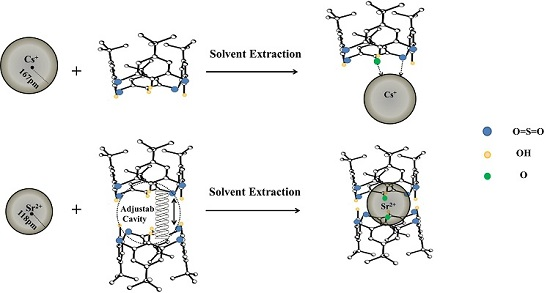
Extraction Property of p-tert-Butylsulfonylcalix[4]arene Possessing Irradiation Stability towards Cesium(I) and Strontium(II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Extraction Procedure
2.3. Irradiation Procedure and Thermogravimetric Analysis
3. Results and Discussion
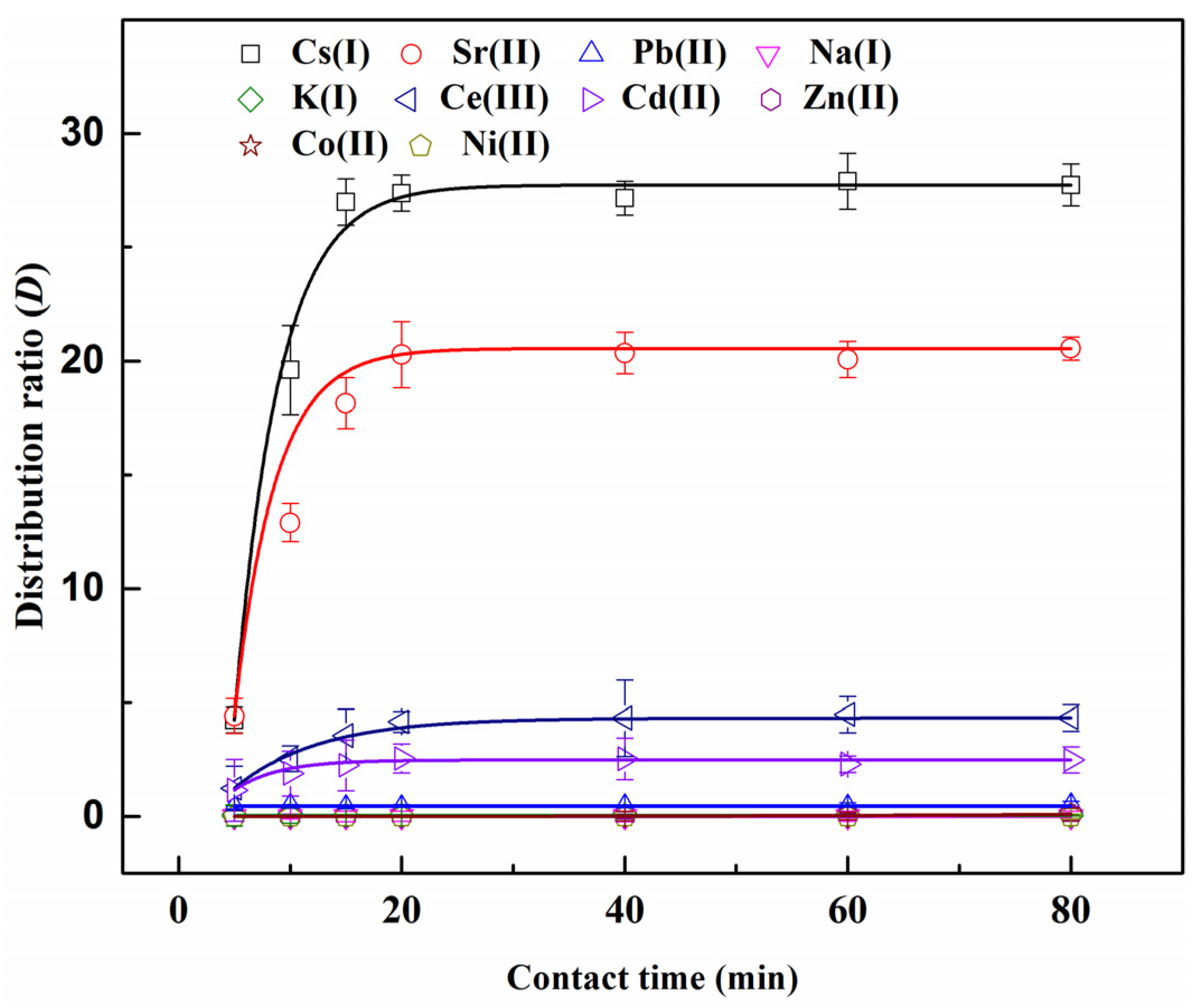
3.1. Dependence of Extraction on Contact Time
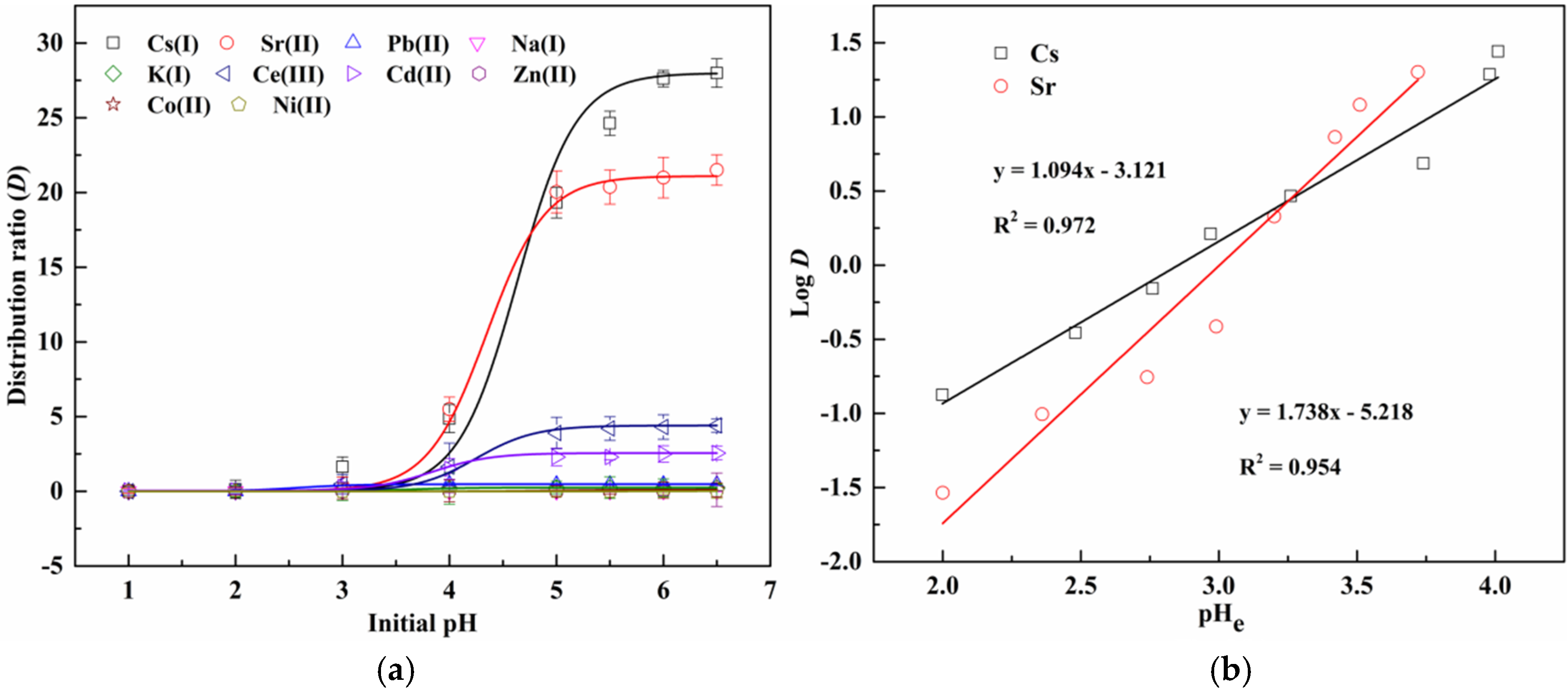
3.2. Dependence of Extraction on pH
3.3. Dependence of Extraction on SC4A Concentration
3.4. Dependence of Extraction on Nitrate Concentration
3.5. Dependence of Extraction on Temperature
3.6. Function of Groups and Coordination Feature
3.7. Effect of Irradiation
3.7.1. Irradiation Stability and Thermal Stability of SC4A
3.7.2. Irradiation Stability of SC4A/CHCl3
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koarashi, J.; Moriya, K.; Atarashiandoh, M.; Matsunaga, T.; Fujita, H.; Nagaoka, M. Retention of potentially mobile radiocesium in forest surface soils affected by the Fukushima nuclear accident. Sci. Rep. 2012, 2, 564–565. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Liu, L.; Zhao, X.; Yang, S.; Komarneni, S.; Yang, D. Capture of radioactive cations from water using niobate nanomaterials with layered and tunnel structures. RSC Adv. 2015, 5, 75354–75359. [Google Scholar] [CrossRef]
- Sheha, R.R. Synthesis and characterization of magnetic hexacyanoferrate (II) polymeric nanocomposite for separation of cesium from radioactive waste solutions. J. Colloid Interface Sci. 2012, 388, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, L. Synthesis and characterization of whisker surface imprinted polymer and selective solid-phase extraction of trace Sr(II) from environment aqueous solution. Desalination Water Treat. 2014, 54, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Mu, W.; Xiang, X.; Liu, B.; Hui, T.; Zhou, G.; Wei, H.; Jian, Y.; Luo, S. Strontium adsorption on tantalum-doped hexagonal tungsten oxide. J. Hazard. Mater. 2014, 264, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, J.N.; Achuthan, P.V.; Hubli, R.C. Selective separation of cesium from simulated high level liquid waste solution using 1,3-dioctyloxy calix[4]arene-benzo-crown-6. J. Radioanal. Nucl. Chem. 2014, 299, 1547–1553. [Google Scholar] [CrossRef]
- Liu, B.; Mu, W.; Xie, X.; Li, X.; Wei, H.; Tan, Z.; Jian, Y.; Luo, S. Enhancing the adsorption capacity of Sr2+ and Cs+ onto hexagonal tungsten oxide by doped niobium. RSC Adv. 2015, 5, 15603–15611. [Google Scholar] [CrossRef]
- Sharma, J.N.; Kumar, A.; Kumar, V.; Pahan, S.; Janardanan, C.; Tessi, V.; Wattal, P.K. Process development for separation of cesium from acidic nuclear waste solution using 1,3-dioctyloxycalix 4 arene-crown-6 plus isodecyl alcohol/n-dodecane solvent. Sep. Purif. Technol. 2014, 135, 176–182. [Google Scholar] [CrossRef]
- Levitskaia, T.G.; Leon, M.; Berkel, G.J.; Van Moyer, B.A. Anion partitioning and ion-pairing behavior of anions in the extraction of cesium salts by 4,5′ ′-Bis(tert-octylbenzo)dibenzo-24-crown-8 in 1,2-dichloroethane. Inorg. Chem. 2007, 46, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Bryan, J.C.; Sachleben, R.A.; Lavis, J.M.; Davis, M.C.; Burns, J.H.; Hay, B.P. Structural Aspects of Rubidium Ion Selectivity by Tribenzo-21-crown-71a. Inorg. Chem. 1998, 37, 2749–2755. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.P.; Dietz, M.L.; Fisher, E.D. Correlation of the extraction of strontium nitrate by a crown ether with the water content of the organic phase. Solvent Extr. Ion Exch. 1990, 8, 199–208. [Google Scholar] [CrossRef]
- Casnati, A.; Pochini, A.; Ungaro, R.; Ugozzoli, F.; Arnaud, F.; Fanni, S.; Schwing, M.-J.; Egberink, R.J.M.; de Jong, F.; Reinhoudt, D.N. Synthesis, complexation, and membrane transport studies of 1,3-alternate calix[4]arene-crown-6 conformers: A new class of cesium selective ionophores. J. Am.Chem. Soc. 1995, 117, 2767–2777. [Google Scholar] [CrossRef]
- Lamare, V.; Dozol, J.F.; Ugozzoli, F.; Casnati, A.; Ungaro, R. X-ray crystal structures and molecular modelling studies of calix[4]dibenzocrowns-6 and their alkali metal cation complexes. Eur. J. Org. Chem. 1998, 1998, 1559–1568. [Google Scholar] [CrossRef]
- Mohapatra, P.K.; Ansari, S.A.; Sarkar, A.; Bhattacharyya, A.; Manchanda, V.K. Evaluation of calix-crown ionophores for selective separation of radio-cesium from acidic nuclear waste solution. Anal. Chim. Acta 2006, 571, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Co-extraction of strontium and cesium from simulated high-level liquid waste (HLLW) by calixcrown and crown ether. J. Nucl. Sci. Technol. 2015, 52, 171–177. [Google Scholar] [CrossRef]
- Riddle, C.L.; Baker, J.D.; Law, J.D.; McGrath, C.A.; Meikrantz, D.H.; Mincher, B.J.; Peterman, D.R.; Todd, T.A. Fission product extraction (FPEX): Development of a novel solvent for the simultaneous separation of strontium and cesium from acidic solutions. Solvent Extr. Ion Exch. 2005, 23, 449–461. [Google Scholar] [CrossRef]
- Huimin, L.; Sheng, D.; Bonnesen, P.V.; Buchanan, A.C.; Holbrey, J.D.; Bridges, N.J.; Rogers, R.D. Extraction of cesium ions from aqueous solutions using calix[4]arene-bis(tert-octylbenzo-crown-6) in Ionic Liquids. Anal. Chem. 2004, 76, 3078–3083. [Google Scholar]
- Yi, R. Novel thermo-responsive hydrogel microspheres with calixcrown host molecules as cross-links for highly specific binding and controllable release of cesium. RSC Adv. 2015, 5, 55277–55284. [Google Scholar] [CrossRef]
- Suzuki, H.; Sasaki, Y.; Sugo, Y.; Apichaibukol, A.; Kimura, T. Extraction and separation of Am(III) and Sr(II) by N,N,N′,N′-tetraoctyl-3-oxapentanediamide (TODGA). Radiochim. Acta 2004, 92, 463–466. [Google Scholar] [CrossRef]
- Ali, S.M. Dual mode of extraction for Cs+ and Na+ ions with dicyclohexano-18-crown-6 and bis(2-propyloxy)calix[4]crown-6 in ionic liquids: Density functional theoretical investigation. Rsc Adv. 2014, 4, 22911–22925. [Google Scholar] [CrossRef]
- Talanov, V.S.; Talanova, G.G.; Gorbunova, M.G.; Bartsch, R.A. Novel caesium-selective, 1,3-alternate calix[4]arene-bis(crown-6-ethers) with proton-ionizable groups for enhanced extraction efficiency. J. Chem. Soc. Perkin Trans. 2002, 2, 209–215. [Google Scholar] [CrossRef]
- Talanov, V.S.; Talanova, G.G.; Bartsch, R.A. New proton-ionizable, cesium-selective calix[4]arene-bis(crown-6-ethers) with markedly enhanced extraction efficiency. Tetrahedron Lett. 2000, 41, 8221–8224. [Google Scholar] [CrossRef]
- Liu, S.M.; Liu, H.H.; Huang, Y.J.; Yang, W.J. Solvent extraction of rubidium and cesium from salt lake brine with t-BAMBP-kerosene solution. Trans. Nonferr. Met. Soc. China 2015, 25, 329–334. [Google Scholar] [CrossRef]
- Kocherginsky, N.M.; Zhang, Y.K.; Stucki, J.W. D2EHPA based strontium removal from strongly alkaline nuclear waste 1. Desalination 2002, 144, 267–272. [Google Scholar] [CrossRef]
- Dong-Sheng, G.; Yu, L. Calixarene-based supramolecular polymerization in solution. Chem. Soc. Rev. 2012, 41, 5907–5921. [Google Scholar]
- Chinta, J.P.; Ramanujam, B.; Rao, C.P. Structural aspects of the metal ion complexes of the conjugates of calix[4]arene: Crystal structures and computational models. Coord. Chem. Rev. 2012, 256, 2762–2794. [Google Scholar] [CrossRef]
- Duncan, N.C.; Roach, B.D.; Williams, N.J.; Bonnesen, P.V.; Rajbanshi, A.; Moyer, B.A. N, N′-dicyclohexyl-N″-isotridecylguanidine as suppressor for the next generation caustic side solvent extraction (NG-CSSX) Process. Sep. Sci. Technol. 2007, 95, 2074–2087. [Google Scholar]
- Izatt, R.M.; Lamb, J.D.; Hawkins, R.T.; Brown, P.R.; Izatt, S.R.; Christensen, J.J. Selective M+-H+ coupled transport of cations through a liquid membrane by macrocycle calixarene ligands. J. Am. Chem. Soc. 1983, 14, 1782–1785. [Google Scholar] [CrossRef]
- Izatt, S.R.; Hawkins, R.T.; Christensen, J.J.; Izatt, R.M. Cation transport from multiple alkali cation mixtures using a liquid membrane system containing a series of calixarene carriers. J. Am. Chem. Soc. 1985, 107, 63–66. [Google Scholar] [CrossRef]
- Casnati, A.; Barboso, S.; Rouquette, H.; Schwing-weill, M.; Arnaud-Neu, F.; Dozol, J.-F.; Ungaro, R. New efficient calixarene amide ionophores for the selective removal of strontium ion from nuclear waste: Synthesis, complexation, and extraction properties. J. Am. Chem. Soc. 2001, 123, 12182–12190. [Google Scholar]
- Iki, N.; Kumagai, H.; Morohashi, N.; Ejima, K.; Hasegawa, M.; Miyanari, S.; Miyano, S. Selective oxidation of thiacalix[4]arenes to the sulfinyl- and sulfonylcalix[4]arenes and their coordination ability to metal ions. Tetrahedron Lett. 1998, 39, 7559–7562. [Google Scholar] [CrossRef]
- Lhoták, P. Regioselective and stereoselective oxidation of thiacalix[4]arene tetraacetate: Synthesis of all possible sulfinylcalix[4]arenes. Tetrahedron 2001, 57, 4775–4779. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- De Namor, A.F.D.; Cleverley, R.M.; Zapata-Ormachea, M.L. Thermodynamics of calixarene chemistry. Chem. Rev. 1998, 98, 2495–2526. [Google Scholar] [CrossRef]
- Iki, N. High complexation ability of thiacalixarene with transition metal ions. The effects of replacing methylene bridges of tetra(p-t-butyl)calix[4]arenetetrol by epithio groups. Bull. Chem. Soc. Jpn. 1998, 71, 1597–1603. [Google Scholar] [CrossRef]
- Dozol, J.F.; Dozol, M.; Macias, R.M. Extraction of strontium and cesium by dicarbollides, crown ethers and functionalized calixarenes. J. Incl. Phenom. 2000, 38, 1–22. [Google Scholar] [CrossRef]
- Morohashi, N.; Iki, N.; Sugawara, A.; Miyano, S. Selective oxidation of thiacalix[4]arenes to the sulfinyl and sulfonyl counterparts and their complexation abilities toward metal ions as studied by solvent extraction. Tetrahedron 2001, 57, 5557–5563. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Gurung, M.; Kawakita, H.; Ohto, K. Cation complexation with p-tert-butylcalix[5]arene pentacarboxylic acid derivative: An allosteric regulation of the first metal ion for stepwise extraction of the second ion. Analyst 2011, 136, 3758–3769. [Google Scholar] [CrossRef] [PubMed]
- Arnaud-Neu, F.; Collins, E.M.; Deasy, M.; Ferguson, G.; Harris, S.J.; Kaitner, B.; Lough, A.J.; McKervey, M.A.; Marques, E. Synthesis, x-ray crystal structures, and cation-binding properties of alkyl calixaryl esters and ketones, a new family of macrocyclic molecular receptors. J. Am. Chem. Soc. 1989, 111, 8681–8691. [Google Scholar] [CrossRef]
- Adhikari, B.B.; Gurung, M.; Chetry, A.B.; Kawakita, H.; Ohto, K. Highly selective and efficient extraction of two Pb2+ ions with a p-tert-butylcalix[6]arene hexacarboxylic acid ligand: An allosteric effect in extraction. RSC Adv. 2013, 3, 25950–25959. [Google Scholar] [CrossRef]
- Dietz, M.L.; Jensen, M.P. EXAFS investigations of strontium complexation by a polymer-supported crown ether. Talanta 2004, 62, 109–113. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic-radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Antonio, M.R.; Dietz, M.L.; Jensen, M.P.; Soderholm, L.; Horwitz, E.P. EXAFS studies of cesium complexation by dibenzo-crown ethers in tri-n-butyl phosphate. Inorg. Chim. Acta 1997, 255, 13–20. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, X.; Song, C. The study of radiolytic stability of 25,27-bis(2-propyloxy)calix[4]-26,28-crown-6(iPr-C[4]C-6). J. Radioanal. Nucl. Chem. 2012, 293, 851–855. [Google Scholar] [CrossRef]
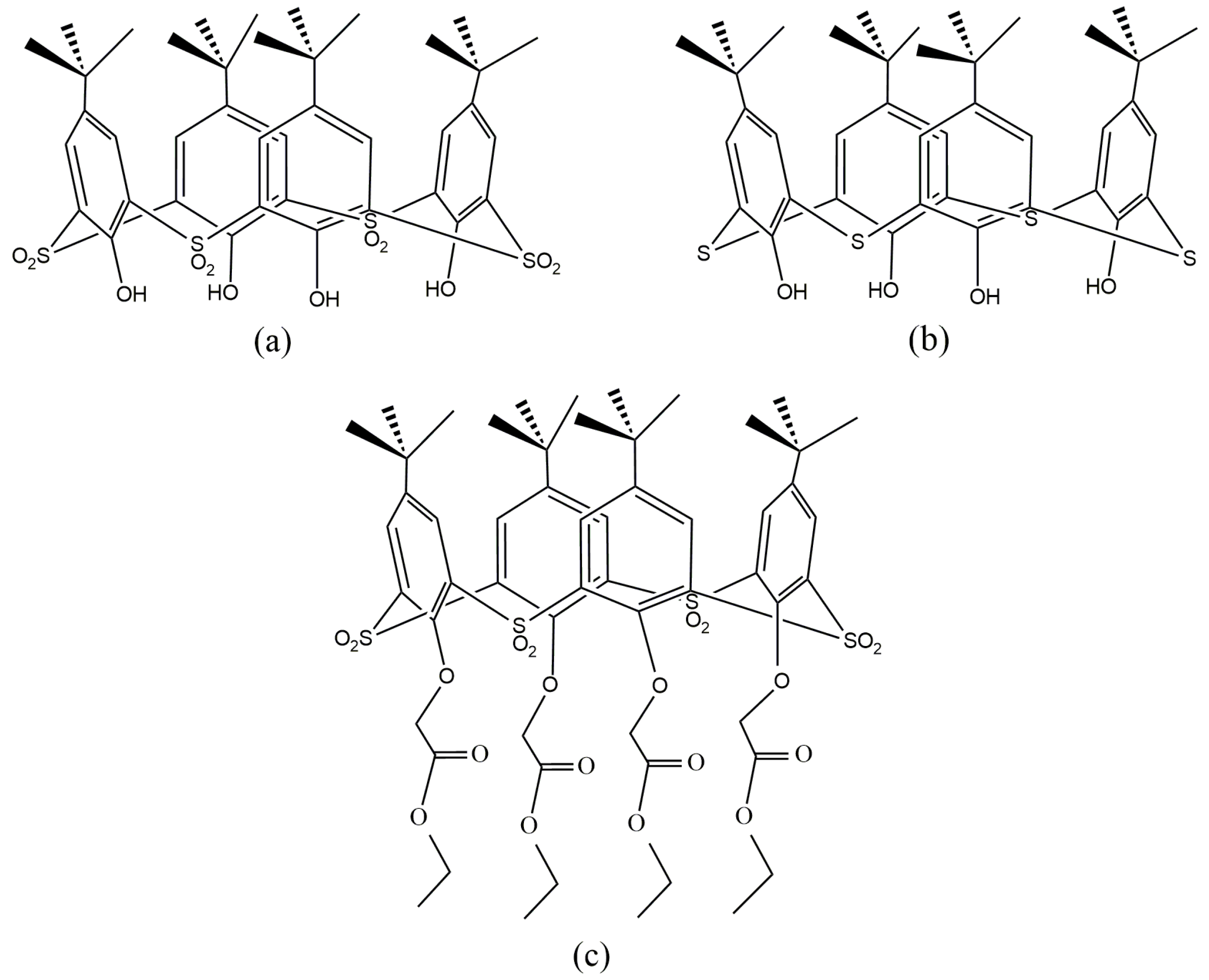
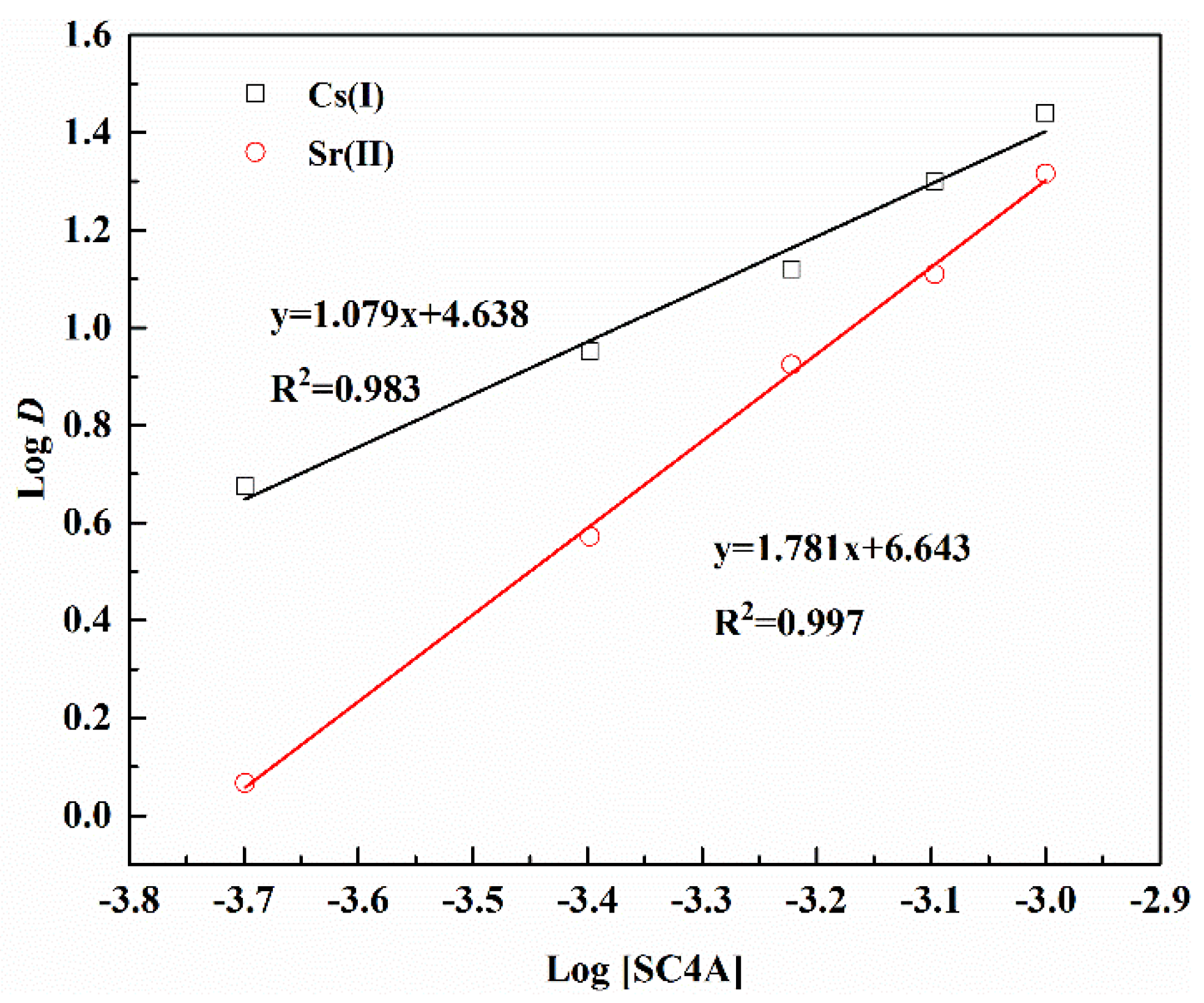
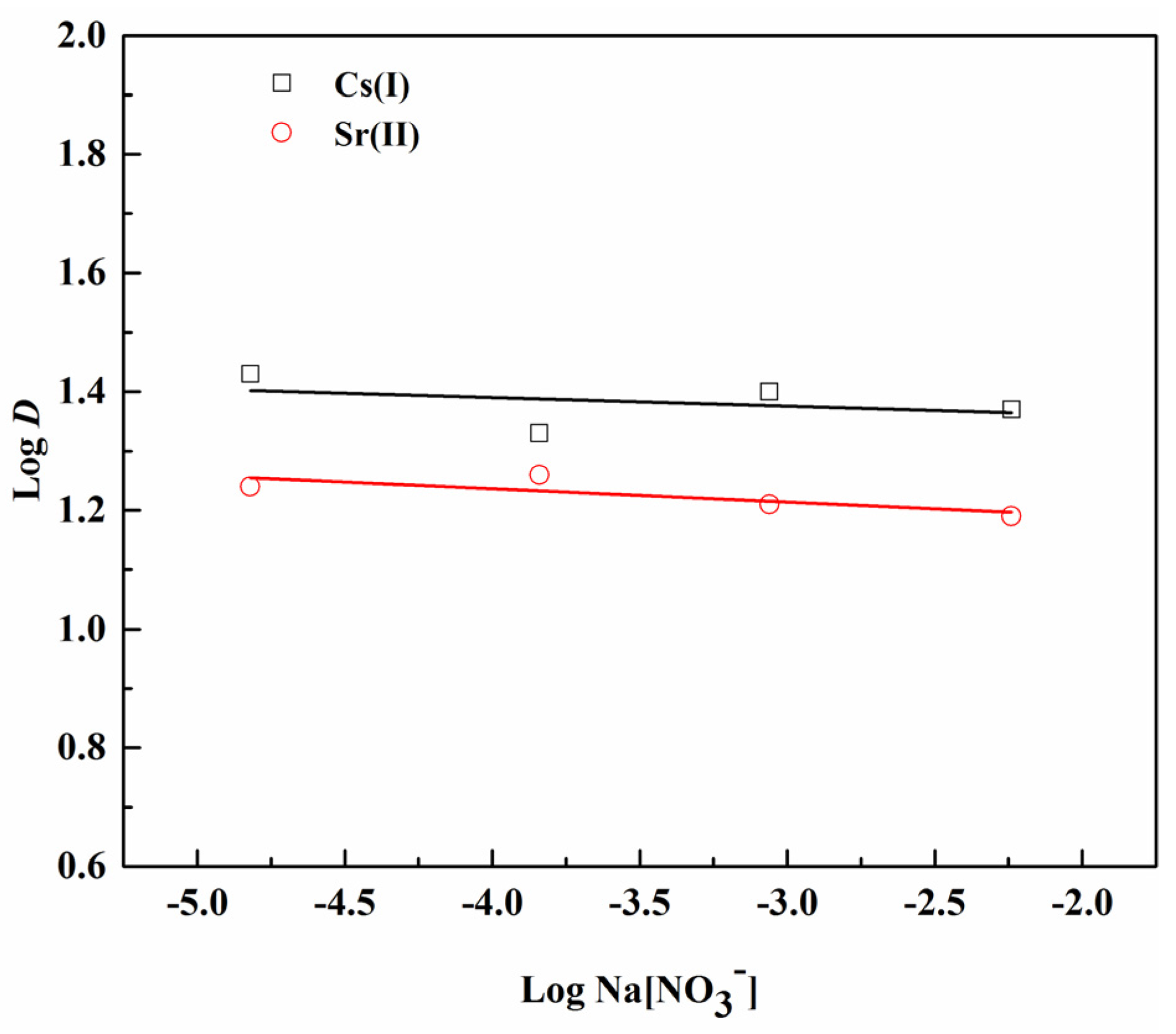
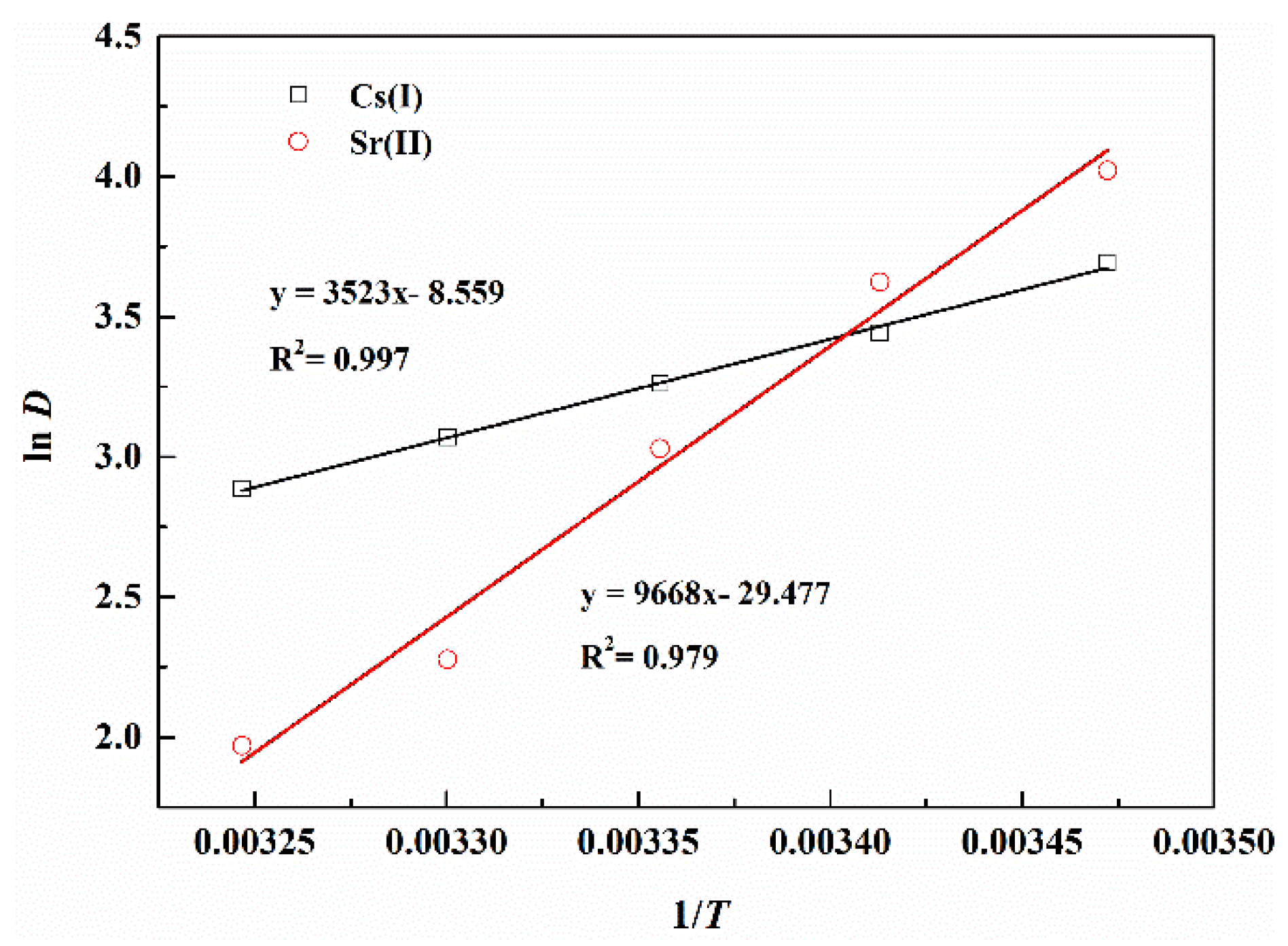
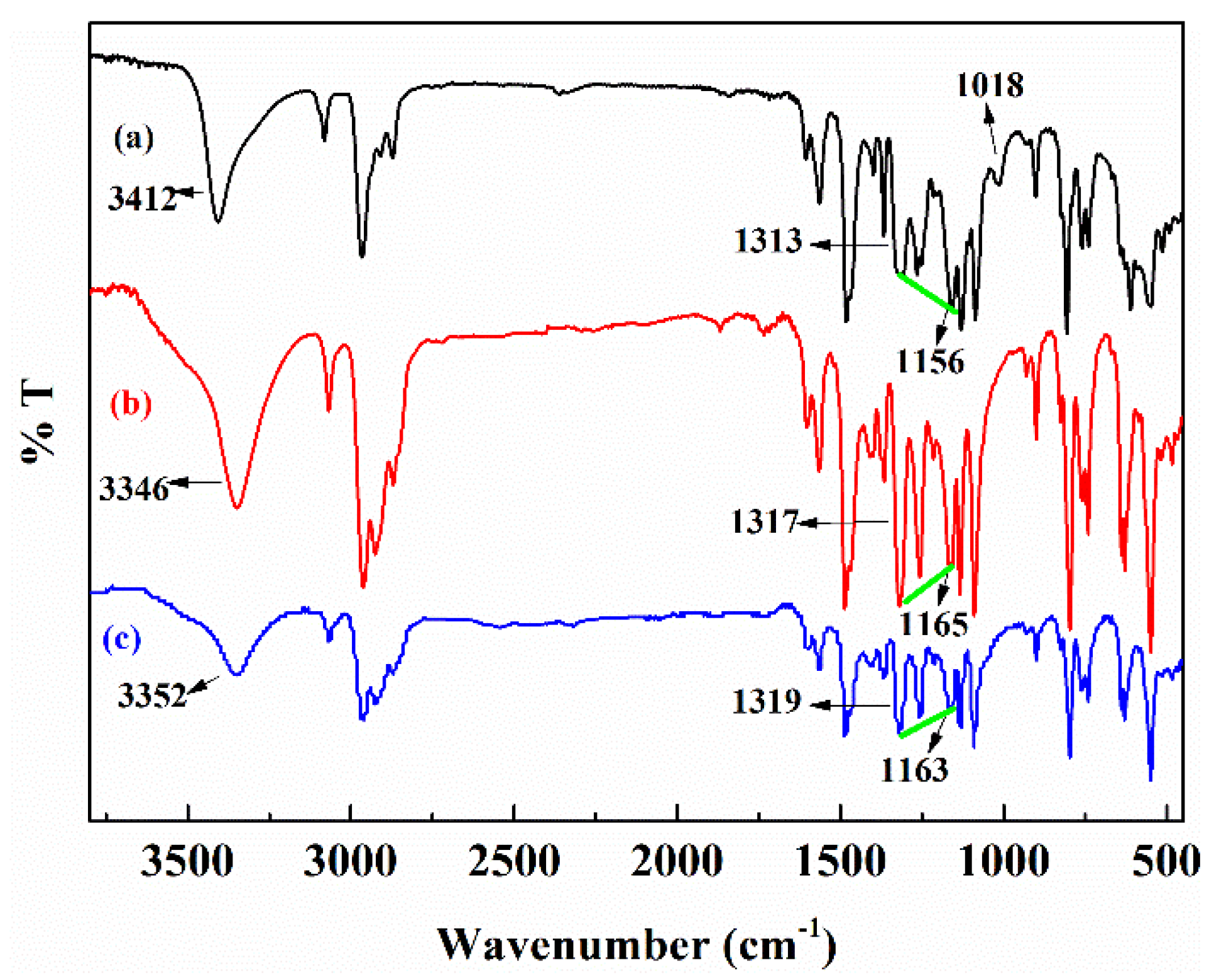

| Separation Factors | pH (±0.05) | ||||
|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | |
| SFCs/Na | - | 1.94 | 28.36 | 64.85 | 58.94 |
| SFCs/K | 9.14 | 25.89 | 24.71 | 77.90 | 115.4 |
| SFCs/Pb | 96.37 | 0.60 | 10.24 | 42.75 | 61.05 |
| SFCs/Ce | 21.51 | 0.10 | 1.37 | 3.94 | 6.28 |
| SFCs/Cd | 1.12 | 0.88 | 2.56 | 8.42 | 10.81 |
| SFCs/Zn | - | 160 | 162 | >162 | >162 |
| SFCs/Co | - | - | 121 | >121 | >121 |
| SFCs/Ni | - | - | - | >103 | >103 |
| SFSr/Na | - | 1.35 | 8.36 | 42.25 | 44.17 |
| SFSr/K | 7.64 | 17.95 | 7.28 | 14.72 | 14.72 |
| SFSr/Pb | 80.60 | 0.42 | 3.02 | 38.07 | 45.74 |
| SFSr/Ce | 17.99 | 0.07 | 0.40 | 3.74 | 4.70 |
| SFSr/Cd | 0.94 | 0.61 | 0.76 | 7.59 | 8.10 |
| SFSr/Zn | - | 39.4 | 183 | >183 | >183 |
| SFSr/Co | - | - | 137 | >137 | >137 |
| SFSr/Ni | - | - | - | >103 | >103 |
| Conditions | Cs(I) | Sr(II) | ||||
|---|---|---|---|---|---|---|
| Temperature | ΔH° (kJ·mol−1) | ΔS° (kJ·mol−1 K−1) | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (kJ·mol−1 K−1) | ΔG° (kJ·mol−1) |
| 288 K | −3.49 | −6.44 | ||||
| 293 K | −3.03 | −4.85 | ||||
| 298 K | −29.29 | −0.09 | −2.56 | −80.38 | −0.26 | −3.51 |
| 303 K | −2.09 | −2.08 | ||||
| 308 K | −1.62 | −0.69 | ||||
| Conditions | DCs | DSr | ||||
|---|---|---|---|---|---|---|
| pH (±0.05) | SC4A | TC4A | SC4AOEt | SC4A | TC4A | SC4AOEt |
| 2 | 0.13 | 0.07 | 0.07 | 0.04 | 0 | 0.25 |
| 3 | 1.60 | 0.06 | 0.09 | 0.39 | 0 | 0.28 |
| 4 | 4.86 | 0.04 | 0.10 | 5.49 | 0.01 | 0.30 |
| 5 | 19.32 | 0.02 | 0.12 | 20.04 | 0 | 0.31 |
| 6 | 27.62 | 0.04 | 0.17 | 20.67 | 0 | 0.35 |
| Irradiated Samples | Dose Rate (Gy/min) | Irradiation Dose (Gy) |
|---|---|---|
| A | 0 | 0 |
| B | 30 | 1.0 × 104 |
| C | 30 | 1.0 × 105 |
| D | 50 | 5.0 × 105 |
| E | 75 | 1.1 × 106 |
| Irradiation Dose (Gy) | 0 | 1.0 × 104 | 1.0 × 105 | 5.0 × 105 | 1.1 × 106 |
|---|---|---|---|---|---|
| DCs | 27.62 | 27.48 | 27.42 | 28.37 | 31.27 |
| DSr | 20.74 | 20.18 | 21.14 | 21.54 | 20.94 |
| Irradiationdose (Gy) | 0 | 1.0 × 104 | 1.0 × 105 | 5.0 × 105 | 1.1 × 106 |
|---|---|---|---|---|---|
| DCs | 27.59 | 26.92 | 27.55 | 20.67 | 16.27 |
| DSr | 20.86 | 21.13 | 20.62 | 15.36 | 11.51 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Zhang, D.; Zhao, L.; Zhang, P.; Lu, X.; He, S. Extraction Property of p-tert-Butylsulfonylcalix[4]arene Possessing Irradiation Stability towards Cesium(I) and Strontium(II). Appl. Sci. 2016, 6, 212. https://doi.org/10.3390/app6080212
Liu C, Zhang D, Zhao L, Zhang P, Lu X, He S. Extraction Property of p-tert-Butylsulfonylcalix[4]arene Possessing Irradiation Stability towards Cesium(I) and Strontium(II). Applied Sciences. 2016; 6(8):212. https://doi.org/10.3390/app6080212
Chicago/Turabian StyleLiu, Cong, Dongxiang Zhang, Liting Zhao, Peng Zhang, Xin Lu, and Shengnan He. 2016. "Extraction Property of p-tert-Butylsulfonylcalix[4]arene Possessing Irradiation Stability towards Cesium(I) and Strontium(II)" Applied Sciences 6, no. 8: 212. https://doi.org/10.3390/app6080212
APA StyleLiu, C., Zhang, D., Zhao, L., Zhang, P., Lu, X., & He, S. (2016). Extraction Property of p-tert-Butylsulfonylcalix[4]arene Possessing Irradiation Stability towards Cesium(I) and Strontium(II). Applied Sciences, 6(8), 212. https://doi.org/10.3390/app6080212