A Label-Free Aptamer-Based Fluorescent Assay for Cadmium Detection
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Chemicals
2.2. Instrumentation
2.3. Fluorescence Detection of Cd2+
3. Results and Discussion
3.1. Working Mechanism
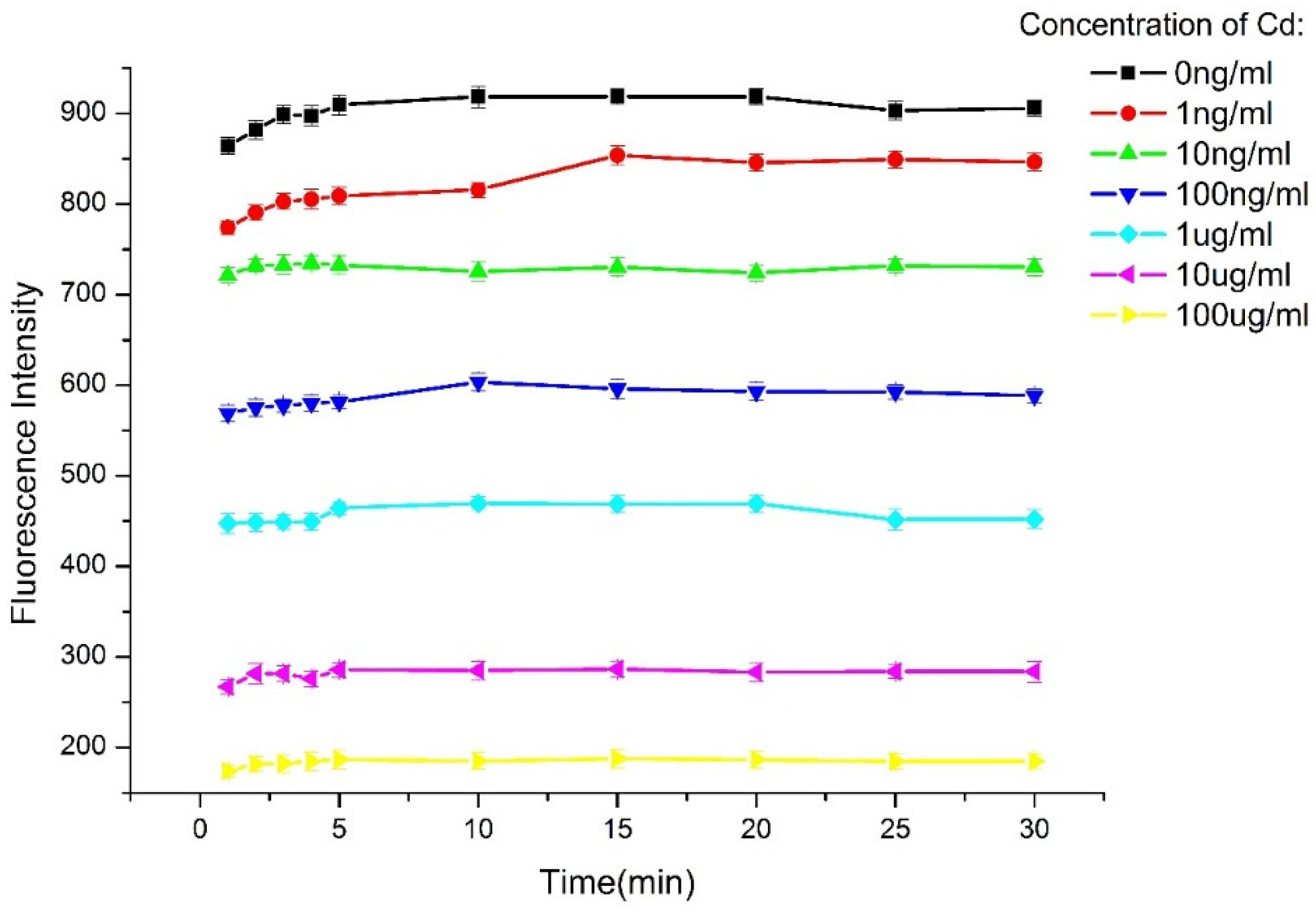
3.2. Optimization of the Experimental Parameters
3.3. Detection Sensitivity
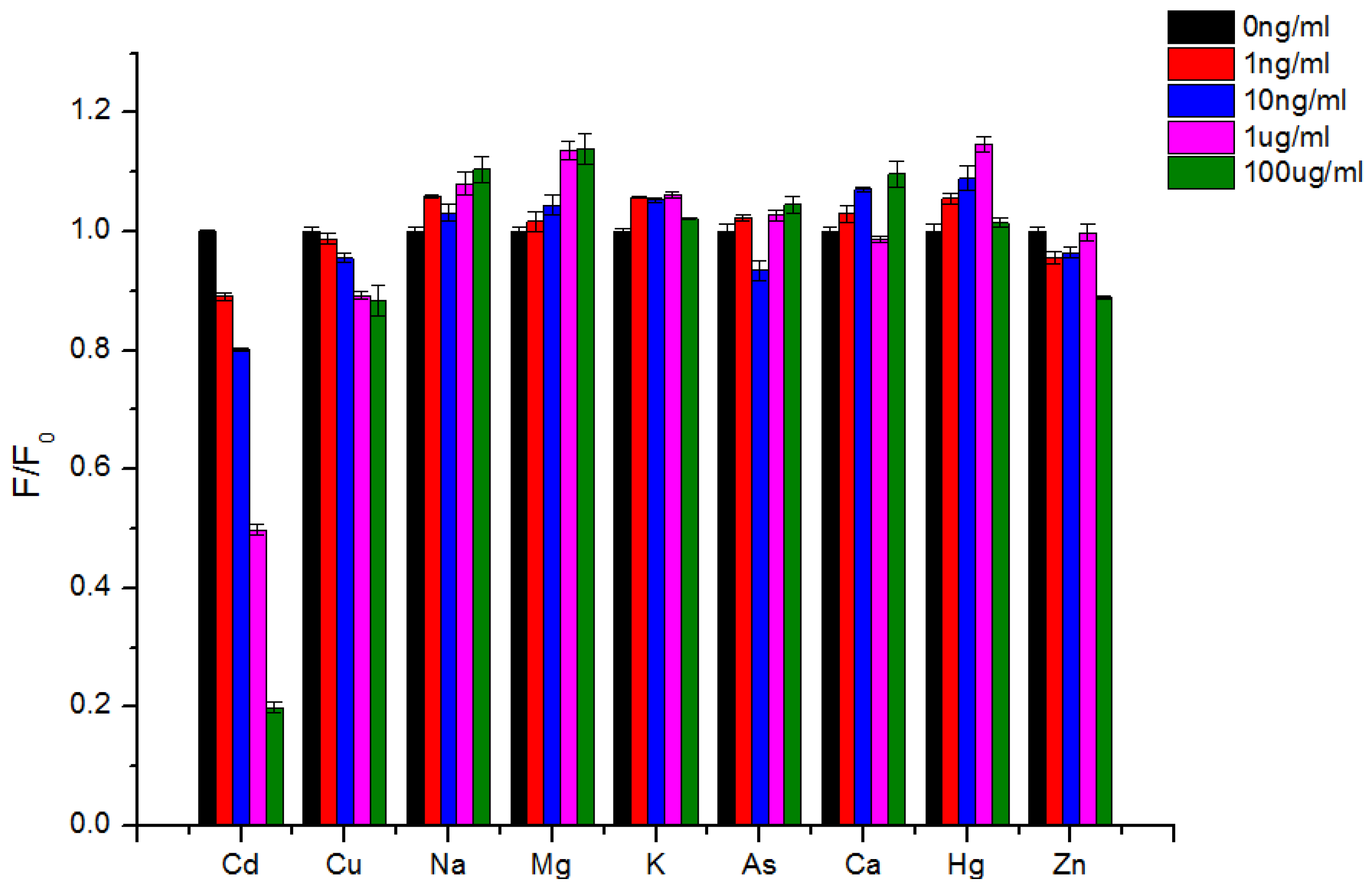
3.4. Detection Selectivity
3.5. Practical Application
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, T.; Zhu, X.F.; Zhou, S.H.; Yang, G.; Gan, W.; Yuan, Q.H. DNA derived fluorescent bio-dots for sensitive detection of mercury and silver ions in aqueous solution. Appl. Surf. Sci. 2015, 347, 505–513. [Google Scholar] [CrossRef]
- Hua, Z.L.; Yang, B.; Chen, W.; Bai, X.; Xu, Q.J.; Gu, H.X. Surface functionalized magnetic PVA microspheres for rapid naked-eye recognizing of copper (II) ions in aqueous solutions. Appl. Surf. Sci. 2014, 317, 226–235. [Google Scholar] [CrossRef]
- The ATSDR Substance Priority List. Available online: http://www.atsdr.cdc.gov/spl/ (accessed on 7 May 2014).
- Houk, R.S. Elemental and isotopic analysis by inductively coupled plasma mass spectrometry. Acc. Chem. Res. 1994, 11, 333–339. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Saraswathi, R. Electrochemical sensing of cadmium and lead ions at zeolite-modified electrodes: Optimization and field measurements. Sens. Actuators B Chem. 2009, 141, 65–75. [Google Scholar] [CrossRef]
- Cheng, T.; Xu, Y.; Zhang, S.; Zhu, W.; Qian, X.; Duan, L. A highly sensitive and selective OFF–ON fluorescent sensor for cadmium in aqueous solution and living cell. J. Am. Chem. Soc. 2008, 48, 16160–16161. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Du, J.; Fan, J.; Wang, J.; Wu, Y.; Zhao, J.; Sun, S.; Xu, T. A selective fluorescent sensor for imaging Cd2+ in living cells. J. Am. Chem. Soc. 2007, 129, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yang, M.; Fan, X.; Wang, H. Turn-on electrochemiluminescence sensing of Cd2+ based on CdTe quantum dots. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 133, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Soo Oh, S.; Nie, J.; Stewart, R.; Eisenstein, M.; Chambers, J.; Marth, J.D.; Walker, F.; Thomson, J.A.; Soh, H.T. Quantitative selection and parallel characterization of aptamers. Proc. Natl. Acad. Sci. USA 2013, 110, 18460–18465. [Google Scholar] [CrossRef] [PubMed]
- Dragan, A.I.; Casas-Finet, J.R.; Bishop, E.S.; Strouse, R.J.; Schenerman, M.A.; Geddes, C.D. Characterization of PicoGreen Interaction with dsDNA and the Origin of Its Fluorescence Enhancement upon Binding. Biophys. J. 2010, 99, 3010–3019. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhan, S.; Wang, L.; Zhou, P. Selection of a DNA aptamer for cadmium detection based on cationic polymer mediated aggregation of gold nanoparticles. Analyst 2014, 139, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cao, Z.; Kai, M.; Lu, J. Label-free aptamer-based chemiluminescence detection of adenosine. Talanta 2009, 79, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.L.; Chen, F.S.; Zhang, J.; Ren, S.B.; Liang, H.D.; Li, S.S. On-line flame AAS determination of traces Cd(II) and Pb(II) in water samples using thiol-functionalized SBA-15 as solid phase extractant. J. Ind. Eng. Chem. 2015, 27, 362–367. [Google Scholar] [CrossRef]
- Borges, S.S.D.; Beinner, M.A.; Silva, J.B.B. Direct Method for Determination of Al, Cd, Cu, and Pb in Beers In Situ Digested by GF AAS Using Permanent Modifiers. Biol. Trace Elem. Res. 2011, 167, 155–163. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.E.; Watson, R.J.; Butler, E.C. An ICP-MS procedure to determine Cd, Co, Cu, Ni, Pb and Zn in oceanic waters using in line flow injection with solid-phase extraction for preconcentration. Talanta 2013, 115, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Karami, H.; Mousavi, M.F.; Yamini, Y.; Shamsipur, M. On-line preconcentration and simultaneous determination of heavy metal ions by inductively coupled plasma atomic emission spectrometry. Anal. Chim. Acta 2004, 509, 89–94. [Google Scholar] [CrossRef]
- Queirolo, F.; Stegen, S.; Restovic, M.; Paz, M.; Ostapczuk, P.; Schwuger, M.J.; Muñoz, L. Total arsenic, lead, and cadmium levels in vegetables cultivated at the Andean villages of northern Chile. Sci. Total Environ. 2000, 255, 75–84. [Google Scholar] [CrossRef]
- Xi, T.; Tao, L.H.; Deliang, L.; Qiufen, H.; Guangyu, Y.; Jiayuan, Y. Determination of Heavy Metal Element in Water Samples by Solid Phase Extraction Concentration High Efficiency Liquid Phase Chromatography. Arid Environ. Monit. 2004, 18, 65–68. [Google Scholar]


| Detection Methods | Detection Limit | Time | Application | Reference |
|---|---|---|---|---|
| FAAS a | 0.11 µg/L | >1 h | Environmental water samples | [14] |
| GF-AAS b | 0.006 µg/L | >1 h | Beer | [15] |
| ICP-MS c | 3 × 10−4 µg/L | >1 h | Ocean water | [16] |
| ICP-AES d | 0.001 µg/L | >1 h | Environmental water samples | [17] |
| ASV e | 2.4 × 10−3 µg/L | 30 min | Vegetables | [18] |
| HPLC f | 0.0015 µg/L | >1 h | Environmental water samples | [19] |
| Fluorescence | 0.038 µg/L | 25 min | Environmental water samples | This work |
| Sample | Spiked Cd2+ (ng·mL−1) | Cd2+ (ng·mL−1) | Recovery (%) | R.S.D. a (%) |
|---|---|---|---|---|
| 1 | 100 | 89.93 | 89.93 | 4.11 |
| 2 | 50 | 49.05 | 98.1 | 5.54 |
| 3 | 10 | 9.03 | 90.3 | 4.79 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Y.; Lu, A.; Chen, J.; Fu, H.; Xu, L. A Label-Free Aptamer-Based Fluorescent Assay for Cadmium Detection. Appl. Sci. 2016, 6, 432. https://doi.org/10.3390/app6120432
Luan Y, Lu A, Chen J, Fu H, Xu L. A Label-Free Aptamer-Based Fluorescent Assay for Cadmium Detection. Applied Sciences. 2016; 6(12):432. https://doi.org/10.3390/app6120432
Chicago/Turabian StyleLuan, Yunxia, Anxiang Lu, Jiayi Chen, Hailong Fu, and Li Xu. 2016. "A Label-Free Aptamer-Based Fluorescent Assay for Cadmium Detection" Applied Sciences 6, no. 12: 432. https://doi.org/10.3390/app6120432
APA StyleLuan, Y., Lu, A., Chen, J., Fu, H., & Xu, L. (2016). A Label-Free Aptamer-Based Fluorescent Assay for Cadmium Detection. Applied Sciences, 6(12), 432. https://doi.org/10.3390/app6120432






