Abstract
Volatile organic compounds (VOCs) released by plant growth-promoting rhizobacteria (PGPR) are involved in promoting growth and triggering systemic resistance (ISR) in plants. Importantly, the release of VOCs by some PGPR strains confers improved plant uptake of nutrient elements from the soil. However, the underlying mechanisms of VOCs-regulated nutrient acquisition remain elusive. In this study, VOCs were extracted and identified from Bacillus amyloliquefaciens (strain BF06) using gas chromatography–mass spectrometry (GC–MS). BF06 VOCs exposure significantly promoted the growth and photosynthesis of Arabidopsis plants. To explore how microbial VOCs stimulate growth in plants, gene expression profiles of Arabidopsis seedlings exposed to BF06 VOCs were examined using transcriptomic analyses. In screening differentially expressed genes (DEGs), most upregulated DEGs were found to be related to amino acid transport, iron (Fe) uptake and homeostasis, and sulfate transport. Furthermore, BF06 VOCs significantly enhanced Fe absorption in plants under Fe-limited conditions. However, when nitric oxide (NO) synthesis was inhibited, BF06 VOCs exposure could not substantially augment Fe acquisition in plants under alkaline stress, indicating that VOCs-mediated plant uptake of Fe was required for induction of root NO accumulation. In addition, BF06 VOCs exposure led to a marked increase in some genes encoding for sulfate transporters, and further increased Se accumulation in plants. Intriguingly, BF06 VOCs exposure failed to increase Se uptake in sultr1;2 mutants, which may indicate that high-level transcription of these sulfate transporters induced by BF06 VOCs was essential for enhancing Se absorption by plants. Taken together, our results demonstrated the potential of VOCs released by this strain BF06 to increase Fe and Se uptake in plants.
1. Introduction
Being sessile organisms, plants live in soils for growth, development, and completing the life cycle. A variety of soil-borne microorganisms naturally occur in the plant rhizosphere, many of which can assist with plant growth and adaptation to adverse conditions, especially nutrient limitation [1,2,3]. Beneficial soil microbes are usually called plant growth-promoting rhizobacteria (PGPR). It has previously been indicated that plant roots can secrete several organic compounds that are utilized by soil bacteria as an important nutrient source [4]. Root exudates can also inhibit soil-borne plant pathogens, and shape the composition and diversity of local soil microbiota [5]. Certain PGPR strains can release some important substances such as non-volatile (phytohormones, antibiotics, siderophores, etc.) and volatile organic compounds (VOCs), which offer great benefits to plants [1,6,7,8,9,10]. Since their discovery in the late 18th century, soil inoculation with PGPR has been widely applied in agriculture [11,12,13]. Emerging evidence indicates that application of PGPR remarkably enhances plant uptake of macro- and micro-elements by increasing the availability of soil nutrients and activating the mechanisms of nutrient acquisition in host plants [14,15,16]. Hence, PGPR can be exploited as biofertilizers to effectively improve nutrient assimilation by plants.
Previous studies have shown that PGPR can mediate many physiological processes of host plants without being in physical contact with the roots by release of VOCs [9,10]. Microbial VOCs (mVOCs) have recently been considered as a novel type of regulators in plant–microbe interactions [17]. In spite of different types of VOCs being emitted by a wide array of bacterial species and strains, a common characteristic is that VOCs can be released by various soil microbes [18,19,20]. Intriguingly, mVOCs do not contain any well-known plant growth hormones (such as auxin and cytokine) and iron-chelators (siderophores), whereas endogenous hormones and iron (Fe) uptake in plants can be positively regulated by differential mVOCs emission [21,22,23]. mVOCs can function as signal molecules for root–microbe communication, and mediate plant growth and resistance to phytopathogens [18,19,20,21,22,23]. Hitherto, a large number of bacterial species such as Paenibacillus [14], Bacillus [22], and Pseudomonas [24] have been known to produce VOCs, which play diverse roles during plant–microbe interactions. 2,3-butanediol is a major VOC released by Bacillus subtilis GB03 [9] and Pseudomonas chlororaphis O6 [25], and can regulate plant growth and stress tolerance by modulation of various metabolic pathways. Plants inoculated with Bacillus strains and plants treated with exogenous butanediol exhibit induced systemic resistance (ISR). In addition, a sulfur (S)-containing volatile compound, dimethyl disulfide (DMDS), from Bacillus sp. strain B5 obviously recovers plant growth and enhances S assimilation under S deprivation [26].
Recently, the release of VOCs by some PGPR strains has been shown to promote Fe absorption in plants, indicating that mVOCs are involved in regulating plant Fe deficiency responses [22,27]. Fe is an essential micro-element for plant growth and development, and Fe deficiency has increasingly become one of the wide-ranging abiotic stresses that severely affect diverse processes such as chlorophyll formation, respiration, and photosynthesis [28]. Despite a high abundance of Fe in the earth’s crust, it is not easily utilized by plants due to its low availability in alkaline or calcareous soils [29]. Plants often display typical symptoms of chlorotic leaves under Fe-limited conditions [30]. To cope with Fe deficiency, the strategy I plants, dicots and monocots, can acidify the rhizosphere for increasing the solubility of Fe (III) by the Arabidopsis plasma membrane H+-ATPase isoform 2 (AHA2) [31], and the reduction of Fe (III) to Fe (II) involves the ferric chelate reductase (FRO2), encoding the Fe (III) chelate reductase [32]. Then, Fe (II) is transported across the plasma membrane of roots via the divalent metal transporter, IRT [33]. However, the Fe acquisition mechanisms of the strategy I plants, such as the rhizospheric acidification and FCR activity, are largely hampered by high pH conditions [34]. Importantly, inoculation with certain PGPR strains, such as Paenibacillus polymyxa BFKC01 [14] and B. subtilis GB03 [22], significantly enhances Fe uptake in Arabidopsis plants with upregulation of the Fe deficiency-induced transcription factor 1 (FIT1), which is responsible for inducing the expression of IRT1 and FRO2 [35]. Moreover, GB03 VOCs have been demonstrated to regulate plant Fe acquisition through FIT1-dependent pathways [22]. However, it remains unclear how GB03 VOCs activates the FIT1-mediated Fe acquisition in Arabidopsis.
In the past decade, Fe deficiency in plants has become one of the most crucial issues for human health. Over 50% of the world’s population is anemic, primarily due to Fe deficiency [36]. Therefore, understating mVOCs-induced plant uptake of Fe is beneficial to increase the source of Fe in foods for humans. Besides Fe, improved selenium (Se) absorption by plants has recently attracted considerable attention. Although Se is not essential for plant growth, it can enhance the tolerance of plants to various abiotic stresses by activation of antioxidant defense systems [37]. For both animals and humans, Se is an essential mineral element require for growth, immune functions, and metabolism [38]. Se in soil can be directly taken up by plants and enter into the food chain. The Se content of plant foods is closely related to soil Se levels, and a low availability of Se in soils has been reported in many countries such as China, Korea, Japan, the USA, and Australia [39]. Thus, it is imperative to develop efficient strategies to improve plant Se absorption. It has increasingly been recognized that several Bacillus strains such as B. cereus YAP6, B. licheniformis YAP7, and B. pichinotyi YAM2 exhibit great potential to increase Se uptake in wheat plants [40,41]. Nevertheless, whether mVOCs affect plant Se assimilation and its underlying mechanisms has not been determined to date.
In the present study, a novel strain of Bacillus amyloliquefaciens (strain BF06) was isolated from calcareous soils. A blend of VOCs was released by BF06 to trigger plant growth without physical contact with the plant roots. BF06 VOCs not only induced a marked increase of biomass, but also augmented the content of Fe in plants with promoted synthesis of photosynthetic machinery and activities. Our results indicated that microbial-induced accumulation of Fe depended on VOCs-mediated cellular NO synthesis in Arabidopsis. Furthermore, the production of VOCs by BF06 remarkably enhanced plant Se uptake with upregulation of some putative sulfate transporter genes. Our results indicated that BF06 VOCs could improve plant nutrient status by invoking various metabolic pathways such as response to Fe, Fe homeostasis, and sulfate transport.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Arabidopsis thaliana (ecotype Col-0) and the sulrt1;2 (sel1-8 allele) mutants harboring the I551T ethylmethylsulfone (EMS)-induced mutation in At1g78000 were used in this study. The seeds were initially surface sterilized for 5 min using 0.1% HgCl2, followed by three rinses with sterile water. Then, the sterilized seeds were placed on a half-strength (1/2) Murashige and Skoog (MS) agar medium for seven days. All cultures were kept at 23 °C with 16 h light/8 h dark cycle, and 130 μmol·m−2·s−1 light intensity. Lastly, seven-day-old seedlings were transferred to 1/2 MS agar medium and subjected to various treatments for 10 days.
2.2. Assays of Microbial VOCs
B. amyloliquefaciens BF06 was isolated from rhizospheric soils of cultivated maize plants and identified by 16S rDNA sequencing (Genebank No. KY235778). This BF06 strain was cultured in 10 mL solid phase micro-extraction (SPME) vials. Then, the vials were conditioned at 50 °C with SPME fibers, and the fibers were inserted into the headspace above bacterial samples for 1 h. Subsequently, the fibers were desorbed and injected in a Gas Chromatography–Mass Spectrometer (GC–MS) (Agilent 5975C, Santa Rosa, CA, USA) according to the method described by Miyazawa et al. [42]. The compositions of VOCs were analyzed using a U.S. National Institute of Standards and Technology (NIST) 2.0 mass spectra database search.
2.3. Measurement of Total Chlorophyll Content and Chlorophyll Fluorescence
To measure the amount of chlorophyll, approximately 200 mg of leaf samples was ground with 5 mL 80% (v/v) aqueous acetone, followed by centrifugation at 12,000× g for 10 min. The absorbance values of supernatant were recorded by spectrophotometer (Thermo Fisher Scientific, Chelmsford MA, USA) at 645 and 663 nm, respectively. Chlorophyll amounts were calculated using the formula previously described by Porra [43]: 20.21 × A645 + 8.02 × A663. In addition, chlorophyll fluorescence parameters including photosystem II (PSII) maximum quantum yield (Fv/Fm), effective quantum yield (ΦPSII), and non-photochemical fluorescence quenching (NPQ) were measured as recently described by Du et al. [44].
2.4. Determination of Fe and Se Content
The samples were washed and digested, and then were used to determine the total Fe content using inductively coupled plasma spectrometry (ICP-AES, Perkin Elmer Optimal 2100DV, Norwalk, NY, USA) according to the methods reported by Lei et al. [45]. To analyze soluble Fe content, the samples was first ground and extracted with deionized water. After centrifugation at 12,000× g for 10 min, the supernatant was used to determine the concentration of soluble Fe, as previously reported by Cassin et al. [46]. Cell wall Fe was examined according to the method described by Lei et al. [45]. In addition, the Se content in plants was determined as reported by Lindblom et al. [47]. About 100 mg of dried shoot and root samples were digested in 1 mL of concentrated nitric acid for 1.5 h at 60 °C, and then 5 h at 130 °C. The digested samples were used to analyze the Se content by ICP-AES.
2.5. In Vivo Localization of NO in Plant Roots
Root NO accumulation was monitored using 4-amino-5-methylamino-2′, 7′-difluorofluorescein diacetate (DAF–FM DA) probes [48]. The samples were incubated in the solution containing 1.5 μM DAF–FM DA for 15 min, followed by three rinses with phosphate buffer saline (PBS, pH 7.4). Lastly, these samples were used to detect the fluorescence by a Leica Scanning Confocal Microscope (SP2-AOBS, Leica, Wetzlar, Germany) with a filter set (excitation: 490 nm, emission: 515 nm).
2.6. RNA-Sequencing (RNA-Seq) Analysis
Total RNA from the whole plants was extracted using TruSeq RNA Sample Preparation Kit (Illumina, San Diego, CA, USA). Then, the genomic DNA contamination in RNA samples was digested by DNase (Promega, Madison, WI, USA). The RNA quality and quantity was checked by Bioanalyzer 2100 systems (Agilent, Santa Clara, CA, USA). The RNA from three independent plants in each group was pooled to construct two cDNA libraries. Cluster generation of the cDNA libraries was conducted using the cBot (Illumina, San Diego, CA, USA) and sequenced by the Hiseq 2500 platform (Illumina, San Diego, CA, USA). 120-bp paired-end reads were generated from the two libraries. Raw RNA-Seq reads were deposited in the NCBI SRA database (accession number: SRR5122892 and SRR5122894). Analyses of RNA-Seq data were carried out as reported by Peng et al. [49]. Differentially expressed genes (DEGs) between the control and VOCs-exposed plants were identified using DEGseq at FDR-adjusted p value < 0.05. Gene ontology (GO) term (http://www.geneontology.org/) was assigned to DEGs based on the GO annotations for biological process, molecular function, and cellular component.
2.7. Quantitative Real-Time Polymerase Chain Reaction (qRT–PCR) Analyses
Total RNA from the whole plants was isolated using Trizol reagent (Invitrogen, Carlsbad, CA, USA). About 500 ng of total RNA was reversely transcribed using PrimeScript RT reagent kit according to the manufacturer’s instructions (Takara, Dalian, China). qRT–PCR was conducted in an ABI 7500 real-time PCR machine according to the method reported by Zhu et al. [50]. Arabidopsis actin2 was used as an internal control for normalization. Experiments were performed in triplicate with different cDNA samples from independent biological replicates. The primers used in this study are listed in Table S1.
2.8. Ferric-Chelate Reductase (FCR) Activity
Root FCR activity was analyzed as previously reported by Lei et al. [45]. Root samples were separated from the whole plants, and the samples were immersed in a solution containing 0.2 mM Ferrozine, 0.1 mM Fe(III)-EDTA, and 0.5 mM CaSO4 and incubated for 3 h at 21 °C in dark conditions. The Fe(II) concentration was determined by a spectrophotometer at 562 nm based on a molar extinction coefficient of 28.6 mM−1·cm−1.
2.9. Statistical Analyses
Each experiment was conducted in triplicate. Tukey’s test was used for statistical analysis. The bars indicate the mean and standard deviation (SD) of three biological replicates. Different letters above each bar indicate significant differences among the different groups at p < 0.05.
3. Results
3.1. Effects of VOCs Released by BF06 on Plant Growth and Photosynthesis
To investigate whether the release of VOCs by BF06 could regulate plant growth, the composition of mVOCs was initially extracted and identified using headspace SPME–GC–MS. Our results revealed that the strain BF06 could release 21 different types of volatile compounds (Table S2). The effects of BF06 VOCs on plant growth were further evaluated. The Arabidopsis seedlings were first cultured on 1/2 MS medium for seven days. Then, the plants were transferred to two separate petri dishes with or without exposure to BF06 VOCs. After 10 days of BF06 VOCs exposure, the growth phenotype and several physiological parameters were analyzed in both the non-exposed (control) and VOCs-exposed plants. The VOCs-exposed plants appeared to show better growth performance than the controls (Figure 1A,B). Shoot and root fresh weights were notably higher in the VOCs-exposed plants than in the controls (Figure 1C). Similar results were observed for dry weights (Figure 1D). In addition, the number of rosette leaves was greater in the VOCs-exposed plants than the controls (Figure 1E). The VOCs-exposed plants also exhibited a larger size of rosette leaves compared with the controls (Figure 1F).

Figure 1.
The release of VOCs by B. amyloliquefaciens (BF06) improved the growth of Arabidopsis plants. Seven-day-old seedlings were treated with or without exposure to BF06 VOCs for 10 days, then these plants were selected to analyze their growth phenotypes (A,B); fresh weight (C); dry weight (D); rosette leaf number (E); and total leaf areas (F). Different letters represent significant difference between the control and VOCs-exposed plants at p < 0.05.
BF06 VOCs exposure markedly increased total chlorophyll content to over 28% as compared to the controls with 1.55 ± 0.16 mg·Chl·g−1 FW (Figure 2A). We further examined net photosynthetic rate (Pn) and chlorophyll fluorescence parameters in leaves of both the control and VOCs-exposed plants. The VOCs-exposed plants exhibited higher values of Pn than the controls (Figure 2B). The maximum photosystem II (PSII) quantum ration of variable to maximum fluorescence (Fv/Fm) in the VOCs-exposed plants was remarkably higher than that in the controls (Figure 2C). A similar trend of the effective quantum yields of PSII photochemistry (ΦPSII) was seen in the tested plants (Figure 2D). In addition, the values of NPQ were notably higher in the VOCs-exposed plants than in the controls (Figure 2E), indicating that mVOCs exposure reduced excitation pressure on PSII by thermal energy dissipation.
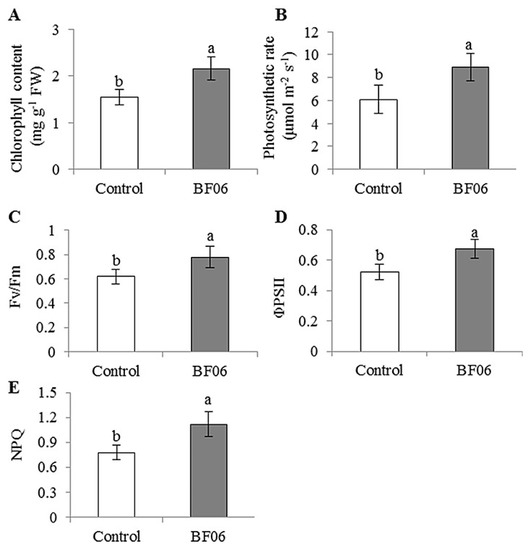
Figure 2.
The release of VOCs by BF06 increased chlorophyll content and photosynthetic efficiency in Arabidopsis. After 10 days of exposure to microbial VOCs, we examined chlorophyll content (A); and photosynthetic parameters including photosynthetic rate (B); Fv/Fm (C); ΦPSII (D); and NPQ (E) in plants. Different letters represent a significant difference between the control and VOCs-exposed plants at p < 0.05.
3.2. Transcriptomic Analysis of BF06 VOCs-Regulated Gene Profiles
To decipher the mechanisms underlying the release of VOCs by BF06 regulated plant growth, whole genome expression profiling of Arabidopsis plants was performed using RNA-Seq. For this purpose, seven-day-old Arabidopsis plants were grown on 1/2 MS medium for 10 days with or without exposure to BF06 VOCs. Changes in gene transcription were examined by comparing the control and VOCs-exposed plants. To evaluate the accuracy of RNA-Seq analyses, the original sequences were firstly filtered to ensure the quality of reads. The RNA-Seq generated about 35.5 million raw reads, and approximately 98% of the raw reads were mapped to the Arabidopsis genome. The RNA-Seq analyses further revealed that 761 genes were differentially expressed in the VOCs-exposed plants. Compared with the controls, 335 genes were upregulated and 426 genes were downregulated in the VOCs-exposed plants (Table S3). To further explore the biological processes in Arabidopsis plants that were mediated by VOCs released by BF06, gene ontology (GO) analysis was applied to the above DEGs, and enrichment analysis was carried out by using an FDR-adjusted p value < 0.05 as the cutoff. The GO enrichment analysis revealed the major functional categories including biological processes (BP), cellular components (CC), and molecular function (MF) in the upregulated (Ur) and downregulated (Dr) DEGs. The most overrepresented GO terms in the BP domain of Ur DEGs related to some important metabolic pathways such as amino acid transport, Fe homeostasis, response to Fe, and sulfate transport (Figure 3). Furthermore, the most overrepresented categories in the BP domain of Dr DEGs were closely associated with the abscisic acid (ABA)-related pathways including response to ABA, response to various stresses such as water deprivation, cold, salt and wounding, and glutathione, hydrogen peroxide, and toxin catabolic processes (Figure S1).
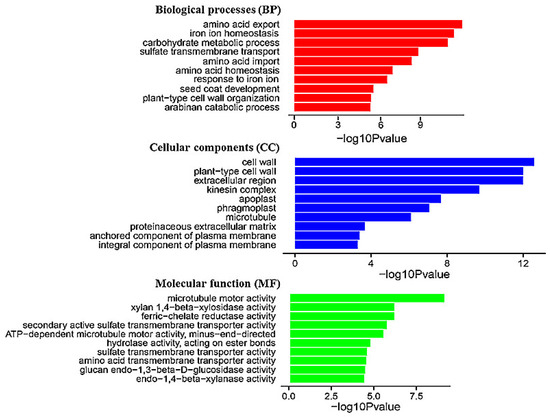
Figure 3.
List of the top 10 significant GO terms for upregulated differentially expressed genes (DEGs) of VOCs-exposed Arabidopsis plants based on GO classifications. GO terms were categorized into three functional groups including biological process (BP), cellular component (CC), and molecular function (MF). The p value indicates the significance of the comparison between the control and VOCs-exposed plants.
Plant roots can secrete abundant metabolites such as amino acids, sugars and organic acids, which serve as a carbon source for most soil-borne bacteria [2,4]. Herein, BF06 VOCs sufficiently activated the expression of some genes encoding amino acid transporters (Table S4), suggesting that soil microbes could release VOCs to induce root exudates such as amino acids for attracting them colonizing in the rhizosphere, which was not required for physical contact with host plants. We also found that BF06 VOCs markedly upregulated some genes associated with the response of plants to Fe and its homeostasis (Table S4). Recent studies have indicated that Fe deficiency is the major limiting factor for the rate of photosynthesis, and the enhanced ability of plants to absorb Fe is conducive to elevating photosynthesis [21,22]. As mentioned above, the VOCs-exposed plants exhibited higher photosynthetic efficiency compared with the controls. Thus, plant Fe acquisition was positively regulated by the release of VOCs by BF06. In addition, the transcription of several sulfate transporter genes was significantly increased in the VOCs-exposed plants compared with the controls (Table S4). These sulfate transporter genes have been demonstrated to play essential roles in Se assimilation in Arabidopsis plants [51,52,53,54]. Thus, the VOCs-induced expression of sulfate transporter genes might participate in the regulation of Se accumulation in plants.
3.3. BF06 VOCs Enhance the Tolerance of Arabidopsis to Fe Deficiency
The solubility of soil Fe in the plant rhizosphere is much lower under high pH conditions, which contributes to Fe deficiency-induced chlorosis in plants [30]. Fe deficiency often caused a reduction of chlorophyll content, and severely interfered with the structure and functions of chloroplasts [14]. In this study, we explored the effects of BF06 VOCs on the responses of plants to alkaline stress. Seven-day-old Arabidopsis seedlings were grown on an alkaline MS medium (pH = 7.6) under different Fe conditions, for 10 days with or without exposure to BF06 VOCs. We found that VOCs exposure evidently alleviated the leaf chlorosis caused by Fe deficiency (Figure 4A), indicating increased Fe levels in the VOCs-exposed plants. Total chlorophyll content was much higher in the VOCs-exposed plants than in the controls under alkaline stress (Figure 4B). Accordantly, BF06 VOCs exposure obviously improved photosynthetic capacity in plants. Several photosynthetic parameters including Pn, Fv/Fm, and ΦPSII were significantly elevated in the VOCs-exposed plants compared with the controls (Figure 4C–E). Considering that Fe is an essential element in photosynthesis, the levels of Fe might be correlated with BF06 VOCs exposure. Thus, we measured the content of Fe in both the control and VOCs-exposed plants under alkaline stress. Our data revealed that BF06 VOCs exposure induced a great elevation of Fe content in alkaline-treated plants (Figure 5A). Especially at 5 μM Fe, total Fe content of VOCs-exposed plants was about double that of the controls.
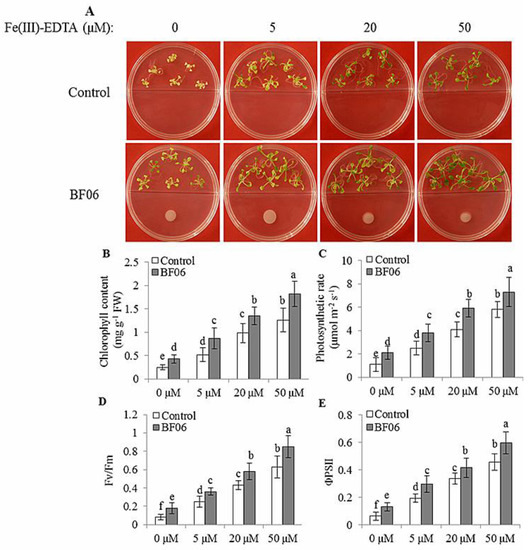
Figure 4.
BF06 VOCs promoted the growth of Arabidopsis plants under Fe-limited conditions. Seven-day-old seedlings were cultured on an alkaline MS medium containing different Fe levels (0, 5, 20, or 50 μM Fe(III)-EDTA) for 10 days with or without exposure to BF06 VOCs. The control and VOCs-exposed plants were used to analyze plant growth phenotype (A); chlorophyll content (B); photosynthetic rate (C); Fv/Fm (D); and ΦPSII (E). Different letters represent the significant difference between the control and VOCs-exposed plants at p < 0.05.
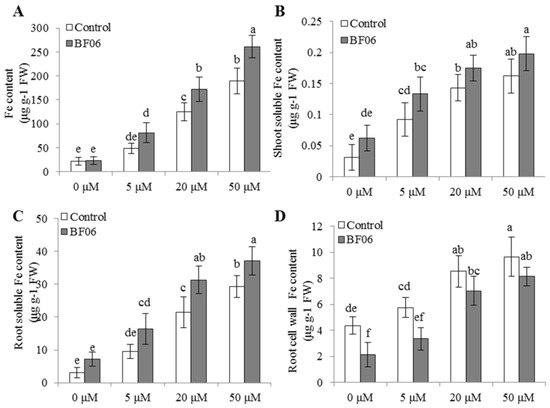
Figure 5.
BF06 VOCs increased Fe content in Arabidopsis under alkaline stress condition. Seven-day-old seedlings were cultured on an alkaline MS medium containing different Fe levels (0, 5, 20, and 50 μM) with or without exposure to BF06 VOCs. After 10 days, these plants were used to measure the content of total Fe (A); shoot (B); and root (C) soluble Fe; and root cell wall Fe (D). Different letters represent the significant difference between the control and VOCs-exposed plants at p < 0.05.
When plants were grown on the medium without the Fe source, BF06 VOCs exposure obviously ameliorated plant Fe deficiency. However, no significant difference in total Fe content was observed between the control and VOCs-exposed plants (Figure 5A). To account for this, the soluble and cell wall Fe was analyzed in the control and VOCs-exposed plants. The soluble Fe content of shoots or roots in the controls was markedly lower than in the VOCs-exposed plants (Figure 5B,C). It has previously been indicated that the cell wall plays an important role in Fe stores in plants under Fe-deficient conditions [45]. Thus, we assessed whether BF06 VOCs exposure promoted the release of root cell wall Fe under Fe-limited conditions. In this study, the cell wall of control roots had less bound Fe than the VOCs-exposed roots (Figure 5D). Similar phenomena were observed where VOCs exposure remarkably increased the soluble Fe in plants under different Fe conditions (Figure 5B,C). Our results implied that the VOCs-induced increase of soluble Fe contributed to enhancing the availability of cell wall Fe.
Furthermore, ultrastructural observation of mesophyll cells revealed that few grana lamellae rudimentary grana occurred in the chloroplasts of control plants, displaying the classic traits of thylakoid disorganization caused by Fe deficiency (Figure 6A–D). By contrast, when plants grown under alkaline stress were exposed to BF06 VOCs, the number of normal grana stacking and grana lamellae was markedly increased (Figure 6E,H). For example, no starch granules and less grana stacking was observed in the chloroplasts of control plants grown at 20 or 50 μM Fe(III)-EDTA, whereas the chloroplasts of VOCs-exposed plants exhibited better development with more grana stacking and starch granules.
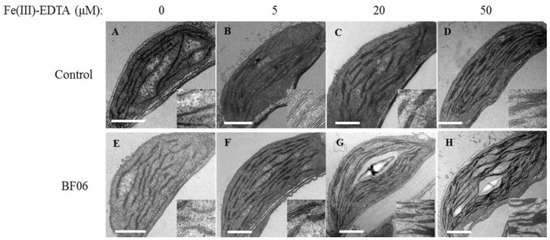
Figure 6.
Ultrastructural analyses of chloroplasts in the control and VOCs-exposed plants. Seven-day-old seedlings were cultured on an alkaline MS medium containing different concentrations of Fe(III)-EDTA (0, 5, 20, or 50 μM) with or without exposure to BF06 VOCs. Then, transmission electron micrographs of chloroplasts were observed in both the control (A–D) and VOCs-exposed (E–H) plants when plants were treated with 0, 5, 20, or 50 μM Fe(III)-EDTA, respectively. Scale bar = 1 μm.
3.4. VOCs-Induced NO Accumulation Promotes Fe Absorption and Availability
NO functions as a reciprocal signaling molecule in plant–microbe interactions, involving modulation of various physiological processes such as bacterial biofilm, plant growth, and development [55,56,57]. NO has been demonstrated to play an essential role in controlling Fe absorption in plants under Fe-limited conditions [58,59,60]. Here, DAF–FM DA was first used to analyze the accumulation of VOCs-induced NO in Arabidopsis roots. As shown in Figure 7, only a slight NO-associated fluorescence was detected in the roots of control plants grown on an alkaline MS medium containing 50 μM Fe(III)-EDTA, whereas the VOCs-exposed roots exhibited stronger fluorescence than the controls. Moreover, the VOCs-induced accumulation of root NO significantly decreased after treatment with the nitric oxide synthase (NOS) inhibitors, Nω-nitro-l-arginine methyleste (L-NAME, 5 mM), or the NO scavenger, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (c-PTIO,1 mM).
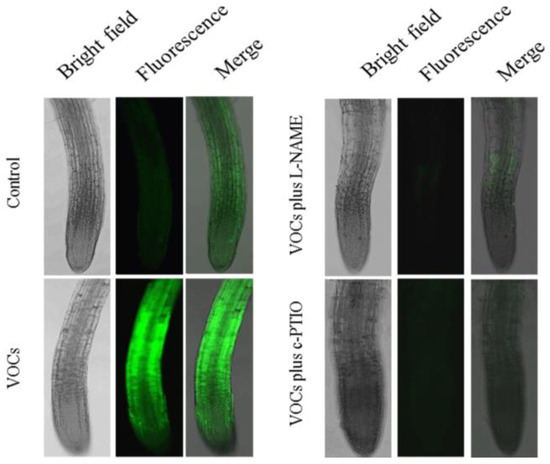
Figure 7.
Nitric oxide (NO) accumulation in Arabidopsis roots after exposure to BF06 VOCs. Arabidopsis plants were grown on an alkaline MS medium (pH = 7.6) for seven days, then transferred to 1/2 MS medium containing 50 μM Fe(III)-EDTA with or without exposure to BF06 VOCs for 10 days. Roots were incubated with the NO fluorescent probe DAF–FM DA. NO-associated fluorescence was detected in roots of non VOCs-exposed (control), VOCs-exposed, VOCs + L-NAME-treated, and VOCs + c-PTIO-treated plants. Left lines were obtained under bright-field microscopy, middle lines were imaged under green fluorescence, and the bright and fluorescence images were then merged.
NO has been demonstrated to be involved in the regulation of plant responses and tolerance to Fe-deficient conditions [59]. Moreover, NO exposure can promote the formation of nitrosyl–Fe complexes, thereby increasing the mobilization of Fe and delivery of NO to different tissues [61]. Reduced production of NO influences FIT-regulated Fe deficiency responses in plants. The Arabidopsis FIT1 plays a vital role in mediating Fe deficiency responses, which is responsible for upregulating IRT1 and FRO2 [35]. In this study, BF06 VOCs exposure significantly enhanced the expression of FIT1, IRT1 and FRO2 in plants under alkaline stress. As shown in Figure 8A–C, after 10 days of exposure to BF06 VOCs, the expression of FIT1 increased at least 6-fold in plants grown on an alkaline medium containing different Fe concentrations. The transcription of IRT1 and FRO2 was elevated more than 6-fold in the VOCs-exposed plants compared with the controls. Moreover, the activity of FCR in the VOCs-exposed plants was markedly higher than in the controls. However, the activity of FCR was significantly decreased in plants treated with L-NAME or c-PTIO. Similarly, the transcription levels of FIT1, FRO2, and IRT1 were also markedly lower in the non-treated plants than in the L-NAME- and c-PTIO-treated plants.
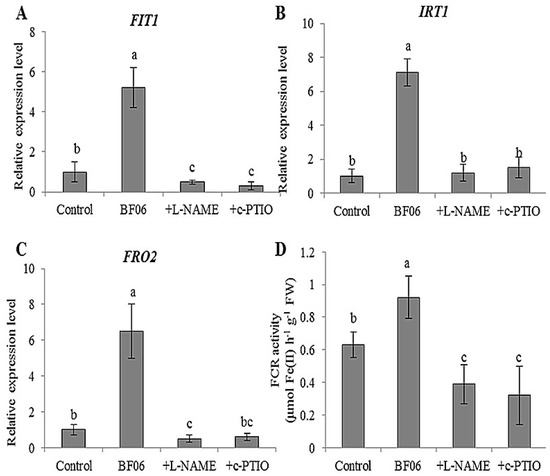
Figure 8.
Expression of Fe acquisition-related genes and FCR activity in Arabidopsis plants after exposure to BF06 VOCs. Arabidopsis plants were grown on an alkaline MS medium (pH = 7.6) for seven days, then transferred to a 1/2 MS medium containing 50 μM Fe(III)-EDTA with or without exposure to BF06 VOCs for 10 days. qRT-PCR analyses of the expression of FIT1 (A), IRT1 (B), and FRO2 (C) among non VOCs-exposed (control), VOCs-exposed, VOCs + L-NAME-treated, and VOCs + c-PTIO-treated plants, and their FCR activities were further examined (D). Different letters represent a significant difference between the control and VOCs-exposed plants at p < 0.05.
We addressed the question of whether microbial VOCs-regulated plant Fe absorption was attributable to the action of NO. Seven-day-old Arabidopsis seedlings were grown on an alkaline MS medium under different Fe conditions for 10 days with or without exposure to BF06 VOCs. Our results indicated that BF06 VOCs exposure failed to enhance the ability of plants to absorb Fe, or to mitigate chlorotic symptoms under Fe-limited conditions when plants were treated with 5 mM L-NAME or 1 mM c-PTIO (Figure 9).
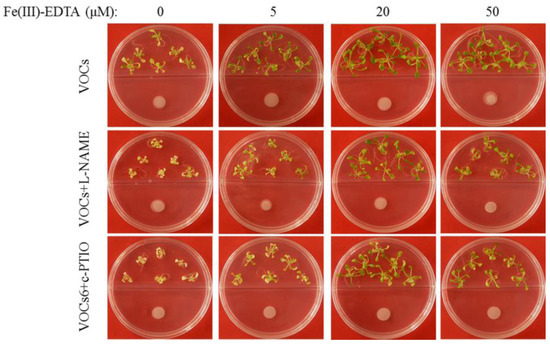
Figure 9.
Phenotype of the BF06 VOCs-exposed Arabidopsis plants after treatment with L-NAME or c-PTIO. Arabidopsis plants were grown on an alkaline MS medium (pH = 7.6) for seven days, then transferred to 1/2 MS medium containing different Fe levels (0, 5, 20, or 50 μM Fe(III)-EDTA) with exposure to BF06 VOCs for 10 days. However, treatment with L-NAME or c-PTIO diminished the positive effects of BF06 VOCs on alleviating plant Fe deficiency.
Additionally, the total chlorophyll content of BF06 VOCs-exposed plants was markedly decreased after treatment with L-NAME or c-PTIO (Figure 10A), which was in accordance with the phenotype observed. Furthermore, we determined the total Fe content in both the non-treated and treated plants. Treatment with L-NAME or c-PTIO abolished the promoting effects of VOCs on Fe uptake in plants. Consistently, the treated plants had less total Fe content than the non-treated plants, but no significant difference was observed under the conditions of no Fe source (Figure 10B). Moreover, we examined soluble Fe levels in the VOCs-exposed plants with or without treatment with L-NAME or c-PTIO. As shown in Figure 10C,D, the shoots and roots of non-treated plants accumulated more soluble Fe content than L-NAME or c-PTIO-treated plants under different Fe conditions. Conversely, the level of root cell wall Fe was observed to be notably lower in the non-treated plants compared with the L-NAME- or c-PTIO-treated plants (Figure 10E). Collectively, these results indicated that VOCs-induced root NO accumulation was essential for increasing the soluble Fe content and Fe absorption in plants.
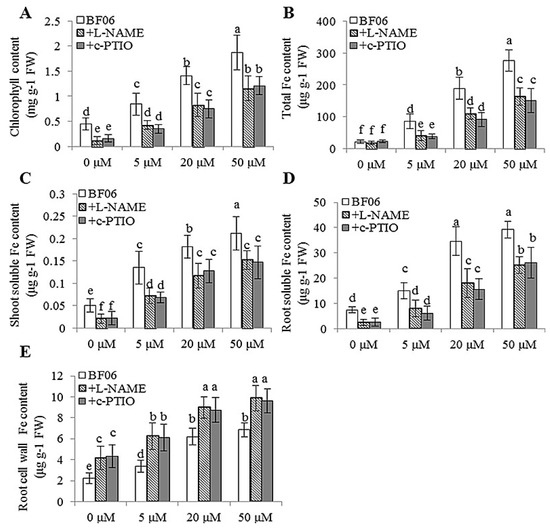
Figure 10.
Treatment with L-NAME or c-PTIO reduced the content of chlorophyll and Fe in Arabidopsis plants after exposure to BF06 VOCs. Arabidopsis plants were grown on an alkaline MS medium (pH = 7.6) for seven days, then transferred to a 1/2 MS medium containing different Fe concentrations (0, 5, 20, or 50 μM Fe(III)-EDTA) with exposure to BF06 VOCs for 10 days. Subsequently, these plants were sampled to measure the content of chlorophyll (A), total Fe (B), shoot (C) and root (D) soluble Fe, and root cell wall Fe (E). Different letters represent a significant difference between the control and VOCs-exposed plants at p < 0.05.
3.5. Effects of BF06 VOCs on Se Accumulation in Plants
Previous studies have indicated that Se can be transported into root cells via some sulfate transporter genes [62]. Activating sulfate transporter is conducive to promoting Se uptake and translocation from roots to shoots in plants [51,52,53,54]. In this study, the data of RNA-Seq revealed that the release of VOCs released by BF06 induced the expression of sulfate transporter genes including SULRT1;1, SULRT1;2, SULTR2;1, SULTR2;2 and SULTR3;5, indicating that BF06 VOCs was likely involved in the regulation of Se uptake in Arabidopsis. Furthermore, analyses of qRT-PCR revealed that the transcription levels of these sulfate transporter genes were markedly upregulated in Arabidopsis after exposure to BF06 VOCs (Figure 11A).

Figure 11.
Effects of BF06 VOCs on the expression of some sulfate transporter genes, and Se accumulation in Arabidopsis plants. Seven-day-old seedlings were treated with or without exposure to BF06 VOCs for 10 days, then these plants were sampled to analyze the expression of sulfate transporter genes including SULRT1;1, SULRT1;2, SULRT2;1, SULRT2;2, and SULRT3;5 (A); Furthermore, the content of Se was examined in shoots and roots of both the WT and sulrt1;2 mutants after 10 days of exposure to BF06 VOCs, respectively (B). Different letters represent the significant difference of the comparisons at p < 0.05.
To further determine the endogenous content of Se in wild-type (WT) plants, seven-day-old seedlings were firstly grown on a 1/2 MS medium, then transferred to a 1/2 MS medium containing 10 or 30 μM Se and grown for 10 days with or without exposure to BF06 VOCs. Our data showed that the content of Se in the VOCs-exposed WT shoots was increased by about 23% compared with that in non VOCs-exposed (control) plants, and the roots of VOCs-exposed plants displayed higher Se accumulation than the controls (Figure 11B). However, the content of Se was significantly higher in shoots of VOCs-exposed WT plants than the VOCs-exposed WT roots. Moreover, the effects of BF06 VOCs on the Se accumulation in sultr1;2 mutants were analyzed. As shown in Figure 11B, the shoots of sultr1;2 mutants accumulated less Se than WT plants after two weeks of exposure to BF06 VOCs. Similar results were observed for the roots. These results indicated that the VOCs-induced expression of sulfate transporter genes was responsible for improving Se accumulation in plants.
4. Discussion
In natural environments, diverse microbial communities colonize in the rhizosphere of plants, and affect plant growth and fitness [1,2,3]. PGPR form mutually beneficial associations with plants by a complicated exchange of chemical metabolites [4,5,6]. Depending on bacterial relationships with plants, PGPR can be classified into two groups: symbiotic and free-living bacteria species. PGPR can regulate multiple physiological processes in plants by different mechanisms, such as synthesizing plant growth-regulating compounds, increasing the solubility of soil nutrients, and activating a plant’s defense system [4,5,6,7,8]. Several free-living PGPR strains can facilitate plants’ acquisition of nutrients from their surroundings, and help them resist soil-borne pathogens [1,2]. Ample evidence has indicated that the microbial release of VOCs triggers plant growth without physical contact with the roots [9,10,11]. The shift of soil microbes to belowground space is physically constrained by the soil, whereas mVOCs can easily diffuse into the rhizosphere and reach the aboveground tissues of plants [9,10]. Rhizospheric conditions are beneficial to VOCs-mediated communication in plant–microbe interactions. Importantly, mVOCs have been demonstrated to play an important role in modulating nutrient absorption by plants [22]. Herein, we demonstrated that the release of VOCs by BF06 induced the expression of some Fe uptake-related genes, thereby enhancing plants’ Fe acquisition machinery. Such regulation of Fe acquisition mechanisms by microbial VOCs was previously shown to improve plant growth under Fe deficiency. Additionally, BF06 VOCs also markedly enhanced Se accumulation in plants via the upregulation of sulfate transporter genes.
4.1. A Promoting Effect of BF06 VOCs on Plant Growth
B. amyloliquefaciens is a PGPR well known for its ability to regulate plant growth and increase the resistance of plants to abiotic or biotic stresses [62,63,64]. In this study, we investigated the effects of B. amyloliquefaciens BF06 VOCs on the growth of Arabidopsis plants. Our data showed that the blend of VOCs produced by BF06 significantly improved plant growth, with increased biomass. The results of GC-MS analyses showed that BF06 could produce about 21 types of VOCs, including 2,3-butanediol. Ryu et al. [10] have reported that the release of 2,3-butanediol by GB03 is involved in promoting plant growth and activating ISR in plants. Several other PGPR strains such as P. chlororaphis O6 can produce 2,3-butanediol, which induces systemic resistance and enhanced drought tolerance in plants by regulation of stomatal closure and water loss rate [10,25]. In addition, application of 2,3-butanediol has been shown to enhance the generation of NO, and inhibition of NO synthesis impairs 2,3-butanediol-triggered stress resistance [65]. This indicated a key role for NO signaling in improving stress tolerance conferred by exposure to 2,3-butanediol.
To clarify the mechanism of BF06 VOCs-regulated plant growth, comparative transcriptomic analysis was performed to identify the DEGs of the VOCs-exposed plants. The results of RNA-Seq indicated that the expression of some Fe acquisition-related genes such as FIT1, IRT1, and FRO2 was markedly upregulated in the VOCs-exposed plants. Zhang et al. [22] have indicated that GB03 VOCs activate Fe acquisition and enhance Fe accumulation in plants via upregulation of FIT1. In fit1 mutants, GB03 VOCs failed to induction of FIT1-mediated Fe acquisition gene expression Therefore, the release of VOCs by BF06 induced the expression of FIT1 in plants, possibly due to Fe absorption. Enhanced Fe uptake has been reported to increase chlorophyll content and photosynthesis in plants [22]. As observed in this study, the VOCs-exposed plants exhibited higher chlorophyll content and photosynthetic capacity. Similar results were shown in Arabidopsis plants exposed to GB03 VOCs. Intriguingly, the expression of some ABA biosynthetic and responsive genes was obviously downregulated in plants exposed to BF06 VOCs (Table S4). Zhang et al. [66] have reported that treatment with ABA abolishes GB03 VOCs-induced increase of photosynthesis and chlorophyll levels in plants. Moreover, GB03 VOCs exposure enhances photosynthetic efficiency in Arabidopsis via the inhibition of ABA signaling pathways.
4.2. NO Is Essential for mVOCs-Induced Plant Fe Acquisition
Considerable attention has been paid to plant Fe deficiency due to the low solubility of soil Fe [30]. The bioavailability of Fe is extremely low in alkaline soils, and Fe forms insoluble Fe3+ oxy-hydroxides [29]. In this study, we assessed the ability of VOCs-exposed plants to take up Fe under alkaline conditions. When plants were cultured on an alkaline medium containing different Fe levels, the VOCs-exposed plants exhibited better growth traits with a high level of chlorophyll content. Previous studies have indicated that chlorophyll is the major component of the chloroplasts, which is closely associated with the Fe content of leaves [28]. Fe deficiency results in a significant decrease in chlorophyll content and massive degradation of chloroplast proteins, thereby damaging the structural and functional integrity of chloroplasts [14]. In this study, the control plants exhibited chlorotic leaves under Fe-limited conditions, and fewer photosynthetic lamellae occurred in the chloroplasts. Nevertheless, BF06 VOCs exposure apparently promoted normal grana stacking and increased the number of grana lamellae in the chloroplasts, and thus alleviated chlorotic symptoms. Consistently, total Fe content was significantly higher in the VOCs-exposed plants than in the controls, but this was not observed in plants grown at 0 μM Fe(III)-EDTA. Interestingly, the VOCs-exposed plants grown with no Fe also displayed greener leaves with better chloroplast development under alkaline stress. Thus, BF06 VOCs exposure could promote reutilization of cell wall Fe in plants under Fe-limited conditions. To verify the hypothesis, the content of soluble and cell wall Fe was examined in both the control and VOCs-exposed plants. Our results showed that VOCs exposure led to higher levels of soluble Fe in plants, and a significant decrease of root cell wall Fe. This raised a question as to how BF06 VOCs regulated Fe assimilation and reutilization of cell wall Fe in plants.
NO is an essential regulator that participates in Fe deficiency responses in plants [58]. NO not only activates FER or FIT1-dependent signaling pathways [55], but also functions as an Fe chelator to increase the mobilization of cell wall Fe [60]. Intriguingly, the emission of VOCs by some bacterial strains, such as 2R or 3R-butanediol, induces NO synthesis in plants [65]. In our experiments, BF06 could produce VOCs, containing 2R or 3R-butanediol. Thus, we further investigated whether the VOCs-induced increase of total or soluble Fe content contributed to enhancing NO synthesis. Our data reveled that VOCs exposure resulted in a greater increase of root NO accumulation, but this induction was notably curtailed by the application of L-NAME or c-PTIO. It is well demonstrated that NO regulates Fe deficiency responses via the induction of FRO2 and IRT1 expression regulated by FIT1 [59]. Here, we found that treatment with L-NAME largely abrogated the expression of VOCs-induced FIT1, IRT1, and FRO2. It was also observed that total or soluble Fe content was not pronouncedly increased in the VOCs-exposed plants when treated with L-NAME or c-PTIO under alkaline stress. Consistently, the VOCs-induced reduction of cell wall Fe was not found in the NO-deficient plants. Therefore, the enhanced reutilization of cell wall Fe and Fe uptake was dependent on the VOCs-induced NO production in roots.
4.3. BF06 VOCs Regulates Plant Uptake of Se by Upregulating of Sulfate Transporter Genes
Recently, some PGPR strains have been shown to promote Se accumulation in plants [40,41], implying that the system of Se uptake in plants may be triggered by certain microbial signals. In this study, combined analyses of RNA-Seq and qRT-PCR confirmed that the release of VOCs by BF06 distinctly upregulated the expression of several sulfate transporter genes including SULRT1;1, SULRT1;2, SULTR2;1, SULTR2;2, and SULTR3;5, which are involved in the regulation of Se uptake and translocation [51,52,53,54]. For this purpose, we investigated the effects of microbial VOCs on Se absorption in Arabidopsis plants. The results showed that the accumulation of Se was significantly increased in the VOCs-exposed plants compared with the controls, whereas the Se content was evidently higher in roots than in shoots. Previous studies suggested that Se in soils can be taken up by the roots through high-affinity sulfate transporters (HASTs), such as AtSULRT1;1 and AtSULRT1;2 transporters [67]. The transcription levels of genes encoding homologs of SULRT1;1 and SULRT1;2 markedly increases in the Se non-accumulator plant species when tissue Se concentration is elevated [68]. However, these genes are constitutively expressed at a relatively high level in the roots of Se hyperaccumulator species [69], accounting for their higher Se capacity.
Moreover, the enhanced expression of genes encoding HASTs, particularly SULTR1;1, confers improved Se uptake in plants [70,71]. In this study, we found that a higher level of Se accumulates in VOCs-exposed Arabidopsis roots, while VOCs exposure could not induce an increase of Se in the sultr1;1 mutants. Thus, the VOCs-regulated SULRT1;1 expression was responsible for high accumulation of Se in plants. In addition, the shoots of VOCs-exposed plants also accumulated more Se than those of the controls, indicating that microbial VOCs might activate xylem or phloem transport mechanisms, in turn facilitating Se’s translocation from roots to shoots. It has previously been indicated that some sulfate transporters, including SULTR2;1, SULTR2;2, and SULTR3;5, participate in the long-distance xylem transport of Se in Arabidopsis [60]. The low-affinity sulfate transporters AtSULTR2;1 and AtSULTR2;2 have also been found to catalyze Se uptake into the stele cells, and the activity of AtSULTR2;1 can be positively regulated by the AtSULTR3;5 that does not directly catalyze Se transport [72]. Additionally, increased Se availability can induce the expression of SULTR2;1 and SULTR2;2 in Arabidopsis [60,73,74]. Intriguingly, the transcription levels of SULRT2 are greater in the roots of Se hyperaccumulator species than non-Se hyperaccumulator species, indicating that high-level expression of SULRT2 contributed to the increased Se flux from roots to shoots in the hyperaccumulator species [75]. In this study, the expression of SULTR2;1, SULTR2;2, and SULTR3;5 was observably enhanced by exposure to BF06 VOCs. Thus, upregulation of these genes by VOCs leads to efficient translocation of Se from roots to shoots, thereby elevating shoot Se accumulation.
5. Conclusions
Based on the data shown in this study, the following model was proposed linking the release of VOCs by BF06 to activation of Fe and Se uptake in plants (Figure 12). BF06 VOCs triggered the accumulation of NO in roots, thereby stimulating Fe acquisition systems. Moreover, BF06 VOCs exposure also induced a great increase of Se accumulation in plants via the activation of some sulfate transporters. This research provides evidence that the production of VOCs by BF06 can regulate plant uptake of Fe and increase its bioavailability under alkaline conditions, and also demonstrates its potential roles in promoting Se assimilation in plants.
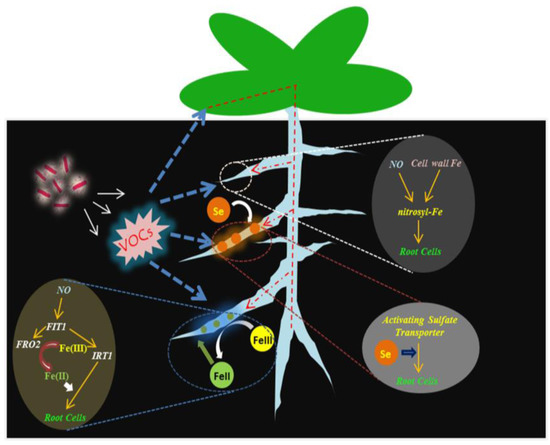
Figure 12.
A proposed model of BF06 VOCs-induced Fe and Se absorption in plants. The release of VOCs by BF06 can induce root NO accumulation, thereby increasing the availability of cell wall Fe and activating FIT1-mediated Fe acquisition. Moreover, BF06 VOCs has been shown to improve plant uptake of Se through the regulation of some important sulfate transporters.
Supplementary Materials
The following are available online at http://www.mdpi.com/2076-3417/7/1/85/s1, Table S1: Primers used in this study; Table S2: Volatile profile of Bacillus amyloliqefaciens BF06, using headspace solid-phase micro extraction (SPME) combined with gas chromatography–mass spectrometry (GC–MS). Different letters represent a significant difference between the control and VOCs-exposed plants at p < 0.05; Table S3: Upregulated and downregulated genes (more than 2-fold) differentially expressed in the VOCs-exposed plants; Table S4: Upregulated or downregulated differentially expressed genes (DEGs) including some amino acid transport-, Fe acquisition-, and sulfate transport-related genes in the VOCs-exposed plants; Figure S1: List of the top 10 significant GO terms in biological process (BP) domain for downregulated differentially expressed genes (DEGs) in the VOCs-exposed plants based on GO classifications. The p value indicates a significant difference between the control and VOCs-exposed plants.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31600210), the Research Foundation of the Ministry of Agriculture (BOFC2015KB02), the National Sparking Plan Project (2015GA710013, 2015GA710014), the Key Research Project of the Anhui Science and Technology Committee (1301032151, 15CZZ03102), the Natural Science Foundation of Anhui Province (1508085QD74, 1608085MC59), the Public Technological Application Project of the Anhui Science and Technology Committee (1604f0704045), and the Research Foundation of Anhui Science and Technology University (ZRC2014403).
Author Contributions
Jianfei Wang and Cheng Zhou conceived and designed the experiment; Cheng Zhou and Lin Zhu performed the experiment; Cheng Zhou, Xin Xiao, Yue Xie, and Zhongyou Ma analyzed the data; Cheng Zhou wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Kloepper, J.W.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Rodriguez-Kabana, R.; Zehnder, G.W.; Murphy, J.; Sikora, E.; Fernandez, C. Plant root-bacterial interactions in biological control of soil borne diseases and potential extension to systemic and foliar diseases. Aust. J. Plant Pathol. 1999, 28, 27–33. [Google Scholar] [CrossRef]
- Lin, W.; Okon, Y.; Hardy, R.W.F. Enhanced mineral uptake by Zea mays and Sorghum bicolor roots inoculated with Azospirillum brasilense. Appl. Environ. Microbiol. 1983, 45, 1775–1779. [Google Scholar] [PubMed]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant roots. Annu. Rev. Plant Phys. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Driouich, A.; Follet-Gueye, M.L.; Vicré-Gibouin, M.; Hawes, M. Root border cells and secretions as critical elements in plant host defense. Curr. Opin. Plant Biol. 2013, 16, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Loper, J.E.; Schroth, M.N. Influence of bacterial sources of indole-3-acetic acid on root elongation of sugar beet. Phytopathology 1986, 76, 386–389. [Google Scholar] [CrossRef]
- MacDonald, E.M.S.; Powell, G.K.; Regier, D.A.; Glass, N.L.; Roberto, F.; Kosuge, T.; Morris, R.O. Secretion of zeatin, ribosylzeatin and ribosyl-1″-methylzeatin by Pseudomonas savastanoi plasmid coded cytokinin biosynthesis. Plant Physiol. 1986, 82, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, S.; Nicander, B.; Granhall, U.; Tillberg, E. Cytokinin production by Paenibacillus polymyxa. Soil Biol. Biochem. 1999, 31, 1847–1852. [Google Scholar] [CrossRef]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Wei, H.X.; Paré, P.W.; Kloepper, J.W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. USA 2003, 100, 4927–4932. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.M.; Farag, M.A.; Hu, C.H.; Reddy, M.S.; Kloepper, J.W.; Paré, P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Coleman-Derr, D.; Tringe, S.G. Building the crops of tomorrow: Advantages of symbiont-based approaches to improving abiotic stress tolerance. Front. Microbiol. 2014, 5, 283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, Z.Y.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Wang, J.F. Rhizobacterial strain Bacillus megaterium BOFC15 induces cellular polyamine changes that improve plant growth and drought resistance. Int. J. Mol. Sci. 2016, 17, 976. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, L.; Orlando, J.; Rodríguez, M.I.; Chulze, S.; Etcheverry, M. Biocontrol of Bacillus subtilis against Fusarium verticillioides in vitro and at the maize root level. Res. Microbiol. 2005, 156, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Guo, J.S.; Zhu, L.; Xiao, X.; Xie, Y.; Zhu, J.; Ma, Z.Y.; Wang, J.F. Paenibacillus polymyxa BFKC01 enhances plant iron absorption via improved root systems and activated iron acquisition mechanisms. Plant Physiol. Biochem. 2016, 105, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Grayston, S.; Chanway, C. N2-fixation and seedling growth promotion of lodgepole pine by endophytic Paenibacillus polymyxa. Microb. Ecol. 2013, 66, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Pii, Y.; Mimmo, T.; Tomasi, N.; Terzano, R.; Cesco, S.; Crecchio, C. Microbial interactions in the rhizosphere: beneficial influences of plant growth-promoting rhizobacteria on nutrient acquisition process. Rev. Biol. Fertil. Soils 2015, 51, 403–415. [Google Scholar] [CrossRef]
- Wenke, K.; Kai, M.; Piechulla, B. Belowground volatiles facilitate interactions between plant roots and soil organisms. Planta 2010, 231, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Effmert, U.; Kalderás, J.; Warnke, R.; Piechulla, B. Volatile mediated interactions between bacteria and fungi in the soil. J. Chem. Ecol. 2012, 38, 665–703. [Google Scholar] [CrossRef] [PubMed]
- Kanchiswamy, C.N.; Malnoy, M.; Maffei, M.E. Chemical diversity of microbial volatiles and their potential for plant growth and productivity. Front. Plant Sci. 2015, 6, 151. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Farag, M.A.; Park, H.B.; Kloepper, J.W.; Lee, S.H.; Ryu, C.M. Induced resistance by a long-chain bacterial volatile: Elicitation of plant systemic defense by a C13 volatile produced by Paenibacillus polymyxa. PLoS ONE 2012, 7, e48744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kim, M.S.; Krishnamachari, V.; Payton, P.; Sun, Y.; Grimson, M.; Farag, M.A.; Ryu, C.M.; Allen, R.; Melo, I.S.; et al. Rhizobacterial volatile emissions regulate auxin homeostasis and cell expansion in Arabidopsis. Planta 2007, 226, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, Y.; Xie, X.; Kim, M.S.; Dowd, S.E.; Paré, P.W. A soil bacteria regulates plant acquisition of iron via deficiency-inducible mechanisms. Plant J. 2009, 58, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Zhang, H.; Ryu, C.M. Dynamic chemical communication between plants and bacteria through airborne signals: Induced resistance by bacterial volatiles. J. Chem. Ecol. 2013, 39, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.S.; Dutta, S.; Ann, M.; Raaijmakers, J.M.; Park, K. Promotion of plant growth by Pseudomonas fluorescens strain SS101 via novel volatile organic compounds. Biochem. Biophys. Res. Commun. 2015, 461, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.Y.; Lee, Y.H.; Cho, B.H.; Yang, K.Y.; Ryu, C.M.; Kim, Y.C. 2R,3R-butanediol, a bacterial volatile produced by Pseudomonas chlororaphis O6, is involved in induction of systemic tolerance to drought in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Meldau, D.G.; Meldau, S.; Hoang, L.H.; Underberg, S.; Wunsche, H.; Baldwin, I.T. Dimethyl disulfide produced by the naturally associated bacterium Bacillus spB55 promotes Nicotiana attenuata growth by enhancing sulfur nutrition. Plant Cell 2013, 25, 2731–2747. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Mosqueda Mdel, C.; Macías-Rodríguez, L.; Santoyo, G.; Farías-Rodríguez, R.; Valencia-Cantero, E. Medicago truncatula increases its iron-uptake mechanisms in response to volatile organic compounds produced by Sinorhizobium meliloti. Folia Microbiol. 2013, 58, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Abadia, J. Function of iron in chloroplasts. J. Plant Nutr. 1986, 9, 609–646. [Google Scholar] [CrossRef]
- Guerinot, M.L.; Yi, Y. Iron: Nutritious, noxious, and not readily available. Plant Physiol. 1994, 104, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Abadía, J.; Vázquez, S.; Rellán-Álvarez, R.; El-Jendoubi, H.; Abadía, A.; Álvarez-Fernández, A.; López-Millán, A.F. Towards a knowledge-based correction of iron chlorosis. Plant Physiol. Biochem. 2011, 49, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Santi, S.; Schmidt, W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 2009, 183, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Campbell, N.H.; Grotz, N.; Prichard, C.L.; Guerinot, M.L. Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol. 2003, 133, 1102–1110. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Fett, J.P.; Guerinot, M.L. Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation. Plant Cell 2002, 14, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Ohwaki, Y.; Sugahara, K. Active extrusion of protons and exudation of carboxylic acids in response to iron deficiency by roots of chickpea (Cicer arietinum L.). Plant Soil 1997, 189, 49–55. [Google Scholar] [CrossRef]
- Colangelo, E.P.; Guerinot, M.L. The essential basic helix–loop–helix protein FIT1 is required for the iron deficiency response. Plant Cell 2004, 16, 3400–3412. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B.; Lotfi, M.; Dalmiya, N.; Sethuraman, K.; Deitchler, M.; Geibel, S.; Gillenwater, K.; Gilman, A.; Mason, K.; Mock, N. The Micronutrient Report: Current Progress and Trends in the Control of Vitamin A, Iodine, and Iron Deficiencies; The Micronutrient Initiative/International Development Research Centre: Ottawa, ON, Canada, 2001. [Google Scholar]
- Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Tahir, M.N.; Zulfiqar, B.; Salahuddin, M.; Shabbir, R.N.; Aslam, M. Selenium supplementation affects physiological and biochemical processes to improve fodder yield and quality of maize (Zea mays L.) under water deficit conditions. Front. Plant Sci. 2016, 7, 1438. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Selinus, O.; Fordyce, F. Selenium deficiency and toxicity in the environment. In Essentials of Medical Geology; Springer: Heidelberg, Germany, 2013; pp. 375–416. [Google Scholar]
- Yasin, M.; El-Mehdawi, A.F.; Pilon-Smits, E.A.; Faisal, M. Selenium-fortified wheat: Potential of microbes for biofortification of selenium and other essential nutrients. Int. J. Phytoremediumtion 2015, 17, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Yasin, M.; El-Mehdawi, A.F.; Anwar, A.; Pilon-Smits, E.A.; Faisal, M. Microbial-enhanced selenium and iron biofortification of wheat (Triticum aestivum L.)—Applications in phytoremediumtion and biofortification. Int. J. Phytoremediumtion 2015, 17, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Naznin, H.A.; Kiyohara, D.; Kimura, M.; Miyazawa, M.; Shimizu, M.; Hyakumachi, M. Systemic resistance induced by volatile organic compounds emitted by plant growth-promoting fungi in Arabidopsis thaliana. PLoS ONE 2014, 9, e86882. [Google Scholar] [CrossRef] [PubMed]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Zhan, C.Y.; Lu, Y.; Cui, H.R.; Wang, X.Y. The conservative cysteines in transmembrane domain of AtVKOR/LTO1 are critical for photosynthetic growth and photosystem II activity in Arabidopsis. Front. Plant. Sci. 2015, 6, 238. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.J.; Zhu, X.F.; Wang, Z.W.; Dong, F.; Dong, N.Y.; Zheng, S.J. Abscisic acid alleviates iron deficiency by promoting root iron reutilization and transport from root to shoot in Arabidopsis. Plant Cell Environ. 2014, 37, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Cassin, G.; Mari, S.; Curie, C.; Briat, J.F.; Czernic, P. Increased sensitivity to iron deficiency in Arabidopsis thaliana over accumulating nicotianamine. J. Exp. Bot. 2009, 60, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Lindblom, S.D.; Fakra, S.C.; Landon, J.; Schulz, P.; Tracy, B.; Pilon-Smits, E.A.H. Inoculation of Astragalus racemosus and Astragalus convallarius with selenium-hyperaccumulator rhizosphere fungi: Effects on growth and accumulation of selenium and other elements. Planta 2012, 237, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.F.; Wang, B.; Song, W.F.; Zheng, S.J.; Shen, R.F. Putrescine alleviates iron deficiency via NO-dependent reutilization of root cell-wall Fe in Arabidopsis. Plant Physiol. 2016, 170, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; He, X.; Gao, J.; Ma, H.; Zhang, Z.; Shen, Y.; Pan, G.; Lin, H. Transcriptomic changes during maize roots development responsive to Cadmium (Cd) pollution using comparative RNAseq-based approach. Biochem. Biophys. Res. Commun. 2015, 464, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Guo, J.; Zhu, J.; Zhou, C. Enhanced expression of EsWAX1 improves drought tolerance with increased accumulation of cuticular wax and ascorbic acid in transgenic Arabidopsis. Plant Physiol. Biochem. 2014, 75, 24–35. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef] [PubMed]
- Shinmachi, F.; Buchner, P.; Stroud, J.L.; Parmar, S.; Zhao, F.J.; McGrath, S.P.; Hawkesford, M.J. Influence of sulfur deficiency on the expression of specific sulfate transporters and the distribution of sulfur, selenium, and molybdenum in wheat. Plant Physiol. 2010, 153, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Gigolashvili, T.; Kopriva, S. Transporters in plant sulphur metabolism. Front. Plant Sci. 2014, 5, 422. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Watanabe-Takahashi, A.; Smith, F.W.; Blake-Kalff, M.; Hawkesford, M.J.; Saito, K. The roles of three functional sulphate transporters involved in uptake and translocation of sulphate in Arabidopsis thaliana. Plant J. 2000, 23, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.P.; Hossain, S.; Xu, Y.; Boon, E.M. Nitric oxide regulation of bacterial biofilms. Biochemistry 2015, 54, 3717–3728. [Google Scholar] [CrossRef] [PubMed]
- Blanquet, P.; Silva, L.; Catrice, O.; Bruand, C.; Carvalho, H.; Meilhoc, E. Sinorhizobium meliloti controls nitric oxide-mediated post-translational modification of a Medicago truncatula nodule protein. Mol. Plant Microbe Interact. 2015, 28, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Hichri, I.; Boscari, A.; Castella, C.; Rovere, M.; Puppo, A.; Brouquisse, R. Nitric oxide: A multifaceted regulator of the nitrogen-fixing symbiosis. J. Exp. Bot. 2015, 66, 2877–2887. [Google Scholar] [CrossRef] [PubMed]
- Graziano, M.; Beligni, M.V.; Lamattina, L. Nitric oxide improves internal iron availability in plants. Plant Physiol. 2002, 130, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Graziano, M.; Lamattina, L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J. 2007, 52, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Yang, J.L.; Qin, C.; Jin, C.W.; Mo, J.H.; Ye, T.; Zheng, S.J. Nitric oxide acts downstream of auxin to trigger root ferric-chelate reductase activity in response to iron deficiency in Arabidopsis. Plant Physiol. 2010, 154, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Simontacchi, M.; Buet, A.; Lamattina, L.; Puntarulo, S. Exposure to nitric oxide increases the nitrosyl-iron complexes content in sorghum embryonic axes. Plant Sci. 2012, 183, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.S.; Yang, H.J.; Oh, B.J.; Anderson, A.J.; Kim, Y.C. Biological control potential of Bacillus amyloliquefaciens KB3 isolated from the feces of Allomyrina dichotoma Larvae. Plant Pathol. J. 2016, 32, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, R.; Waqas, M.; Khan, A.L.; Asaf, S.; Khan, M.A.; Kang, S.M.; Yun, B.W.; Lee, I.J. Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 2016, 106, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.M.; Kim, Y.H.; Anderson, A.J.; Kim, Y.C. Nitric oxide and hydrogen peroxide production are involved in systemic drought tolerance induced by 2R,3R-butanediol in Arabidopsis thaliana. Plant Pathol. J. 2013, 29, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Barberon, M.; Berthomieu, P.; Clairotte, M.; Shibagaki, N.; Davidian, J.C.; Gosti, F. Unequal functional redundancy between the two Arabidopsis thaliana high-affinity sulphate transporters SULTR1;1 and SULTR1;2. New Phytol. 2008, 180, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Inostroza-Blancheteau, C.; Reyes-Díaz, M.; Alberdi, K.; Godoy, Y.; Rojas-Lillo, P.; Cartes, P.; Mora, M.L. Influence of selenite on selenium uptake, differential antioxidant performance and gene expression of sulfate transporters in wheat genotypes. Plant Soil 2013, 369, 47–59. [Google Scholar] [CrossRef]
- Schiavon, M.; Pilon, M.; Malagoli, M.; Pilon-Smits, E.A.H. Exploring the importance of sulphate transporters and ATPsulphurylases for selenium hyperaccumulation—A comparison of Stanleya pinnata and Brassica juncea (Brassicaceae). Front. Plant Sci. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Bowen, H.C.; Parmaguru, P.; Fritz, M.; Spracklen, W.P.; Spiby, R.E.; Meacham, M.C.; Mead, A.; Harriman, M.; Trueman, L.J.; et al. Interactions between selenium and sulphur nutrition in Arabidopsis thaliana. J. Exp. Bot. 2004, 55, 1927–1937. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Bowen, H.C.; Marshall, B.; Broadley, M.R. Extraordinarily high leaf selenium to sulphur ratios define ‘Se-accumulator’ plants. Ann. Bot. 2007, 100, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T.; Hayashi, N.; Yamaya, T.; Takahashi, H. Root-to-shoot transport of sulfate in Arabidopsis. Evidence for the role of SULTR3;5 as a component of low-affinity sulfate transport system in the root vasculature. Plant Physiol. 2004, 136, 4198–4204. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Uraji, M.; Shimoishi, Y.; Mori, I.C.; Nakamura, Y.; Murata, Y. Mechanisms of the selenium tolerance of the Arabidopsis thaliana knockout mutant of sulfate transporter SULTR1;2. Biosci. Biotechnol. Biochem. 2012, 76, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Buchner, P.; Parmar, S.; Kriegel, A.; Carpentier, M.; Hawkesford, M.J. The sulfate transporter family in wheat: Tissue-specific gene expression in relation to nutrition. Mol. Plant 2010, 3, 374–389. [Google Scholar] [CrossRef] [PubMed]
- Cabannes, E.; Buchner, P.; Broadley, M.R.; Hawkesford, M.J. A comparison of sulfate and selenium accumulation in relation to the expression of sulfate transporter genes in Astragalus species. Plant Physiol. 2011, 157, 2227–2239. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).