Biodegradation of Unsymmetrical Dimethylhydrazine in Solution and Soil by Bacteria Isolated from Activated Sludge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Enrichment Procedure and Isolation of Microorganisms
2.3. Strain Identification
2.4. Inoculum Preparation
2.5. Studies on Unsymmetrical Dimethylhydrazine (UDMH) Degradation in Mineral Salt Medium (MSM)
2.6. Studies on UDMH Degradation in Soil
2.7. Sample Pretreatment for Analyses
2.8. Chemical Analyses
3. Results
3.1. The Biodegradation of UDMH by Activated Sludge
3.2. Isolation and Identification of M12 and P4
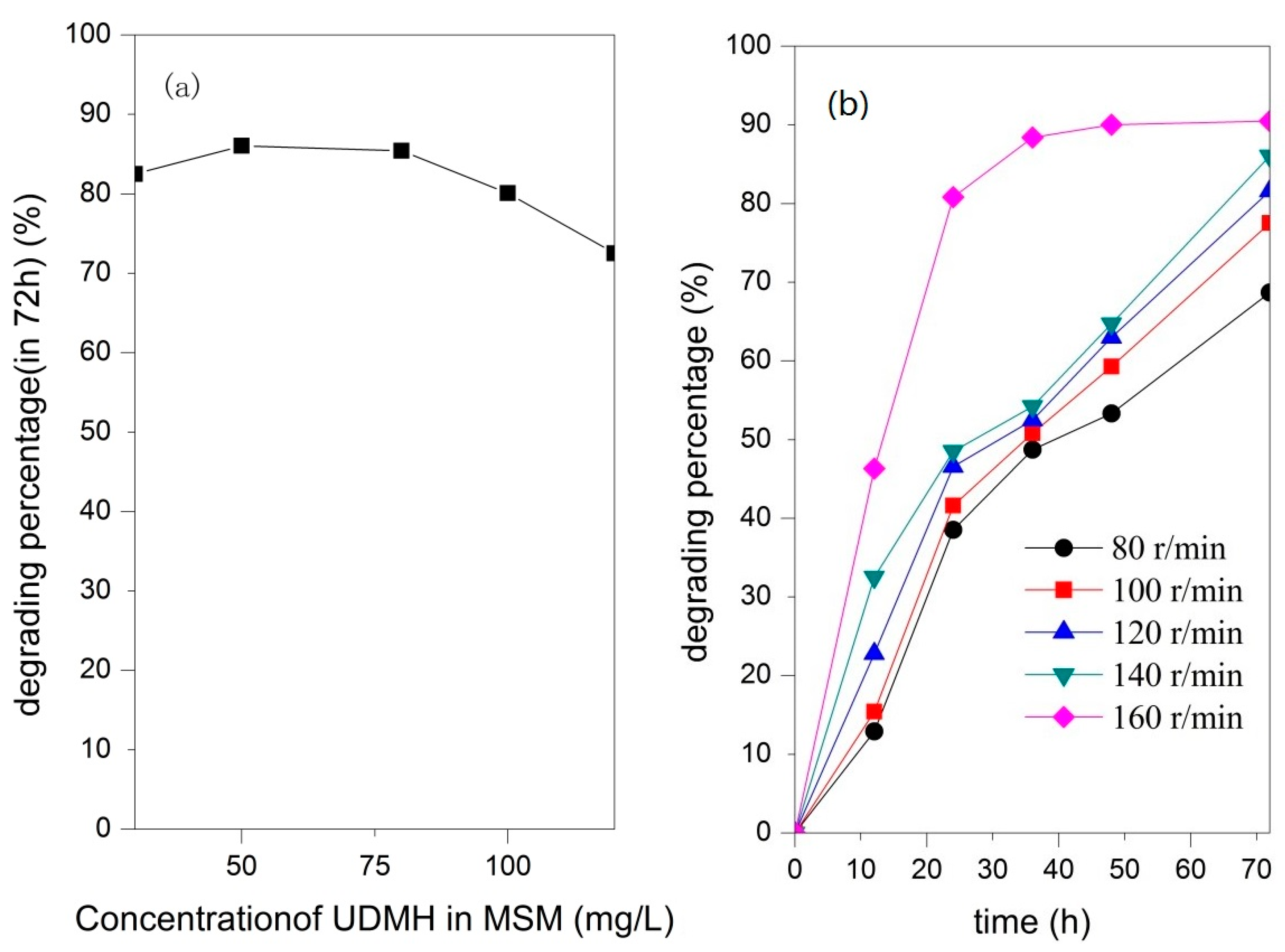
3.3. Biodegradation of UDMH in Aqueous MSM
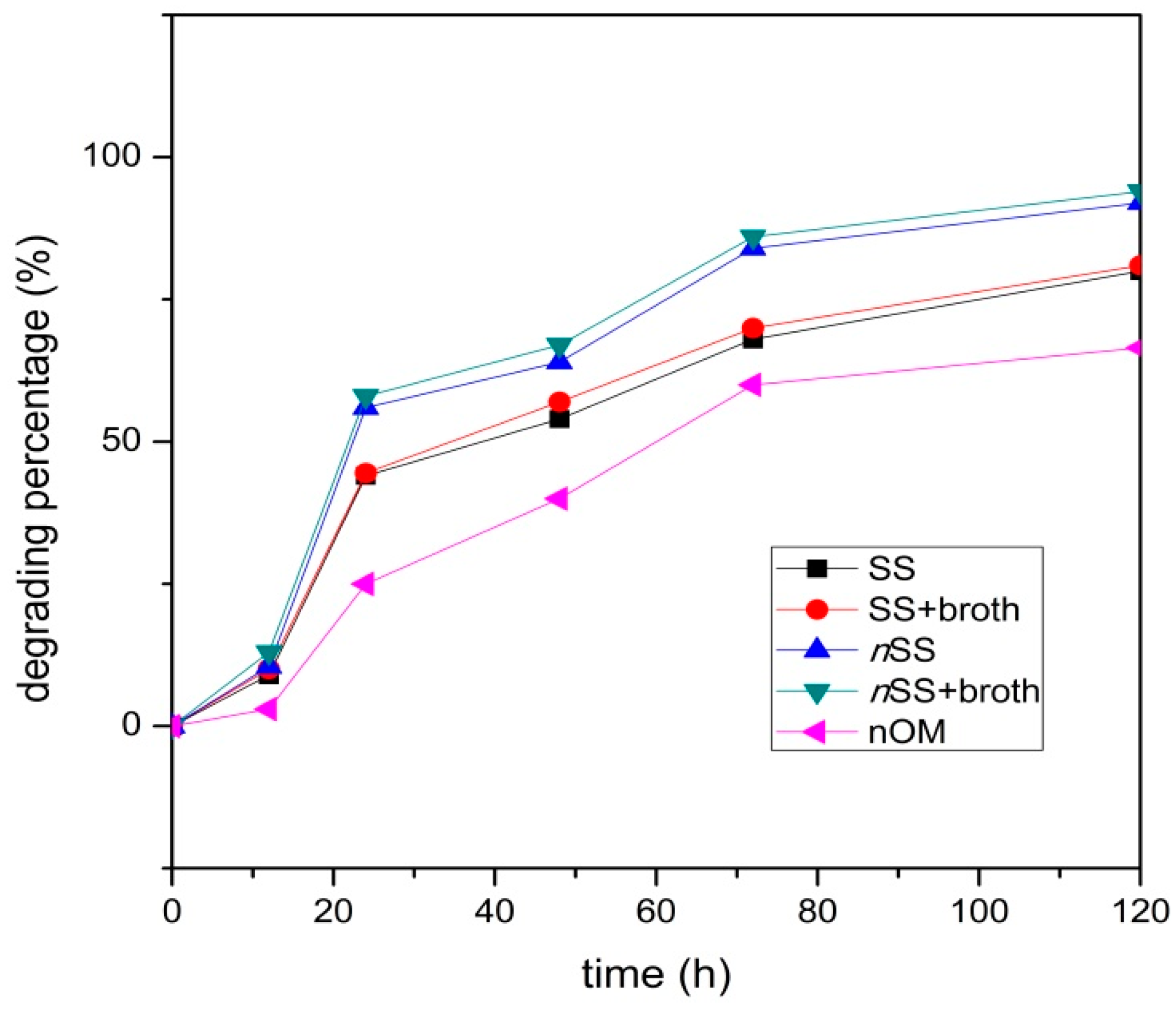
3.4. Biodegradation of UDMH in Soil
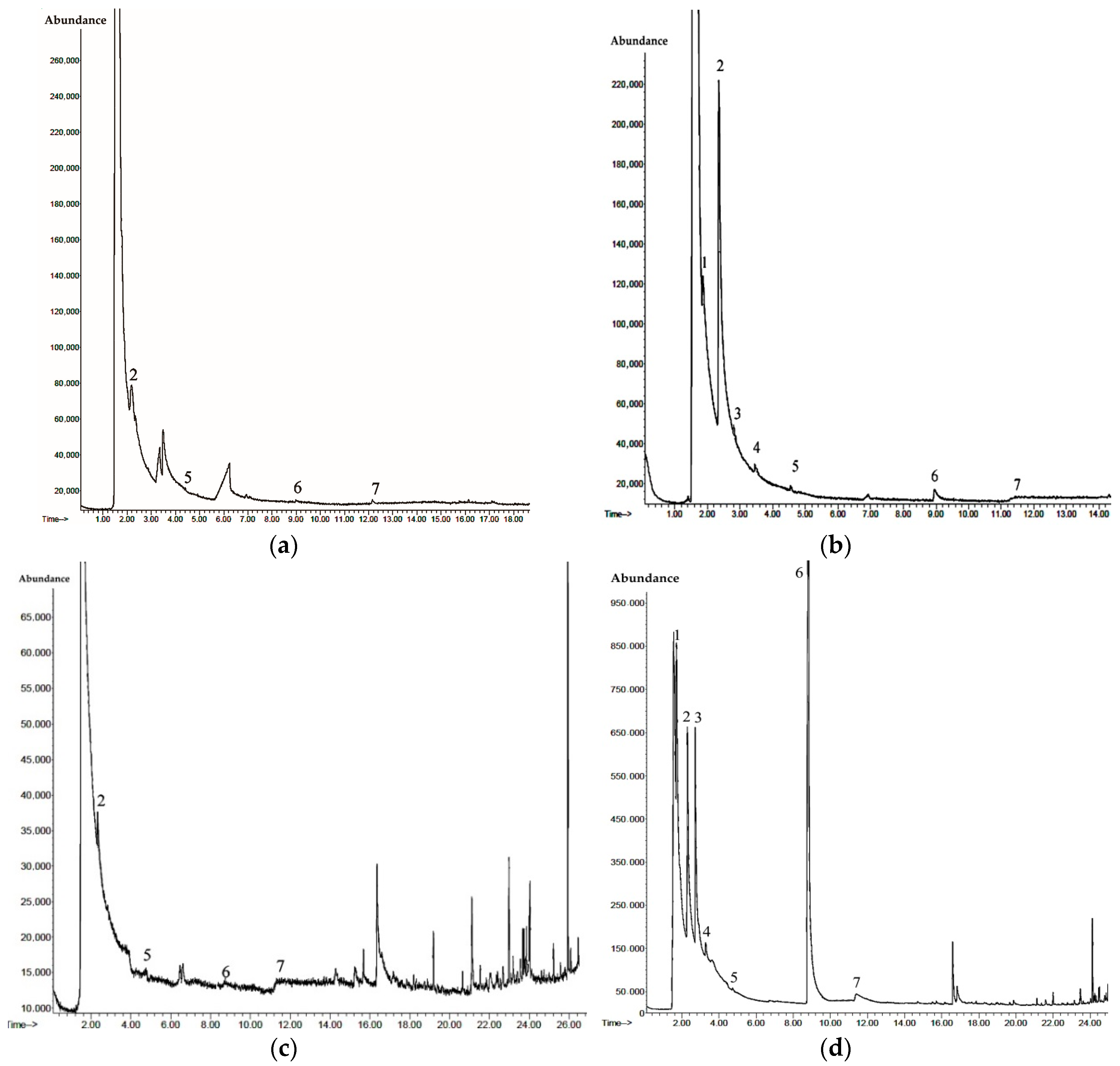
3.5. Degradation Intermediates
4. Discussion
4.1. Capability of Biodegradation UDMH
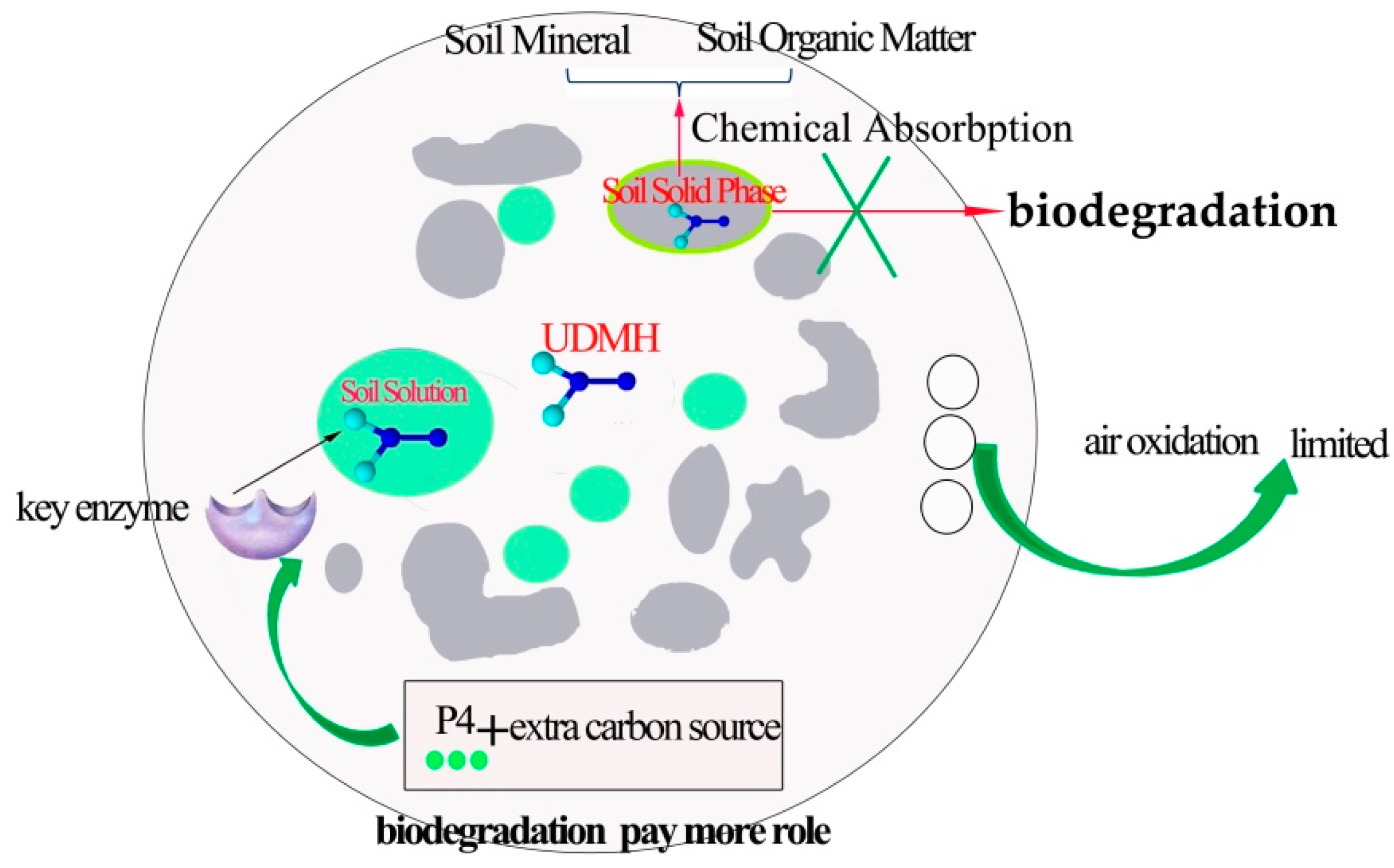
4.2. The Complexity of UDMH Biodegradation in Soil
4.3. The Influencing Factors on Microbial Degradation of UDMH
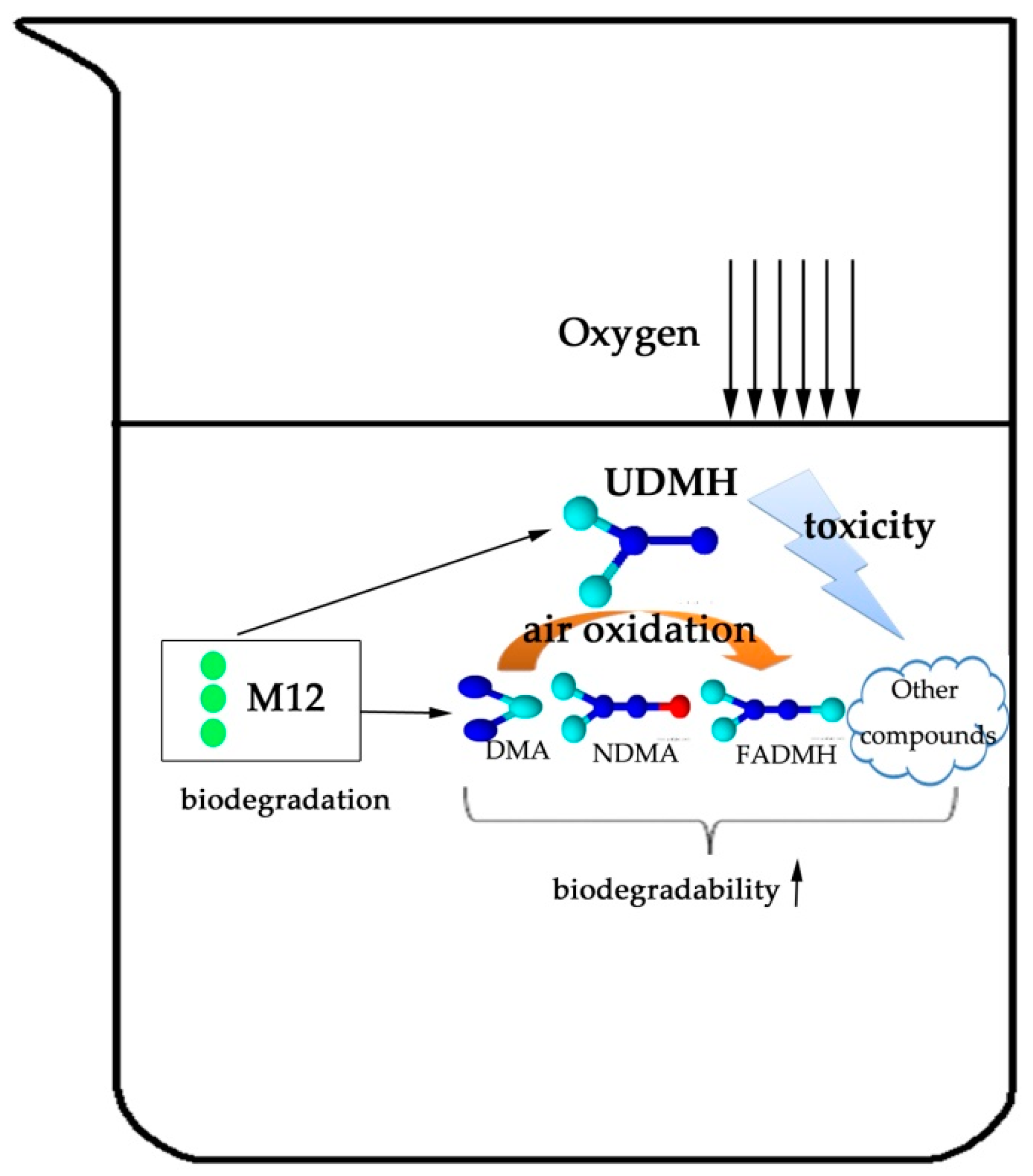
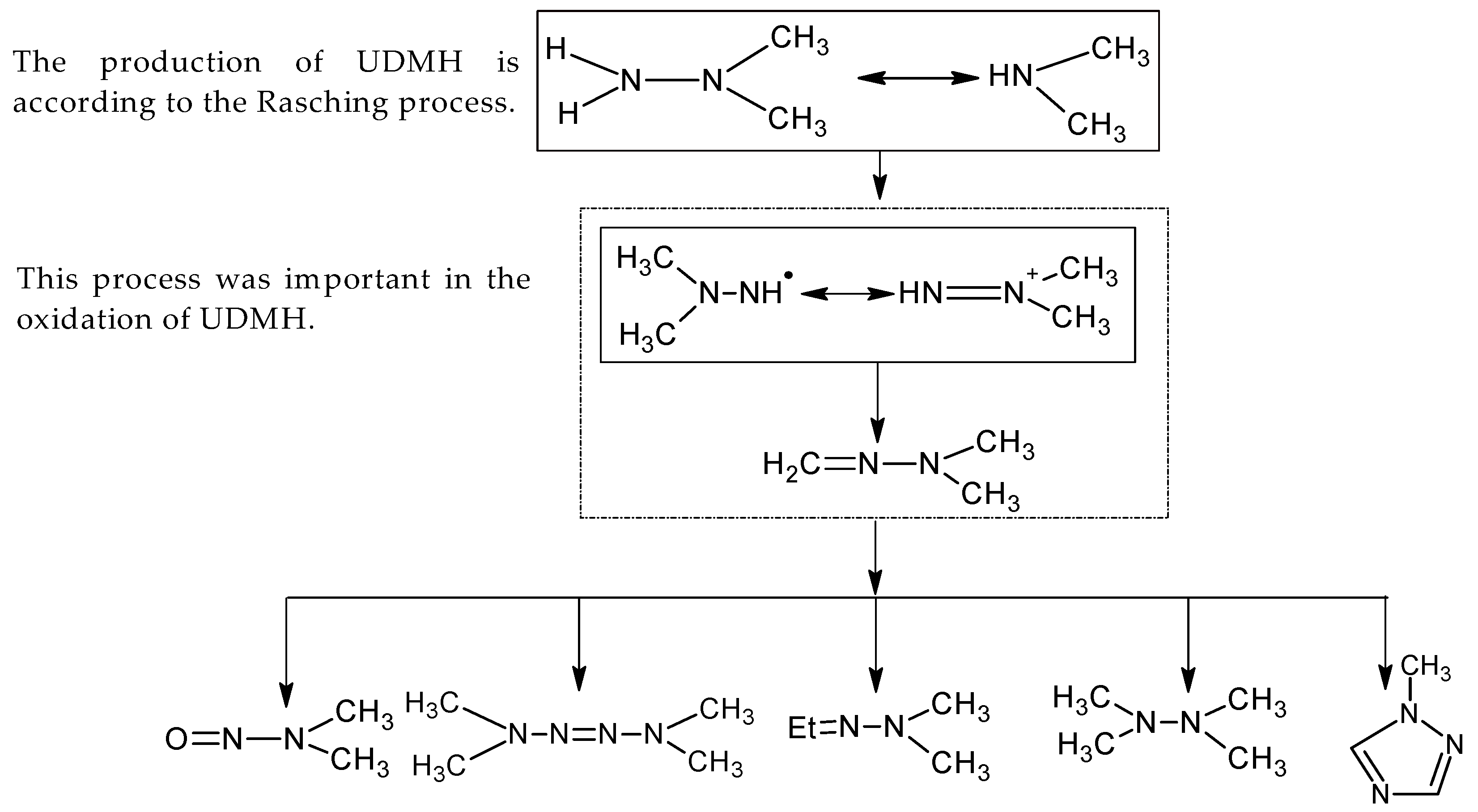
4.4. Degradation Pathway
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kenessov, B.N.; Koziel, J.A.; Grotenhuis, T.; Carlsen, L. Screening of transformation products in soils contaminated with unsymmetrical dimethylhydrazine using headspace spme and GC-MS. Anal. Chim. Acta 2010, 674, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, L.; Kenessov, B.N.; Batyrbekova, S.Y.; Kolumbaeva, S.; Shalakhmetova, T.M. Assessment of the mutagenic effect of 1,1-dimethyl hydrazine. Environ. Toxicol. Pharmacol. 2009, 28, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Roe, F.; Grant, G.; Millican, D. Carcinogenicity of hydrazine and 1,1-dimethylhydrazine for mouse lung. Nature 1967, 216, 375–376. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Skibutani, M.; Toyoda, K.; Shoda, T.; Takada, K.; Uneyama, C.; Takahashi, M.; Hirose, M. Tumor-promoting activities of hydroquinone and 1,1-dimethylhydrazine after initiation of newborn mice with 1-methyl-1-nitrosourea. Cancer Lett. 1999, 143, 71–80. [Google Scholar] [CrossRef]
- Choudhary, G.; IIansen, H.; Donkin, S.; Kirman, C. Toxicological profile for hydrazines. US Dep. Health Hum. Serv. 1997, 5, 1–185. [Google Scholar]
- USA Environmental Protection Agency (EPA). 1,1-dimethylhydrazine. Available online: http://www.epa.gov/ttn/atw/hlthef/dimethyl.html#ref7 (accessed on 3 May 2014).
- Fedorov, L.A. Liquid missile propellants in the former Soviet Union. Environ. Pollut. 1999, 105, 157–161. [Google Scholar]
- Buryak, A.K.; Serdyuk, T.M.; Ul’yanov, A.V. Investigation of the reaction products of unsymmetrical dimethylhydrazine with potassium permanganate by gas chromatography-mass spectrometry. Theor. Found. Chem. Eng. 2011, 45, 550–555. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Li, B. Discharge Standard of Water Pollutant and Standard of Analytical Methed for Space Propellant; The Ministry of Environmental Protection of the People Republic of China: Beijing, China, 1993; p. 22.
- Moliner, A.M.; Street, J. Decompostion of hydrazine in aqueous solutions. J. Environ. Qual. 1989, 18, 483–487. [Google Scholar] [CrossRef]
- Carlsen, L.; Kenessov, B.N.; Batyrbekova, S.Y. A QSAR/QSTR study on the environmental health impact by the rocket fuel 1,1-dimethyl hydrazine and its transformation products. Environ. Health Insights 2008, 1. [Google Scholar] [CrossRef]
- Carlsen, L.; Kenesova, O.A.; Batyrbekova, S.E. A preliminary assessment of the potential environmental and human health impact of unsymmetrical dimethylhydrazine as a result of space activities. Chemosphere 2007, 67, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Giles, J. Study links sickness to Russian launch site. Nature 2005, 433. [Google Scholar] [CrossRef] [PubMed]
- The International Science and Technology Center (ISTC). System Analysis of Environmental Objects in the Territories of Kazakhstan, which Suffered Negative Influence through Baikonur Space Port Activity; Final Technical Report of ISTC k451.2; Center of Physical-Chemical Methods of Analysis, Al-Farabi Kazakh National University in Almaty: Almaty, Kazakhstan, 2006. [Google Scholar]
- Ismagilov, Z.R.; Kerzhentsev, M.A.; Ismagilov, I.Z.; Sazonov, V.A.; Parmon, V.N.; Elizarova, G.L.; Pestunova, O.P.; Shandakov, V.A.; Zuev, Y.L.; Eryomin, V.N.; et al. Oxidation of unsymmetrical dimethylhydrazine over heterogeneous catalysts: Solution of environmental problems of production, storage and disposal of highly toxic rocket fuels. Catal. Today 2002, 75, 277–285. [Google Scholar] [CrossRef]
- Pestunova, O.P.; Elizarova, G.L.; Ismagilov, Z.R.; Kerzhentsev, M.A.; Parmon, V.N. Detoxication of water containing 1,1-dimethylhydrazine by catalytic oxidation with dioxygen and hydrogen peroxide over Cu- and Fe-containing catalysts. Catal. Today 2002, 75, 219–225. [Google Scholar] [CrossRef]
- Makhotkina, O.; Kuznetsova, E.; Preis, S. Catalytic detoxification of 1,1-dimethylhydrazine aqueous solutions in heterogeneous fenton system. Appl. Catal. B Environ. 2006, 68, 85–91. [Google Scholar] [CrossRef]
- Greene, B.; McClure, M.B.; Johnson, H.T. Destruction or decomposition of hypergolic chemicals in a liquid propellant testing laboratory. Chem. Health Saf. 2004, 11, 6–13. [Google Scholar] [CrossRef]
- Kolinko, P.A.; Kozlov, D.V.; Vorontsov, A.V.; Preis, S.V. Photocatalytic oxidation of 1,1-dimethyl hydrazine vapours on TiO2: FTIR in situ studies. Catal. Today 2007, 122, 178–185. [Google Scholar] [CrossRef]
- Ismagilov, I.; Michurin, E.; Sukhova, O.; Tsykoza, L.; Matus, E.; Kerzhentsev, M.; Ismagilov, Z.; Zagoruiko, A.; Rebrov, E.; Decroon, M. Oxidation of organic compounds in a microstructured catalytic reactor. Chem. Eng. J. 2008, 135, S57–S65. [Google Scholar] [CrossRef]
- Brubaker, K.L.; Bonilla, J.V.; Boparai, A.S. Products of the Hypochlorite Oxidation of Hydrazine fuels. Available online: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA213557 (accessed on 1 March 2016).
- Megharaj, M.; Ramakrishnan, B.; Venkateswarlu, K.; Sethunathan, N.; Naidu, R. Bioremediation approaches for organic pollutants: A critical perspective. Environ. Int. 2011, 37, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.P.; Bae, H.; Kim, I.H.; Kwon, S.T. Cloning, expression, and PCR application of DNA polymerase from the hyperthermophilic archaeon, Thermococcus celer. Biotechnol. Lett. 2011, 33, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lane, D. 16S/23S rRNA sequencing. Nucleic Acid Tech. Bact. Syst. 1991, 5, 125–175. [Google Scholar]
- Shunhong, H. Characteristics of Chromium Pollution at Chromium-Containing Slag Site and Chromium (VI) Bioremediation in the Contaminated Soil; Zhongnan University: Wuhan, China, 2009. [Google Scholar]
- Smolenkov, A.D.; Smirnov, R.S.; Rodin, I.A.; Tataurova, O.G.; Shpigun, O.A. Effect of sample preparation conditions on the determination of the total concentrations of unsymmetrical dimethylhydrazine in soils. J. Anal. Chem. 2011, 67, 6–13. [Google Scholar] [CrossRef]
- Rodin, I.; Smirnov, R.; Smolenkov, A.; Krechetov, P.; Shpigun, O. Transformation of unsymmetrical dimethylhydrazine in soils. Eurasian Soil Sci. 2012, 45, 386–391. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Y.; Liao, Q.L. Analysis of degradation products for water containing UDMH in different time by SPME-GC/MS method. Mod. Instrum. 2006, 12, 71–72. [Google Scholar]
- Zhang, G.Y.; Peng, P.T.; Xu, W.G.; Liao, Q.L. GC/MS determination of N-nitrosodimethylamine in waste water containing 1,1-dimethylhydrazine (in Chinese). Phys. Test. Chem. Anal. 2008, 44, 11–12. [Google Scholar]
- Cao, Y.; Wang, L.; Han, Z.-Z.; Zhang, G.-Y.; Liu, J.-G. GC-MS determination of unsymmetrical dimethylhydrazine in air (in Chinese). Phys. Test. Chem. Anal. 2010, 46, 1184–1186. [Google Scholar]
- Xi, H.; Xu, Z.; Wang, L. Water Quality-Determination of Asymmetrical Dimethyl Hydrazine-Amino Ferrocyanide Sodium Spectrophotometric Method; GB/T14376; The Ministry of Environmental Protection of the People Republic of China: Beijing, China, 1993.
- London, S.A. Relative Toxicity of Hydrazine Propellants to a Soil bacterium. Available online: http://www.dtic.mil/dtic/tr/fulltext/u2/a080646.pdf (accessed on 1 March 2016).
- ISO. Water Quality–Determination of the Chemical Oxygen Demand, 2nd ed.; ISO 6060-1989; International Standards Organization: Geneva, Swltzerland, 1989; p. 7. [Google Scholar]
- Ziagova, M.; Kyriakou, G.; Liakopoulou-Kyriakides, M. Co-metabolism of 2,4-dichlorophenol and 4-Cl-m-cresol in the presence of glucose as an easily assimilated carbon source by Staphylococcus xylosus. J. Hazard. Mater. 2009, 163, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Street, J. Hydrazine degradation and its effect on microbial activity in soil. Bull. Environ. Contam. Toxicol. 1987, 38, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, R.S.; Rodin, I.A.; Smolenkov, A.D.; Shpigun, O.A. Determination of the products of the transformation of unsymmetrical dimethylhydrazine in soils using chromatography/mass spectrometry. J. Anal. Chem. 2010, 65, 1266–1272. [Google Scholar] [CrossRef]
- Carlsen, L.; Kenessov, B.N.; Batyrbekova, S.Y. A QSAR/ASTR study on the human health impact of the rocket fuel 1,1-dimethyl hydrazine and its transformation products multicriteria hazard ranking based on partial order methodologies. Environ. Toxicol. Pharmacol. 2009, 27, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Mitch, W.A.; Sharp, J.O.; Trussell, R.R.; Valentine, R.L.; Alvarez-Cohen, L.; Sedlak, D.L. N-nitrosodimethylamine (NDMA) as a drinking water contaminant: A review. Environ. Eng. Sci. 2003, 20, 389–404. [Google Scholar] [CrossRef]
- Fleming, E.C.; Pennington, J.C.; Wachob, B.G.; Howe, R.A.; Hill, D.O. Removal of N-nitrosodimethylamine from waters using physical-chemical techniques. J. Hazard. Mater. 1996, 51, 151–164. [Google Scholar] [CrossRef]
- Rodin, I.A.; Anan’eva, I.A.; Smolenkov, A.D.; Shpigun, O.A. Determination of the products of the oxidative transformation of unsymmetrical dimethylhydrazine in soils by liquid chromatography/mass spectrometry. J. Anal. Chem. 2010, 65, 1405–1410. [Google Scholar] [CrossRef]
- Smolenkov, A.D.; Krechetov, P.P.; Pirogov, A.V.; Koroleva, T.V.; Bendryshev, A.A.; Shpigun, O.A.; Martynova, M.M. Ion chromatography as a tool for the investigation of unsymmetrical hydrazine degradation in soils. Int. J. Environ. Anal. Chem. 2005, 85, 1089–1100. [Google Scholar] [CrossRef]
- Mantel, C.; London, S. Adaptation of a soil bacterium to hydrazine propellants. Bull. Environ. Contam. Toxicol. 1980, 25, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.A.; Williamson, K.J. Bacterial Toxicity and Metabolism of Three Hydrazine fuels. Available online: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA099514 (accessed on 1 March 2016).
- Kuch, D.J. Bioremediation of Hydrazine: A Literature Review. Available online: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA323822 (accessed on 1 March 2016).
- Li, W. The Aerobic Biodegradation of UDMH Wastewater and Its Kinetics Research. Master’s Thesis, Chongqing University, Chongqing, China, 15 June 2005. [Google Scholar]
- Zhao, H.; Wang, L.; Xia, B.L.; Tan, S.Y.; Liu, Y.; Liao, Q.L. Treatment of water containing unsymmetrical dimethylhydrazine using electrolyzed oxidizing water combined with a membrane bioreactor. Fresen. Environ. Bull. 2013, 22, 3577–3583. [Google Scholar]
- Nwankwoala, A.U.; Egiebor, N.O.; Nyavor, K. Enhanced biodegradation of methylhydrazine and hydrazine contaminated NASA wastewater in fixed-film bioreactor. Biodegradation 2001, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kenessov, B.; Alimzhanova, M.; Sailaukhanuly, Y.; Baimatova, N.; Abilev, M.; Batyrbekova, S.; Carlsen, L.; Tulegenov, A.; Nauryzbayev, M. Transformation products of 1,1-dimethylhydrazine and their distribution in soils of fall places of rocket carriers in central Kazakhstan. Sci. Total. Environ. 2012, 427–428, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Street, J.; Johnston, C.; Mansell, R.; Bloom, S. Environmental Interactions of Hydrazine Fuels in Soil/Water Systems. Available online: http://www.dtic.mil/dtic/tr/fulltext/u2/a206244.pdf (accessed on 1 March 2016).
- Shelton, D.R.; Doherty, M.A. A model describing pesticide bioavailability and biodegradation in soil. Soil Sci. Soc. Am. J. 1997, 61, 1078–1084. [Google Scholar] [CrossRef]
- Cosser, R.; Tompkins, F. Heterogeneous decomposition of hydrazine on tungsten films. Trans. Faraday Soc. 1971, 67, 526–544. [Google Scholar] [CrossRef]
- Zavilgelsky, G.B.; Kotova, V.Y.; Manukhov, I.V. Action of 1,1-dimethylhydrazine on bacterial cells is determined by hydrogen peroxide. Mutat. Res. 2007, 634, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Lunn, G.; Sansone, E.B. Oxidation of 1,1-dimethylhydrazine (UDMH) in aqueous solution with air and hydrogen peroxide. Chemosphere 1994, 29, 1577–1590. [Google Scholar] [CrossRef]
- Blum, P.; Sagner, A.; Tiehm, A.; Martus, P.; Wendel, T.; Grathwohl, P. Importance of heterocylic aromatic compounds in monitored natural attenuation for coal tar contaminated aquifers: A review. J. Contam. Hydrol. 2011, 126, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Boon, N.; Goris, J.; de Vos, P.; Verstraete, W.; Top, E.M. Bioaugmentation of activated sludge by an indigenous 3-chloroaniline-degrading comamonas testosteroni strain, I2gfp. Appl. Environ. Microbiol. 2000, 66, 2906–2913. [Google Scholar] [CrossRef] [PubMed]
- Sierka, R.A.; Cowen, W.F. The Ozone Oxidation of Hydrazine Fuels. Available online: http://www.dtic.mil/cgi-bin/GetTRDoc?AD=ADA065829 (accessed on 1 March 2016).
- Guangyou, Z.; Li, W.; Yafei, X.; Chunhua, F.; Rongshu, X. The quantum chemical investigation on the important middle product dimethyldiazene in the degredation process of UDMH and •OH. Procedia Environ. Sci. 2011, 10, 703–708. [Google Scholar] [CrossRef]
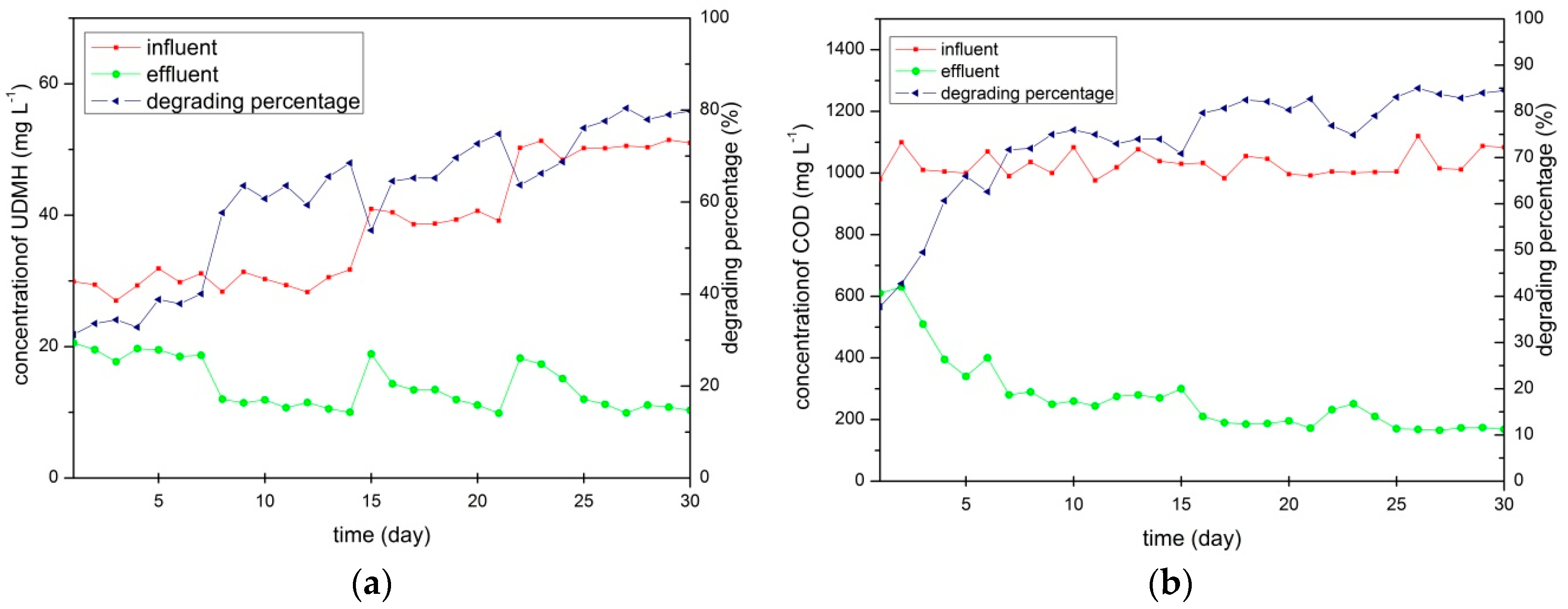
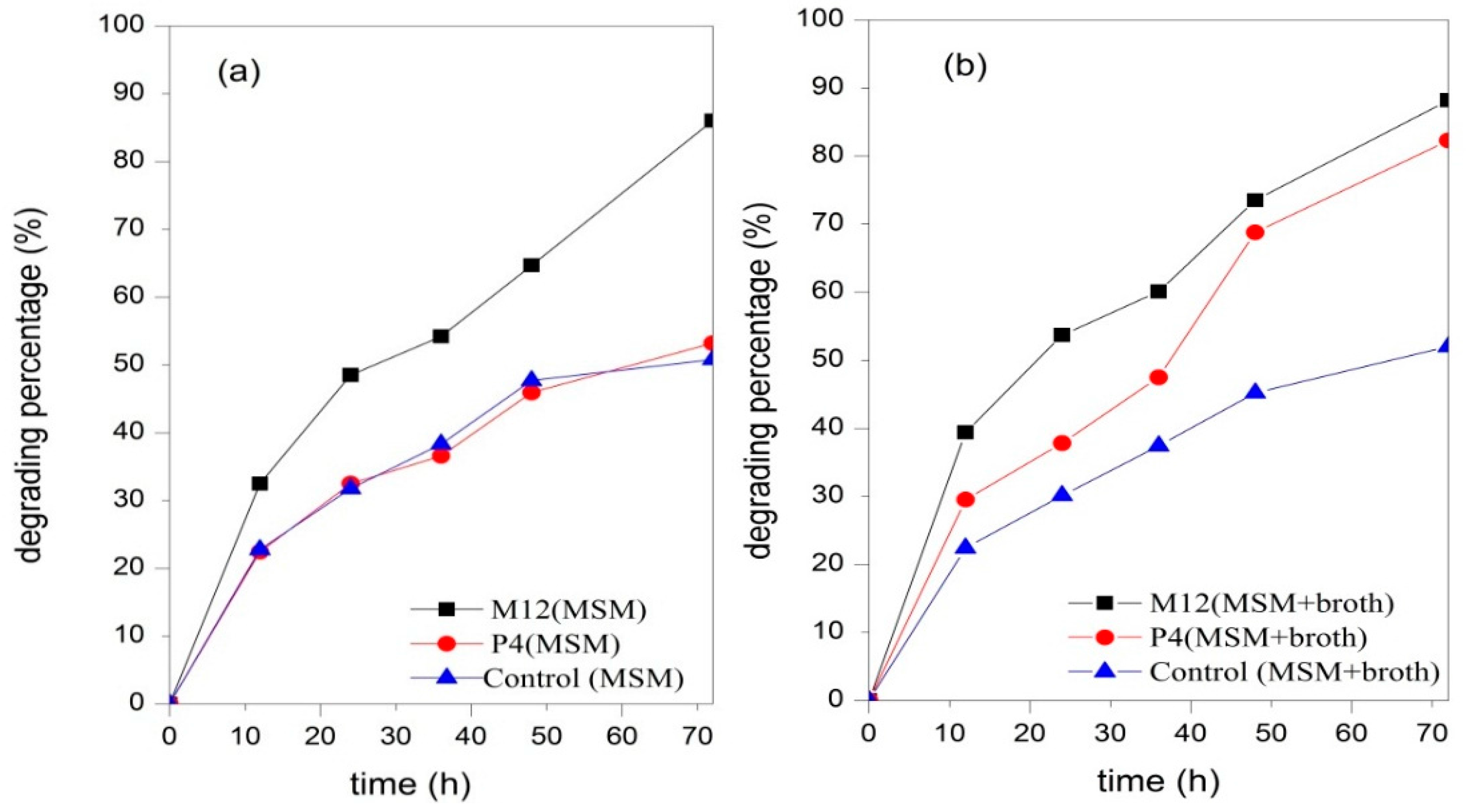
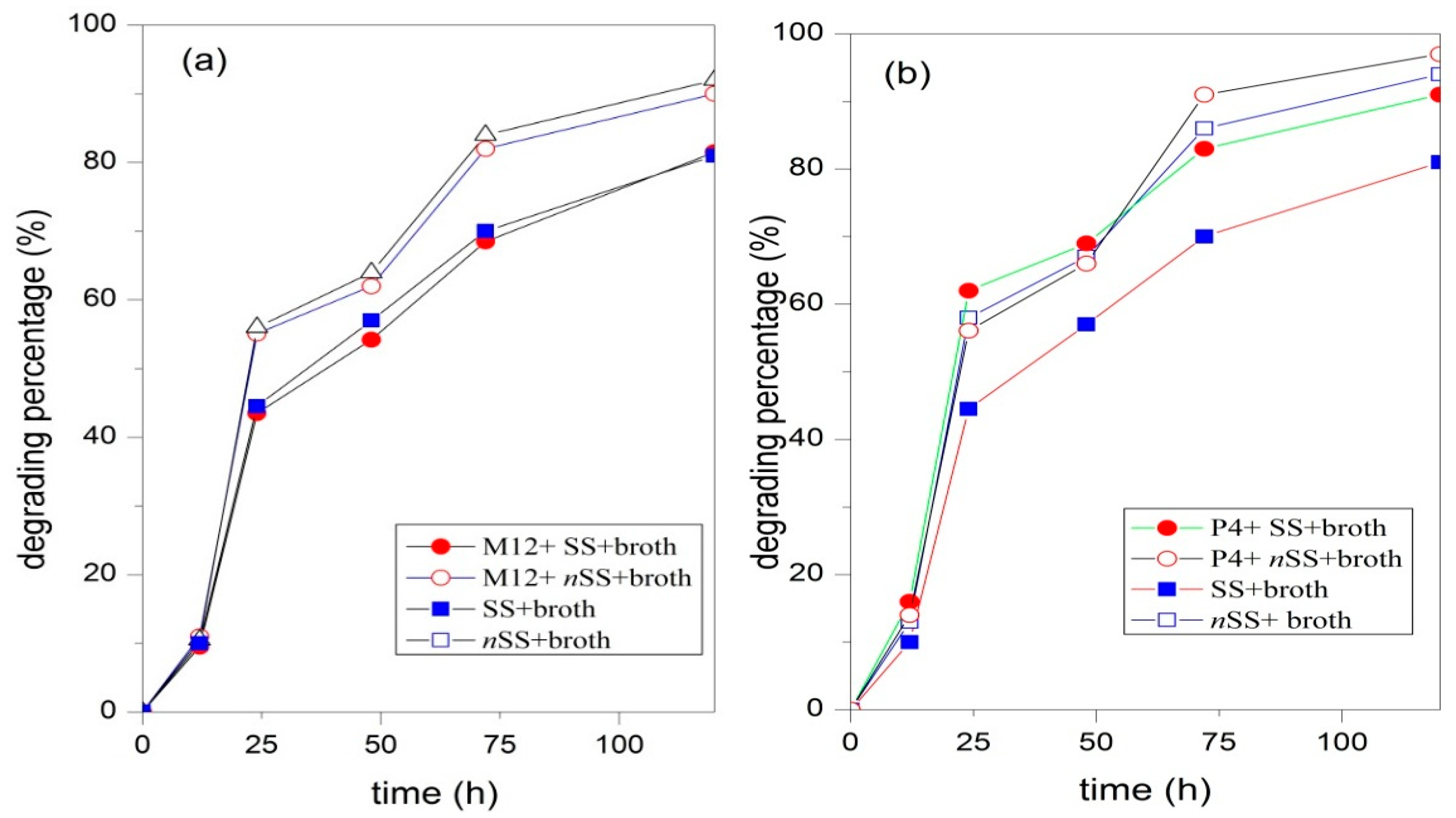
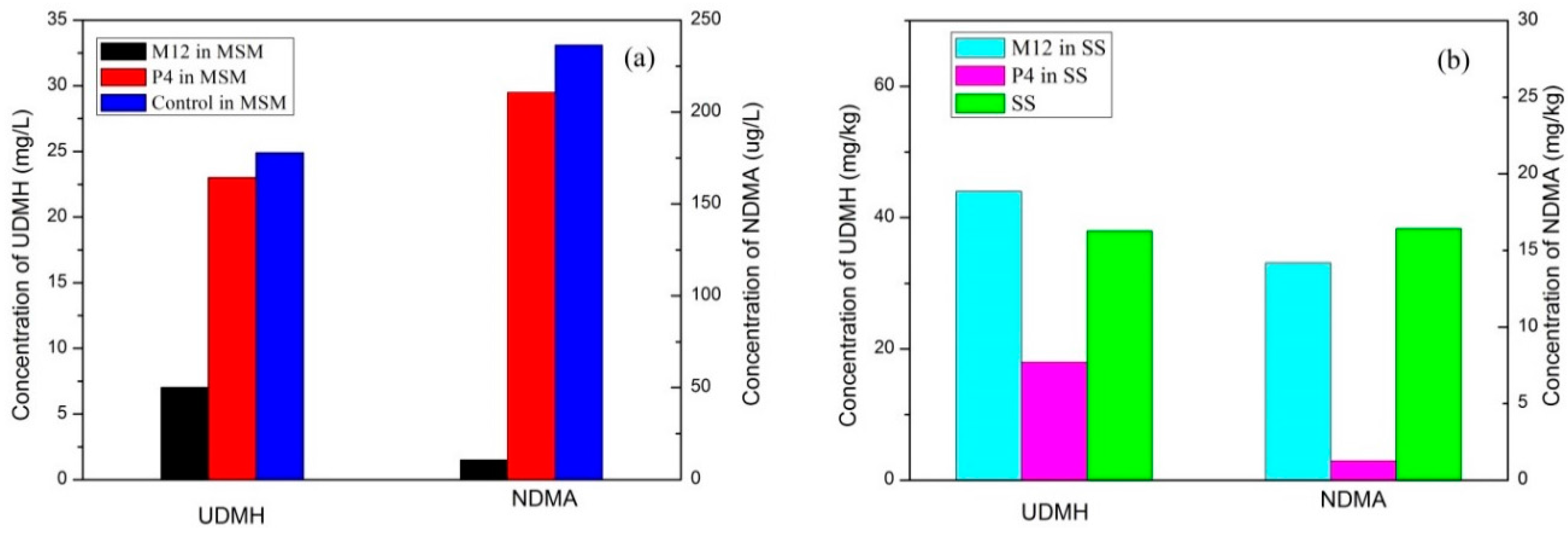

| Parameter | Values |
|---|---|
| Sand (50–2000 μm) (%) | 48.83 |
| Silk (2–50 μm) (%) | 35.47 |
| Clay (<2 μm) (%) | 15.70 |
| Density (g·cm−3) | 1.55 |
| pH (in water) | 7.63 |
| Water-holding capacity WHC (%) | 23.04 |
| Organic carbon (%) | 1.26 |
| Total nitrogen content (%) | 0.11 |
| No. | Compound Name | CAS No. | Formula | Characteristic Ions, m/z (Relative Abundance, %) |
|---|---|---|---|---|
| 1 | Dimethylamine | 124-40-3 | C2NH7 | 44 (100); 45 (56); 28 (26); 46 (3) |
| 2 | Formaldehyde dimethylhydrazone | 2035-89-4 | C3N2H8 | 72 (100); 71 (71); 42 (61); 57 (15) |
| 3 | Tetramethylhydrazine | 6415-12-9 | C4N2H12 | 88 (100); 73 (89); 44 (73); 42 (63) |
| 4 | Acetaldehyde dimethylhydrazone | 7422-90-4 | C4N2H10 | 86 (100); 44 (51); 42 (48); 85 (34) |
| 5 | N-Nitrosodimethylamine | 62-75-9 | C2N2H6O | 74 (100); 42 (37); 43 (15); 41 (4) |
| 6 | 1,1,4,4-Tetramethyltetrazene | 6130-87-6 | C4N4H12 | 116 (100); 43 (53); 72 (42); 42 (32) |
| 7 | 1-methyl-1H-1,2,4-triazole | 6086-21-1 | C3N3H5 | 83 (100); 56 (29); 84 (5); 40 (4) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Q.; Feng, C.; Wang, L. Biodegradation of Unsymmetrical Dimethylhydrazine in Solution and Soil by Bacteria Isolated from Activated Sludge. Appl. Sci. 2016, 6, 95. https://doi.org/10.3390/app6040095
Liao Q, Feng C, Wang L. Biodegradation of Unsymmetrical Dimethylhydrazine in Solution and Soil by Bacteria Isolated from Activated Sludge. Applied Sciences. 2016; 6(4):95. https://doi.org/10.3390/app6040095
Chicago/Turabian StyleLiao, Qili, Changgen Feng, and Li Wang. 2016. "Biodegradation of Unsymmetrical Dimethylhydrazine in Solution and Soil by Bacteria Isolated from Activated Sludge" Applied Sciences 6, no. 4: 95. https://doi.org/10.3390/app6040095
APA StyleLiao, Q., Feng, C., & Wang, L. (2016). Biodegradation of Unsymmetrical Dimethylhydrazine in Solution and Soil by Bacteria Isolated from Activated Sludge. Applied Sciences, 6(4), 95. https://doi.org/10.3390/app6040095






