Rheological Properties with Temperature Response Characteristics and a Mechanism of Solid-Free Polymer Drilling Fluid at Low Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Instruments
2.3. Experimental Methods
2.3.1. Measurement of Rheological Parameters
2.3.2. Evaluation of the Rheological Model
2.3.3. Microscopic Analysis of the Drilling Fluid
3. Results
3.1. Rheological Model of KL, PAM, and XC Drilling Fluids
3.2. Determination of the Quantities of KL, PAM, and XC
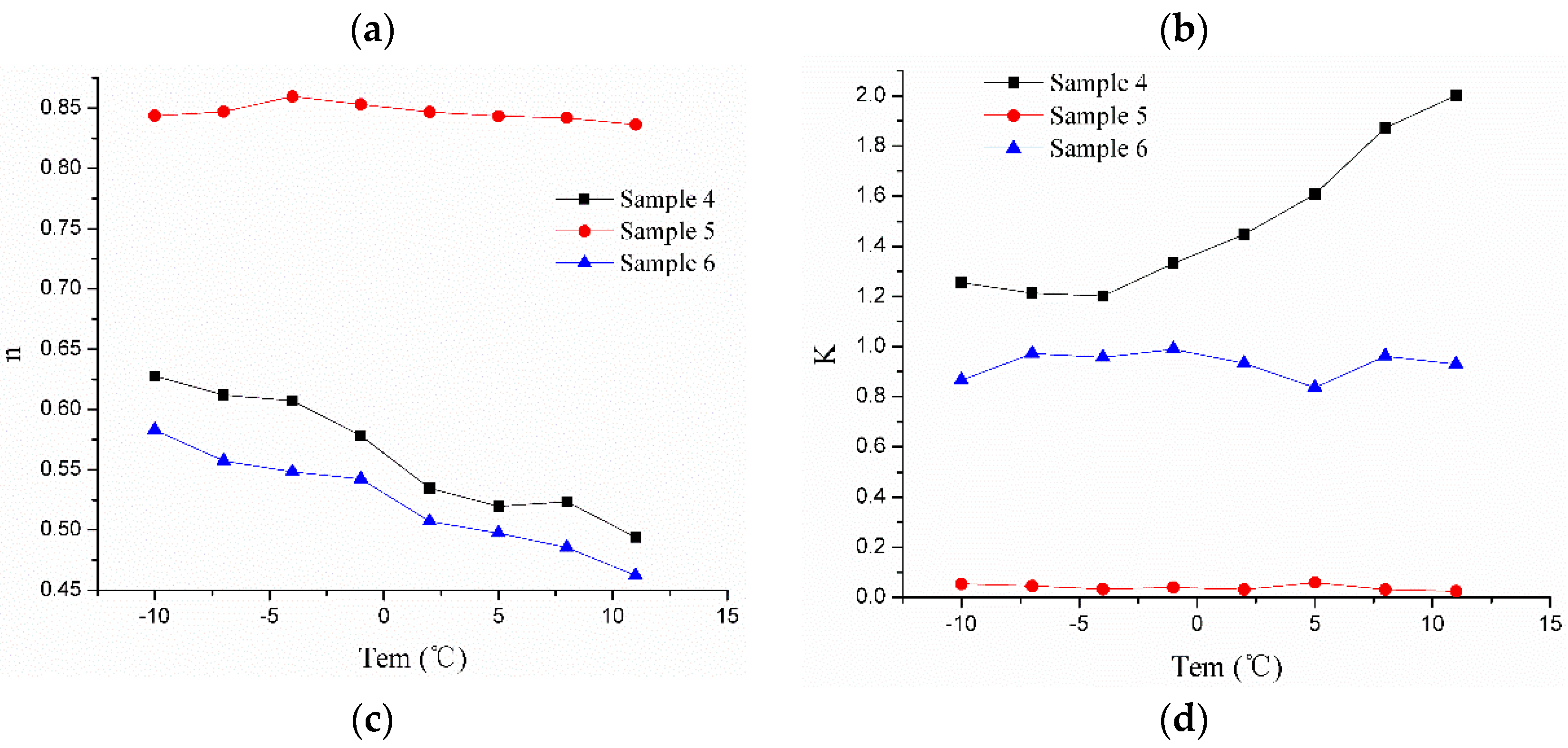
3.3. Rheological Properties with Temperature Response Characteristics of SFPDF
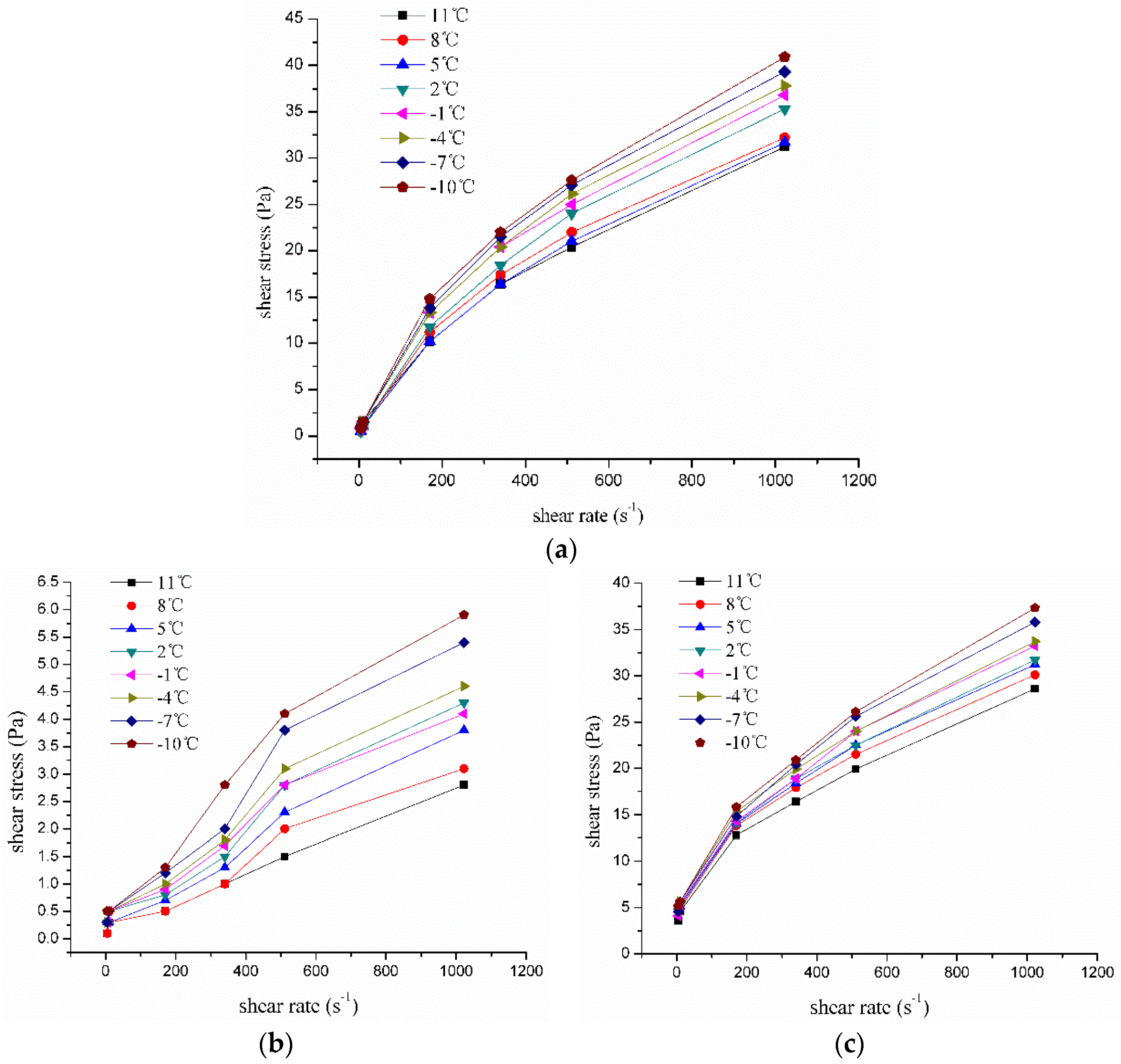
3.3.1. KL, PAM, and XC Drilling Fluid and Rheological Properties with Temperature Response Characteristics of SFPDF
- Sample 1: 1L H2O + 15% NaCl + 0.5‰ NaOH + 0.8% KL;
- Sample 2: 1L H2O + 15% NaCl + 0.5‰ NaOH +0.4% PAM;
- Sample 3: 1L H2O + 15% NaCl + 0.5‰ NaOH +0.8% XC;
3.3.2. Addition of GC Treatment Agent and the Rheological Properties with Temperature Response Characteristics of SFPDF
- Sample 4: 1 L H2O + 15% NaCl + 0.5‰ NaOH + 0.8% KL + 0.6% GC
- Sample 5: 1 L H2O + 15% NaCl + 0.5‰ NaOH + 0.4% PAM + 0.6% GC
- Sample 6: 1 L H2O + 15% NaCl + 0.5‰ NaOH + 0.8% XC + 0.6% GC
3.4. Rheological Properties with Temperature Response Mechanisms in SFPDF
3.4.1. Fourier Transform Infrared Analysis
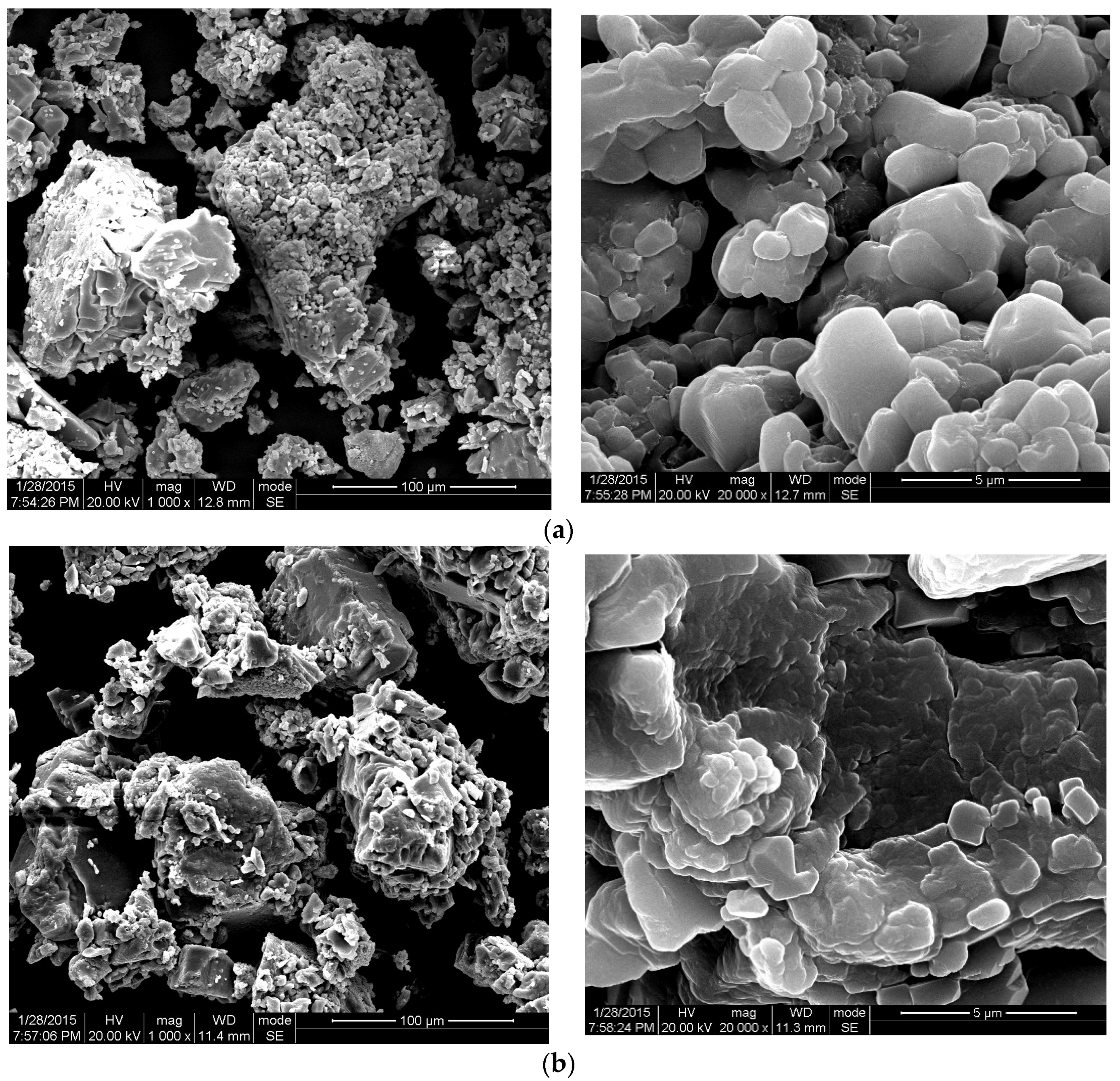
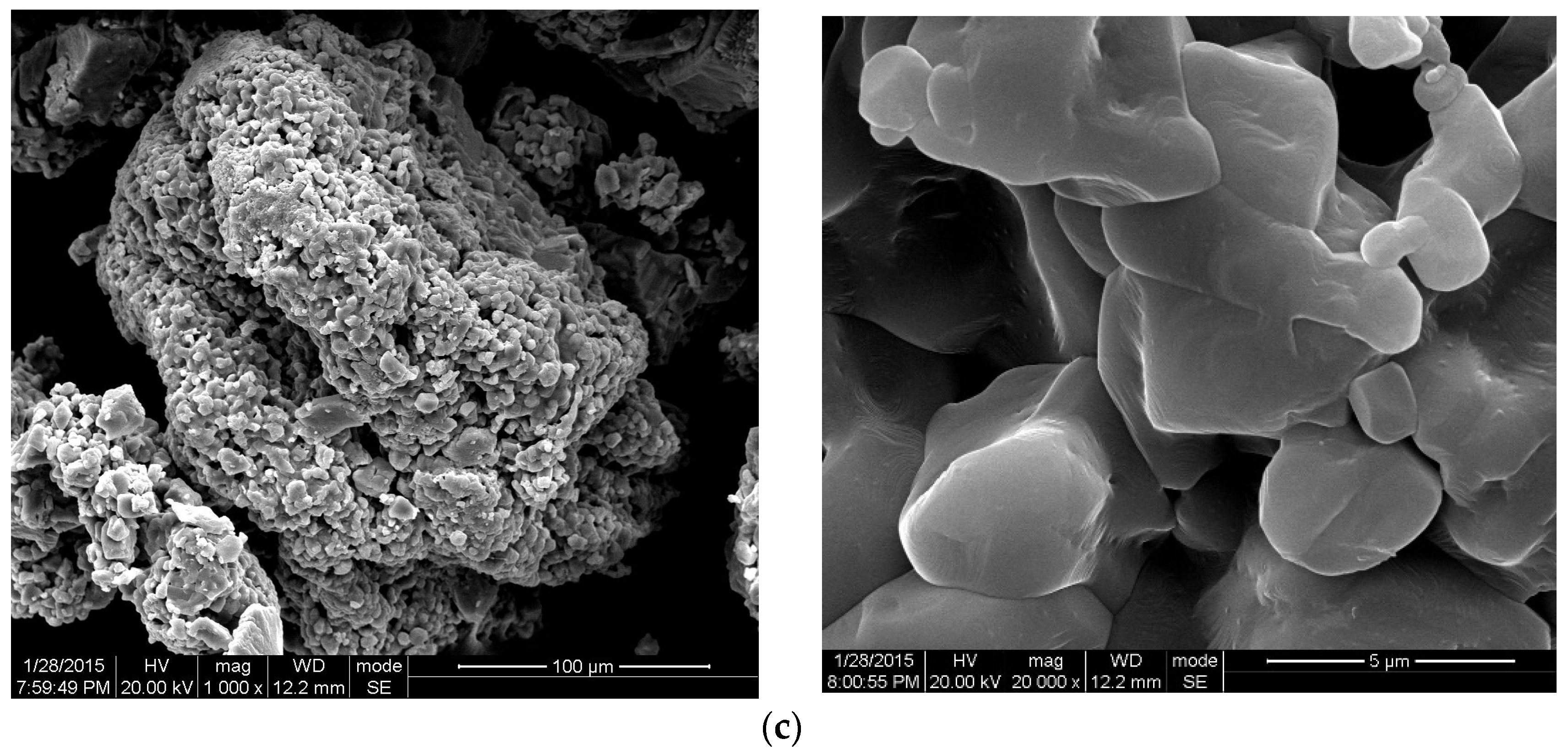
3.4.2. SEM Analysis
3.4.3. Discussion of Rheological Properties with Temperature Response Mechanisms
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sloan, E.D. Introductory Overview: Hydrate Knowledge Development. Am. Mineral. 2004, 89, 1155–1161. [Google Scholar] [CrossRef]
- Ecker, C.; Nur, A.M.; Dvorkin, J. Estimating the Amount of Gas Hydrate and Free Gas from Marine Seismic Data. Geophysics 2000, 65, 565–573. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Jiang, X.; Li, X. Hot-brine Injection for the Dissociation of Natural Gas Hydrates. Petroleum Sci. Technol. 2013, 31, 1320–1326. [Google Scholar] [CrossRef]
- Sloan, E.D. Fundamental Principles and Applications of Natural Gas Hydrates. Nature 2003, 426, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.L.; Zhang, S.D.; Jiang, G.S.; Кудряшов, Б.Б.; Wu, X.; Dou, B.; Gao, H. Dependence of Hole Temperature Distribution upon Drilling Fluid while Drilling in Frozen Formations Containing Natural Gas Hydrate. Geol. Sci. Technol. Inf. 2002, 21, 96–100. [Google Scholar]
- Hege, E.; Majeed, Y.; Eirik, S. Hydrate Control during Deepwater Drilling: Overview and New Drilling-Fluids Formulations. SPE Drill. Complet. 2001, 6, 19–26. [Google Scholar]
- Watson, P.; Kolstad, E.; Borstmayer, R.; Pope, T.; Reseigh, A. An Innovative Approach to Development Drilling in the Deepwater Gulf of Mexico. World Oil 2004, 225, 41–48. [Google Scholar]
- Rojas, J.C.; Daugherty, W.T.; Irby, R.D.; Bern, P.A.; Romo, L.A.; Dye, W.M.; Greene, B.; Trotter, R.N. New Constant-Rheology Synthetic-Based Fluid Reduces Downhole Losses in Deepwater Environments; SPE Annual Technical Conference and Exhibition; SPE: Anaheim, CA, USA, 2007. [Google Scholar]
- Feng, Z.; Xu, H.W. Experimental Study on Ethylene Alcohol Multiplicity Polymer Drilling Fluid with Low Temperature Resistance. Glob. Geol. 2008, 27, 95–99. [Google Scholar]
- Ning, F.L.; Wu, X.; Zhang, L. Experimental study on performance of water-based drilling fluid used to drill formations with gas hydrate. Nat. Gas Ind. 2006, 26, 52–55. [Google Scholar]
- Wang, S.; Chen, L.Y.; Zhang, Y.Q. Development of Solids-free Low Temperature Drilling Fluid System: To be used for Natural Gas Hydrate Drilling in Tibet Plateau Permafrost. Nat. Gas Ind. 2009, 29, 59–62. [Google Scholar]
- Kelland, M.A.; Mønig, K.; Iversen, J.E.; Lekvam, K. Feasibility Study for the Use of Kinetic Hydrate Inhibitors in Deep-Water Drilling Fluids. Energy Fuels 2008, 22, 2405–2410. [Google Scholar] [CrossRef]
- Mehta, A.P.; Hebert, P.B.; Cadena, E.R.; Weatherman, J.P. Fulfilling the Promise of Low-Dosage Hydrate Inhibitors: Journey from Academic Curiosity to Successful Field Implementation. SPE Prod. Facil. 2003, 18, 73–79. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yin, D.Y. Preparation of a New Lignosulfonate-Based Thinner: Introduction of Ferrous Ions. Colloids Surf. A Physicochem. Eng. Aspects 2002, 210, 12–21. [Google Scholar] [CrossRef]
- Zhang, G.B.; Wang, S.J.; Shi, P.Q. Researches and Applications of Viscosity Reducers for Drilling Fluids in China. Oilfield Chem. 2003, 17, 78–81. [Google Scholar]
- Chen, L.; Wang, S.; Zhang, Y. Low Temperature Mud Basis Liquid of Gas Hydrate Drilling in Plateau Permafrost. Adv. Earth Sci. 2008, 23, 469–473. [Google Scholar]
- Zhang, L.; Jiang, G.S.; Cai, J.H.; Dou, B.; Ning, F.L.; Tu, Y.Z. Overview of low temperature formation characteristics while drilling and available drilling fluid technology. Drill. Fluid Complet. Fluid 2006, 23, 73–76. [Google Scholar]


| Rheological Model | Bingham Plastic Model | Power Law Model | Herschel–Bulkley Model | Carson Model |
|---|---|---|---|---|
| Formula | τ = τ0 + μpγ | τ = Kγn | τ = τy + Kγn | Τ0.5= τ∞0.5 + ηc0.5 γ0.5 |
| Parameter elucidation | τ0: Yield stress μp: Plastic viscosity | n: Flow behavior index K: Consistency coefficient | τy: Yield stress n: Flow behavior indices K: consistency coefficient | τ∞: Extremely high shear stress ηc: Carson dynamic shear |
| Concentration | KL (%) | PAM (%) | XC (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.2 | 0.4 | 0.6 | 0.8 | 0.2 | 0.4 | 0.6 | 0.8 | 0.2 | 0.4 | 0.6 | 0.8 | ||
| Bingham plastic model | R2 1 | 0.9931 | 0.9925 | 0.9961 | 0.9263 | 0.9843 | 0.9881 | 0.9889 | 0.9986 | 0.9981 | 0.9821 | 0.9763 | 0.9227 |
| SSE 2 | 0.042 | 0.055 | 0.396 | 66.22 | 0.051 | 0.029 | 0.011 | 0.008 | 0.011 | 1.063 | 4.155 | 27.789 | |
| Power law model | R2 | 0.9934 | 0.9870 | 0.9986 | 0.9958 | 0.9785 | 0.9772 | 0.9793 | 0.9812 | 0.9796 | 0.9805 | 0.9885 | 0.9902 |
| SSE | 0.045 | 0.041 | 0.143 | 3.667 | 0.05 | 0.075 | 0.134 | 0.128 | 0.133 | 1.3805 | 1.998 | 3.258 | |
| Herschel–Bulkley model | R2 | 0.9958 | 0.9995 | 0.9989 | 0.9993 | 0.9958 | 0.9985 | 0.9975 | 0.9983 | 0.9979 | 0.9978 | 0.9926 | 0.9972 |
| SSE | 0.039 | 0.013 | 0.127 | 0.257 | 0.038 | 0.026 | 0.013 | 0.007 | 0.01 | 1.03 | 1.015 | 0.657 | |
| Carson model | R2 | 0.9933 | 0.9791 | 0.9983 | 0.9692 | 0.9615 | 0.9682 | 0.9901 | 0.9818 | 0.9703 | 0.9813 | 0.9843 | 0.9846 |
| SSE | 0.045 | 0.012 | 0.174 | 27.084 | 0.053 | 0.056 | 0.063 | 0.057 | 0.063 | 1.194 | 0.947 | 5.48 | |
| Fluid | Concentration | AV 1 (mPa·s) | YP 2 (Pa) | n 3 | K 4 (Pa·sn) | R2 5 | SSE 6 (Pa2) |
|---|---|---|---|---|---|---|---|
| KL 7 | 0.2% | 3.3 | 0.1 | 0.9 | 0.1 | 0.9958 | 0.039 |
| KL | 0.4% | 6.0 | 0.1 | 0.9 | 0.1 | 0.9995 | 0.013 |
| KL | 0.6% | 13.5 | 0.3 | 0.9 | 0.1 | 0.9989 | 0.127 |
| KL | 0.8% | 38.0 | 0.3 | 0.6 | 0.7 | 0.9993 | 0.257 |
| PAM 8 | 0.2% | 2.0 | 0.1 | 0.7 | 0.1 | 0.9958 | 0.038 |
| PAM | 0.4% | 2.8 | 0.1 | 0.9 | 0.1 | 0.9985 | 0.026 |
| PAM | 0.6% | 3.5 | 0.3 | 0.9 | 0.1 | 0.9975 | 0.013 |
| PAM | 0.8% | 3.8 | 0.3 | 1.0 | 0.1 | 0.9983 | 0.007 |
| XC 9 | 0.2% | 3.5 | 0.3 | 0.9 | 0.1 | 0.9979 | 0.01 |
| XC | 0.4% | 10.5 | 0.5 | 0.7 | 0.1 | 0.9978 | 1.03 |
| XC | 0.6% | 18.5 | 1.0 | 0.9 | 0.1 | 0.9926 | 1.015 |
| XC | 0.8% | 28.8 | 3.6 | 0.6 | 0.4 | 0.9972 | 0.657 |
| Temperature (°C) | 10 | 7 | 4 | 1 | –2 | –5 | –8 | –11 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample 1 1 | BP model 4 | R2 7 | 0.9383 | 0.9163 | 0.9351 | 0.9269 | 0.9125 | 0.9171 | 0.9145 | 0.9176 |
| SSE 8 | 68.988 | 69.126 | 77.344 | 80.103 | 33.530 | 50.966 | 37.524 | 54.071 | ||
| PL model 5 | R2 | 0.9991 | 0.9954 | 0.9976 | 0.9961 | 0.9960 | 0.9966 | 0.9965 | 0.9965 | |
| SSE | 2.656 | 2.568 | 3.216 | 2.988 | 0.539 | 2.471 | 1.300 | 2.580 | ||
| HB model 6 | R2 | 0.9994 | 0.9993 | 0.9993 | 0.9993 | 0.9995 | 0.9991 | 0.9990 | 0.9992 | |
| SSE | 0.247 | 0.295 | 0.388 | 0.143 | 0.222 | 0.185 | 0.157 | 0.338 | ||
| Carson model | R2 | 0.9793 | 0.9663 | 0.9757 | 0.9704 | 0.9657 | 0.9676 | 0.9665 | 0.9675 | |
| SSE | 27.168 | 27.006 | 30.772 | 31.184 | 11.195 | 20.684 | 14.064 | 21.534 | ||
| Sample 2 2 | BP model | R2 | 0.9921 | 0.9657 | 0.9755 | 0.9737 | 0.9656 | 0.9636 | 0.9547 | 0.9486 |
| SSE | 0.029 | 0.186 | 0.109 | 0.295 | 0.282 | 0.329 | 0.699 | 0.776 | ||
| PL model | R2 | 0.9815 | 0.9611 | 0.9653 | 0.9645 | 0.9534 | 0.9573 | 0.9616 | 0.9551 | |
| SSE | 0.076 | 0.212 | 0.205 | 0.446 | 0.339 | 0.387 | 0.659 | 0.533 | ||
| HB model | R2 | 0.9914 | 0.9549 | 0.9677 | 0.9697 | 0.9594 | 0.9562 | 0.9508 | 0.9495 | |
| SSE | 0.027 | 0.184 | 0.109 | 0.292 | 0.219 | 0.266 | 0.565 | 0.417 | ||
| Carson model | R2 | 0.9851 | 0.9636 | 0.9672 | 0.9736 | 0.9664 | 0.9631 | 0.9603 | 0.9584 | |
| SSE | 0.056 | 0.201 | 0.160 | 0.357 | 0.242 | 0.293 | 0.597 | 0.483 | ||
| Sample 3 3 | BP model | R2 | 0.9224 | 0.9206 | 0.9033 | 0.9246 | 0.9226 | 0.9153 | 0.9319 | 0.9425 |
| SSE | 27.789 | 33.022 | 30.995 | 34.747 | 37.963 | 42.130 | 39.438 | 39.173 | ||
| PL model | R2 | 0.9903 | 0.9883 | 0.9887 | 0.9892 | 0.9922 | 0.9924 | 0.9903 | 0.9825 | |
| SSE | 3.258 | 3.498 | 6.148 | 3.909 | 4.249 | 3.628 | 5.663 | 7.212 | ||
| HB model | R2 | 0.9976 | 0.9997 | 0.9955 | 0.9993 | 0.9981 | 0.9993 | 0.9991 | 0.9967 | |
| SSE | 0.658 | 0.336 | 0.582 | 0.096 | 0.602 | 0.323 | 0.260 | 0.368 | ||
| Carson model | R2 | 0.9847 | 0.9865 | 0.9781 | 0.9863 | 0.9843 | 0.9822 | 0.9882 | 0.9911 | |
| SSE | 5.480 | 6.477 | 5.229 | 6.321 | 7.482 | 8.516 | 6.721 | 5.925 |
| Parameter | AV 1 | R2 2 | SSE 3 | ty 4 | R2 | SSE | n 5 | R2 | SSE | K 6 | R2 | SSE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
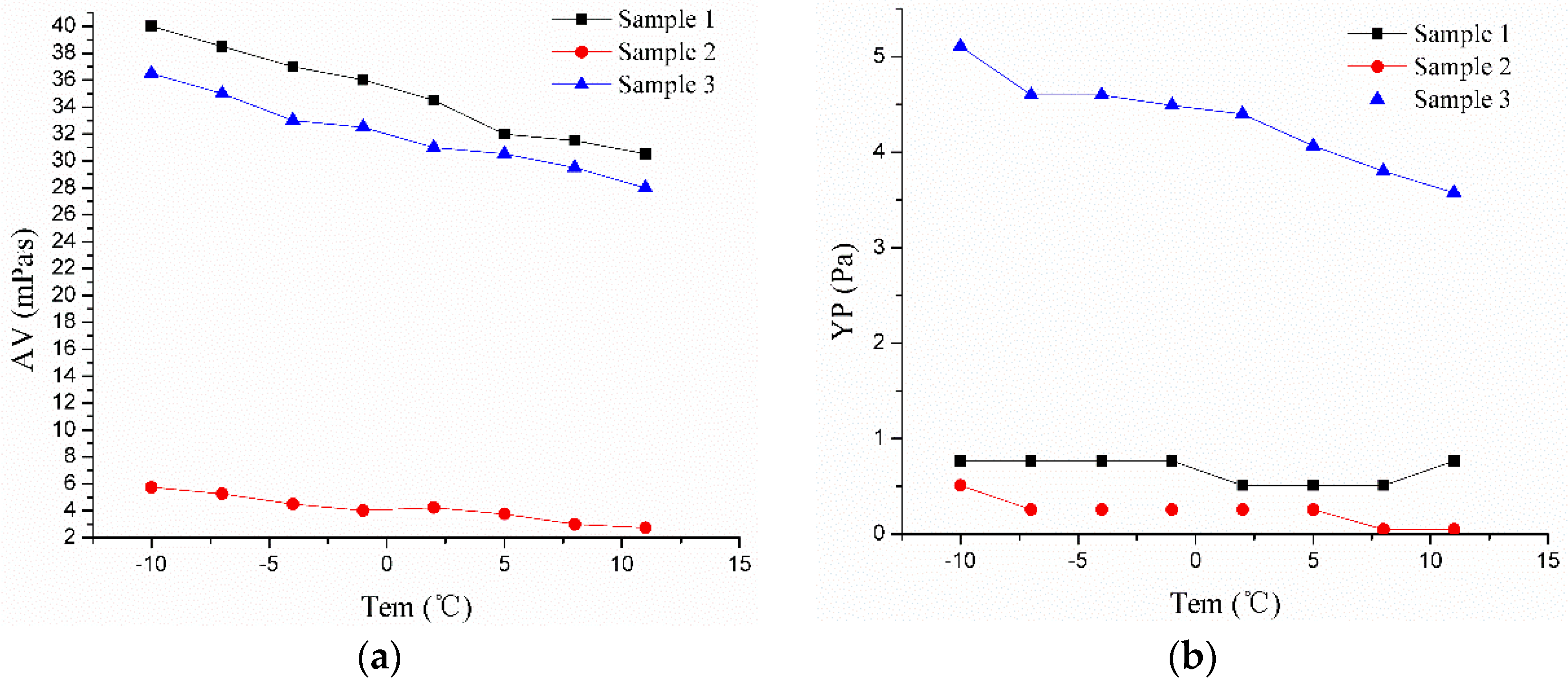
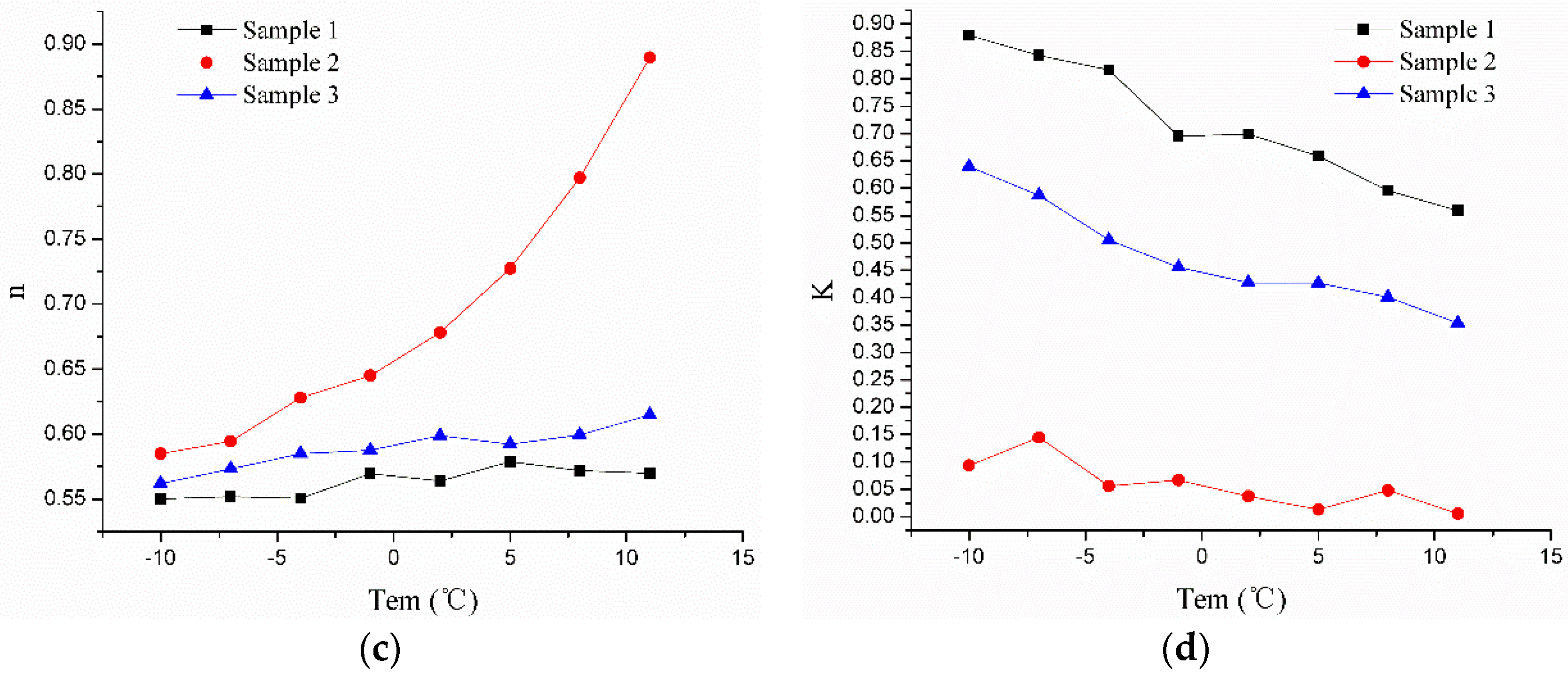
| Sample 1 | y = 35.23 − 0.47x | 0.985 | 1.11 | y = 0.68 − 0.01x | 0.913 | 0.09 | y = 0.57 + 0.01x + 4x2 − 1.35x3 | 0.983 | 0.78 | y = 0.73 − 0.02x | 0.961 | 0.003 |
| Sample 2 | y = 4.22 − 0.14x | 0.939 | 0.38 | y = 0.24 − 0.02x | 0.969 | 0.04 | y = 0.66 + 0.01x + 6.38x2 + 3.04x3 | 0.938 | 0.26 | y = 0.06 − 0.01x | 0.961 | 0.005 |
| Sample 3 | y = 32.19 − 0.38x | 0.976 | 1.14 | y = 4.36 − 0.07x | 0.934 | 0.09 | y = 0.59 + 9.6x − 5.64x2 + 1.44x3 | 0.988 | 0.38 | y = 0.48 − 0.013x | 0.913 | 0.004 |
| Parameter | AV 1 | R2 2 | SSE 3 | ty 4 | R2 | SSE | n 5 | R2 | SSE | K 6 | R2 | SSE |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
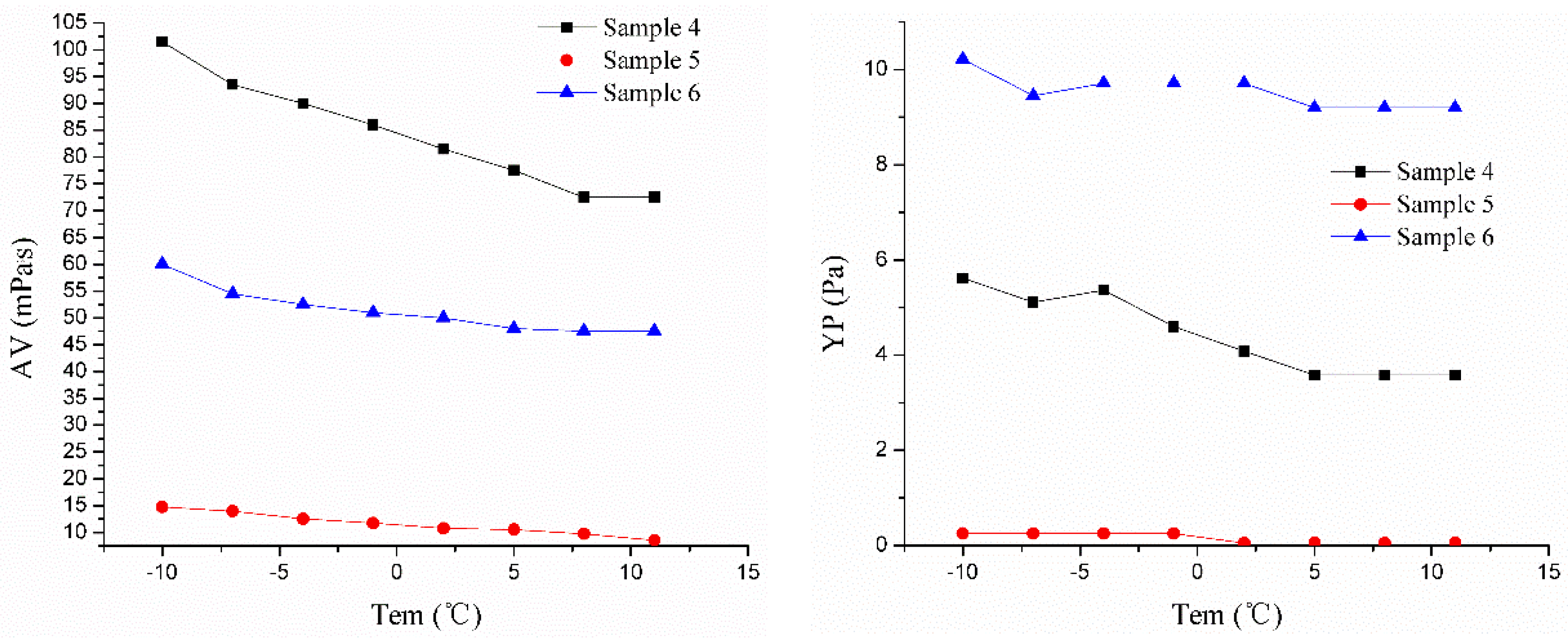
| Sample 4 | y = 85.07 − 1.39x | 0.97 | 1.21 | y = 4.49 − 0.11x | 0.929 | 0.47 | y = 0.57 − 0.01x − 7.97x2 + 2.34x3 | 0.938 | 0.03 | y = 1.47 + 0.04x | 0.985 | 0.09 |
| Sample 5 | y = 11.71 − 0.29x | 0.978 | 0.61 | y = 0.16 − 0.01x | 0.972 | 0.02 | y = 0.85 + 0.01x − 1.21x2 + 1.05x3 | 0.9711 | 0.06 | y = 0.04 − 8x | 0.951 | 0.02 |
| Sample 6 | y = 51.65 − 0.54x | 0.984 | 1.15 | y = 9.57 − 0.04x | 0.955 | 0.31 | y = 0.52 + 0.01x − 1.2x2 + 2.49x3 | 0.963 | 0.03 | y = 0.93 − 8.06x | 0.962 | 0.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Yuan, C.; Zhang, C.; Chen, L.; Liu, J. Rheological Properties with Temperature Response Characteristics and a Mechanism of Solid-Free Polymer Drilling Fluid at Low Temperatures. Appl. Sci. 2017, 7, 18. https://doi.org/10.3390/app7010018
Wang S, Yuan C, Zhang C, Chen L, Liu J. Rheological Properties with Temperature Response Characteristics and a Mechanism of Solid-Free Polymer Drilling Fluid at Low Temperatures. Applied Sciences. 2017; 7(1):18. https://doi.org/10.3390/app7010018
Chicago/Turabian StyleWang, Sheng, Chaopeng Yuan, Chuan Zhang, Liyi Chen, and Jiancheng Liu. 2017. "Rheological Properties with Temperature Response Characteristics and a Mechanism of Solid-Free Polymer Drilling Fluid at Low Temperatures" Applied Sciences 7, no. 1: 18. https://doi.org/10.3390/app7010018
APA StyleWang, S., Yuan, C., Zhang, C., Chen, L., & Liu, J. (2017). Rheological Properties with Temperature Response Characteristics and a Mechanism of Solid-Free Polymer Drilling Fluid at Low Temperatures. Applied Sciences, 7(1), 18. https://doi.org/10.3390/app7010018






