Abstract
Global interest towards lactic acid production has recently significantly increased because lactic acid can be used as raw material for the production of polylactic acid (PLA), a polymer used in biodegradable plastics for its special, environmentally-friendly properties. However, the high production costs have hindered the large-scale application of PLA due to the high price of lactic acid. Here we evaluated the potential of pear pomace and ricotta cheese whey (RCW) as a low-cost source of nutrients for lactic acid fermentation of Lactobacillus casei and Lactobacillus farciminis in microaerophilic conditions and mild sterility. After an initial lab-scale screening of 19 lactic acid bacteria (LAB) strains to select the highest producer of lactic acid, we reported the 1L-batch scale-up to test process efficiency and productivity of the most promising LAB strains. Batch fermentation of a 25:75 mixture of pear pomace and RCW, respectively, reached an overall yield factor of 90% and a volumetric productivity of 0.42 g/L·h.
1. Introduction
Due to the limited use and strong environmental impact of petroleum, bio-based chemicals are gaining strength as concrete sustainable alternatives to the fossil oil-based economy [1]. In fact, the products obtained by fermentation are expanding beyond the traditional high-value low-volume compounds, such as pharmaceuticals, and are beginning to compete with the traditional high-volume synthetic production of commodity chemicals [2]. However, to fully win the battle, products based on renewable resources should be more competitive in terms of quality, technical performance, environmental impact, and at least be comparable for price [3]. Thus, it becomes strictly necessary to maximize productivity and minimize production costs. Whereas productivity has been greatly improved due to biotechnological research [4,5], cost-efficiency depends on technological variables and the cost of raw materials [6]. Pure nutrients (i.e., glucose and amino acids) are expensive and make most of the developed biotechnological processes economically unfeasible. Therefore, great attention has been focussed on the biotechnological potential of agro-industrial waste, such as cassava bagasse [7], sugarcane bagasse [8], sugar beet pulp [9], coffee husk and pulp [10], apple pomace [11], oil-cakes [12], wheat/rice bran [13] etc., for their use as raw materials in the production of several value-added products. Special attention must be paid to product recovery and purification costs [14].
Taking into account the actual market volumes and relevance of current or future biorefinery operations, lactic acid, together with glycerol, malonic and propionic acids, is largely considered at the top of the list of potential C3 building-block chemicals obtainable from renewable resources [15]. Due to its versatile applications as an acidulant, flavour enhancer and preservative, lactic acid has occupied a prime position in food, pharmaceutical, cosmetic and other chemical industries. Recently, use of lactic acid has expanded; it has been used as monomer in polymerization to form polylactic acid (PLA), a polymer of great interest because it is biodegradable [16]. Unfortunately, PLA production is now restricted worldwide due to the high production costs, although the increasing market demand is expected to reach 2170 billion USD by 2020 [17]. It has been reported that the cost of raw materials for the fermentative production of lactic acid accounts for about 35% of the total manufacturing costs [18]. Thus, the efficiency and economics of the up-stream fermentation is still a key factor, and the nutrients supply plays a vital role in the improvement of overall process sustainability. There have been several attempts to produce lactic acid from low-cost resources, such as rice bran [19], whey permeate [20], solid waste [21] and green microalgae [22]. Nowadays, lignocellulosic and starchy raw materials represent the most attractive substrates as feedstock for the production of lactic acid due to their abundance and cheapness [23]. Up to now, the principal factor, which limits their utilization on a large scale, is that these materials are not directly available for fermentation. Expensive pretreatments are necessary to remove lignin, separate cellulose and hemicellulose in order to increase the accessible surface area to the hydrolytic enzymes before microbial fermentation [24].
Up to now, lactic acid bacteria (LAB) have been the most common microorganisms used for the production of lactic acid at an industrial scale [25] since they are able to convert numerous mono- (both hexose and pentose) and di-saccharides [26], are tolerant to broad conditions of temperature (ranging from 20 to 55 °C) and pH (they can survive at pH 5 and lower), and are highly versatile. Depending on the metabolic pathways, they can produce l- or d-lactic acid (by homo-, hetero- or mixed-acid fermentation) [27]. In spite of the wide use and advantages of LAB strains for lactic acid production, economic feasibility often remains problematic. Since LAB have limited potential to biosynthesize amino acids, nucleotides, and/or vitamins, supplementation of these nutrients is necessary for optimal growth. These complex nutritional requirements limit the development of cost-effective lactic acid production due to the high costs of medium preparation (up to 38% of the total fermentation costs) [28]. Together with inexpensive carbon and nitrogen sources, low-cost operation processes are also essential to reducing overall production costs. For industrial fermentation, the use of nonsterile conditions as well as the possibility of fermenting without supplying or completely removing oxygen reduces equipment needs, energy consumption, and labour costs [29].
The aim of this study was to set up a facility for l-lactic acid production at a lab-scale. In this paper we report the use of local fruit processing residues (pear pomace) and ricotta cheese whey (RCW) as a low-cost source of nutrients for lactic acid fermentation in microaerophilic conditions and mild sterility. RCW is a by-product of ricotta cheese production and shows different characteristics. In particular, it is deprived of albumin and usually added with salts. It is estimated that Italian RCW production amounts to about 1000 tons/year [30], thus determining significant environmental problems related to its disposal. In fact, nowadays it is definitely considered a waste and is not reused in any way, although it contains a significant quantity of lactose and residual proteins that can be exploited as fermentation substrate. Unfortunately, due to its low dry matter content, it is a highly perishable material and needs to be reused rapidly and as close as possible to the site of production. Pear pomace, which belongs to the large family of fruit processing waste, could be an interesting alternative among the different raw substrates used to attempt lactic acid production by LAB, and in particular among the lignocellulosic materials, because of the presence of promptly fermentable sugars that do not need any further hydrolytic pretreatments. On the other hand, the narrow seasonal availability of pear pomace could represent limitations in its utilization, unless its use is integrated into an annual plan in which it is supplied together with other agri-food waste products and where RCW is used as continuous baseload.
After an initial lab-scale screening of 19 LAB strains to select the highest producer of lactic acid, we reported the 1L-batch scale-up to test process efficiency and productivity of the most promising LAB strains.
Our interest was principally focused on LAB, Lactobacilli in particular, since they are easily accessible and generally considered safe in industrial production, considering their long history of exploitation without any known adverse health effects on people nor in production workers. Moreover, commercially available LAB strains, similar to those used in this study, are particularly useful due to their acid tolerance and general robustness to stress conditions [31]. In fact, the rationale behind this study was not to search for “the best” lactic acid production process overall, but rather to obtain the best compromise between the urgent need to limit costs, on the one side, and maximize efficiency, on the other. The best fermentation conditions are not necessarily the most favourable from a financial point of view. Therefore, the purpose of this study was to provide producers with a convincing alternative to waste disposal, focusing their attention on the added value of lactic acid instead of the current value loss (environmental and economical) of waste.
2. Materials and Methods
2.1. LAB Strains and Chemicals
The 19 LAB strains belong to our lab bank (Table 1). The master cell bank is maintained at −20 °C in cryovials in a standard semisintetic De Man, Rogosa and Sharpe (MRS) medium (1 mL) mixed with glycerol (0.5 mL) as acrioprotectant agent. The standard MRS medium (Fluka Analytical) contained glucose 20 g/L, bacteriological peptone 10 g/L, meat extract 8 g/L, yeast extract 4 g/L, CH3COONa·3H2O 5 g/L, K2HPO4 2 g/L, ammonium citrate tribasic 2 g/L, MgSO4·7H2O 0.2 g/L, MnSO4·4H2O 0.05 g/L [32]. The working cell bank was conserved at 4 °C in MRS-agar slants for 6 months and used for seed cultures. All chemicals were purchased from Fluka Analytical (Steinheim, Germany) unless otherwise stated.

Table 1.
Lactic acid bacteria (LAB) screened as lactic acid producers.
2.2. Preparation of Raw Materials
The pear residues, supplied by the local fruit-processing industry, consisted of a mixture of unripe, overripe or damaged pears not suitable for market and classified by the producers as agri-food waste. Ricotta cheese whey (RCW) is the final liquid residue of dairy product production, obtained after the removal of ricotta cheese from milk whey. Pear pomace was mixed using a food blender up to obtain a thick puree and then roughly filtered to eliminate the coarser fibrous component, as well as skin residues, to avoid clogging of sample pipeline in the fermenter. Raw materials were stored in plastic tank at −18 °C, to be analyzed in the laboratory and used as substrate for fermentation.
2.3. LAB Screening Conditions
A loopful of the selected LAB cells was inoculated in 50 mL Erlenmeyer flasks containing 10 mL of sterile MRS medium. The pH was adjusted at 6.2–7.0 (depending on the optimum for each strain tested) and temperature maintained either at 30 °C (LAB 1–13, in Table 1) or 37 °C (LAB 14–19, in Table 1), respectively. Each microrganism was inoculated in three Erlenmeyer flasks, closed with cotton plugs and submitted to different air concentrations: (a) aerobic given by agitation at 130 rpm; (b) microaerophilic without agitation; (c) strictly anaerobic by use of AnaeroGen™ Compact boxes equipped with active carbon to absorb O2 residue and a CO2 generator. All tests were carried out in triplicate for statistical significance. Samples were withdrawn every 12 h up to 72 h of fermentation, immediately analyzed for biomass content and then filtered and stored at −20 °C until being analyzed. Results of chemical characterization of pear pomace and RCW are reported in Table 2.

Table 2.
Effect of oxygen supply on lactic acid yield factor (YLA), specific cell growth rate (μ), substrate uptake (Suptake), specific rate of glucose consumption (qS) and specific productivity (qLA) for the 19 LAB strains tested. Time of fermentation was 48 h and initial glucose concentration 20.00 g/L.
2.4. 1L-Scale Batch Fermentation Conditions
All batch fermentations were carried out in a thermoregulated autoclavable MiniforsTM bioreactor (Infors, Basel, Switzerland) (1 L of working volume, 1.5 L of overall capacity), equipped with probes for pH, temperature and O2 concentration monitoring (Mettler Toledo, Columbus, USA). The pH value of the cultures was automatically maintained at 6.2 by adding 1N NaOH solution. The inoculum in MRS medium (100 mL, 10% v/v) was incubated at 130 rpm for 24 h on a shaking thermostatic incubator before the addition to the fermenter. Temperature and pH of inoculum were initially set up at 30 °C and pH 6.2. Before inoculation, the fermenter was filled with a mixture of filtered pear pomace and RCW (ratio 25:75), without any other nutrients added. The culture was maintained at 50 rpm by a mechanical stirrer. An airflow of 0.5 L/min was fluxed on the head space of the fermenter, as needed to maintain a slight overpressure. Before each fermentation, the fermenter was sanitized under steam stripping conditions (100 °C for 30 min). Batch processes were followed for 72 h, and samples collected every 12 h.
2.5. Analytical Methods
Sugars (glucose, fructose, galactose, lactose) and sorbitol were analyzed by HPLC (Jasco, Easton, MD, USA) equipped with a refractive index detector (Jasco, Oklahoma City, OK, USA) and ion-exclusion column Aminex HPX-87H 300 mm × 7.8 mm (Bio-Rad Laboratories, Hercules, CA, USA). Isocratic elution was carried out at 65 °C with 6 mL/min of 5.0 mM H2SO4. l-lactic acid and d-lactic acid concentration was quantified using K-DLATETM enzymatic assay kit (Megazyme, Chicago, IL, USA), based on spectrophotometric determination of NADH (Nicotinamide Adenine Dinucleotide Hydrogenated) at 340 nm. Before the HPLC analysis, samples were placed at 80 °C for 10 min to eliminate possible interferences due to microbial enzymes. Nitrogen content was measured by means of FLASH 2000 Series CHNS/O analyzer (ThermoFischer Scientific, Waltham, MA, USA). Protein content was derived multiplying nitrogen concentration for the coefficient 6.25 [33]. Metals (Na, K, Ca, Mg, Fe) were measured with Analyst 800 instrument (Perkin-Elmer, Waltham, MA, USA). Biomass was monitored by measuring the turbidity at 600 nm. Biomass concentration (dry weight, DW) was determined gravimetrically after drying it overnight at 105 °C on a pre-weighed 0.2 μm filter.
3. Results and Discussion
3.1. LAB Screening and Selection
Screening tests were carried out in order to select the best lactic acid producers and investigate the effect of the presence of oxygen on microbial growth.
It is well known that LAB in general are facultatively anaerobic, and in presence of glucose they are able to produce lactic acid [34]. Thinking in terms of low-cost lactic acid production, the economic impact of air supply or air removal devices could be relevant, the best choice being to invest on microaerofilic LAB, which tolerate the presence of oxygen in the environment, reaching a sustainable combination of yield and productivity. For this reason, LAB were grown in aerobic, microaerophilic and strictly anaerobic conditions in order to compare cell growth and lactic acid production, as well as glucose depletion and glucose depletion rate. The results of the fermentation tests are shown in Table 2. As expected, anaerobic conditions appeared to favour lactic acid production and substrate consumption, whereas biomass growth rate was higher overall in the presence of oxygen [35].
Lactobacillus alimentarius, Lactobacillus curvatus and Lactobacillus reuteri 20015 were promptly excluded because they partially consumed glucose without producing lactic acid in any of the tested conditions. In anaerobic and microaerophilic conditions the majority of the strains completely depleted glucose in 72 h, whereas only four strains (Lactobacillus casei, Lactobacillus farciminis, Lactobacillus fermentum 20052 and Lactobacullus kadleri) consumed glucose in an aerobic environment although with a lower qs. Generally, except for a few cases, lactic acid yield factors (YLA) and specific productivity (qLA) were low, but comparable to those obtained in other batch fermentations on glucose without pH control [36]. The best lactic acid producers were L. casei and L. farciminis with YLA > 90% in all conditions, although YLA went down to 79% and 71%, respectively, with the proportional increase in oxygen availability.
L. casei is reported to be a facultative anaerobic microorganism with a homofermentative nature [37]. Consequently, the microorganism grows better in a static culture where the fermentation conditions favour low oxygen concentrations. Furthermore, L. casei is known to be acidotolerant with an optimum pH of 5.5 and it is relatively insensitive to product inhibition by lactic acid. In microaerophilic conditions, L. casei achieved a final lactic acid concentration of 15.89 ± 0.56 g/L compared to 20.00 ± 0.20 g/L of glucose, versus 19.50 ± 0.79 g/L obtained in anaerobic conditions. L. farciminis, known as a homofermentant microrganism isolated from meat and meat products [38], in our experimental conditions produced 18.37 ± 0.94 g/L of lactic acid in anaerobiosis and 15.71 ± 0.54 g/L in microaerophilia.
Several tested LAB were reported to produce both l-lactic and d-lactic acid stereoisomers in the different experimental conditions (Table 3). In particular, as shown in Figure 1, while L. casei, with increasing dissolved oxygen, proportionally increased the amount of d-lactic acid at the expense of l-lactic acid, L. farciminis only produced l-lactic acid in microaerophilic conditions. This could be an interesting point, because the stereochemistry of the monomer lactic acid has a great influence on the properties of the polylactide. In fact, the stereoregularity is the key factor that makes polylactic acid (PLA) a highly crystalline (pure l-lactic acid), fully amorphous or semicristalline polymer (by the inclusion of different percentages of the d-isomer) [39]. After recovery from the culture broth and cell biomass, the possibility to directly use the l-lactic acid produced without any further steps with respect to optical purification, could be of some interest.

Table 3.
Total lactic acid (l + d-LA), d-lactic acid (d-LA) and l-lactic acid (l-LA) concentrations obtained by the screening of 19 LAB strains in different oxygen supply conditions.
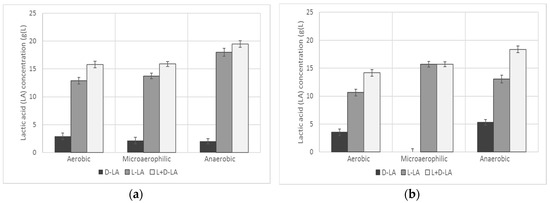
Figure 1.
Concentration of l-lactic, d-lactic and the sum of d + l-lactic for L. casei (a) and L. farciminis (b) with different oxygen supply (p < 0.05).
3.2. Waste Chemical Characterization and Fermentation Mash Preparation
The chemical characterization of pear pomace and RCW used in this study are reported in Table 4.

Table 4.
Chemical composition of pear pomace and ricotta cheese whey (RCW).
Pear pomace puree used as raw material in these experiments was too dense to be fermented without dilution. The possibility of using water was taken into account only in the first lab test, but then discarded when the scale up to the fermenter was set up for the obvious impact of using fresh tap water on the overall environmental and economic balance of the process. Therefore, RCW was used both as a further supplier of nutrients and as a diluting agent up to the desired total fermentable sugar (TFS) concentration. Different mixtures were tested to reach the optimal substrate characteristics, which were identified in the 25:75 of pear pomace:RCW ratio, corresponding to a mixture containing 57.70 g/L of TFS (distributed as 4.67 g/L of glucose, 16.10 g/L of fructose, 0.56 g/L of sorbitol and 35.71 g/L of lactose) and an average N content of 0.86 g/L.
3.3. 1L Scale Batch Fermentation
Lactic acid is most commonly produced in the batch mode [40], but some examples of continuous culture exist [41], as well as several examples of fed-batch fermentation [42]. Batch strategy generally gives high lactic acid concentrations and yields, provided all carbon substrate is depleted. However, it provides lower productivity compared to continuous or fed-batch fermentation, due to unavoidable idle times (i.e., load and discharge) and consequently to a sub-optimal utilization of the bioreactor. In our experimental conditions, where simplicity and low managing cost were the main drives, batch mode was chosen for its simplicity. This closed system has the advantage to reduce the risk of contamination and to assure the complete consumption of the substrate. On the other hand, either substrate and/or product inhibition could limit the fermentation performance. Previous studies on lactic acid production have reported the highest lactic acid yield up to 96% in batch fermentation by LAB on glucose [43]. The fermentation mixture, constituted by a ratio of 25:75 of pear pomace and RCW, respectively, contained a total amount of fermentable sugars of 31.60 g/L, characterized by 2.10 g/L of glucose, 4.30 g/L of fructose, 24.20 g/L of lactose and 1.00 g/L of sorbitol.
3.3.1. Fermentation of L. casei
As reported in Figure 2, in 72 h complete depletion of fermentable sugars occurred, with l-lactic acid production of 30.0 g/L. In this experiment in particular, we did not measure the concentration of d-lactic acid. As expected, sorbitol was not consumed at all as a carbon source, since most Lactobacilli lack the proper metabolic pathway to break it down, except under certain genetic conditions [44]. It is worthwhile noting that, in experimental conditions similar to ours, L. casei has been reported to express diauxic growth [45], with uptake of lactose starting only when monosaccharides are completely depleted and after a lag phase of a few hours to allow the rearrangement of enzymatic assets. The trend of lactic acid concentration reflected the change in the metabolic pathway of carbon source utilization. Glucose and fructose were promptly depleted in 20 h. No accumulation of galactose and glucose was observed from lactose hydrolysis, indicating that both were produced and immediately consumed.
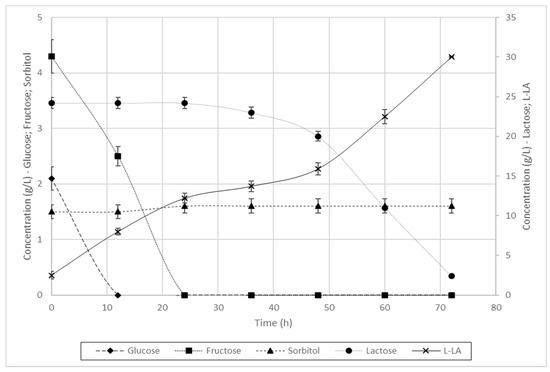
Figure 2.
Batch fermentation of L. casei in the presence of glucose, fructose, lactose and sorbitol, derived from pear pomace and RCW mixtures, for the production of l-lactic acid.
Overall, in 72 h, uptake of almost 97% of the sugars present in the waste mixtures occurred with L. casei to produce l-lactic acid with a yield factor (YLA/S) of 90%, which became 87% when calculated considering also sorbitol. Sugar uptake rates were 100% (Table 5). Ninety percent of yield factor for the sole l-lactic acid could be likely explained taking into account the unavoidable small quantity of d-lactic produced by L. casei in microaerophilic conditions.

Table 5.
Best-fit kinetic parameters calculated from experimental data and statistical analysis.
In 1L batch fermentation, the overall volumetric rate of l-lactic acid was 0.42 grams per hour, while the specific productivity was 0.17 grammes of product per gramme of cell per hour. Yield factor for biomass was 8%, corresponding to 2.50 g/L of cells.
In the waste mixture, 2.5 g/L of l-lactic acid was already present, probably derived from side fermentation occurring in the non-sterile RCW by other LAB.
3.3.2. Fermentation of L. farciminis
When L. farciminis was exposed to fermentation at the same conditions and in the same waste mixture of pear pomace and RCW, the results were surprisingly different. Figure 3 shows the sugar depletion and l-lactic acid production profiles in the 72 h of the process. Interestingly, lactose and sorbitol uptake did not occur with L. farciminis in the same conditions. This meant that, out of the total 31.60 g/L theoretically available in the waste, only 6.4 g/L were effectively consumed to produce the product of interest. Moreover, L. farciminis was shown to prefer glucose and only when this was completely depleted did it switch to fructose. Biomass yield factor was 15%, corresponding to 0.96 g/L of cells.
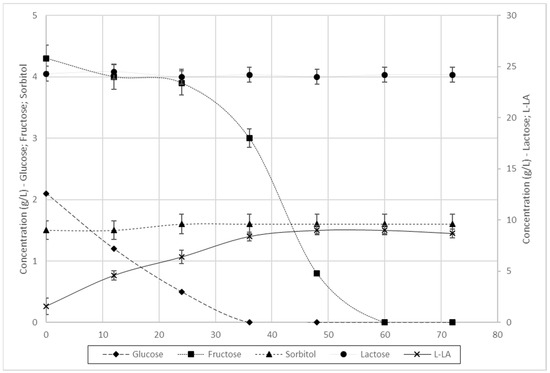
Figure 3.
Batch fermentation of L. farciminis in the presence of glucose, fructose, lactose and sorbitol, derived from pear pomace and RCW mixtures, for the production of l-lactic acid.
Considering the previous results and taking into account the high yield factor (calculated on the total amount of sugars consumed, YLA/S), it is reasonable to conclude that all the lactic acid produced was the l-enantiomer. In our study, optical purity was not measured and was not considered as a key point since recent research has reported that the stereocomplex PLA, which is composed of both poly-l- and -d-lactic acid, has been attracting a lot of attention due to its high thermostability. Stereocomplex-type polymers show a melting point (ca. 230 °C) that is approximately 50 °C higher than that of the respective single polymers [46]. Therefore, d-lactic acid, in addition to l-lactic acid, which has been the focus of production to date, is becoming increasingly important [47].
Overall, L. farciminis was shown to be inappropriate for fermenting pear pomace together with RCW, pear pomace alone, or waste containing, in a broader perspective, only monosaccharides as principal carbon sources. Thus, the need to find a low-cost diluting agent for pear pomace, besides fresh water, obliged us, at this stage of our research, to pay special attention to more versatile microrganisms, such as L. casei.
Even in the case of L. casei, overall productivity values were lower in our experimental conditions compared to those reported in the literature and the reason could be principally due to the batch strategy chosen for the experiments. To limit operating costs, as well as minimizing the risk of contamination, continuous fermentation must surely be excluded, but a fed-batch process might be the best compromise to reach effective process performance.
4. Conclusions
Here we attempted to produce l-lactic acid, a monomer of important added value in bioplastic production, by exploiting agri-food waste. To reach this goal we performed a screening of several Lactobacilli, which are recognized as being among the highest producers of lactic acid. Microaerophilic fermentation, batch strategy and mild sterilization were the principal variables that guaranteed the realization of a cost-effective viable process. Lab-scale experiments with the two best strains selected showed that only L. casei had the potential to be used in the fermentation of a 25:75 mixture of pear pomace and RCW, respectively, reaching an overall yield factor of 90% and volumetric productivity of 0.42 g/L·h, obtained in the most cost-effective conditions possible. These values might be improved by further optimizing certain aspects of the process, i.e., the fermentation strategy or the use of other feedstock. Before being applicable on an industrial scale, these results need to be scaled up and all variables re-tested to obtain maximum productivity. Moreover, it should be not forgotten that the principal bottlenecks of bio-based productions are often represented by the subsequent purification process (known as downstream processing), which could comprise up to 80% of the entire production costs.
Acknowledgments
The authors gratefully acknowledge the financial support provided by Emilia Romagna Region for the GLV Green Lab Valley Prototype Project 2015–2017, under the Regional Framework Programme for Research and Innovation.
Author Contributions
Giovanni Dedenaro, Stefania Costa and Irene Rugiero performed all the experiments and carried out all the analytical assays, giving also a great contribution to the discussion. Elena Tamburini conceived and designed the experiments. As supervisor of the research group, Paola Pedrini has defined the general research statement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pleissner, D.; Qi, Q.; Gao, C.; Rivero, C.P.; Webb, C.; Lin, C.S.K.; Venus, J. Valorization of organic residues for the production of added value chemicals: A contribution to the bio-based economy. Biochem. Eng. J. 2015, 116, 3–16. [Google Scholar] [CrossRef]
- John, R.P.; Nampoothiri, K.M.; Pandey, A. Fermentative production of lactic acid from biomass: An overview on process developments and future perspectives. Appl. Microbiol. Biotechnol. 2007, 74, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, J.E.; de Klerk-Engels, B.; Struik, P.C.; Rabbinge, R. Securing renewable resource supplies for changing market demands in a bio-based economy. Ind. Crops Prod. 2005, 21, 129–144. [Google Scholar] [CrossRef]
- Paes, B.G.; Almeida, J.R. Genetic improvement of microorganisms for applications in biorefineries. Chem. Biol. Technol. Agric. 2014, 1, 1–21. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.U. Systems strategies for developing industrial microbial strains. Nat. Biotechnol. 2015, 33, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q. From a co-production design to an integrated single-cell biorefinery. Biotechnol. Adv. 2014, 32, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T. Biotechnological potential of agro-industrial residues. I: Sugarcane bagasse. Bioresour. Technol. 2000, 74, 69–80. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Soccol, V.T.; Vandenberghe, L.P.; Mohan, R. Biotechnological potential of agro-industrial residues. II: Cassava bagasse. Bioresour. Technol. 2000, 74, 81–87. [Google Scholar] [CrossRef]
- Xue, M.; Liu, D.; Zhang, H.; Qi, H.; Lei, Z. A pilot process of solid state fermentation from sugar beet pulp for the production of microbial protein. J. Ferment. Bioeng. 1997, 73, 203–205. [Google Scholar] [CrossRef]
- Antier, P.; Minjares, A.; Roussos, S.; Raimbault, M.; Viniegra-Gonzalez, G. Pectinase-hyperproducing mutants of Aspergillus niger C28B25 for solid-state fermentation of coffee pulp. Enzyme Microb. Technol. 1993, 15, 254–260. [Google Scholar] [CrossRef]
- Joshi, V.K.; Parmar, M.; Rana, N.S. Pectin esterase production from apple pomace in solid-state and submerged fermentations. Food Technol. Biotechnol. 2006, 44, 213–217. [Google Scholar]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Chen, H. Single cell oil production in solid-state fermentation by Microsphaeropsis sp. from steam-exploded wheat straw mixed with wheat bran. Bioresour. Technol. 2008, 99, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass—Volume I; U.S. Department of Energy: Oak Ridge, TN, USA, 2004.
- Martinez, F.A.C.; Balciunas, E.M.; Salgado, J.M.; González, J.M.D.; Converti, A.; de Souza Oliveira, R.P. Lactic acid properties, applications and production: A review. Trends Food Sci. Technol. 2013, 30, 70–83. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Global Lactic Acid and Poly Lactic Acid (PLA) Market by Application (Packaging, Agriculture, Transport, Electronics, Textiles) Expected to Reach USD 4,312.2 Million and USD 2,169.6 Million Respectively by 2020: Grand View Research, Inc. Available online: https://www.grandviewresearch.com/press-release/global-lactic-acid-and-poly-lactic-acid-market (accessed on 25 July 2016).
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Makino, M.; Kaku, N.; Koyama, M.; Nakamura, K.; Sasano, K. Fermentative l-lactic acid production from non-sterilized rice washing drainage containing rice bran by a newly isolated lactic acid bacteria without any additions of nutrients. J. Biosci. Bioeng. 2013, 115, 449–452. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Srikanth, K.; Limaye, A.M.; Sivaprakasam, S. Homo-fermentative production of d-lactic acid by Lactobacillus sp. employing casein whey permeate as a raw feed-stock. Biotechnol. Lett. 2014, 36, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- McCaskey, T.A.; Zhou, S.D.; Britt, S.N.; Strickland, R. Bioconversion of municipal solid waste to lactic acid by Lactobacillus species. Appl. Biochem. Biotechnol. 1994, 45, 555–563. [Google Scholar] [CrossRef]
- Nguyen, C.M.; Kim, J.S.; Hwang, H.J.; Park, M.S.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Kim, J.C. Production of l-lactic acid from a green microalga, Hydrodictyon reticulum, by Lactobacillus paracasei LA104 isolated from the traditional Korean food, makgeolli. Bioresour. Technol. 2012, 110, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Qiao, H.; Zheng, Z.; Chu, Q.; Li, X.; Yong, Q.; Ouyang, J. Lactic acid production from pretreated hydrolysates of corn stover by a newly developed bacillus coagulans strain. PLoS ONE 2016, 11, e0149101. [Google Scholar] [CrossRef] [PubMed]
- Hassan, N.; Idris, A. Simultaneous saccharification and fermentation of lactic acid from empty fruit bunch at high solids loading. BioResources 2016, 11, 3799–3812. [Google Scholar] [CrossRef]
- Mazzoli, R.; Bosco, F.; Mizrahi, I.; Bayer, E.A.; Pessione, E. Towards lactic acid bacteria-based biorefineries. Biotechnol. Adv. 2014, 32, 1216–1236. [Google Scholar] [CrossRef] [PubMed]
- Kandler, O. Carbohydrate metabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1983, 49, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Van de Guchte, M.; Serror, P.; Chervaux, C.; Smokvina, T.; Ehrlich, S.D.; Maguin, E. Stress responses in lactic acid bacteria. Antonie Van Leeuwenhoek 2002, 82, 187–216. [Google Scholar] [CrossRef] [PubMed]
- Altaf, M.; Naveena, B.J.; Reddy, G. Use of inexpensive nitrogen sources and starch for l (+) lactic acid production in anaerobic submerged fermentation. Bioresour. Technol. 2007, 98, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xie, N.; Guo, L.; Wang, L.; Yu, B.; Ma, Y. Efficient open fermentative production of polymer-grade l-lactate from sugarcane bagasse hydrolysate by thermotolerant Bacillus sp. strain P38. PLoS ONE 2014, 9, e107143. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, S.; Curcio, S.; Calabrò, V.; Iorio, G. Bio-ethanol production by fermentation of ricotta cheese whey as an effective alternative non-vegetable source. Biomass Bioenergy 2009, 33, 1687–1692. [Google Scholar] [CrossRef]
- Silva, J.; Carvalho, A.S.; Ferreira, R.; Vitorino, R.; Amado, F.; Domingues, P.; Gibbs, P.A. Effect of the pH of growth on the survival of Lactobacillus delbrueckii subsp. bulgaricus to stress conditions during spray-drying. J. Appl. Bacteriol. 2005, 98, 775–782. [Google Scholar] [CrossRef] [PubMed]
- DeMan, C.J.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Jones, D.B. Factors for Converting Percentages of Nitrogen in Foods and Feeds into Percentages of Proteins; US Department of Agriculture: Washington, DC, USA, 1941; pp. 1–22.
- Condon, S. Aerobic metabolism of lactic acid bacteria. Irish J. Food Sci. Technol. 1983, 7, 15–25. [Google Scholar]
- Condon, S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol. Rev. 1987, 3, 269–280. [Google Scholar] [CrossRef]
- Hofvendahl, K.; Hahn-Hagerdal, B. Factors affecting the fermentative lactic acid production from renewable resources. Enzyme Microb. Technol. 2000, 26, 87–107. [Google Scholar] [CrossRef]
- Sheeladevi, A. Lactic acid production using lactic acid bacteria under optimized conditions. Int. J. Pharm. Biol. Arch. 2011, 2, 1686–1691. [Google Scholar]
- Reuter, G. Lactobacillus alimentarius sp. nov., nom rev. and Lactobacillus farciminis sp. nov., nom. rev. Syst. Appl. Microbiol. 1983, 4, 277–279. [Google Scholar] [CrossRef]
- Mehta, R.; Kumar, V.; Bhunia, H.; Upadhyay, S.N. Synthesis of poly (lactic acid): A review. J. Macromol. Sci. C Polym. Rev. 2005, 45, 325–349. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendo, T.; Sakai, K.; Sonomoto, K. Highly efficient l-lactic acid production from xylose in cell recycle continuous fermentation using Enterococcus mundtii QU 25. RSC Adv. 2016, 6, 17659–17668. [Google Scholar] [CrossRef]
- Bai, D.M.; Wei, Q.; Yan, Z.H.; Zhao, X.M.; Li, X.G.; Xu, S.M. Fed-batch fermentation of Lactobacillus lactis for hyper-production of l-lactic acid. Biotechnol. Lett. 2003, 25, 1833–1835. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.K.; Wee, Y.J.; Choi, G.W. A novel lactic acid bacterium for the production of high purity l-lactic acid, Lactobacillus paracasei subsp. paracasei CHB2121. J. Biosci. Bioeng. 2012, 114, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Yebra, M.J.; Pérez-Martínez, G. Cross-talk between the l-sorbose and d-sorbitol (d-glucitol) metabolic pathways in Lactobacillus caseia. Microbiology 2002, 148, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Van Dedem, G.; Moo-Young, M. A model for diauxic growth. Biotechnol. Bioeng. 1975, 17, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Ikada, Y.; Jamshidi, K.; Tsuji, H.; Hyon, S.-H. Stereocomplex formation between enantiomeric poly(lactides). Macromolecules 1987, 20, 904–906. [Google Scholar] [CrossRef]
- Okano, K.; Zhang, Q.; Shinkawa, S.; Yoshida, S.; Tanaka, T.; Fukuda, H.; Kondo, A. Efficient production of optically pure d-lactic acid from raw corn starch by using a genetically modified l-lactate dehydrogenase gene-deficient and α-amylase-secreting Lactobacillus plantarum strain. Appl. Environ. Microbiol. 2009, 75, 462–467. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).