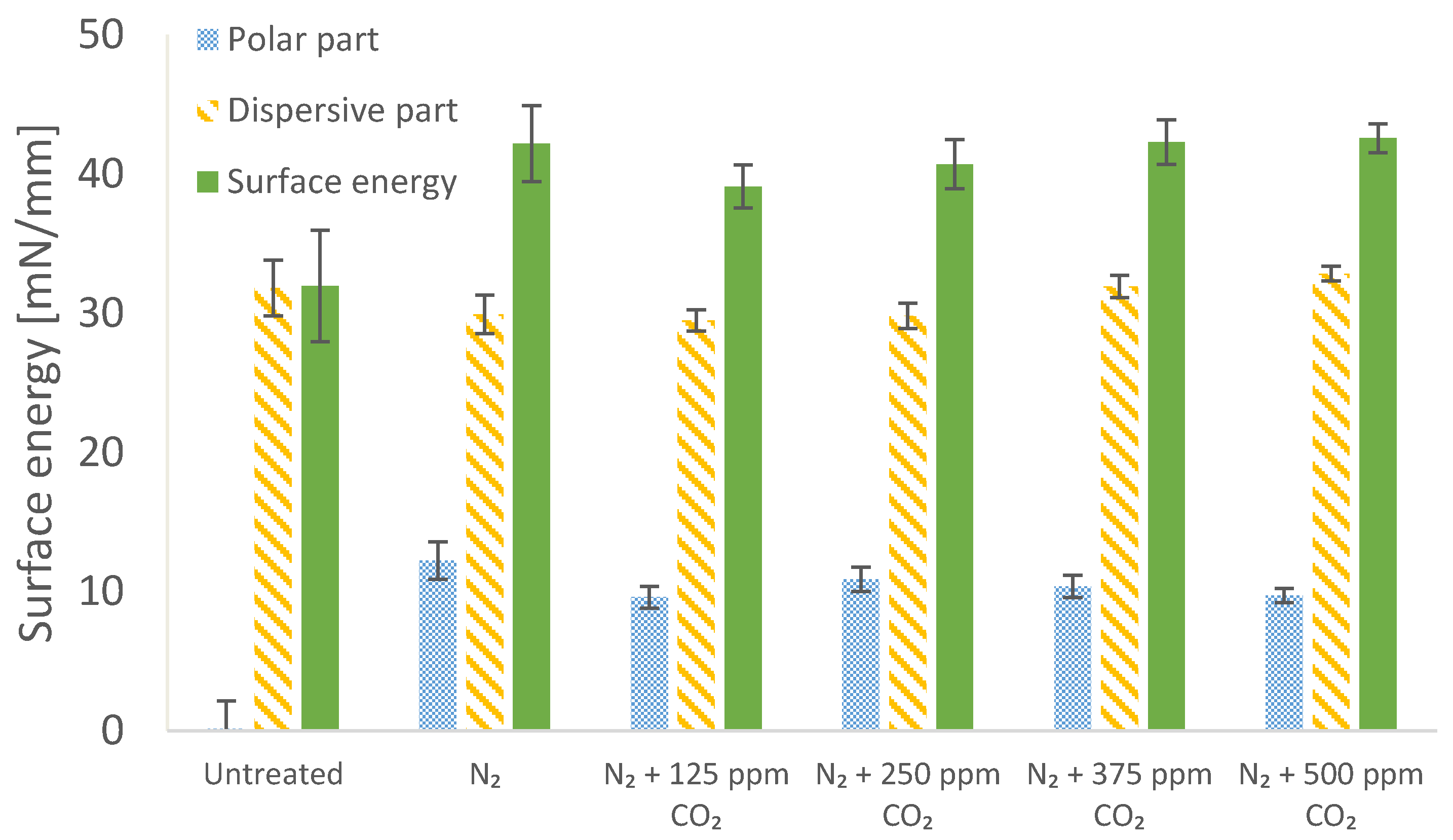
2.5. Treatment of BOPP Film in Nitrogen with Admixtures
2.6. X-ray Photoelectron Spectroscopy Analysis of Surface Chemistry
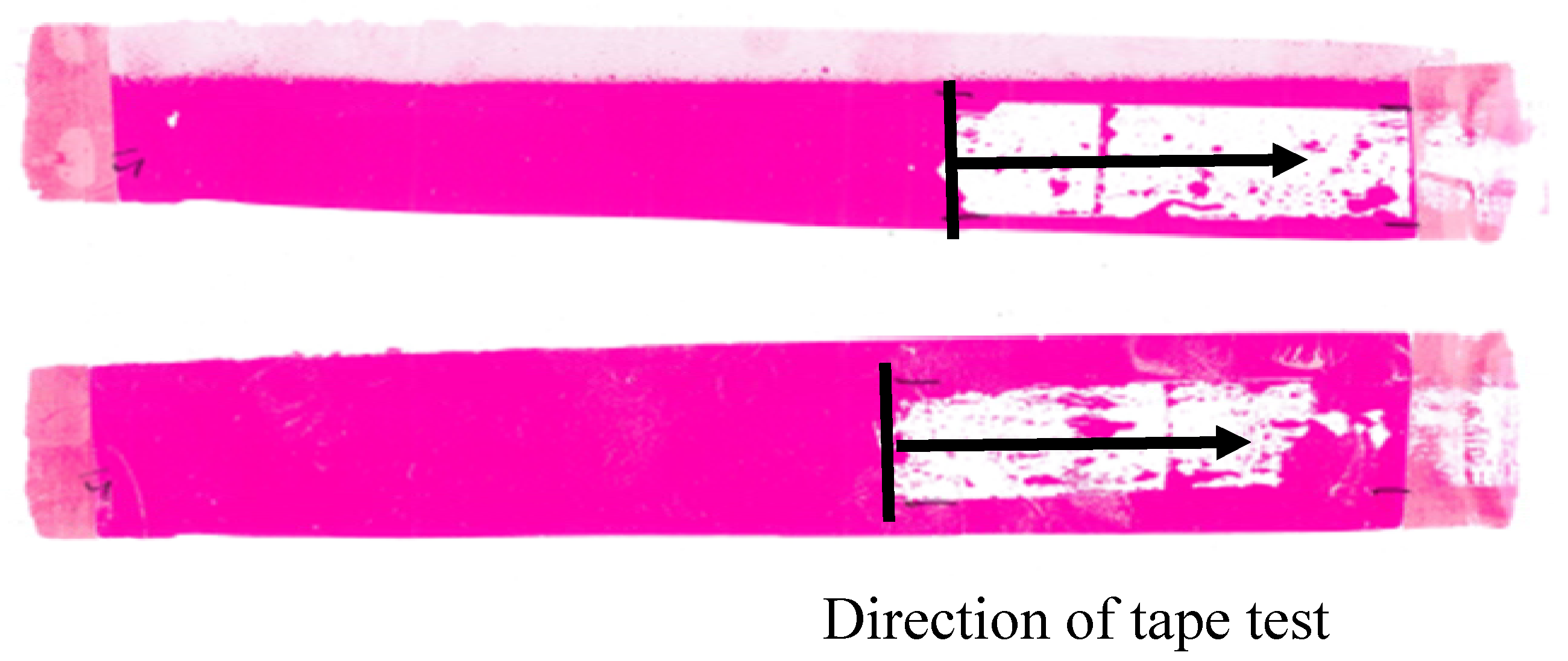
2.7. Dyne Pen and Ink Adhesion Tests
3. Results and Discussion
3.1. Characterisation of the DBD
3.2. Effect of Nitrogen Treatment on Water Contact Angle
3.3. Effect of Nitrogen Treatment with Admixtures on Surface Energy
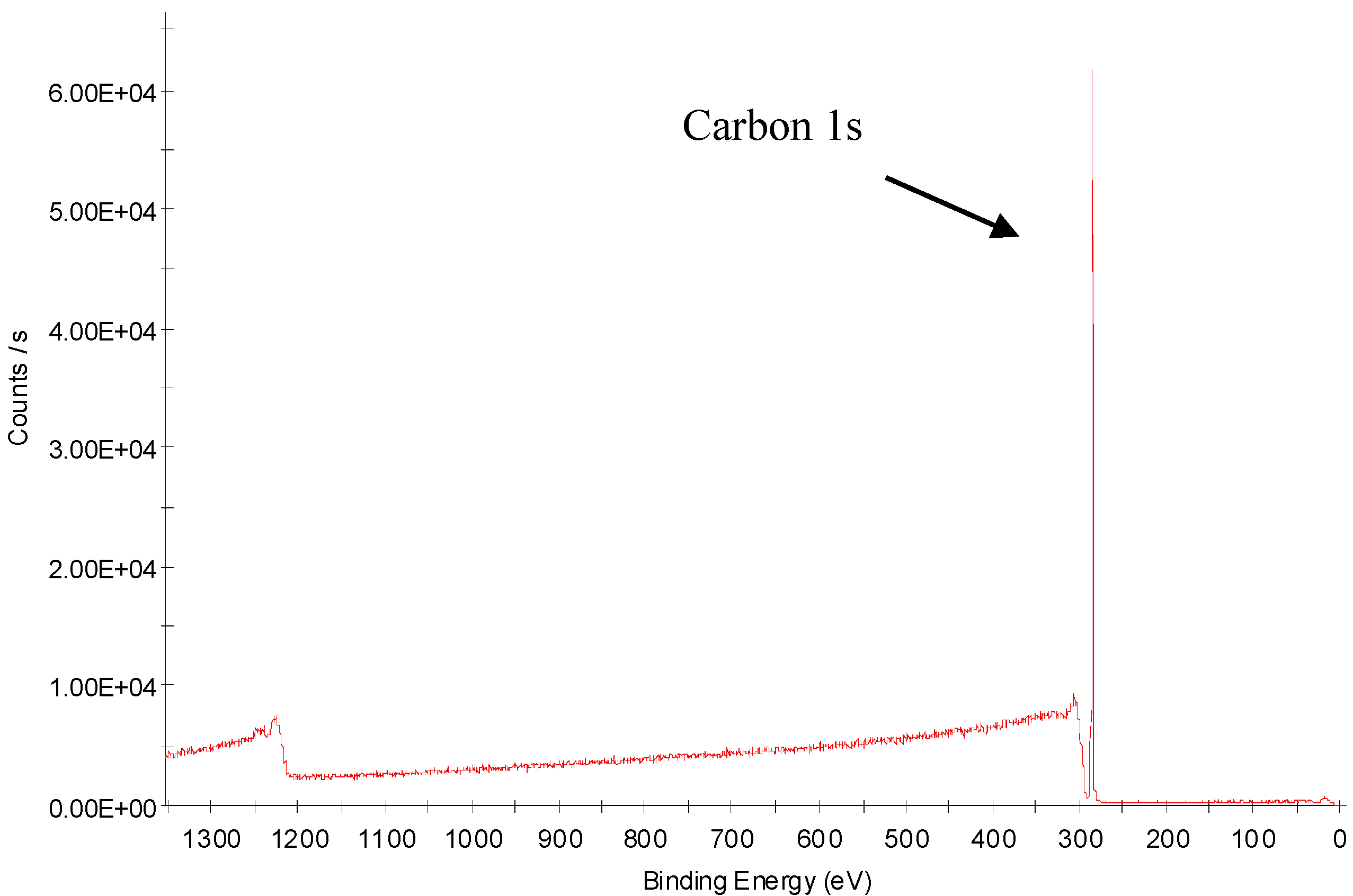
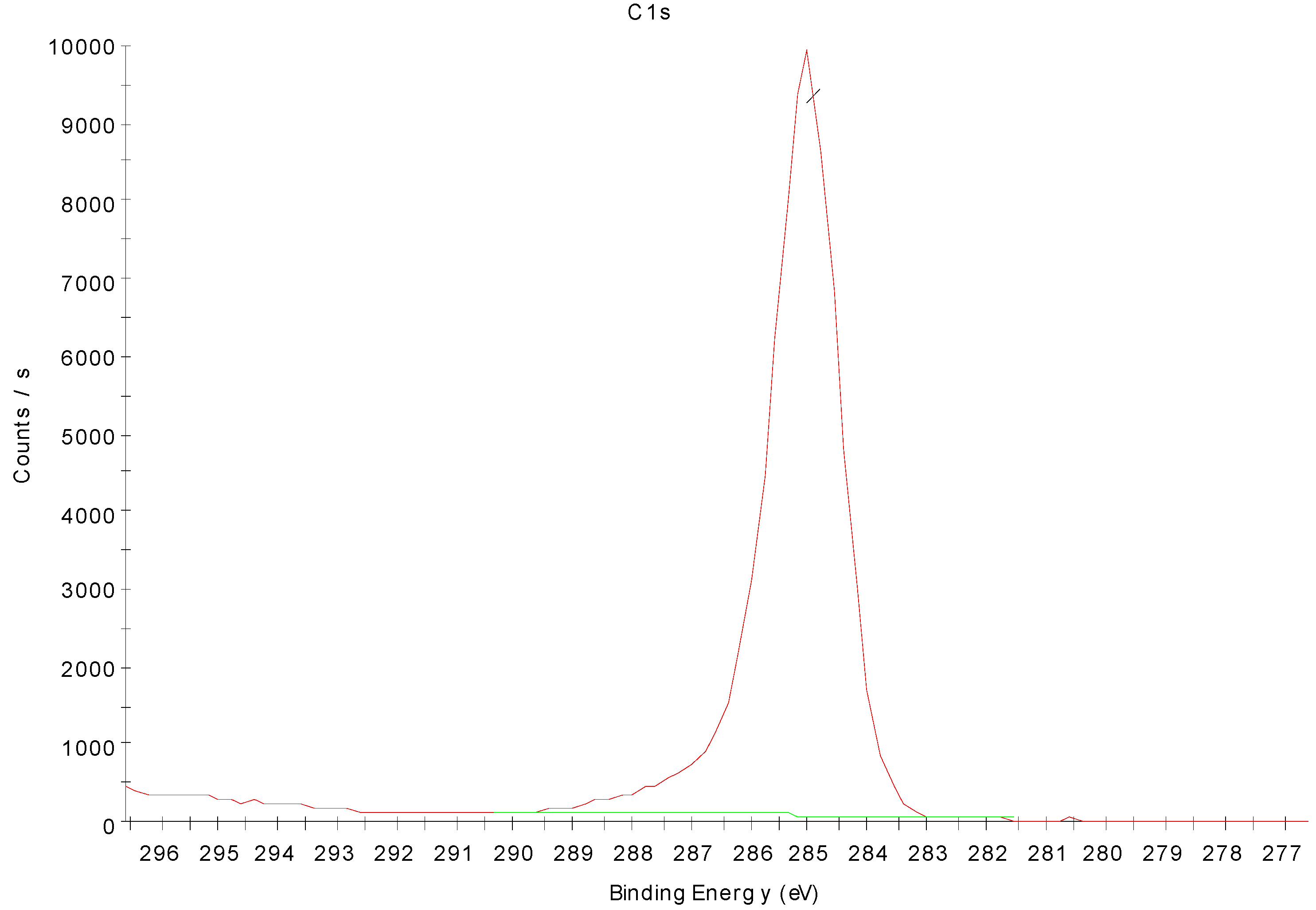
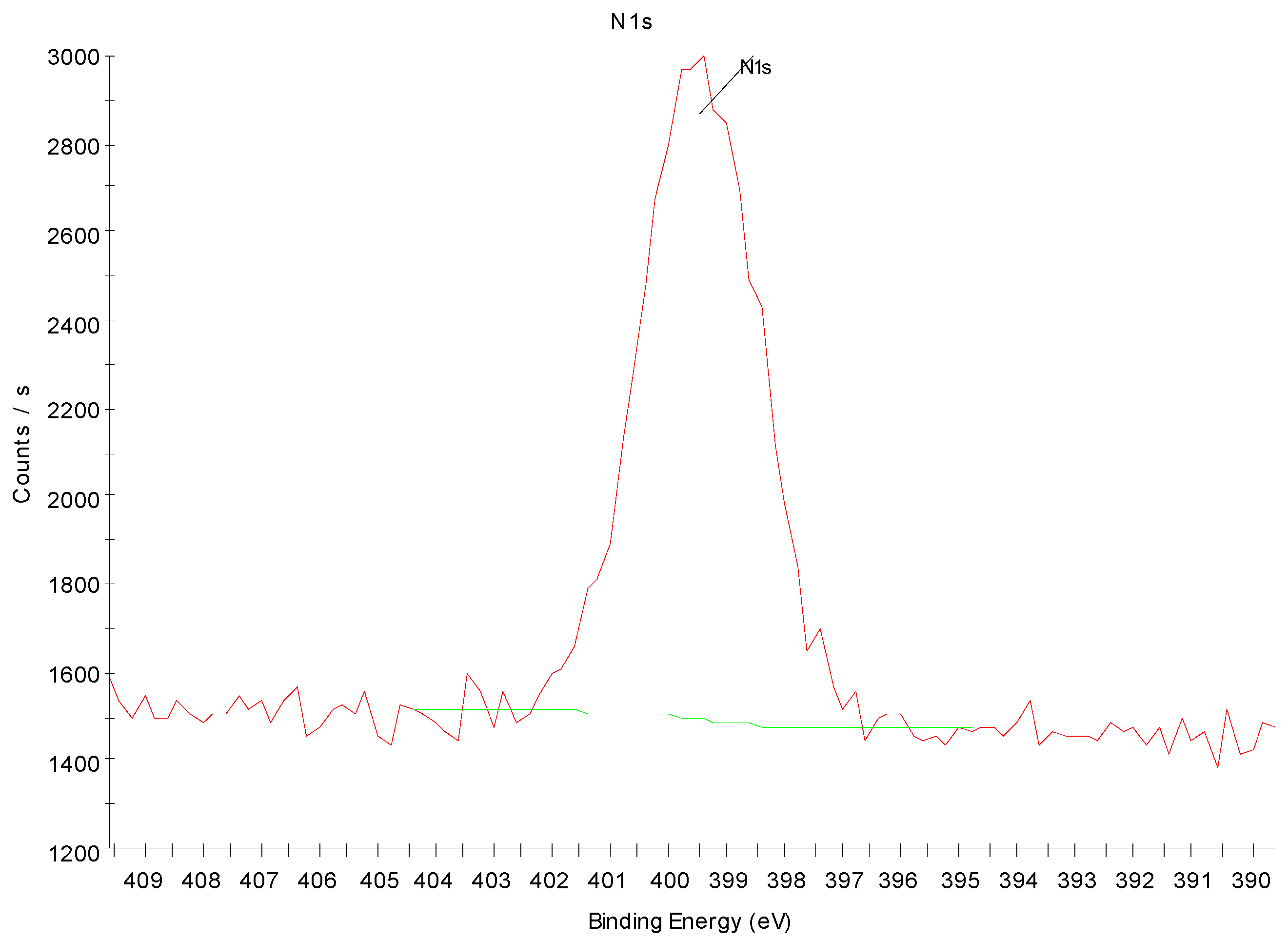
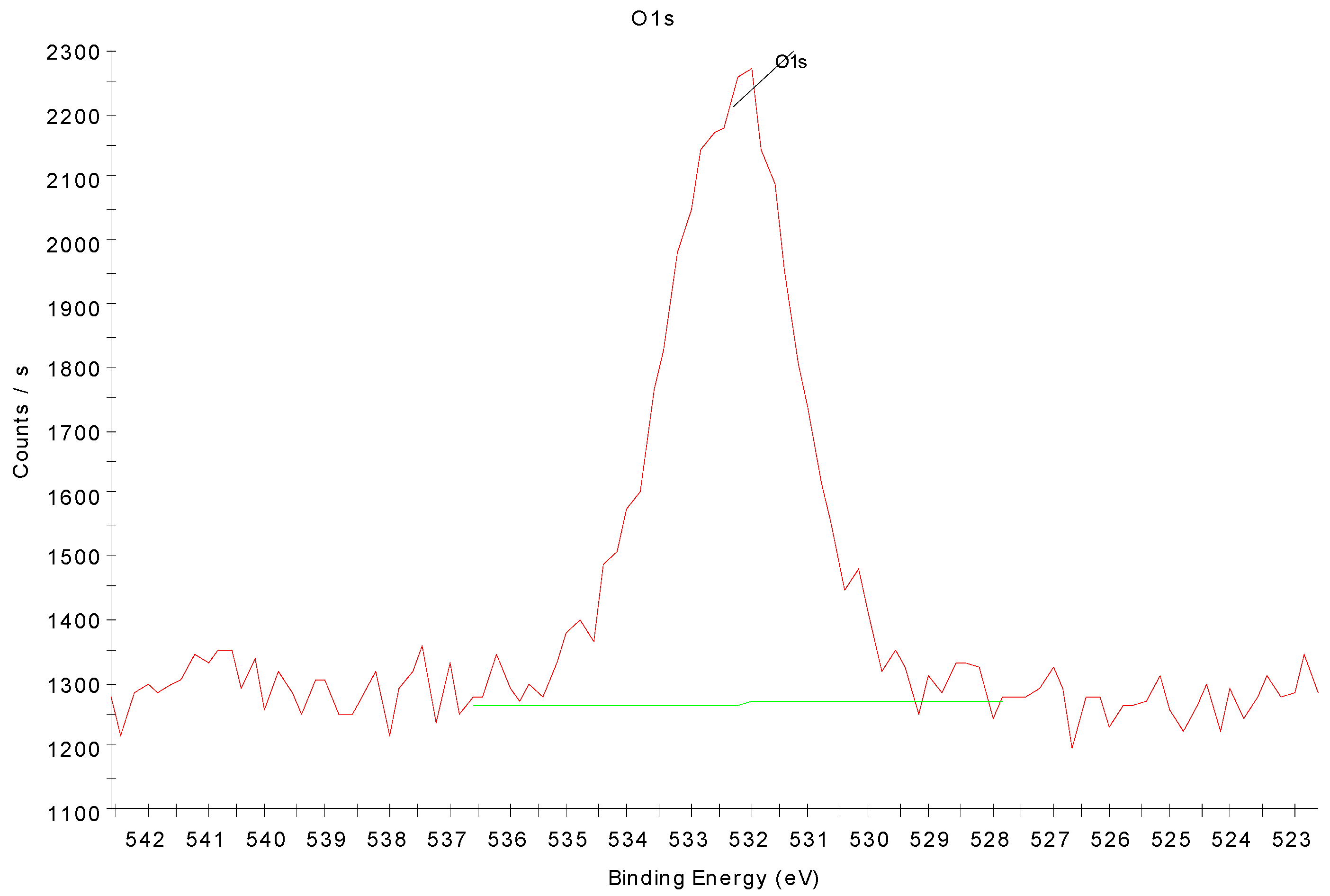
3.4. X-ray Photoelectron Spectroscopy Analysis of Nitrogen-Treated BOPP Film
3.5. Dyne Pen and Ink Adhesion Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wolf, R.A. Atmospheric Pressure Plasma for Surface Modification; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Thomas, M.; Mittal, K.L. Atmospheric Pressure Plasma Treatment of Polymers: Relevance to Adhesion; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Shaw, D.; West, A.; Bredin, J.; Wagenaars, E. Mechanisms behind surface modification of polypropylene film using an atmospheric-pressure plasma jet. Plasma Sources Sci. Technol. 2016, 25, 065018. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, D.; Wadsworth, L.C. Corona Treatment of Polyolefin Films—A Review. Adv. Polym. Technol. 1999, 18, 171–180. [Google Scholar]
- Ruddy, A.C. The Effect of Atmospheric Glow Discharge (APGD) Treatment on Polyetherimide, Polybutyleneterephthalate, and Polyamides. J. Plast. Film Sheet. 2006, 22, 103. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-barrier discharges: Their history, discharge physics, and industrial applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Aerts, R.; Tu, X.; Van Gaens, W.; Whitehead, J.C.; Bogaerts, A. Gas Purification by Nonthermal Plasma: A Case Study of Ethylene. Environ. Sci. Technol. 2013, 47, 6478–6485. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.D.; Jung, J.S. Effective corona discharge and ozone generation from a wire-plate discharge system with a slit dielectric barrier. J. Electrost. 2007, 65, 660–666. [Google Scholar] [CrossRef]
- Ueno, H.; Kawahara, S.; Nakayama, H. Fundamental Study of Barrier Discharge and Ozone Generation Characteristics for Multiple Needles to Plane Configuration. Ozone Sci. Eng. 2011, 33, 98–105. [Google Scholar] [CrossRef]
- Helmke, A.; Wandke, D.; Mahmoodzada, M.; Weltmann, K.D.; Viol, W. Impact of Electrode Design, Supply Voltage and Interelectrode Distance on Safety Aspects and Characteristics of a Medical DBD Plasma Source. Contrib. Plasma Phys. 2013, 53, 623–638. [Google Scholar] [CrossRef]
- Schwabedissen, A.; Łaciński, P.; Chen, X.; Engemann, J. PlasmaLabel—A New Method to Disinfect Goods Inside a Closed Package Using Dielectric Barrier Discharges. Contrib. Plasma Phys. 2007, 47, 551–558. [Google Scholar]
- Kogelschatz, U.; Eliasson, B.; Egli, W. From ozone generators to flat television screens: History and future potential of dielectric-barrier discharges. Pure Appl. Chem. 1999, 71, 1819–1828. [Google Scholar] [CrossRef]
- Bobkova, E.; Rybkin, V. Peculiarities of Energy Efficiency Comparison of Plasma Chemical Reactors for Water Purification from Organic Substances. Plasma Chem. Plasma Process. 2015, 35, 133–142. [Google Scholar] [CrossRef]
- Rodriguez-Mendez, B.G.; López-Callejas, R.; Olguín, M.T.; Hernández-Arias, A.N.; Valencia-Alvarado, R.; Peña-Eguiluz, R.; Mercado-Cabrera, A.; Alcántara-Díaz, D.; Muñoz-Castro, A.E.; de la Piedad-Beneitezet, A. Bacterial inactivation in water by means of a combined process of pulsed dielectric barrier discharge and silver modified natural zeolite. J. Phys. D Appl. Phys. 2104, 47. [Google Scholar] [CrossRef]
- Shibata, T.; Nishiyama, H. Acetic Acid Decomposition in a Coaxial Dielectric Barrier Discharge Tube with Mist Flow. Plasma Chem. Plasma Process. 2014, 34, 1331. [Google Scholar] [CrossRef]
- Massines, F.; Sarra-Bournet, C.; Fanelli, F.; Naudé, N.; Gherardi, N. Atmospheric Pressure Low Temperature Direct Plasma Technology: Status and Challenges for Thin Film Deposition. Plasma Process. Polym. 2012, 9, 1041–1073. [Google Scholar] [CrossRef]
- Premkumar, P.A.; Starostin, S.A.; Creatore, M.; de Vries, H.; Paffen, R.M.J.; Koenraad, P.M.; van de Sanden, M.C.M. Smooth and Self-Similar SiO2-like Films on Polymers Synthesized in Roll-to-Roll Atmospheric Pressure-PECVD for Gas Diffusion Barrier Applications. Plasma Process. Polym. 2010, 7, 635–639. [Google Scholar] [CrossRef]
- Schäfer, J.; Horn, S.; Foest, R.; Brandenburg, R.; Vašina, P.; Weltmann, K.-D. Complex analysis of SiOxCyHz films deposited by an atmospheric pressure dielectric barrier discharge. Surf. Coat. Technol. 2011, 205, S330–S334. [Google Scholar] [CrossRef]
- Vinogradov, I.; Shakhatre, M.; Lunk, A. Spectroscopic Diagnostics of DBD Remote Plasma in Ar/Fluorocarbon Mixtures—Correlation Between Plasma Parameters and Thin Film Properties. Plasma Process. Polym. 2007, 4, S797–S800. [Google Scholar] [CrossRef]
- Niu, J.; Liu, D.; Wu, Y. Large-area and uniform surface modification of polymers by barrier discharge plasmas. Surf. Coat. Technol. 2011, 205, 3434–3437. [Google Scholar] [CrossRef]
- Papageorghiou, L.; Panousis, E.; Loiseau, J.F.; Spyrou, N.; Held, B. Two-dimensional modelling of a nitrogen dielectric barrier discharge (DBD) at atmospheric pressure: Filament dynamics with the dielectric barrier on the cathode. J. Appl. Phys. D Appl. Phys. 2009, 42, 105201. [Google Scholar] [CrossRef]
- Kanazawa, S.; Kogoma, M.; Moriwaki, T.; Okazaki, S. Stable glow plasma at atmospheric pressure. J. Appl. Phys. D Appl. Phys. 1988, 21, 838. [Google Scholar] [CrossRef]
- Fanelli, F. Thin film deposition and surface modification with atmospheric pressure dielectric barrier discharges. Surf. Coat. Technol. 2010, 205, 1536–1543. [Google Scholar] [CrossRef]
- Liston, E.; Martinu, L.; Wertheimer, W. Plasma surface modification of polymers for improved adhesion: A critical review. J. Adhes. Sci. Technol. 1993, 7, 1091–1127. [Google Scholar] [CrossRef]
- Cáceres, C.A.; Mazzola, N.; França, M.; Canevarolo, S.V. Controlling in-line the energy level applied during the corona treatment. Polym. Test. 2012, 31, 505–511. [Google Scholar] [CrossRef]
- Reichen, P. Plasma Surface Modification in the Afterglow of Micro-barrier Discharges. Ph.D. Dissertation, ETH Zürich, Zürich, Switzerland, 2009; p. 212. [Google Scholar]
- Mangolini, L.; Anderson, C.; Heberlein, J.; Kortshagen, U. Effects of current limitation through the dielectric in atmospheric pressure glows in helium. J. Appl. Phys. D Appl. Phys. 2004, 37, 1021. [Google Scholar] [CrossRef]
- Borcia, G.; Anderson, C.A.; Brown, N.M.D. Using a nitrogen dielectric barrier discharge for surface treatment. Plasma Sources Sci. Technol. 2005, 14, 259. [Google Scholar] [CrossRef]
- Seidelmann, L.J.W. Atmospheric Pressure Dielectric Barrier Discharges for the Surface Modification of Polypropylene. Ph.D. Thesis, Manchester Metropolitan University, Manchester, UK, 2015. [Google Scholar]
- Petrie, E.M. Theories of Adhesion. In Handbook of Adhesives and Sealants; McGraw-Hill: New York, NY, USA, 2007; pp. 39–58. [Google Scholar]
- Leroux, F.; Campagne, C.; Perwuelz, A.; Gengembre, L. Polypropylene film chemical and physical modifications by dielectric barrier discharge plasma treatment at atmospheric pressure. J. Colloid Interface Sci. 2008, 328, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Šíra, M.; Trunec, D.; Stahel, P.; Buršíková, V.; Navrátil, Z.; Buršík, J. Surface modification of polyethylene and polypropylene in atmospheric pressure glow discharge. J. Appl. Phys. D Appl. Phys. 2005, 38, 621. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Kaelble, D.H. Dispersion-polar surface tension properties of organic solids. J. Adhes. 1970, 2, 66. [Google Scholar] [CrossRef]
- Zenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. Mater. Manuf. Eng. 2007, 24, 137–145. [Google Scholar]
- Kwok, D.Y.; Neumann, A.W. Contact angle measurement and contact angle interpretation. Adv. Colloid Interface Sci. 1999, 81, 167–249. [Google Scholar] [CrossRef]
- ASTM D2578-09. Standard Test Method for Wetting Tension of Polyethylene and Polypropylene Films, ASTM International: West Conshohocken, PA, USA, 2009.
- Chvatalova, L.; Čermák, R.; Mráček, A.; Grulich, O.; Veseld, A.; Ponížil, P.; Minařík, A.; Cvelbard, U.; Beníčeka, L.; Sajdl, P. The effect of plasma treatment on structure and properties of poly(1-butene) surface. Eur. Polym. J. 2012, 48, 866–874. [Google Scholar] [CrossRef]
- Cui, N.Y.; Brown, N.M.D. Modification of the Surface Properties of a Polypropylene (PP) Film using an Air Dielectric Barrier Discharge Plasma. Appl. Surf. Sci. 2002, 189, 31–38. [Google Scholar] [CrossRef]
- Upadhyay, D.J.; Cui, N.Y.; Anderson, C.A.; Brown, N.M.D. Surface oxygenation of polypropylene using an air dielectric barrier discharge: The effect of different electrode–platen combinations. Appl. Surf. Sci. 2004, 229, 352–364. [Google Scholar] [CrossRef]
- Weikart, C.M.; Yasuda, H.K. Modification, degradation, and stability of polymeric surfaces treated with reactive plasmas. J. Polym. Sci. A Polym. Chem. 2000, 38, 3028–3042. [Google Scholar] [CrossRef]
- Guimond, S.; Wertheimer, M.R. Surface degradation and hydrophobic recovery of polyolefins treated by air corona and nitrogen atmospheric pressure glow discharge. J Appl. Polym. Sci. 2004, 94, 1291–1303. [Google Scholar] [CrossRef]
- Guimond, S.; Radu, I.; Czeremuszkin, G.; Carlsson, D.J.; Wertheimer, M.R. Biaxially oriented polypropylene (BOPP) surface modification by nitrogen atmospheric pressure glow discharge (APGD) and by air corona. Plasmas Polym. 2002, 7, 71. [Google Scholar] [CrossRef]


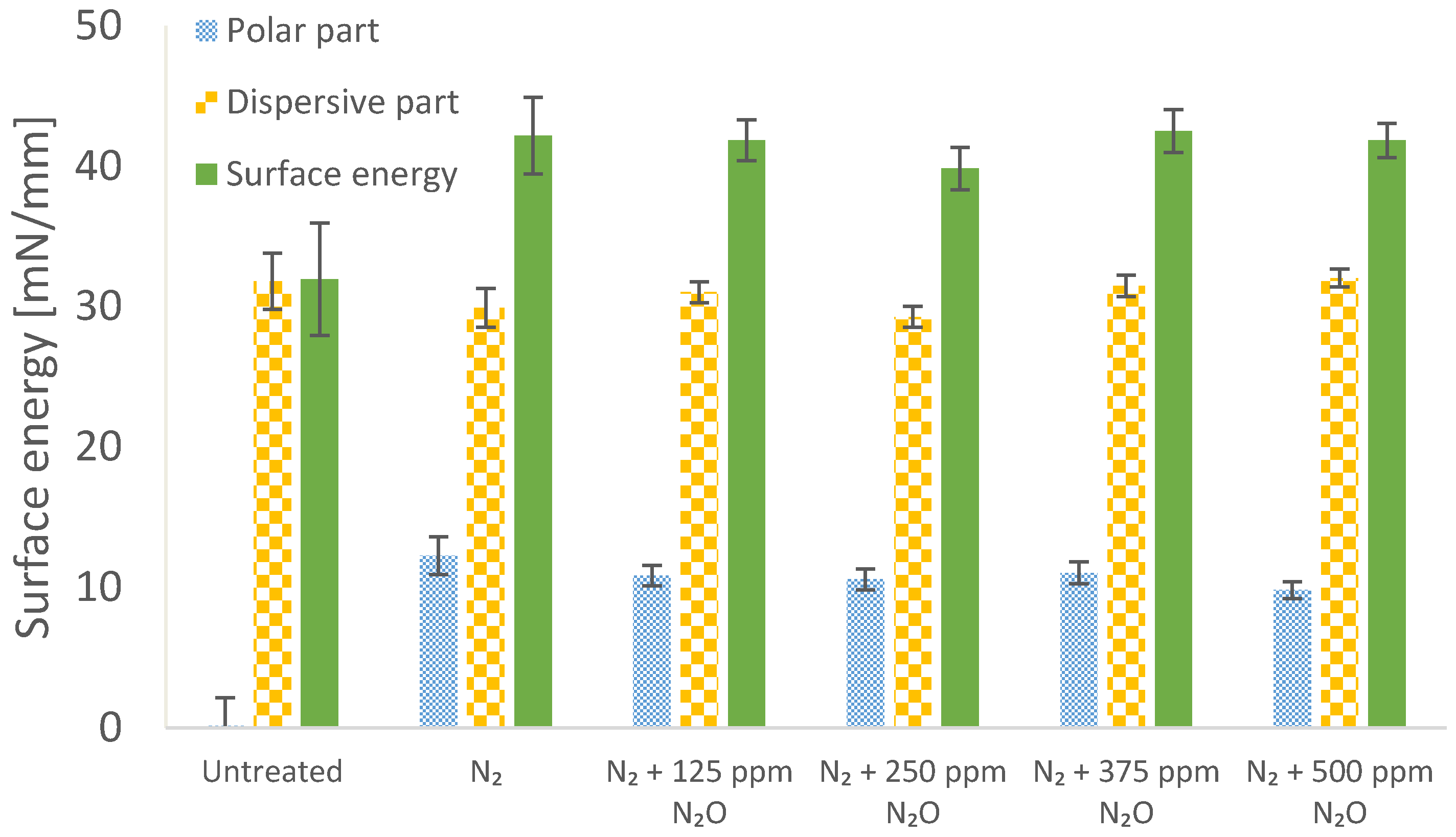


| Number of Experiment | Current [A] | Thickness of the Dielectric [mm] | Size of the Gas Gap [mm] | Optimal Frequency [kHz] |
|---|---|---|---|---|
| 1 | 0.8 | 0.63 | 2.0 | 29.8 |
| 2 | 1.4 | 0.63 | 0.5 | 29.6 |
| 3 | 1.2 | 1.26 | 0.5 | 29.6 |
| 4 | 1.2 | 0.63 | 2.0 | 29.8 |
| 5 | 0.8 | 0.63 | 0.5 | 29.6 |
| 6 | 1.0 | 1.89 | 2.0 | 29.9 |
| 7 | 0.8 | 2.52 | 2.0 | 29.9 |
| 8 | 0.8 | 1.89 | 1.5 | 29.9 |
| 9 | 1.0 | 0.63 | 1.5 | 29.8 |
| 10 | 0.8 | 1.26 | 0.5 | 29.6 |
| 11 | 0.8 | 1.89 | 1.0 | 29.7 |
| 12 | 0.8 | 0.63 | 1.0 | 29.6 |
| 13 | 0.8 | 1.26 | 2.0 | 29.9 |
| 14 | 1.2 | 1.26 | 2.0 | 29.9 |
| 15 | 1.2 | 0.63 | 0.5 | 29.6 |
| 16 | 0.8 | 2.52 | 0.5 | 29.7 |
| Number of Experiment | Discharge Power [W] | Average Water Contact Angle, Degrees |
|---|---|---|
| 1 | 5.5 | 80.4 |
| 2 | 5 | 61.5 |
| 3 | 5.3 | 67.6 |
| 4 | 5 | 74.7 |
| 5 | 5 | 71.7 |
| 6 | 6.8 | 76.9 |
| 7 | 8.5 | 79.8 |
| 8 | 6.7 | 75.8 |
| 9 | 4.6 | 72.2 |
| 10 | 5.4 | 68.3 |
| 11 | 6.8 | 71.5 |
| 12 | 5 | 70.7 |
| 13 | 5.1 | 78.0 |
| 14 | 5.2 | 69.2 |
| 15 | 4.6 | 64.1 |
| 16 | 8.5 | 67.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seidelmann, L.J.; Bradley, J.W.; Ratova, M.; Hewitt, J.; Moffat, J.; Kelly, P. Reel-to-Reel Atmospheric Pressure Dielectric Barrier Discharge (DBD) Plasma Treatment of Polypropylene Films. Appl. Sci. 2017, 7, 337. https://doi.org/10.3390/app7040337
Seidelmann LJ, Bradley JW, Ratova M, Hewitt J, Moffat J, Kelly P. Reel-to-Reel Atmospheric Pressure Dielectric Barrier Discharge (DBD) Plasma Treatment of Polypropylene Films. Applied Sciences. 2017; 7(4):337. https://doi.org/10.3390/app7040337
Chicago/Turabian StyleSeidelmann, Lukas JW, James W Bradley, Marina Ratova, Jonathan Hewitt, Jamie Moffat, and Peter Kelly. 2017. "Reel-to-Reel Atmospheric Pressure Dielectric Barrier Discharge (DBD) Plasma Treatment of Polypropylene Films" Applied Sciences 7, no. 4: 337. https://doi.org/10.3390/app7040337
APA StyleSeidelmann, L. J., Bradley, J. W., Ratova, M., Hewitt, J., Moffat, J., & Kelly, P. (2017). Reel-to-Reel Atmospheric Pressure Dielectric Barrier Discharge (DBD) Plasma Treatment of Polypropylene Films. Applied Sciences, 7(4), 337. https://doi.org/10.3390/app7040337






