Use of Ferritin-Based Metal-Encapsulated Nanocarriers as Anticancer Agents
Abstract
:1. Introduction
2. Experimental
2.1. Cell Culture
2.2. Bacterial Expression and Purification
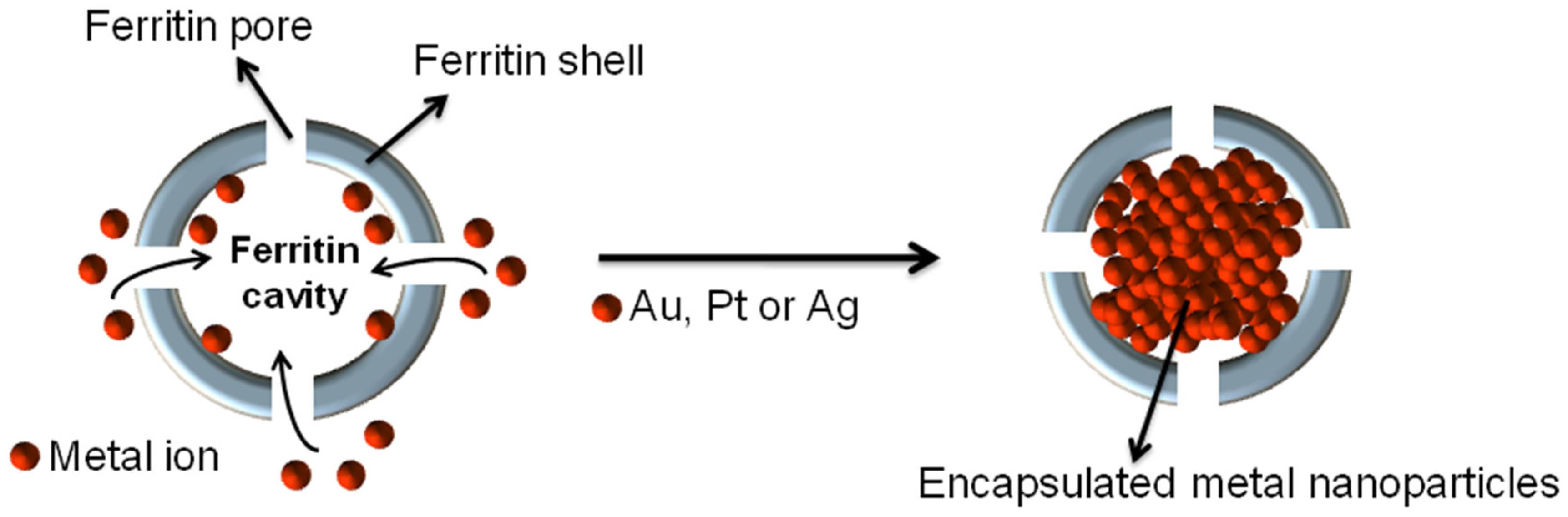
2.3. Metal-Containing Nanocarrier Preparation
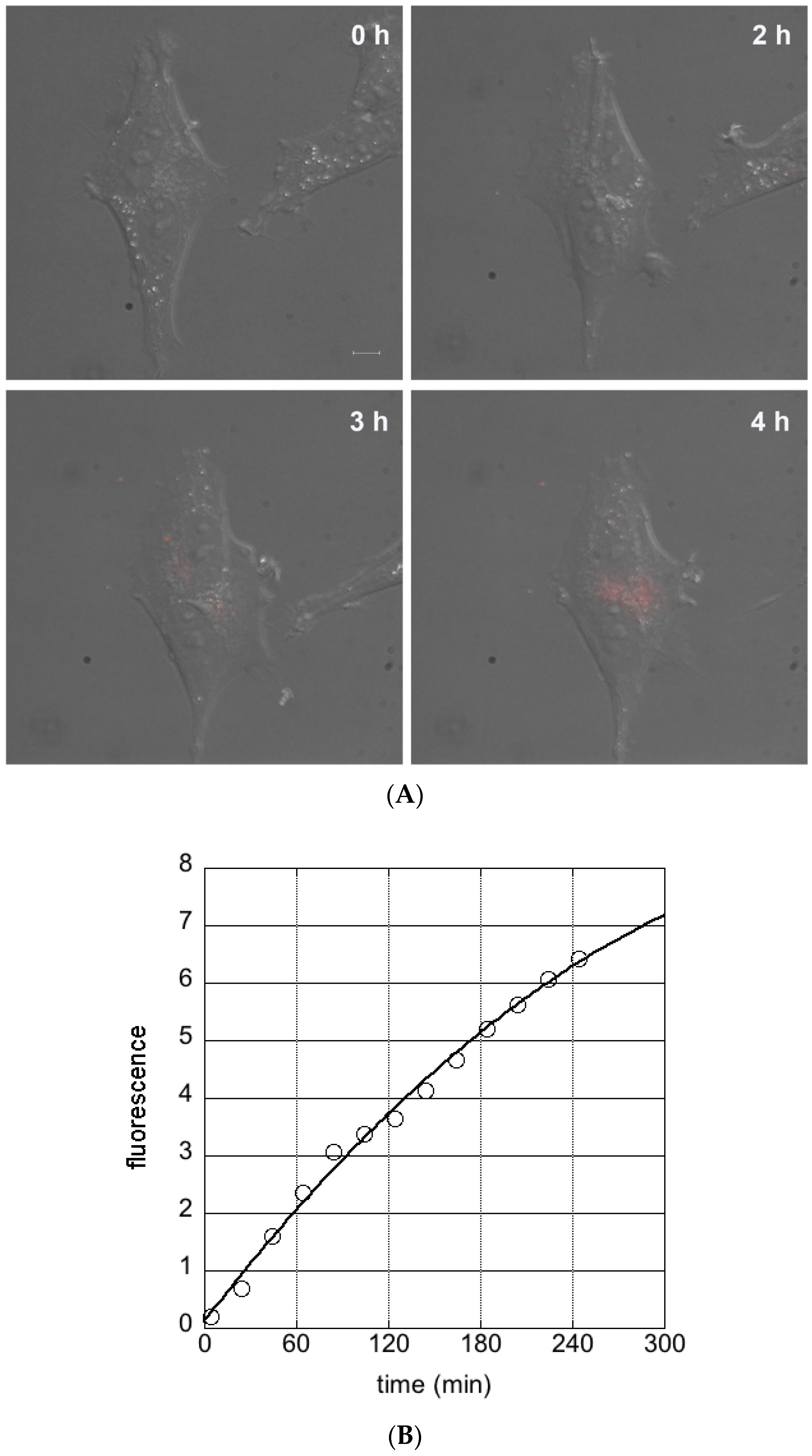
2.4. Fluorescence Microscopy
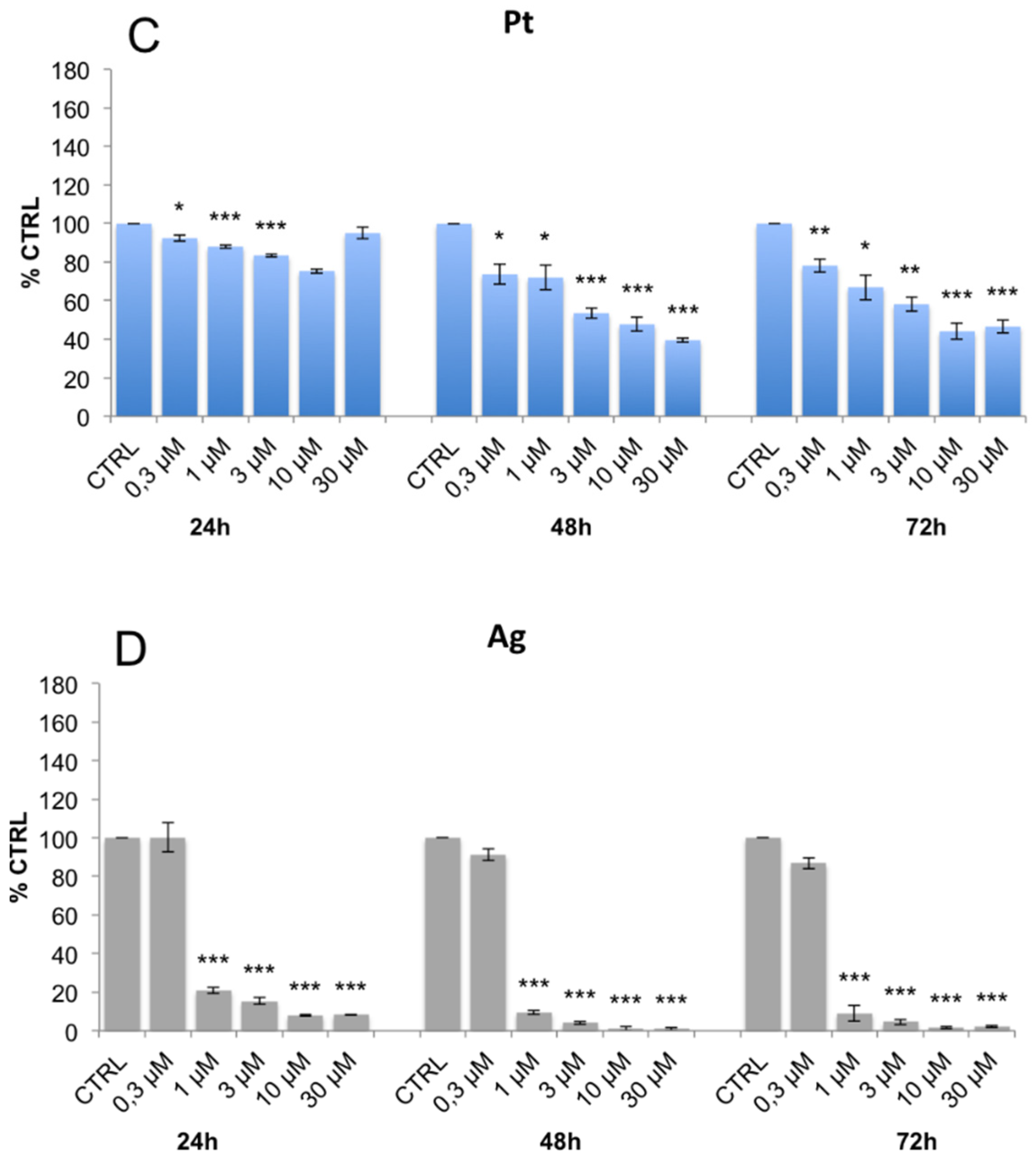
2.5. Determination of Cell Viability (MTT Reduction Assay)
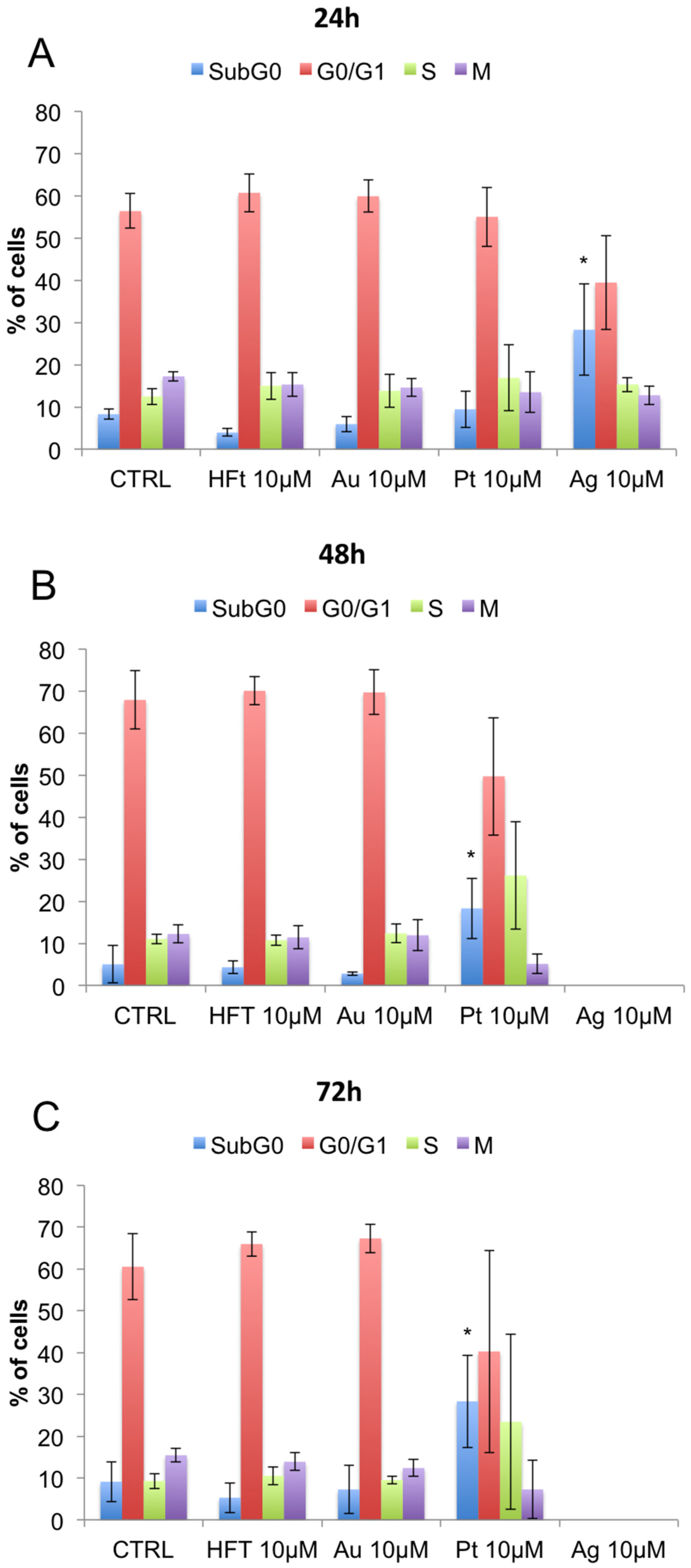
2.6. Cell Cycle Assessment by Flow Cytometry
2.7. Measurement of Intracellular Glutathione Levels
2.8. Measurement of Mitochondrial Membrane Potential
3. Results
3.1. Synthesis
3.2. Cellular Uptake of Ferritin Nanocarriers
3.3. Evaluation of Cell Viability and Cell Cycle Progression in the Presence of Metal-Containing Ferritin Nanocarriers
3.4. Evaluation of Cellular Redox Homeostasis and Mitochondrial Transmembrane Potential in the Presence of Metal-Containing Ferritin Nanocarriers
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| NC | nanocarriers |
| NP | nanoparticles |
| Ft | Ferritin |
| HFt | human ferritin H chain |
| TfR | transferrin receptor |
| HFt NCs | H-subunit Ft-based nanocarriers |
| GSH | glutathione |
| cisPt | cisplatin |
References
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.O.; Chiappini, C.; Ziemys, A.; Faust, A.M.; Kojic, M.; Liu, X.; Ferrari, M.; Tasciotti, E. Engineering multi-stage nanovectors for controlled degradation and tunable release kinetics. Biomaterials 2013, 34, 8469–8477. [Google Scholar] [CrossRef] [PubMed]
- Geninatti Crich, S.; Bussolati, B.; Tei, L.; Grange, C.; Esposito, G.; Lanzardo, S.; Camussi, G.; Aime, S. Magnetic resonance visualization of tumor angiogenesis by targeting neural cell adhesion molecules with the highly sensitive gadolinium-loaded apoferritin probe. Cancer Res. 2006, 66, 9196–9201. [Google Scholar] [CrossRef] [PubMed]
- Jutz, G.; van Rijn, P.; Santos Miranda, B.; Boker, A. Ferritin: A versatile building block for bionanotechnology. Chem. Rev. 2015, 115, 1653–1701. [Google Scholar] [CrossRef] [PubMed]
- Kostiainen, M.A.; Hiekkataipale, P.; Laiho, A.; Lemieux, V.; Seitsonen, J.; Ruokolainen, J.; Ceci, P. Electrostatic assembly of binary nanoparticle superlattices using protein cages. Nat. Nanotechnol. 2013, 8, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Fan, K.; Zhou, M.; Duan, D.; Zheng, J.; Yang, D.; Feng, J.; Yan, X. H-ferritin-nanocaged doxorubicin nanoparticles specifically target and kill tumors with a single-dose injection. Proc. Natl. Acad. Sci. USA 2014, 111, 14900–14905. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xie, J.; Niu, G.; Zhang, F.; Gao, H.; Yang, M.; Quan, Q.; Aronova, M.A.; Zhang, G.; Lee, S.; et al. Chimeric ferritin nanocages for multiple function loading and multimodal imaging. Nano Lett. 2011, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, L.; van Hest, J.C. Functionalization of protein-based nanocages for drug delivery applications. Nanoscale 2014, 6, 7124–7141. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Willits, D.A.; Muller, K.; Willis, A.F.; Jackiw, L.; Jutila, M.; Young, M.J.; Porter, A.E.; Douglas, T. Intracellular distribution of macrophage targeting ferritin-iron oxide nanocomposite. Adv. Mater. 2009, 21, 458–462. [Google Scholar] [CrossRef]
- Falvo, E.; Tremante, E.; Arcovito, A.; Papi, M.; Elad, N.; Boffi, A.; Morea, V.; Conti, G.; Toffoli, G.; Fracasso, G.; et al. Improved doxorubicin encapsulation and pharmacokinetics of ferritin-fusion protein nanocarriers bearing proline, serine, and alanine elements. Biomacromolecules 2016, 17, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Kasyutich, O.; Ilari, A.; Fiorillo, A.; Tatchev, D.; Hoell, A.; Ceci, P. Silver ion incorporation and nanoparticle formation inside the cavity of Pyrococcus furiosus ferritin: Structural and size-distribution analyses. J. Am. Chem. Soc. 2010, 132, 3621–3627. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.M.; Arosio, P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef]
- Levi, S.; Santambrogio, P.; Cozzi, A.; Rovida, E.; Corsi, B.; Tamborini, E.; Spada, S.; Albertini, A.; Arosio, P. The role of the L-chain in ferritin iron incorporation. Studies of homo and heteropolymers. J. Mol. Biol. 1994, 238, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Bucchini, D.; Martin, M.E.; Levi, S.; Arosio, P.; Grandchamp, B.; Beaumont, C. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 2000, 275, 3021–3024. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Menzies, S.; Muckenthaler, M.; Torti, F.M.; Wood, T.; Torti, S.V.; Hentze, M.W.; Beard, J.; Connor, J. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J. Neurosci. Res. 2003, 71, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Chiancone, E.; Ceci, P.; Ilari, A.; Ribacchi, F.; Stefanini, S. Iron and proteins for iron storage and detoxification. Biometals 2004, 17, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Wade, V.J.; Levi, S.; Arosio, P.; Treffry, A.; Harrison, P.M.; Mann, S. Influence of site-directed modifications on the formation of iron cores in ferritin. J. Mol. Biol. 1991, 221, 1443–1452. [Google Scholar] [CrossRef]
- Fisher, J.; Devraj, K.; Ingram, J.; Slagle-Webb, B.; Madhankumar, A.B.; Liu, X.; Klinger, M.; Simpson, I.A.; Connor, J.R. Ferritin: A novel mechanism for delivery of iron to the brain and other organs. Am. J. Physiol. Cell Physiol. 2007, 293, C641–C649. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.B.; Rubin, J.B.; Handrahan, J.V.; Connor, J.R.; Fine, R.E. Receptor-mediated transcytosis of transferrin across the blood-brain barrier. J. Neurosci. Res. 1987, 18, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, R.N. Brain microvasculature in aging. Neurobiol. Aging 1994, 15, 765–766. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebron, J.A.; Bjorkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef] [PubMed]
- Malecki, E.A.; Cook, B.M.; Devenyi, A.G.; Beard, J.L.; Connor, J.R. Transferrin is required for normal distribution of 59Fe and 54Mn in mouse brain. J. Neurol. Sci. 1999, 170, 112–118. [Google Scholar] [CrossRef]
- Tortorella, S.; Karagiannis, T.C. Transferrin receptor-mediated endocytosis: A useful target for cancer therapy. J. Membr. Biol. 2014, 247, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Falvo, E.; Tremante, E.; Fraioli, R.; Leonetti, C.; Zamparelli, C.; Boffi, A.; Morea, V.; Ceci, P.; Giacomini, P. Antibody-drug conjugates: targeting melanoma with cisplatin encapsulated in protein-cage nanoparticles based on human ferritin. Nanoscale 2013, 5, 12278–12285. [Google Scholar] [CrossRef] [PubMed]
- Baiocco, P.; Ilari, A.; Ceci, P.; Orsini, S.; Gramiccia, M.; di Muccio, T.; Colotti, G. Inhibitory effect of silver nanoparticles on trypanothione reductase activity and leishmania infantum proliferation. ACS Med. Chem. Lett. 2011, 2, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Mazzucchelli, S.; Galbiati, E.; Sommaruga, S.; Fiandra, L.; Truffi, M.; Rizzuto, M.A.; Colombo, M.; Tortora, P.; Corsi, F.; et al. Protein nanocages for self-triggered nuclear delivery of DNA-targeted chemotherapeutics in Cancer Cells. J. Control. Release 2014, 196, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Ceci, P.; Chiancone, E.; Kasyutich, O.; Bellapadrona, G.; Castelli, L.; Fittipaldi, M.; Gatteschi, D.; Innocenti, C.; Sangregorio, C. Synthesis of iron oxide nanoparticles in Listeria innocua Dps (DNA-binding protein from starved cells): A study with the wild-type protein and a catalytic centre mutant. Chemistry 2010, 16, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Fantechi, E.; Innocenti, C.; Zanardelli, M.; Fittipaldi, M.; Falvo, E.; Carbo, M.; Shullani, V.; di Cesare Mannelli, L.; Ghelardini, C.; et al. A smart platform for hyperthermia application in cancer treatment: Cobalt-doped ferrite nanoparticles mineralized in human ferritin cages. ACS Nano 2014, 8, 4705–4719. [Google Scholar] [CrossRef] [PubMed]
- Vannucci, L.; Falvo, E.; Failla, C.M.; Carbo, M.; Fornara, M.; Canese, R.; Cecchetti, S.; Rajsiglova, L.; Stakheev, D.; Krizan, J.; et al. In vivo targeting of cutaneous melanoma using an melanoma stimulating hormone-engineered human protein cage with fluorophore and magnetic resonance imaging tracers. J. Biomed. Nanotechnol. 2015, 11, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Alekseenko, A.V.; Waseem, T.V.; Fedorovich, S.V. Ferritin, a protein containing iron nanoparticles, induces reactive oxygen species formation and inhibits glutamate uptake in rat brain synaptosomes. Brain Res. 2008, 1241, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Gatter, K.C.; Brown, G.; Trowbridge, I.S.; Woolston, R.E.; Mason, D.Y. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J. Clin. Pathol. 1983, 36, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, A.; Oliviero, I.; Deaglio, S.; Mariani, G.; Biffoni, M.; Sposi, N.M.; Malavasi, F.; Peschle, C.; Testa, U. Transferrin receptor 2 is frequently expressed in human cancer cell lines. Blood Cells Mol. Dis. 2007, 39, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Noguchi, M.; Mukai, K.; Matsuno, Y.; Sato, Y.; Shimosato, Y.; Monden, Y. Transferrin receptor expression in adenocarcinoma of the lung as a histopathologic indicator of prognosis. Chest 1990, 97, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Prutki, M.; Poljak-Blazi, M.; Jakopovic, M.; Tomas, D.; Stipancic, I.; Zarkovic, N. Altered iron metabolism, transferrin receptor 1 and ferritin in patients with colon cancer. Cancer Lett. 2006, 238, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Ryschich, E.; Huszty, G.; Knaebel, H.P.; Hartel, M.; Buchler, M.W.; Schmidt, J. Transferrin receptor is a marker of malignant phenotype in human pancreatic cancer and in neuroendocrine carcinoma of the pancreas. Eur. J. Cancer 2004, 40, 1418–1422. [Google Scholar] [CrossRef] [PubMed]
- Seymour, G.J.; Walsh, M.D.; Lavin, M.F.; Strutton, G.; Gardiner, R.A. Transferrin receptor expression by human bladder transitional cell carcinomas. Urol. Res. 1987, 15, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Mugler, K.; Hailoo, D.W.; Burke, S.; Nemesure, B.; Torkko, K.; Shroyer, K.R. Differential expression of transferrin receptor (TfR) in a spectrum of normal to malignant breast tissues: implications for in situ and invasive carcinoma. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Paillard, A.; Passirani, C.; Morille, M.; Benoit, J.P.; Betbeder, D.; Garcion, E. Transferrin adsorption onto PLGA nanoparticles governs their interaction with biological systems from blood circulation to brain cancer cells. Pharm. Res. 2012, 29, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.J.; Liu, S.; Perrotti, D.; Marcucci, G.; Lee, R.J. Efficient delivery of a Bcl-2-specific antisense oligodeoxyribonucleotide (G3139) via transferrin receptor-targeted liposomes. J. Control. Release 2006, 112, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Mao, J.; Jiang, Z.; Sun, T.; Hu, Y.; Jiang, Z.; Zhang, C.; Dong, J.; Huang, Q.; Lan, Q. Transferrin-modified Doxorubicin-loaded biodegradable nanoparticles exhibit enhanced efficacy in treating brain glioma-bearing rats. Cancer Biother. Radiopharm. 2013, 28, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.P.; Park, S.C.; Kim, T.H.; Jang, J.Y.; Choi, C.; Jang, M.K.; Nah, J.W. Encapsulation of paclitaxel into lauric acid-O-carboxymethyl chitosan-transferrin micelles for hydrophobic drug delivery and site-specific targeted delivery. Int. J. Pharm. 2013, 457, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lu, Y.; Lee, A.; Pan, X.; Yang, X.; Zhao, X.; Lee, R.J. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J. Pharm. Pharm. Sci. 2007, 10, 350–357. [Google Scholar] [PubMed]
- Yoon, D.J.; Chu, D.S.; Ng, C.W.; Pham, E.A.; Mason, A.B.; Hudson, D.M.; Smith, V.C.; MacGillivray, R.T.; Kamei, D.T. Genetically engineering transferrin to improve its in vitro ability to deliver cytotoxins. J. Control. Release 2009, 133, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Kawabata, H.; Masuda, T.; Uchiyama, T.; Mizumoto, C.; Ohmori, K.; Koeffler, H.P.; Kadowaki, N.; Takaori-Kondo, A. H-ferritin is preferentially incorporated by human erythroid cells through transferrin receptor 1 in a threshold-dependent manner. PLoS ONE 2015, 10, e0139915. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Patton, S.M.; Henderson, R.J.; Connor, J.R. Consequences of expressing mutants of the hemochromatosis gene (HFE) into a human neuronal cell line lacking endogenous HFE. FASEB J. 2007, 21, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Carosio, R.; Zuccari, G.; Orienti, I.; Mangraviti, S.; Montaldo, P.G. Sodium ascorbate induces apoptosis in neuroblastoma cell lines by interfering with iron uptake. Mol. Cancer 2007, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Descamps, L.; Dehouck, M.P.; Torpier, G.; Cecchelli, R. Receptor-mediated transcytosis of transferrin through blood-brain barrier endothelial cells. Am. J. Physiol. 1996, 270, H1149–H1158. [Google Scholar] [PubMed]
- Lajoie, J.M.; Shusta, E.V. Targeting receptor-mediated transport for delivery of biologics across the blood-brain barrier. Ann. Rev. Pharmacol. Toxicol. 2015, 55, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Colotti, G.; Ilari, A.; Boffi, A.; Morea, V. Metals and metal derivatives in medicine. Mini Rev. Med. Chem. 2013, 13, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Misaki, M.; Yamamoto, M.; Shimizu, H.; Ogawa, Y.; Toguchi, H. In vitro cytotoxicities and in vivo distribution of transferrin-platinum(II) complex. J. Pharm. Sci. 1995, 84, 216–221. [Google Scholar] [CrossRef] [PubMed]
- D’Aguanno, S.; D’Alessandro, A.; Pieroni, L.; Roveri, A.; Zaccarin, M.; Marzano, V.; de Canio, M.; Bernardini, S.; Federici, G.; Urbani, A. New insights into neuroblastoma cisplatin resistance: a comparative proteomic and meta-mining investigation. J. Proteome Res. 2011, 10, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.X.; Yang, J.; Wang, P.Y.; Li, Y.J.; Xie, S.Y.; Sun, R.P. Cisplatin regulates SH-SY5Y cell growth through downregulation of BDNF via miR-16. Oncol. Rep. 2013, 30, 2343–2349. [Google Scholar] [PubMed]
- Asharani, P.V.; Hande, M.P.; Valiyaveettil, S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Coccini, T.; Manzo, L.; Bellotti, V.; de Simone, U. Assessment of cellular responses after short- and long-term exposure to silver nanoparticles in human neuroblastoma (SH-SY5Y) and astrocytoma (D384) cells. Sci. World J. 2014, 2014, 259765. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Mattioli, R.; Costantino, P.; Baima, S.; Morelli, G.; Punzi, P.; Giordano, C.; Pinto, A.; Donini, L.M.; d’Erme, M.; et al. Neuroprotective effect of brassica oleracea sprouts crude juice in a cellular model of alzheimer’s disease. Oxid. Med. Cell. Longev. 2015, 2015, 781938. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosca, L.; Falvo, E.; Ceci, P.; Poser, E.; Genovese, I.; Guarguaglini, G.; Colotti, G. Use of Ferritin-Based Metal-Encapsulated Nanocarriers as Anticancer Agents. Appl. Sci. 2017, 7, 101. https://doi.org/10.3390/app7010101
Mosca L, Falvo E, Ceci P, Poser E, Genovese I, Guarguaglini G, Colotti G. Use of Ferritin-Based Metal-Encapsulated Nanocarriers as Anticancer Agents. Applied Sciences. 2017; 7(1):101. https://doi.org/10.3390/app7010101
Chicago/Turabian StyleMosca, Luciana, Elisabetta Falvo, Pierpaolo Ceci, Elena Poser, Ilaria Genovese, Giulia Guarguaglini, and Gianni Colotti. 2017. "Use of Ferritin-Based Metal-Encapsulated Nanocarriers as Anticancer Agents" Applied Sciences 7, no. 1: 101. https://doi.org/10.3390/app7010101
APA StyleMosca, L., Falvo, E., Ceci, P., Poser, E., Genovese, I., Guarguaglini, G., & Colotti, G. (2017). Use of Ferritin-Based Metal-Encapsulated Nanocarriers as Anticancer Agents. Applied Sciences, 7(1), 101. https://doi.org/10.3390/app7010101






