Deranged Dimensionality of Vestibular Re-Weighting in Multiple Chemical Sensitivity
Abstract
:1. Introduction
2. Experimental Section
2.1. Participants And Study Design
2.2. Otoneurological Testing
- -
- Rod and Disc Test (RDT): According to Cousins et al. [25] visual dependency (VD) was evaluated by means of the RDT software (http://www.imperial.ac.uk/medicine/dizzinessandvertigo) on a laptop computer in order to study visual vertical under kinetic visual stimulation [26,27]. Subjects were seated in front of the computer in a darkened room with their heads held against a viewing cone that blocked extraneous visual orientation cues. The diameter of the cone at the subjects’ eyes was 15 cm with a depth of field of 30 cm, subtending a viewing angle of 39°. The visual stimulus consisted of a luminous white 6 cm rod on a black background. The rod rotated 360° in either direction about its midpoint in the central 11° of the visual filed. Outside this central zone, the viewing screen was filled with a collage of 220 off-white dots, each 8 mm (1.5° of visual field) in diameter, randomly distributed on a black background. Subjects controlled the orientation of the rod with a roller mouse. They were instructed to align the rod to their perceived vertical (the subjective visual vertical) under three conditions. In the first test the collage of dots was stationary (N) while in the second and third the collage rotated at 30°/s clockwise (CW) or counter-clockwise (CCW), respectively. Subjects were tested four times per each condition, presenting the latter two conditions randomly after condition 1. During each trial the rod was initially set randomly at ±40° from vertical and the rod tilt per trial was recorded as the degrees’ difference between true vertical and the subjects’ final rod placement.
- -
- Rod and Frame Test (RFT): According to Witkin and Asch [28], in order to study the subjects’ visual vertical tilts under static visual stimulation, a standard RFT square frame device described by Fiori et al. [29] was used. The frame was tilted 33° CW, CCW, or not tilted (0°, N); the rod 11° or 22°, CW or CCW. Thus, 12 randomly presented conditions were achieved, each containing three trials [30]. Errors were calculated as deviation from the gravitational vertical position of the rod.
- -
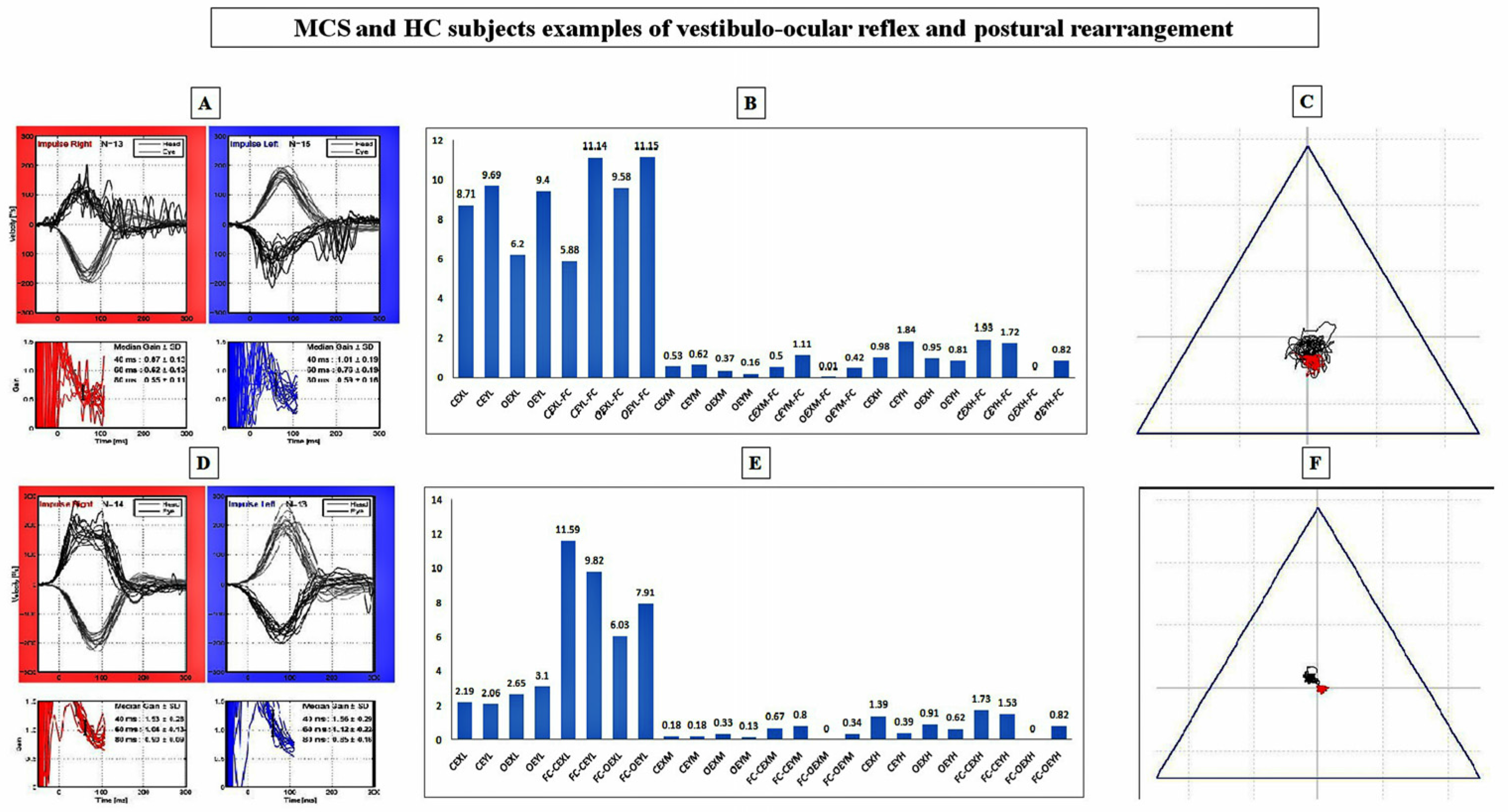
- Video Head Impulse Testing (vHIT): For vestibulo-ocular reflex (VOR) gain measurements, the reliability of vHIT [31] (EyeSeeCamTM System [32]) and the technique proposed by Blodow et al. [33] were used to test the lateral semicircular canal. Thus, anomalies in vHIT outcomes were assessed in case of (1) abnormal gain with respect to the calculated normative data; and (2) the presence of refixation saccades. Amplitudes (°/s) and latencies (ms) of both pinpointed refixation and physiological saccades were computed at peak eye velocity (°/s). According to the manufacturer of the software (OtoAccess TM), both side gain mean and median (med) values recorded at 60 ms as well as regression slope (reslo) were calculated and extracted onto an .xls file. Artefacts possibly related to mask slippage and positioning, calibration, handedness of the operator, patients’ neck stiffness and setting of the laboratory, were controlled by means of a technique proposed by McGarvie et al. [34]. According to the technique proposed by the same group [34] to reach adequate numbers of head values for the lateral semi-circular canal, the operator delivered a range of velocities in a random order and direction to achieve at least 10 artefact-free impulses. By means of this technique, 50–60 impulses were delivered in each canal direction to ensure that the desired number of artefact-free responses was achieved.
- -
- Static Posturography Testing (SPT): The previously instructed subjects maintained an upright position on a standardized platform for static posturography (EDM Euroclinic®) during a 60 s recording period per condition, defined as eyes closed (CE) or opened (OE) while standing on either the stiff platform (SP) or a 6 cm heightened AIREX® Balance-pad plus foam carpet (FC, AIREX AG, 25% compression resistance, 20 kPa; apparent density 55 kg/m3; tensile strength 260 kPa). The sampling frequency was set at 25 Hz [35,36] and the centre of pressure (CoP) was supervised during the test. Classic posturographic parameter evaluations involving the trace length (length), the surface of the ellipse of confidence (surf), and the mean velocity (avspe) of body sways with relative SD (stdspe) were performed, as well as the fast Fourier transform (FFT) elaboration of oscillations on both the X (right-left) and Y (forward-backwards) planes [35,36]. Time-domain oscillation signals (X and Y) were extracted by the original software manufacturer into .txt format and the FFT elaborations were gained through Matlab software. In order to obtain the FFT of X and Y oscillations, a core function (Appendix A) was implemented using as input the.txt file provided by the software manufacturer. A main script was written to acquire the FFT for each patient, related to four different conditions, by accessing to the above mentioned Matlab function. For each condition the script returned a file containing the normalized value of the FFT for X and Y oscillations. Spectral values (power spectra, PS) of body oscillations were quantified on an .xls file, for every frequency from 0.01 to 5.00 Hz [35,36]. According to previous experiences [35,36], the frequency spectrum was clustered into three groups: 0.01–0.70 Hz (low frequency interval); 0.70–1.00 Hz (middle frequency interval); 1.00–5.00 Hz (high frequency interval). Within each group, the spectral intensity was determined by adding the relative PS and the group mean PS (± SD) [35,36].
2.3. Validated Questionnaires (VQ)
- (1)
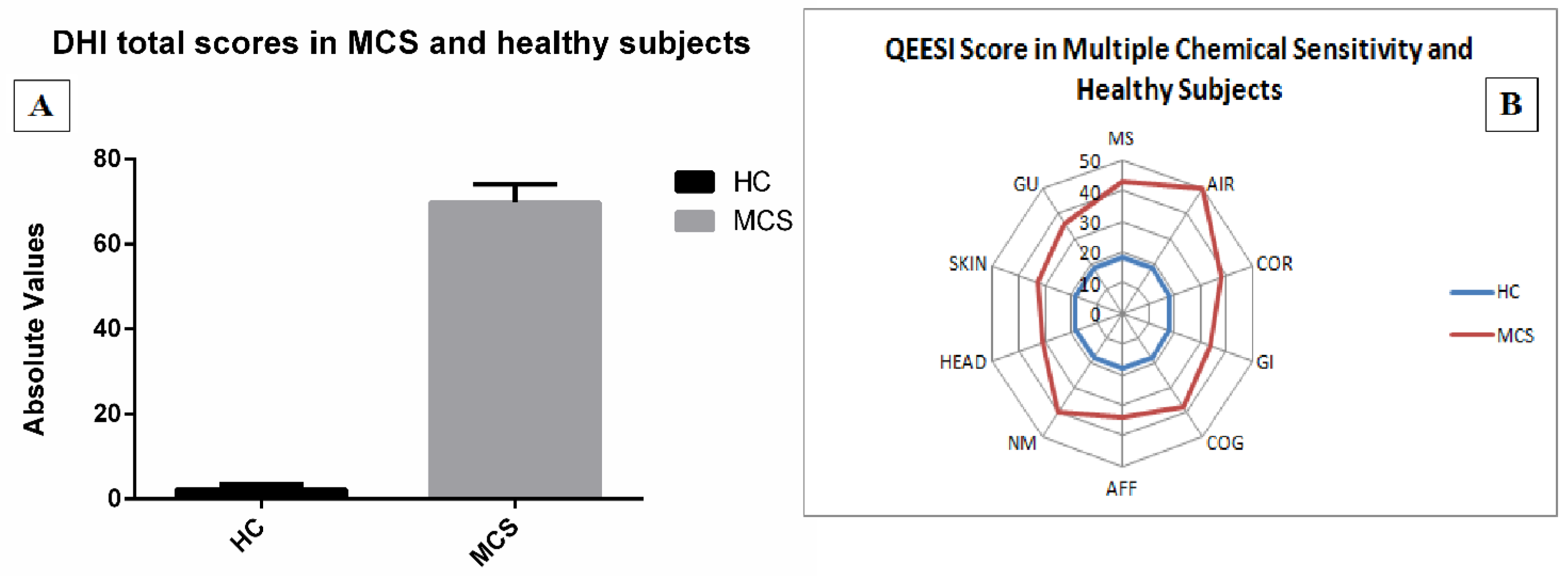
- a modified version of the QEESI Symptom Severity scale [37] in which subjects scored from 1 (low) to 3 (severe) the intensity of head-related (HEAD), cognitive-related (COG), affective-related (AFF), neuromuscular-related (NM), musculoskeletal-related (MS), skin-related (SKIN), genitourinary-related (GU), gastrointestinal-related (GI), heart/chest-related (COR), airway or mucous membrane-related (AIR/MM) symptoms.
- (2)
- the Italian Dizziness Handicap Inventory (DHI), including 25 items aimed at assessing patients’ functional (nine questions), emotional (nine questions) and physical (seven questions) limitations [38] by means a multiple choice scheme: “yes” (4 points), “sometimes” (2 points) and “no” (0 points).
2.4. Data Handling and Statistical Analysis
3. Results
3.1. Subjects
3.2. Validated Questionnaires
3.3. Otoneurological Data
3.4. Inferential Statistical Analysis
3.5. Correlation Analysis
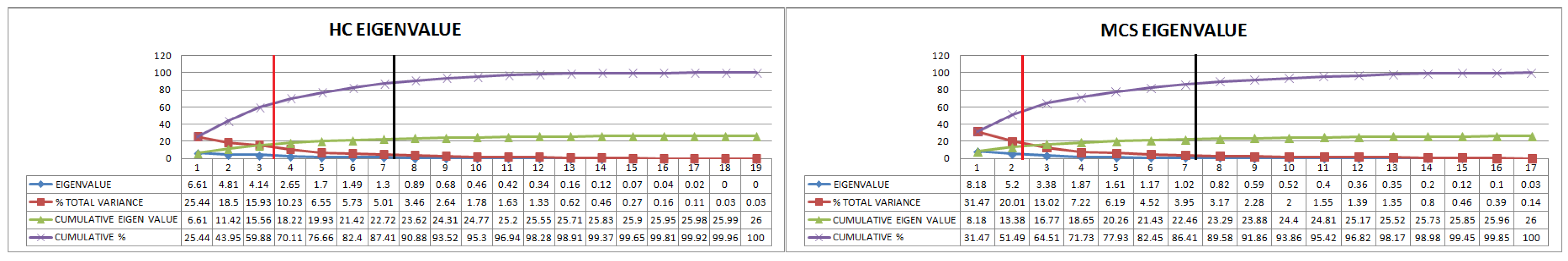
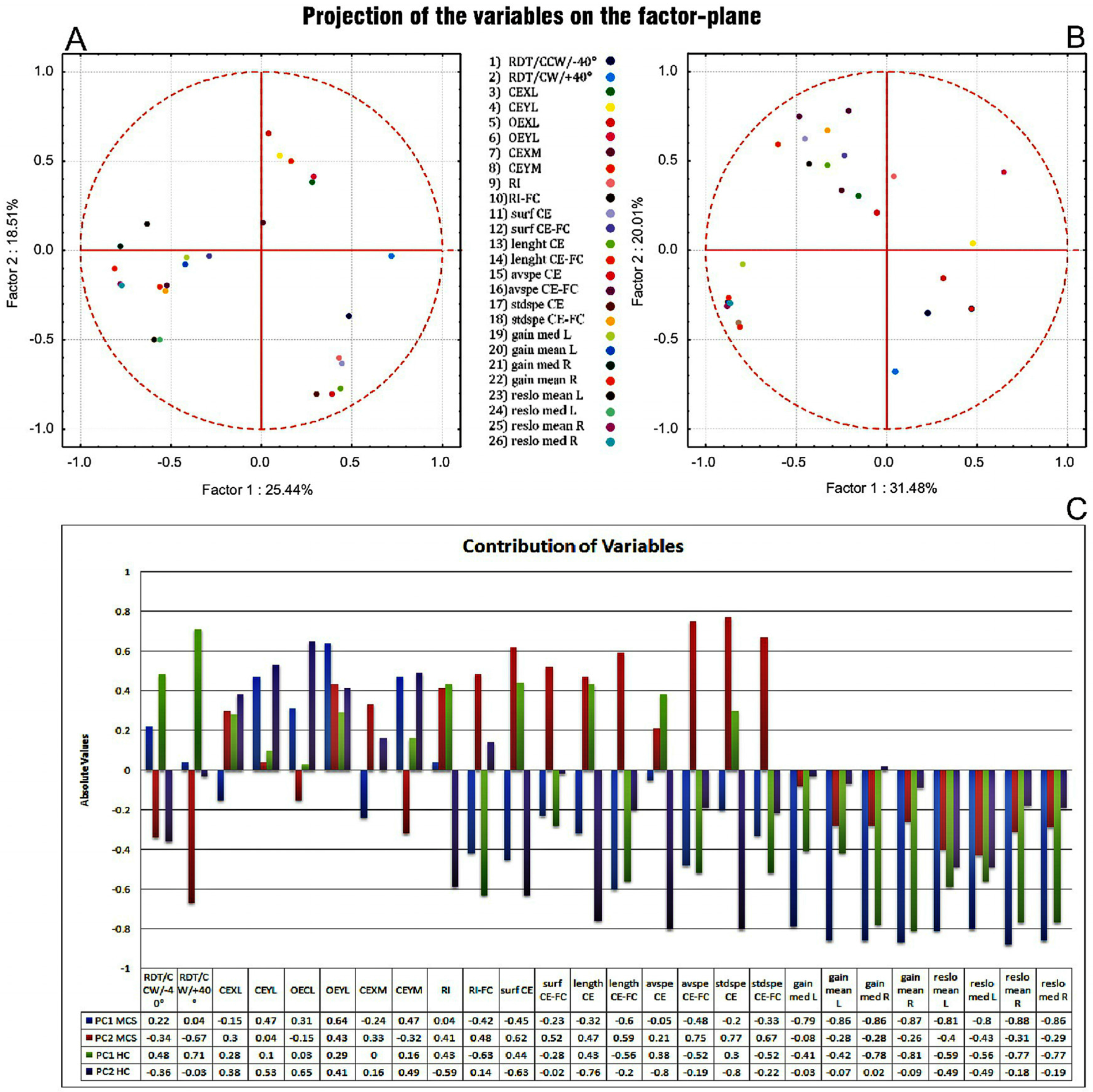
3.6. PCA
4. Discussion
5. Strengths and Limitations of the Study
Author Contributions
Conflicts of Interest
Appendix A
- L = length(s) % s vector of signal values
- Fs = 25; % Sampling frequency
- NFFT = 2^nextpow2(L); % Next power of 2 from length of s
- f = Fs/2*linspace(0,1,NFFT/2 + 1);
- S = fft(s,NFFT)/L; % FFT of a signal s
- modS = 2*abs(S(1:NFFT/2 + 1));
- ms = max(modS);
- S_norm = modS/ms;
References
- Bell, I.R. Clinically relevant EEG studies and psychophysiological findings: Possible neural mechanisms for multiple chemical sensitivity. Toxicology 1996, 111, 101–117. [Google Scholar] [CrossRef]
- Alessandrini, M.; Micarelli, A.; Bruno, E.; Ottaviani, F.; Conetta, M.; Cormano, A.; Genovesi, G. Intranasal administration of hyaluronan as a further resource in olfactory performance in multiple chemical sensitivity syndrome. Int. J. Immunopathol. Pharmacol. 2013, 26, 1019–1025. [Google Scholar] [PubMed]
- Miller, C.S.; Mitzel, H.C. Chemical sensitivity attributed to pesticide exposure versus remodeling. Arch. Environ. Health 1995, 50, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Cullen, M.R. The worker with multiple chemical sensitivities: An overview. Occup. Med. State Art Rev. 1987, 2, 655–661. [Google Scholar]
- Das-Munshi, J.; Rubin, G.J.; Wessely, S. Multiple chemical sensitivities: Review. Curr. Opin. Otolaryngol. Head Neck Surg. 2007, 15, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Bascom, R.; Meggs, W.J.; Frampton, M.; Hudnell, K.; Killburn, K.; Kobal, G.; Medinsky, M.; Rea, W. Neurogenic inflammation: With additional discussion of central and perceptual integration of nonneurogenic inflammation. Environ. Health Perspect. 1997, 105, S531–537. [Google Scholar] [CrossRef]
- Winters, W.; Devriese, S.; Van-Diest, I.; Nemery, B.; Veulemans, H.; Eelen, P.; Van de Woestijne, K.; Van den Bergh, O. Media warnings about environmental pollution facilitate the acquisition of symptoms in response to chemical substances. Psychosom. Med. 2003, 65, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L. Elevated nitric oxide/peroxynitrite theory of multiple chemical sensitivity: Central role of N-methyl-d-aspartate receptors in the sensitivity mechanism. Environ. Health Perspect. 2003, 111, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Miller, C.S.; Schwartz, G.E. An olfactory-limbic model of multiple chemical sensitivity syndrome: Possible relationships to kindling and affective spectrum disorders. Biol. Psychiatry 1992, 32, 218–242. [Google Scholar] [CrossRef]
- Chiaravalloti, A.; Pagani, M.; Micarelli, A.; Di Pietro, B.; Genovesi, G.; Alessandrini, M.; Schillaci, O. Cortical activity during olfactory stimulation in multiple chemical sensitivity: A (18)F-FDG PET/CT study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, M.; Micarelli, A.; Chiaravalloti, A.; Bruno, E.; Danieli, R.; Pierantozzi, M.; Genovesi, G.; Öberg, J.; Pagani, M.; Schillaci, O. Involvement of Subcortical Brain Structures During Olfactory Stimulation in Multiple Chemical Sensitivity. Brain Topogr. 2016, 29, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Hillert, L.; Musabasic, V.; Berglund, H.; Ciumas, C.; Savic, I. Odor processing in Multiple Chemical Sensitivity. Hum. Brain Mapp. 2007, 28, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Uchiyama, I.; Takano, H.; Tanigawa, M.; Azuma, M.; Bamba, I.; Yoshikawa, T. Changes in Cerebral Blood Flow during Olfactory Stimulation in Patients with Multiple Chemical Sensitivity: A Multi-Channel Near-Infrared Spectroscopic Study. PLoS ONE 2013, 8, e80567. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.; Claeson, A.S.; Nyberg, L.; Stenberg, B.; Nordin, S. Brain responses to olfactory and trigeminal exposure in idiopathic environmental illness (IEI) attributed to smells—An fMRI study. J. Psychosom. Res. 2014, 77, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R.; Baldwin, C.M.; Russek, L.G.; Schwartz, G.E.; Hardin, E.E. Early life stress, negative paternal relationships, and chemical intolerance in middle-aged women: Support for a neural sensitization model. J. Womens Health 1998, 7, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Brandt, T. Functional brain imaging of peripheral and central vestibular disorders. Brain 2008, 131, 2538–2552. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, M.; Pagani, M.; Napolitano, B.; Micarelli, A.; Candidi, M.; Bruno, E.; Chiaravalloti, A.; Di Pietro, B.; Schillaci, O. Early and phasic cortical metabolic changes in vestibular neuritis onset. PLoS ONE 2013, 8, e57596. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.S.; Prihoda, T.J. The Environmental Exposure and Sensitivity Inventory (EESI): A standardized approach for measuring chemical intolerances for research and clinical applications. Toxicol. Ind. Health 1999, 15, 370–385. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, E.; Claeson, A.S.; Neely, G.; Stenberg, B.; Nordin, S. Overlap in prevalence between various types of environmental intolerance. Int. J. Hyg. Environ. Health 2014, 217, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Winder, C. Mechanisms of multiple chemical sensitivity. Toxicol. Lett. 2002, 128, 85–97. [Google Scholar] [CrossRef]
- Heinonen-Guzejev, M.; Koskenvuo, M.; Mussalo-Rauhamaa, H.; Vuorinen, H.S.; Heikkilä, K.; Kaprio, J. Noise sensitivity and multiple chemical sensitivity scales: Properties in a population based epidemiological study. Noise Health 2012, 14, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Micarelli, A.; Viziano, A.; Genovesi, G.; Bruno, E.; Ottaviani, F.; Alessandrini, M. Lack of contralateral suppression in transient-evoked otoacoustic emissions in multiple chemical sensitivity: A clinical correlation study. Noise Health 2016, 18, 143–149. [Google Scholar] [PubMed]
- Bartha, L.; Baumzweiger, W.; Buscher, D.S.; Callender, T.; Dahl, K.A.; Davidoff, A.; Donnay, A.; Edelson, S.B.; Elson, B.D.; Elliott, E.; et al. Multiple chemical sensitivity: A 1999 consensus. Arch. Environ. Health 1999, 54, 147–149. [Google Scholar]
- Lacour, M.; Zunder, T.; Schmidtke, K.; Vaith, P.; Scheidt, C. Multiple chemical sensitivity syndrome (MCS)—Suggestions for an extension of the U.S. MCS-case definition. Int. J. Hyg. Environ. Health 2005, 208, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.; Cutfield, N.J.; Kaski, D.; Palla, A.; Seemungal, B.M.; Golding, J.F.; Staab, J.P.; Bronstein, A.M. Visual dependency and dizziness after vestibular neuritis. PLoS ONE 2014, 9, e105426. [Google Scholar] [CrossRef] [PubMed]
- Dichgans, J.; Held, R.; Young, L.R.; Brandt, T. Moving visual scenes influence the apparent direction of gravity. Science 1972, 178, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Guerraz, M.; Yardley, L.; Bertholon, P.; Pollak, L.; Rudge, P.; Gresty, M.A.; Bronstein, A.M. Visual vertigo: Symptom assessment, spatial orientation and postural control. Brain 2001, 124, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Witkin, H.A.; Asch, S.E. Studies in space orientation: Further experiments on perception of the upright with displaced visual fields. J. Exp. Psychol. 1948, 38, 762–782. [Google Scholar] [CrossRef] [PubMed]
- Fiori, F.; David, N.; Aglioti, S.M. Processing of proprioceptive and vestibular body signals and self-transcendence in Ashtanga yoga practitioners. Front. Hum. Neurosci. 2014, 8, 734. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, H.; Treleaven, J.; Johnston, V.; Jull, G. Minimum repetitions for stable measures of visual dependency using the dot version of the computer-based Rod-Frame test. Man. Ther. 2012, 17, 466–469. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, H.G.; Weber, K.P.; McGarvie, L.A.; Halmagyi, G.M.; Curthoys, I.S. The video head impulse test: Diagnostic accuracy in peripheral vestibulopathy. Neurology 2009, 73, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.; Villgrattner, T.; Vockeroth, J.; Bartl, K.; Kohlbecher, S.; Bardins, S.; Ulbrich, H.; Brandt, T. EyeSeeCam: An eye movement-driven head camera for the examination of natural visual exploration. Ann. N. Y. Acad. Sci. 2009, 1164, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Blödow, A.; Pannasch, S.; Walther, L.E. Detection of isolated covert saccades with the video head impulse test in peripheral vestibular disorders. Auris Nasus Larynx 2013, 40, 348–351. [Google Scholar] [CrossRef] [PubMed]
- McGarvie, L.A.; MacDougall, H.G.; Halmagyi, G.M.; Burgess, A.M.; Weber, K.P.; Curthoys, I.S. The Video Head Impulse Test (vHIT) of Semicircular Canal Function—Age-Dependent Normative Values of VOR Gain in Healthy Subjects. Front. Neurol. 2015, 6, 154. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, M.; D’Erme, G.; Bruno, E.; Napolitano, B.; Magrini, A. Vestibular compensation: Analysis of postural re-arrangement as a control index for unilateral vestibular deficit. Neuroreport 2003, 14, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, M.; Lanciani, R.; Bruno, E.; Napolitano, B.; Di Girolamo, S. Posturography frequency analysis of sound-evoked body sway in normal subjects. Eur. Arch. Otorhinolaryngol. 2006, 263, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Schnakenberg, E.; Fabig, K.R.; Stanulla, M.; Strobl, N.; Lustig, M.; Fabig, N.; Schloot, W. A cross-sectional study of self-reported chemical-related sensitivity is associated with gene variants of drug-metabolizing enzymes. Environ. Health 2007, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Nola, G.; Mostardini, C.; Salvi, C.; Ercolani, A.P.; Ralli, G. Validity of Italian adaptation of the Dizziness Handicap Inventory (DHI) and evaluation of the quality of life in patients with acute dizziness. Acta Otorhinolaryngol. Ital. 2010, 30, 190–197. [Google Scholar] [PubMed]
- Ţărmure, V.; Almăşan, O.C.; Buhăţel, D.; Ilea, A.; Jiman, P.; Iancu, M.; Pop, A. Importance of Lateral Cephalogram in Treating Class III. In Proceedings of the International Conference on Advancements of Medicine and Health Care through Technology, Cluj-Napoca, Romania, 5—7 June 2014; Vlad, S., Ciupa, R.V., Eds.; Springer: New York, NY, USA, 2014; pp. 23–26. [Google Scholar]
- Nordin, S.; Palmquist, E.; Claeson, A.; Stenberg, B. The environmental hypersensitivity symptom inventory: Metric properties and normative data from a population-based study. Arch. Public Health 2013, 71, 18. [Google Scholar] [CrossRef] [PubMed]
- Bell, I.R. White paper. Neuropsychiatric aspects of sensitivity to low level chemicals: A neural sensitization model. Toxicol. Ind. Health 1994, 10, 277–312. [Google Scholar] [PubMed]
- Miedema, H.M.; Vos, H. Noise sensitivity and reactions to noise and other environmental conditions. J. Acoust. Soc. Am. 2003, 113, 1492–1504. [Google Scholar] [CrossRef] [PubMed]
- Stansfeld, S.A. Noise, noise sensitivity and psychiatric disorder: Epidemiological and psychophysiological studies. Psychol. Med. Monogr. Suppl. 1992, 22, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Ohashi, N.; Watanabe, Y.; Mizukoshi, K. The contribution of proprioception to posture control in normal subjects. Acta Oto-Laryngol. Suppl. 1993, 504, 112–116. [Google Scholar] [CrossRef]
- Jones, K.D.; King, L.A.; Mist, S.D.; Bennett, R.M.; Horak, F.B. Postural control deficits in people with fibromyalgia: A pilot study. Arthritis Res. Ther. 2011, 13, R127. [Google Scholar] [CrossRef] [PubMed]
- Avni, N.; Avni, I.; Barenboim, E.; Azaria, B.; Zadok, D.; Kohen-Raz, R.; Morad, Y. Brief posturographic test as an indicator of fatigue. Psychiatry Clin. Neurosci. 2006, 60, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Karita, K.; Nakao, M.; Nishikitani, M.; Iwata, T.; Murata, K.; Yano, E. Effect of overtime work and insufficient sleep on postural sway in information-technology workers. J. Occup. Health 2006, 48, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Beraneck, M.; Idoux, E. Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front. Neurol. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Morad, Y.; Azaria, B.; Avni, I.; Barkana, Y.; Zadok, D.; Kohen-Raz, R.; Barenboim, E. Posturography as an indicator of fatigue due to sleep deprivation. Aviat. Space Environ. Med. 2007, 78, 859–863. [Google Scholar] [PubMed]
- Redfern, M.S.; Jennings, J.R.; Martin, C.; Furman, J.M. Attention influences sensory integration for postural control in older adults. Gait Posture 2001, 14, 211–216. [Google Scholar] [CrossRef]
- Lavergne, M.R.; Cole, D.C.; Kerr, K.; Marshall, L.M. Functional impairment in chronic fatigue syndrome, fibromyalgia, and multiple chemical sensitivity. Can. Fam. Physician 2010, 56, e57–e65. [Google Scholar] [PubMed]
- Witkin, H.A. The perception of the upright. Sci. Am. 1959, 200, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, A.M. Visual vertigo syndrome: Clinical and posturography findings. J. Neurol. Neurosurg. Psychiatry 1995, 59, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Bronstein, A.M.; Faldon, M.E.; Mandalà, M.; Murray, K.; Silove, Y. Visual dependence and BPPV. J. Neurol. 2012, 259, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- McGuire, L.M.; Sabes, P.N. Sensory transformations and the use of multiple reference frames for reach planning. Nat. Neurosci. 2009, 12, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Isableu, B.; Ohlmann, T.; Cremieux, J.; Vuillerme, N.; Amblard, B.; Gresty, M.A. Individual differences in the ability to identify, select and use appropriate frames of reference for perceptuo-motor control. Neuroscience 2010, 169, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Bartenstein, P.; Janek, A.; Dieterich, M. Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain 1998, 121, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.O.; Bulthoff, H.H. Merging the senses into a robust percept. Trends. Cogn. Sci. 2004, 8, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Oie, K.S.; Kiemel, T.; Jeka, J.J. Multisensory fusion: Simultaneous re-weighting of vision and touch for the control of human posture. Brain Res. Cogn. Brain Res. 2002, 14, 164–176. [Google Scholar] [CrossRef]
- Ernst, M.O.; Banks, M.S. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 2002, 415, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Seemungal, B.M. The cognitive neurology of the vestibular system. Curr. Opin. Neurol. 2014, 27, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Brandt, T.; Strupp, M.; Dieterich, M. Towards a concept of disorders of “higher vestibular function”. Front. Integr. Neurosci. 2014, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Brodziak-Dopierała, B.; Kwapuliński, J.; Kowol, J.; Sobczyk, K.; Gajda, Z. The Application of Principal Component Analysis to Interpretation of Occurrence of Metals in the Femur Head. Polish J. Environ. Stud. 2010, 1, 49–58. [Google Scholar]
- Bajorath, J. Selected Concepts and Investigations in Compound Classification, Molecular Descriptor Analysis and Virtual Screening. J. Chem. Inf. Comput. Sci. 2001, 41, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G. A critical analysis of multiple chemical sensitivities. Med. Hypotheses 1998, 50, 303–311. [Google Scholar] [CrossRef]
- Karnath, H.O.; Dieterich, M. Spatial neglect—A vestibular disorder? Brain 2006, 129, 293–305. [Google Scholar] [CrossRef] [PubMed]
| Socio-demographical Variables | Total MCS | Reason of Exclusion, Number, Gender | Included MCS |
|---|---|---|---|
| Number | 23 | See below | 18 |
| Gender | 14 female, 9 male | See below | 11 female, 7 male |
| Age | 50.3 ± 8.9 years | - | 49.5 ± 9.3 years |
| Employment | Cosmetologist (n = 4) | psychiatric medications (n = 1, female) | Cosmetologist (n = 3) |
| Hairdresser (n = 5) | - | Hairdresser (n = 5) | |
| Teacher (n = 3) | hypothyroidism (n = 1, female) | Teacher (n = 2) | |
| Nurse (n = 3) | psychiatric medications (n = 1, female) | Nurse (n = 2) | |
| Ex-soldier (n = 3) | alcohol addiction (n = 1, male) | Ex-soldier (n = 2) | |
| Obstetrician (n = 2) | - | Obstetrician (n = 2) | |
| Taxi driver (n = 2) | psychiatric medications (n = 1, male) | Taxi driver (n = 1) | |
| University Professor (n = 1) | - | University Professor (n = 1) |
| Variables | MCS | HC | Variables | MCS | HC | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | Mean | SD | Mean | SD | p Value | ||
| Rod and Disc Test | Fast Fourier Transform | ||||||||||
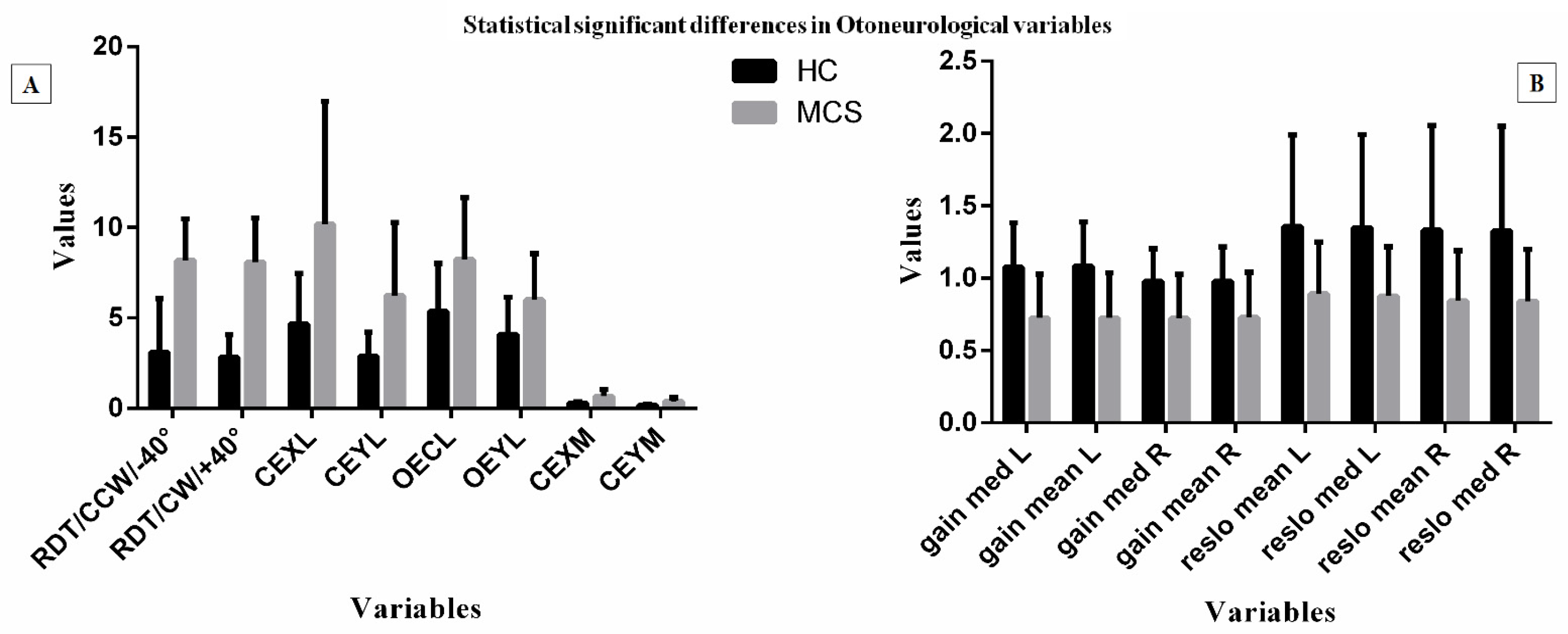
| RDT/N/+40 | 2.39 | 2.05 | 1.63 | 1.82 | p > 0.01 | Low Freqeuncy Interval | |||||
| RDT/CCW/−40 | 8.18 | 2.29 | 3.11 | 1.95 | p < 0.01 | CEXL | 10.17 | 6.8 | 4.68 | 2.77 | p < 0.01 |
| RDT/CW/+40 | 8.09 | 2.43 | 2.83 | 1.24 | p < 0.01 | CEYL | 6.24 | 4.02 | 2.89 | 1.3 | p < 0.01 |
| RDT/N/−40 | 5.3 | 7.67 | 2.05 | 1.89 | p > 0.01 | OEXL | 8.23 | 3.41 | 5.35 | 2.65 | p < 0.01 |
| RDT/CCW/+40 | 4.06 | 2.4 | 3.76 | 4.04 | p > 0.01 | OEYL | 6 | 2.56 | 4.08 | 2.06 | p < 0.01 |
| RDT/CW/−40 | 4.6 | 1.93 | 3.58 | 2.42 | p > 0.01 | CEXL-FC | 8.28 | 2.18 | 9.67 | 1.9 | p > 0.01 |
| Rod and Frame Test | CEYL-FC | 8.24 | 2.55 | 7.51 | 1.79 | p > 0.01 | |||||
| RFT/CW/+11 | 1.608333 | 0.793216 | 1.6125 | 0.43766 | p > 0.01 | OEXL-FC | 7.21 | 3.99 | 6.87 | 2.54 | p > 0.01 |
| RFT/CCW/+11 | 2.238889 | 0.777166 | 2.13 | 0.706437 | p > 0.01 | OEYL-FC | 6.14 | 2.83 | 5.13 | 2.22 | p > 0.01 |
| RFT/CW/−11 | 1.758333 | 0.895947 | 1.58 | 0.88055 | p > 0.01 | Middle Frequency Interval | |||||
| RFT/CCW/−11 | 2.183333 | 0.56983 | 2.1475 | 0.609459 | p > 0.01 | CEXM | 0.67 | 0.37 | 0.28 | 0.07 | p < 0.01 |
| RFT/CW/+22 | 1.536111 | 0.875954 | 1.4925 | 0.739537 | p > 0.01 | CEYM | 0.39 | 0.19 | 0.17 | 0.05 | p < 0.01 |
| RFT/CCW/+22 | 2.319444 | 0.518238 | 2.2225 | 0.713309 | p > 0.01 | OEXM | 0.34 | 0.03 | 0.3 | 0.09 | p > 0.01 |
| RFT/CW/−22 | 2.019444 | 0.500335 | 2.1125 | 0.783695 | p > 0.01 | OEYM | 0.23 | 0.05 | 0.2 | 0.06 | p > 0.01 |
| RFT/CCW/−22 | 1.580556 | 0.75909 | 1.435 | 0.675336 | p > 0.01 | CEXM-FC | 0.84 | 0.29 | 1.02 | 0.5 | p > 0.01 |
| RFT/N/+11 | 1.883333 | 0.582338 | 1.9175 | 0.681962 | p > 0.01 | CEYM-FC | 0.71 | 0.38 | 0.6 | 0.26 | p > 0.01 |
| RFT/N/−11 | 2.125 | 0.53667 | 2.15 | 0.585797 | p > 0.01 | OEXM-FC | 0.01 | 0 | 0.01 | 0 | p > 0.01 |
| RFT/N/+22 | 1.827778 | 0.68368 | 1.905 | 0.657927 | p > 0.01 | OEYM-FC | 0.27 | 0.09 | 0.29 | 0.09 | p > 0.01 |
| RFT/N/−22 | 2.119444 | 0.839142 | 2.0255 | 0.608324 | p > 0.01 | High Frequency Interval | |||||
| Classic Posturography Parameters | CEXH | 1.24 | 0.17 | 1.09 | 0.28 | p > 0.01 | |||||
| RI | 241.29 | 77.61 | 168.73 | 87.85 | p < 0.01 | CEYH | 1.04 | 0.29 | 1.05 | 0.26 | p > 0.01 |
| RI-FC | 348.46 | 101.2 | 251.55 | 135.66 | p < 0.01 | OEXH | 0.96 | 0.32 | 1.01 | 0.28 | p > 0.01 |
| surf OE | 407.25 | 124.89 | 414.06 | 212.59 | p > 0.01 | OEYH | 0.72 | 0.13 | 0.77 | 0.13 | p > 0.01 |
| surf CE | 939.71 | 312.53 | 656.25 | 369.4 | p < 0.01 | CEXH-FC | 1.65 | 0.19 | 1.61 | 0.25 | p > 0.01 |
| surf OE-FC | 831.72 | 243.34 | 740 | 452.75 | p > 0.01 | CEYH-FC | 1.57 | 0.25 | 1.63 | 0.2 | p > 0.01 |
| surf CE-FC | 2769.06 | 750.03 | 1565.61 | 782.82 | p < 0.01 | OEXH-FC | 0 | 0 | 0 | 0 | p > 0.01 |
| length OE | 552.94 | 103.76 | 512.85 | 98.4 | p > 0.01 | OEYH-FC | 0.75 | 0.26 | 0.72 | 0.17 | p > 0.01 |
| length CE | 986.93 | 310.77 | 739.17 | 150.98 | p < 0.01 | Video Head Impulse Test | |||||
| length OE-FC | 822.41 | 285.98 | 722 | 182.44 | p > 0.01 | gain med L | 0.72 | 0.3 | 1.07 | 0.3 | p < 0.01 |
| length CE-FC | 2237.64 | 675.93 | 1694.96 | 642.48 | p < 0.01 | gain mean L | 0.72 | 0.31 | 1.08 | 0.3 | p < 0.01 |
| avspe OE | 9.11 | 1.15 | 8.55 | 1.63 | p > 0.01 | gain med R | 0.72 | 0.3 | 0.97 | 0.22 | p < 0.01 |
| avspe CE | 18.68 | 4 | 12.07 | 2.73 | p < 0.01 | gain mean R | 0.72 | 0.31 | 0.97 | 0.23 | p < 0.01 |
| avspe OE-FC | 13.04 | 2.94 | 12.7 | 5.56 | p > 0.01 | reslo mean L | 0.89 | 0.35 | 1.35 | 0.63 | p < 0.01 |
| avspe CE-FC | 38.12 | 13.63 | 27.71 | 11.28 | p < 0.01 | reslo med L | 0.87 | 0.34 | 1.34 | 0.64 | p < 0.01 |
| stdspe OE | 5.47 | 0.96 | 5.03 | 1.14 | p > 0.01 | reslo mean R | 0.84 | 0.34 | 1.33 | 0.72 | p < 0.01 |
| stdspe CE | 10.68 | 3.24 | 7.61 | 2.09 | p < 0.01 | reslo med R | 0.84 | 0.35 | 1.32 | 0.72 | p < 0.01 |
| stdspe OE-FC | 9.92 | 3.23 | 8.06 | 3.24 | p > 0.01 | ||||||
| stdspe CE-FC | 25.35 | 6.83 | 18.18 | 7.46 | p < 0.01 | ||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micarelli, A.; Viziano, A.; Micarelli, E.; Genovesi, G.; Bruno, E.; Alessandrini, M. Deranged Dimensionality of Vestibular Re-Weighting in Multiple Chemical Sensitivity. Appl. Sci. 2016, 6, 330. https://doi.org/10.3390/app6110330
Micarelli A, Viziano A, Micarelli E, Genovesi G, Bruno E, Alessandrini M. Deranged Dimensionality of Vestibular Re-Weighting in Multiple Chemical Sensitivity. Applied Sciences. 2016; 6(11):330. https://doi.org/10.3390/app6110330
Chicago/Turabian StyleMicarelli, Alessandro, Andrea Viziano, Elisa Micarelli, Giuseppe Genovesi, Ernesto Bruno, and Marco Alessandrini. 2016. "Deranged Dimensionality of Vestibular Re-Weighting in Multiple Chemical Sensitivity" Applied Sciences 6, no. 11: 330. https://doi.org/10.3390/app6110330
APA StyleMicarelli, A., Viziano, A., Micarelli, E., Genovesi, G., Bruno, E., & Alessandrini, M. (2016). Deranged Dimensionality of Vestibular Re-Weighting in Multiple Chemical Sensitivity. Applied Sciences, 6(11), 330. https://doi.org/10.3390/app6110330