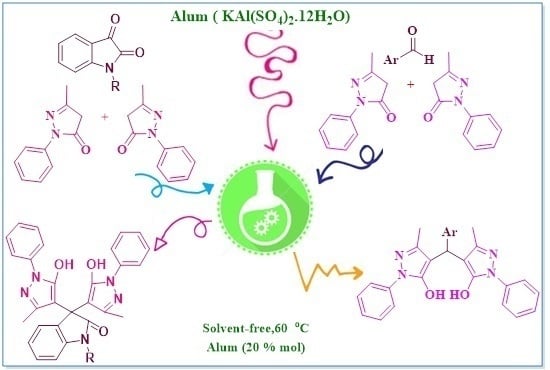
Alum as a Catalyst for the Synthesis of Bispyrazole Derivatives
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. General Procedure for Preparation of Bispyrazol Derivatives
2.3. General Procedure for Catalyst Recovery
2.4. Physical Properties of Compounds 1a–e
2.4.1. 3,3-Bis(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)indolin-2-one (1a, Table 1)
2.4.2. 1-Benzyl-3,3-bis(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)indolin-2-one (1b, Table 1)
| Entry | Compound | Structure | Time (min) | Yield a (%) |
|---|---|---|---|---|
| 1 | 1a | 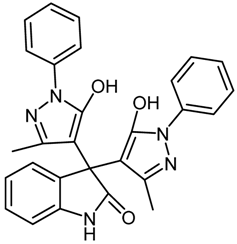 | 20 | 92 |
| 2 | 1b | 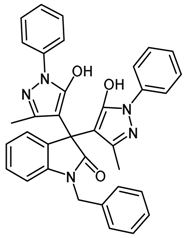 | 25 | 90 |
| 3 | 1c | 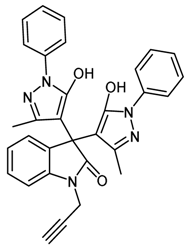 | 20 | 90 |
| 4 | 1d |  | 15 | 87 |
| 5 | 1e | 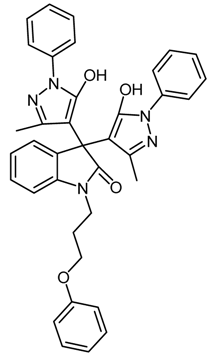 | 30 | 81 |
2.4.3. 3,3-Bis(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)-1-(prop-2-ynyl)indolin-2-one (1c, Table 1)
2.4.4. 3,3-Bis(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)-1-(3-(4-nitrophenoxy)propyl)indolin-2-one (1d, Table 1)
2.4.5. 3,3-Bis(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)-1-(3-(naphthalen-2-yloxy)propyl)indolin-2-one (1e, Table 1)
3. Results and Discussion
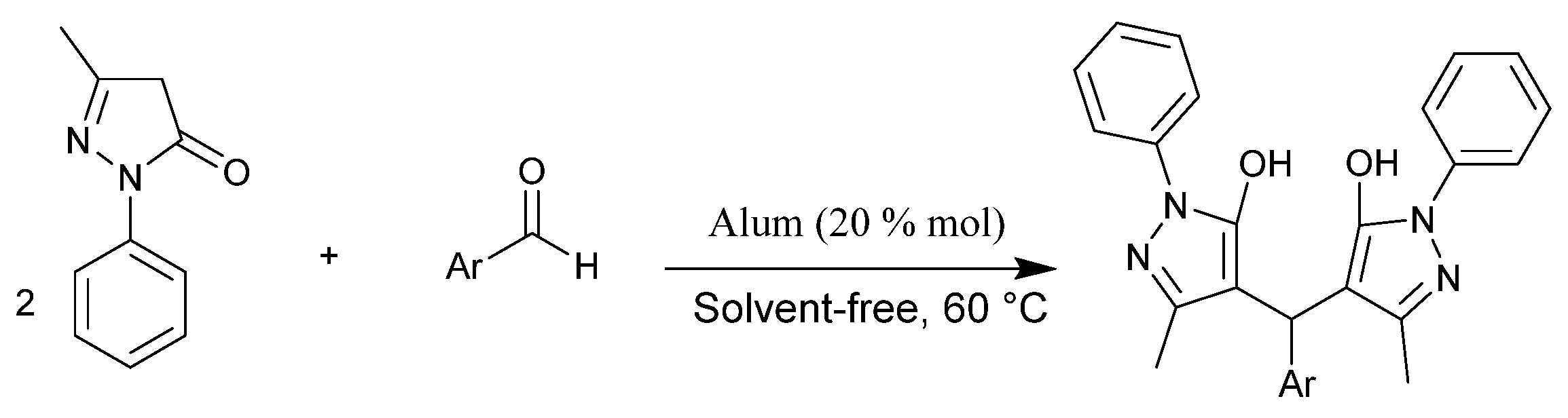
| Entry | Solvent | Amount of Catalyst / mol% | Yield b (%) |
|---|---|---|---|
| 1 | H2O | 20 | 78 |
| 2 | CH3CH2OH | 20 | 79 |
| 3 | CH3CN | 20 | 61 |
| 4 | DMF | 20 | 78 |
| 5 c | none | 20 | 95, 92, 89, 85, 81 |
| 6 | none | 25 | 95 |
| 7 | none | 10 | 78 |
| 8 | none | - | Trace |
| Entry | Ar | Time (min) | Yield a (%) | mp. °C (lit.) | Reference |
|---|---|---|---|---|---|
| 1 | C6H5 | 15 | 86 | 168–170 (169–171) | [13] |
| 2 | 4-ClC6H4 | 25 | 90 | 212–215 (215–217) | [14] |
| 3 | 2-OHC6H4 | 20 | 95 | 226–228 (222–226) | [13] |
| 4 | 2-ClC6H4 | 25 | 87 | 235–236 (235–237) | [14] |
| 5 | 2-MeOC6H4 | 15 | 91 | 211–214 (210–213) | [13] |
| 6 | 4-NO2C6H4 | 60 | 85 | 217–220 (218–220) | [13] |
| 7 | 3-NO2C6H4 | 40 | 90 | 149–150 (153–155) | [13] |
| 8 | 2-Thienyl | 30 | 90 | 185–187 (181–183) | [14] |
| 9 | 2-Furyl | 40 | 88 | 187–190 (188–191) | [20] |
| 10 | 4-MeC6H4 | 25 | 85 | 201-203 (202–204) | [14] |
| 11 | 4-CNC6H4 | 40 | 90 | 215–218 (210–212) | [14] |
| 12 | 2-Naphtyl | 15 | 92 | 201–203 (204–206) | [20] |
| 13 | 4-OHC6H4 | 50 | 89 | 159–162 (155–157) | [20] |
| Entry | Catalyst | Time (min) | Yield a (%) |
|---|---|---|---|
| 1 | Alum | 20 | 95 |
| 2 | Oxalic acid.dihydrate | 25 | 90 |
| 3 | Silicasulfuric acid(sSA) | 60 | 82 |
| 4 | KHSO4 | 60 | 81 |
| 5 | NaHSO4, H2O | 70 | 77 |
| 6 | Fe(HSO4)3 | 65 | 78 |
| 7 | Al(HSO4)3 | 105 | 82 |
| 8 | FeCl3 | 50 | 85 |
| 9 | AlCl3 | 110 | 85 |
| 10 | Citric acid | 75 | 76 |
| 11 | CuFe2O4 | 25 | 89 |
| 12 | MgO | 80 | 62 |
| 13 | ZnO | 70 | 68 |
| 14 | Oxone | 75 | 78 |
| Reaction Condition | Time (min) | Yield b (%) | Reference |
|---|---|---|---|
| Silica-bonded S-sulfonic acid (sBSSA) (0.20 g), EtOH, reflux | 50 | 90 | [14] |
| PEG-400, 110 °C | 150 | 90 | [22] |
| [P4VPy-BuSO3H]HSO4 (0.1 mmol), Ethanol, reflux | 48 | 95 | [23] |
| [Sipmim]HSO4 (0.20 g), Ethanol, reflux condition | 120 | 90 | [24] |
| Alum (20% mol), Solvent-free, 60 °C | 25 | 90 | - c |
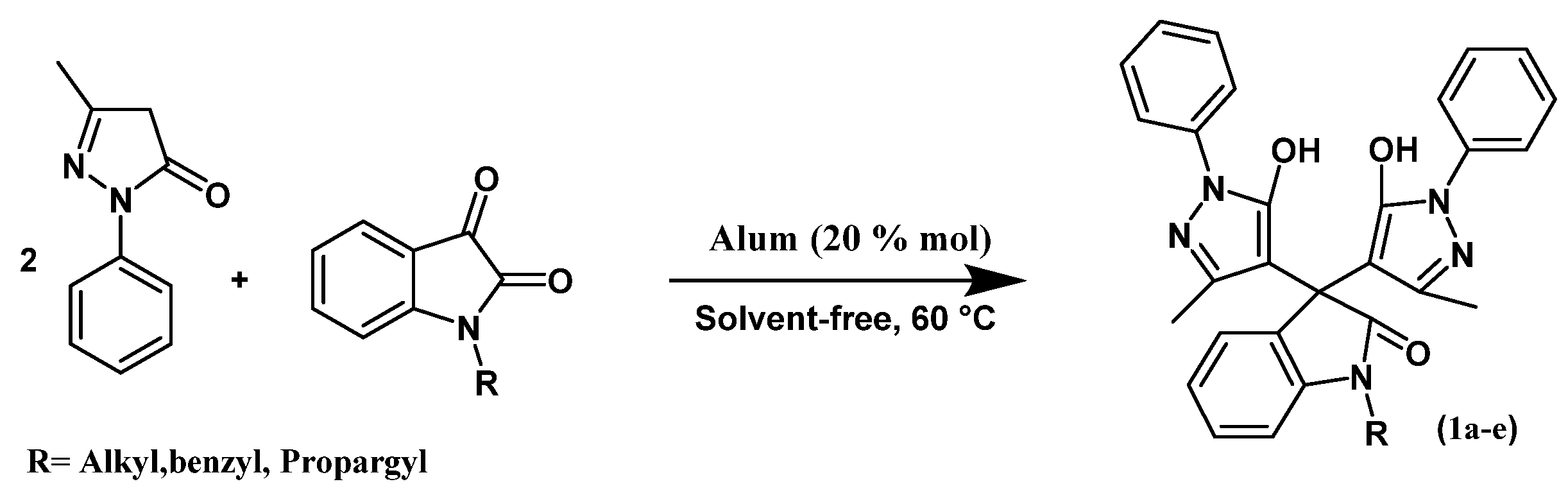
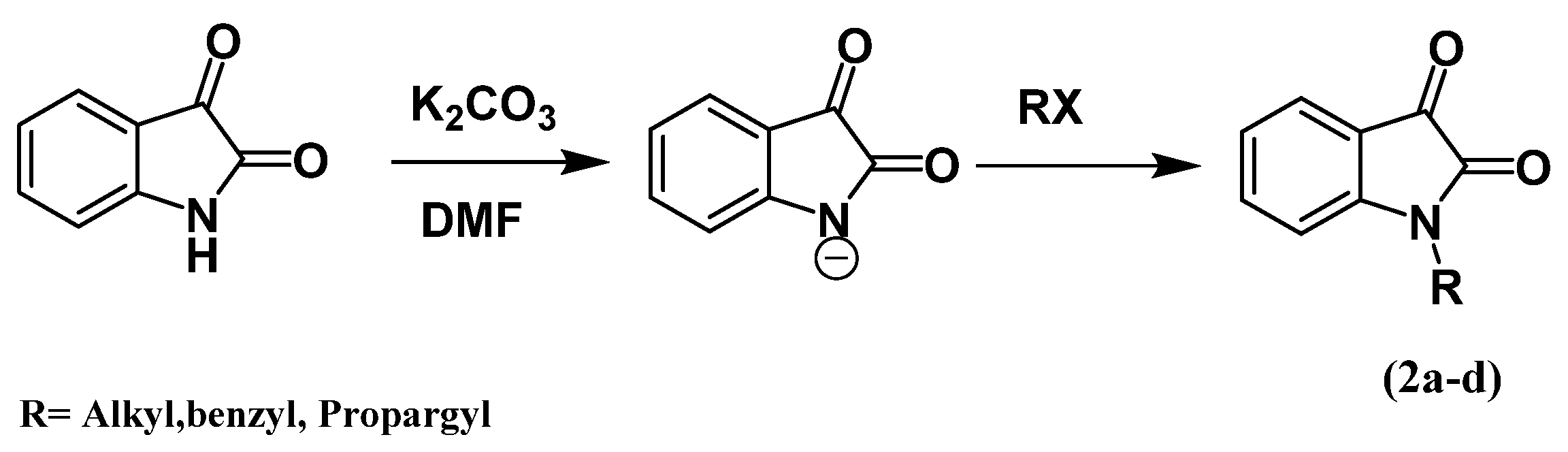
| Compound | R |
|---|---|
| 2a | CH2C≡CH |
| 2b | CH2Ph |
| 2c | 4-NO2C6H4OCH2CH2CH2 |
| 2d | C10H7-2OCH2CH2CH2 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Longhi, K.; Moreira, D.N.; Marzari, M.R.B.; Floss, V.M.; Bonacorso, H.G.; Zanatta, N.; Martins, M.A.P. An efficient solvent-free synthesis of NH-pyrazoles from β-dimethylaminovinylketones and hydrazine on grinding. Tetrahedron Lett. 2010, 51, 3193–3196. [Google Scholar] [CrossRef]
- Sachdeva, H.; Dwivedi, D.; Saroj, R. Alum catalyzed simple, efficient, and green synthesis of 2-[3-amino-5-methyl-5-(pyridin-3-yl)-1,5-dihydro-4H-1,2,4-triazol-4-yl] propanoic acid derivatives in aqueous media. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Sonar, S.S.; Shelke, K.F.; Kakade, G.K.; Shingate, B.B.; Shingare, M.S. Alum: An efficient catalyst for one-pot synthesis of α-aminophosphonates. Chin. Chem. Lett. 2009, 20, 1042–1046. [Google Scholar] [CrossRef]
- Zare, A.; Merajoddin, M.; Abi, F.; Moosavi-Zare, A.R.; Mokhlesi, M.; Zolfigol, M.A.; Asgari, Z.; Khakyzadeh, V.; Hasaninejad, A.; Khalafi-Nezhad, A. Trityl chloride (TrCl): Efficient and homogeneous organocatalyst for the solvent-free synthesis of 14-aryl-14H-dibenzo [a,j] xanthenes by in situ formation of carbocationic system. J. Chin. Chem. Soc. 2012, 59, 860–865. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Mivechi, M.; Kefayati, H. Potassium aluminum sulfate (alum): An efficient catalyst for the one-pot synthesis of trisubstituted imidazoles. Monatsh. Chem. Chem. Mon. 2008, 139, 935–937. [Google Scholar] [CrossRef]
- Azizian, J.; Mohammadi, A.A.; Karimi, A.R.; Mohammadizadeh, M.R. A stereoselective three-component reaction: KAl(SO4)2∙12H2O, an efficient and reusable catalyst for the one-pot synthesis of cis-isoquinolonic acids. J. Org. Chem. 2005, 70, 350–352. [Google Scholar] [CrossRef] [PubMed]
- Dabiri, M.; Baghbanzadeh, M.; Nikcheh, M.S.; Arzroomchilar, E. Eco-friendly and efficient one-pot synthesis of alkyl-or aryl-14H-dibenzo [a,j] xanthenes in water. Bioorg. Med. Chem. Lett. 2008, 18, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Azizian, J.; Mohammadi, A.A.; Karimi, A.R.; Mohammadizadeh, M.R. KAl(SO4)2∙12H2O supported on silica gel as a novel heterogeneous system catalyzed biginelli reaction: One-pot synthesis of di-hydropyrimidinones under solvent-free conditions. Appl. Catal. A Gen. 2006, 300, 85–88. [Google Scholar] [CrossRef]
- Azizian, J.; Mohammadi, A.A.; Bidar, I.; Mirzaei, P. KAl(SO4)2∙12H2O (alum) a reusable catalyst for the synthesis of some 4-substituted coumarins via pechmann reaction under solvent-free conditions. Monatsh. Chem. Chem. Mon. 2008, 139, 805–808. [Google Scholar] [CrossRef]
- Shelke, K.F.; Sapkal, S.B.; Kakade, G.K.; Sadaphal, S.A.; Shingate, B.B.; Shingare, M.S. Alum catalyzed simple and efficient synthesis of 5-arylidene-2,4-thiazolidinedione in aqueous media. Green Chem. Lett. Rev. 2010, 3, 17–21. [Google Scholar] [CrossRef]
- Mahajan, R.N.; Havaldar, F.H.; Fernandes, P.S. Syntheses and biological activity of heterocycles derived from 3-methoxy-1-phenyl-1H-pyrazole-5-carboxylate. J. Indian Chem. Soc. 1991, 68, 245–246. [Google Scholar]
- Selvam, C.; Jachak, S.M.; Thilagavathi, R.; Chakraborti, A.K. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2005, 15, 1793–1797. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Su, Q.; Mo, Y.; Cheng, B. Ionic liquid under ultrasonic irradiation towards a facile synthesis of pyrazolone derivatives. Ultrason. Sonochem. 2011, 18, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Niknam, K.; Saberi, D.; Sadegheyan, M.; Deris, A. Silica-bonded s-sulfonic acid: An efficient and recyclable solid acid catalyst for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols). Tetrahedron Lett. 2010, 51, 692–694. [Google Scholar] [CrossRef]
- Sugiura, S.; Ohno, S.; Ohtani, O.; Izumi, K.; Kitamikado, T.; Asai, H.; Kato, K.; Hori, M.; Fujimura, H. Syntheses and antiinflammatory and hypnotic activity of 5-alkoxy-3-(N-substituted carbamoyl)-1-phenylpyrazoles. J. Med. Chem. 1977, 20, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Rosiere, C.E.; Grossman, M.I. An analog of histamine that stimulates gastric acid secretion without other actions of histamine. Science 1951, 113, 651. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Hansen, P.E.; Hlavac, A.G.; Baizman, E.R.; Pearl, J.; DeFelice, A.F.; Feigenson, M.E. 3,4-diphenyl-1H-pyrazole-1-propanamine antidepressants. J. Med. Chem. 1985, 28, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.M.S.; Singh, S.; Chatterjee, R.K. Antifilarial profiles of substituted pyrazoles: A new class of antifilarial agents. Indian J. Chem. Sect. B Org. Chem. 1993, 32, 858–861. [Google Scholar]
- Londershausen, M. Review: Approaches to new parasiticides. Pestic. Sci. 1996, 48, 269–292. [Google Scholar] [CrossRef]
- Karimi-Jaberi, Z.; Pooladian, B.; Moradi, M.; Ghasemi, E. 1,3,5-Tris(hydrogensulfato) benzene: A new and efficient catalyst for synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol) derivatives. Chin. J. Catal. 2012, 33, 1945–1949. [Google Scholar] [CrossRef]
- Garnovskii, A.D.; Uraev, A.I.; Minkin, V.I. Metal complexes from aryl and hetarylazocompounds. Arkiv. 2004, 3, 29–41. [Google Scholar]
- Hasaninejad, A.; Zare, A.; Shekouhy, M.; Golzar, N. Efficient synthesis of 4,4′-(arylmethylene)-bis (3-methyl-1-phenylpyrazol-5-ol) derivatives in PEG-400 under catalyst-free conditions. Org. Prep. Proced. Int. 2011, 43, 131–137. [Google Scholar] [CrossRef]
- Boroujeni, K.P.; Shojaei, P. Poly (4-vinylpyridine)-supported dual acidic ionic liquid: An environmentally friendly heterogeneous catalyst for the one-pot synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols). Turk. J. Chem. 2013, 37, 756–764. [Google Scholar] [CrossRef]
- Baghernejad, M.; Niknam, K. Synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) using silica-bonded ionic liquid as recyclable catalyst. Int. J. Chem. 2012, 4, 52. [Google Scholar] [CrossRef]
- Da Silva, J.F.; Garden, S.J.; Pinto, A.C. The chemistry of isatins: A review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Shmidt, M.S.; Reverdito, A.M.; Kremenchuzky, L.; Perillo, I.A.; Blanco, M.M. Simple and efficient microwave assisted N-alkylation of isatin. Molecules 2008, 13, 831–840. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zolfigol, M.A.; Khazaei, A.; Karimitabar, F.; Hamidi, M. Alum as a Catalyst for the Synthesis of Bispyrazole Derivatives. Appl. Sci. 2016, 6, 27. https://doi.org/10.3390/app6010027
Zolfigol MA, Khazaei A, Karimitabar F, Hamidi M. Alum as a Catalyst for the Synthesis of Bispyrazole Derivatives. Applied Sciences. 2016; 6(1):27. https://doi.org/10.3390/app6010027
Chicago/Turabian StyleZolfigol, Mohammad Ali, Ardeshir Khazaei, Fatemeh Karimitabar, and Masoud Hamidi. 2016. "Alum as a Catalyst for the Synthesis of Bispyrazole Derivatives" Applied Sciences 6, no. 1: 27. https://doi.org/10.3390/app6010027
APA StyleZolfigol, M. A., Khazaei, A., Karimitabar, F., & Hamidi, M. (2016). Alum as a Catalyst for the Synthesis of Bispyrazole Derivatives. Applied Sciences, 6(1), 27. https://doi.org/10.3390/app6010027