Microwave-Assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-Triazole Moiety
Abstract
:1. Introduction
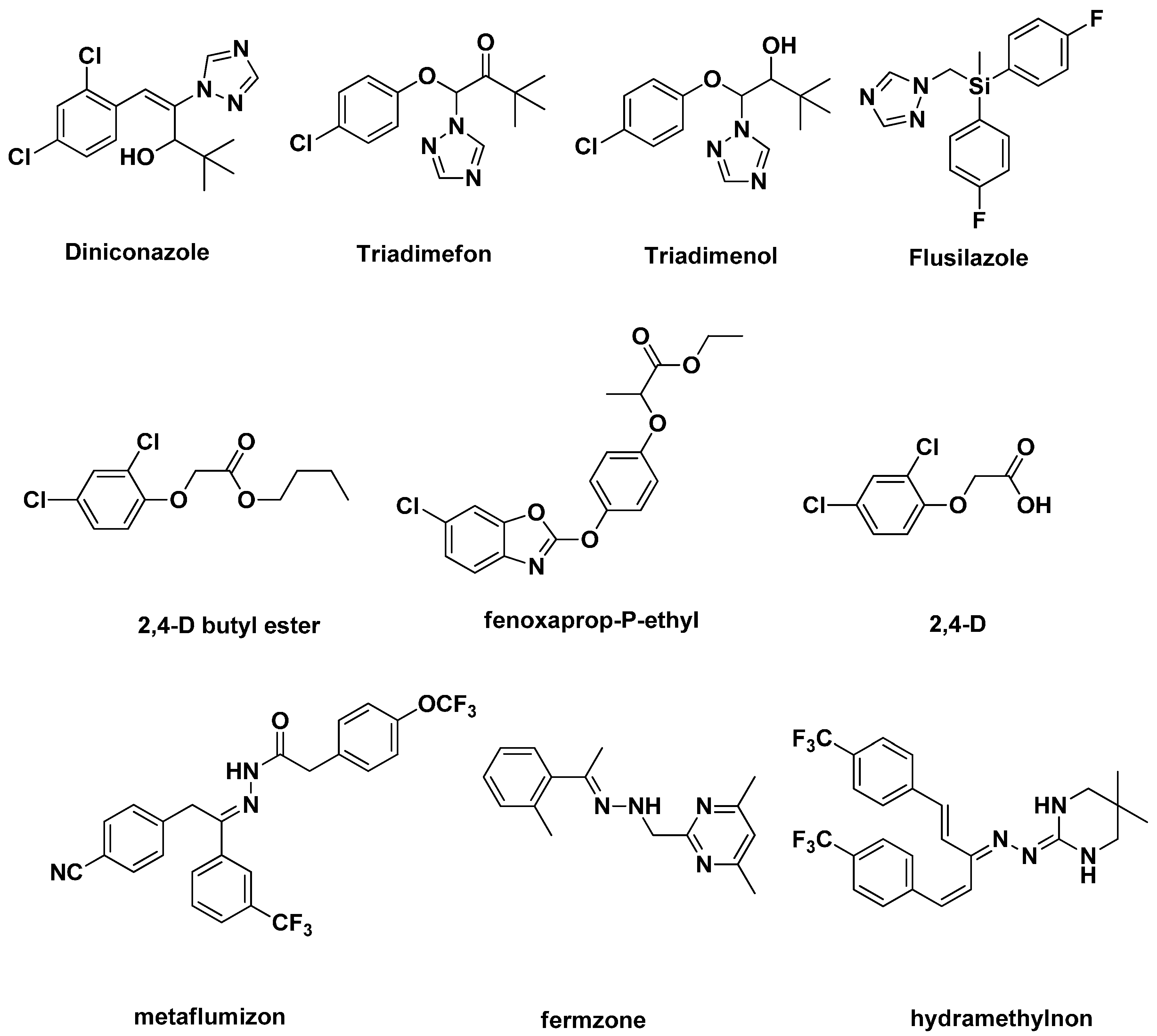
2. Results and Discussion
2.1. Synthesis
| No. | Method | Time | Temperature/°C | Yield/% |
|---|---|---|---|---|
| 5b | No-MW | 24 h | r.t. | 78 |
| 5b | No-MW | 10 min | 90 | 42 |
| 5b | MW | 10 min | 90 | 79 |
| 5b | MW | 15 min | 90 | 81 |
| 5b | MW | 20 min | 90 | 81 |
2.2. Antifungal Activities
| No. | Pythium ultimum | Phytophthora infestans | Corynespora cassiicola | Botrytis cinerea | Rhizoctonia solani |
|---|---|---|---|---|---|
| 5a | 66.7 ± 2.15 | −0.8 ± 0.08 | 4.1 ± 1.02 | −25.4 ± 1.32 | 3.9 ± 0.05 |
| 5b | 55.6 ± 1.65 | −0.8 ± 0.02 | −0.9 ± 0.06 | −11.9 ± 0.98 | 0.0 ± 0.00 |
| 5c | −88.9 ± 2.03 | −0.8 ± 0.09 | 46.2 ± 2.05 | 23.8 ± 1.05 | 6.7 ± 0.07 |
| 5d | −111.1 ± 2.78 | −0.8 ± 0.01 | 46.2 ± 2.12 | 19.9 ± 0.96 | 0.0 ± 0.00 |
| 5e | −55.6 ± 0.89 | −0.8 ± 0.04 | 61.3 ± 1.89 | 19.9 ± 1.01 | 0.0 ± 0.00 |
| 5f | 11.1 ± 0.78 | −0.8 ± 0.03 | 50.9 ± 1.66 | −16.7 ± 0.88 | 0.0 ± 0.00 |
| 5g | 0.0 ± 0.00 | −0.8 ± 0.04 | 41.4 ± 1.15 | −16.7 ± 0.79 | 0.0 ± 0.00 |
| 5h | 55.6 ± 1.35 | −0.5 ± 0.04 | 37.8 ± 1.25 | −56.3 ± 1.22 | 0.0 ± 0.00 |
| 5i | 55.6 ± 1.62 | −0.8 ± 0.02 | 24.9 ± 2.16 | −15.8 ± 1.14 | 0.0 ± 0.00 |
| 5j | −33.3 ± 1.01 | 0.3 ± 0.04 | 42.2 ± 1.14 | −46.6 ± 0.38 | 0.0 ± 0.00 |
| 5k | −122.2 ± 3.05 | 3.3 ± 0.05 | 16.2 ± 1.18 | 1.6 ± 0.06 | 10.6 ± 0.52 |
| Zhongshengmycin | 0.0 ± 0.00 | - | - | - | - |
| Dimethomorph | - | 97.8 ± 2.07 | - | - | - |
| Chlorothalonil | - | - | 45.9 ± 1.77 | - | - |
| Procymidone | - | - | - | −7.6 ± 0.68 | - |
| Validamycin | - | - | - | - | 62.5 ± 2.13 |
3. Experimental Section
3.1. Instruments
3.2. Synthesis
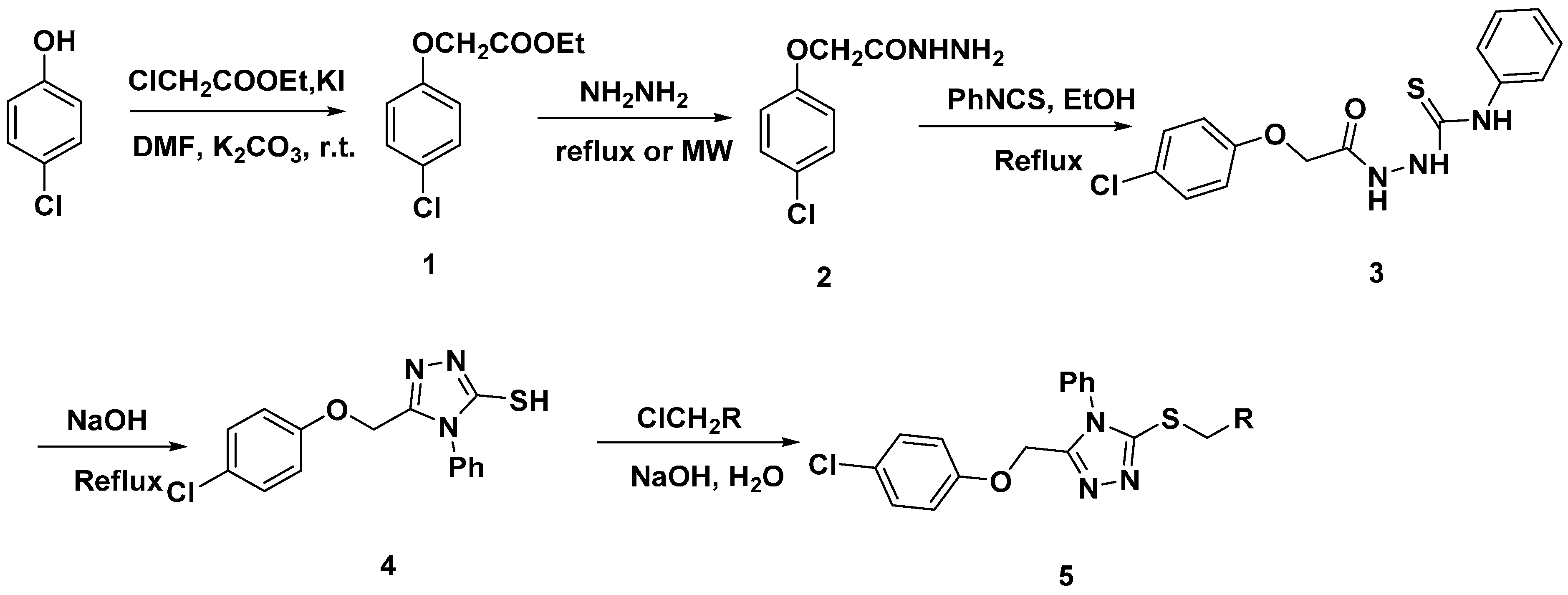
3.2.1. Synthesis of Ethyl 2-(4-chlorophenoxy)acetate (1)
3.2.2. Synthesis of 2-(4-Chlorophenoxy)acetohydrazide (2)
3.2.3. Synthesis of 2-(2-(4-Chlorophenoxy)acetyl)-N-phenylhydrazinecarbothioamide (3)
3.2.4. Synthesis of 5-((4-Chlorophenoxy)methyl)-4-phenyl-4H-1,2,4-triazole-3-thiol (4)
3.2.5. General Procedure for Thioether (5)
3.3. Antifungal Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yan, S.L.; Yang, M.Y.; Sun, Z.H.; Min, L.J.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,2,3-thiadiazole derivatives containing 1,3,4-thiadiazole moiety. Lett. Drug Des. Discov. 2014, 11, 940–943. [Google Scholar] [CrossRef]
- Owen, W.J.; Sullenberger, M.T.; Loso, M.R.; Meyer, K.G.; Slanec, T.J. Synthesis and antifungal activity of 3-aryl-1,2,4-triazin-6-one derivatives. Pest Manag. Sci. 2015, 71, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Yang, M.Y.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Wu, H.K.; Liu, X.H. Synthesis and antifungal activity of 1,3,4-thiadiazole derivatives containing pyridine group. Lett. Drug Des. Discov. 2014, 11, 1107–1111. [Google Scholar] [CrossRef]
- Nugent, B.M.; Buysse, A.M.; Loso, M.R.; Babcock, J.M.; Johnson, T.C.; Oliver, M.P.; Martin, T.P.; Ober, M.S.; Breaux, N.; Robinson, A. Expanding the structure-activity relationship of sulfoxaflor: The synthesis and biological activity of N-heterocyclic sulfoximines. Pest Manag. Sci. 2015, 71, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, dimeric crystal, and fungicidal activity of 1-(4-methylphenyl)-2-(5-((3,5-dimethyl-1H-pyrazol-1-yl)methyl)-4-phenyl-4H-1,2,4-triazol-3-ylthio) ethanone. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 558–564. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kumar, R.; Srivastava, C.; Dureja, P.; Khurana, J.M. Synthesis, biological activities and SAR studies of novel 1-ethyl-7-methyl-4-oxo-1,4-dihydro-[1,8]naphthyridine-3-carboxylic acid based diacyl and sulfonyl acyl hydrazines. Pest Manag. Sci. 201 2014, 70, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Tan, C.X.; Weng, J.Q. Phase transfer-catalyzed, one-potsynthesis of some novel N-pyrimidinyl-N′-picotinylthioureaderivatives. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 552–557. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhai, Z.W.; Sun, Z.H.; Yu, S.J.; Liu, X.H.; Weng, J.Q.; Tan, C.X.; Zhao, W.G. A facile one-pot synthesis of novel 1,2,4-triazolo[4,3-a]pyridine derivativescontaining the trifluoromethyl moiety using microwave irradiation. J. Chem. Res. 2015, 39, 521–523. [Google Scholar] [CrossRef]
- Liu, X.H.; Weng, J.Q.; Wang, B.L.; Li, Y.H.; Tan, C.X.; Li, Z.M. Microwave-assisted synthesis of novel fluorinated 1,2,4-triazole derivatives, and study of their biological activity. Res. Chem. Intermed. 2014, 40, 2605–2612. [Google Scholar] [CrossRef]
- Zhang, L.J.; Yang, M.Y.; Hu, B.Z.; Sun, Z.H.; Liu, X.H.; Weng, J.Q.; Tan, C.X. Microwave-assisted synthesis of novel 8-chloro-[1,2,4]triazolo[4,3-a]pyridinederivatives. Turk. J. Chem. 2015, 39, 867–873. [Google Scholar] [CrossRef]
- Paprocka, R.; Wiese, M.; Eljaszewicz, A.; Helmin-Basa, A.; Modzelewska-Banachiewicz, B.; Gzella, A.; Michalkiewicz, J. Synthesis and anti-inflammatory activity of new 1,2,4-triazole derivatives. Bioorganic Med. Chem. Lett. 2015, 25, 2664–2667. [Google Scholar] [CrossRef] [PubMed]
- Plech, T.; Kaproń, B.; Paneth, A.; Kosikowska, U.; Malm, A.; Strzelczyk, A.; Stączek, P.; Świątek, Ł.; Rajtar, B.; Polz-Dacewicz, M. Determination of the Primary Molecular Target of 1,2,4-Triazole-Ciprofloxacin Hybrids. Molecules 2015, 20, 6254–6272. [Google Scholar] [CrossRef] [PubMed]
- Barbuceanu, S.-F.; Ilies, D.C.; Saramet, G.; Uivarosi, V.; Draghici, C.; Radulescu, V. Synthesis and Antioxidant Activity Evaluation of New Compounds from Hydrazinecarbothioamide and 1,2,4-Triazole Class Containing Diarylsulfone and 2,4-Difluorophenyl Moieties. Int. J. Mol. Sci. 2014, 15, 10908–10925. [Google Scholar] [CrossRef] [PubMed]
- Al-Omair, M.A.; Sayed, A.R.; Youssef, M.M. Synthesis of Novel Triazoles, Tetrazine, Thiadiazoles and Their Biological Activities. Molecules 2015, 20, 2591–2610. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Xu, X.Y.; Tan, C.X.; Weng, J.Q.; Xin, J.H.; Chen, J. Synthesis, crystal structure, herbicidal activities and 3D-QSAR study of some novel 1,2,4-triazolo[4,3-a]pyridine derivatives. Pest Manag. Sci. 2015, 71, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Zhao, W.; Liu, X.H.; Tan, C.X.; Weng, J.Q. Synthesis, crystal structure and antifungal activity of 4-(5-((2,4-dichlorobenzyl)thio)-4-phenyl-4H-1,2,4-triazol-3-yl)pyridine. Chin. J. Struct. Chem. 2015, 34, 203–207. [Google Scholar]
- Liu, X.H.; Sun, Z.H.; Yang, M.Y.; Tan, C.X.; Weng, J.Q.; Zhang, Y.G.; Ma, Y. Microwave assistant one pot synthesis, crystal structure, antifungal activities and 3D-QSAR of novel 1,2,4-triazolo[4,3-a]pyridines. Chem. Biol. Drug Des. 2014, 84, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Sokmen, B.B.; Gumrukcuoglu, N.; Ugras, S.; Sahin, H.; Sagkal, Y.; Ugras, H.I. Synthesis, Antibacterial, Antiurease, and Antioxidant Activities of Some New 1,2,4-Triazole Schiff Base and Amine Derivatives. App. Biochem. Biotechnol. 2015, 175, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Radhika, C.; Venkatesham, A.; Sarangapani, M. Synthesis and antidepressant activity of di substituted-5-aryl-1,2,4-triazoles. Med. Chem. Res. 2012, 21, 3509–3513. [Google Scholar] [CrossRef]
- Liu, X.H.; Pan, L.; Ma, Y.; Weng, J.Q.; Tan, C.X.; Li, Y.H.; Shi, Y.X.; Li, B.J.; Li, Z.M.; Zhang, Y.G. Design, synthesis, biological activities, and 3D-QSAR of new N,N′-diacylhydrazines containing 2-(2,4-dichlorophenoxy)propane moiety. Chem. Biol. Drug Des. 2011, 78, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Polucci, P.; Magnaghi, P.; Angiolini, M.; Asa, D.; Avanzi, N.; Badari, A.; Bertrand, J.; Casale, E.; Cauteruccio, S.; Cirla, A.; et al. Alkylsulfanyl-1,2,4-Triazoles, a New Class of Allosteric Valosine Containing Protein Inhibitors. Synthesis and Structure-Activity Relationships. J. Med. Chem. 2013, 56, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Li, A.R.; Zhang, J.; Greenberg, J.; Lee, T.; Liu, J. Discovery of non-glucoside SGLT2 inhibitors. Bioorganic Med. Chem. Lett. 2011, 21, 2472–2475. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.-B.; Fu, J.-Q.; Weng, J.-Q.; Jin, J.-Z.; Tan, C.-X.; Liu, X.-H. Microwave assisted synthesis, antifungal activity and DFT theoretical study of some novel 1,2,4-triazole derivatives containing the 1,2,3-thiadiazole moiety. Molecules 2013, 18, 12725–12739. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.-X.; Yang, M.-Y.; Shi, Y.-X.; Sun, Z.-H.; Liu, X.-H.; Wu, H.-K.; Li, B.-J.; Zhang, Y.-G. Microwave assistant synthesis, antifungal activity and DFT theoretical study of some novel 1,2,4-triazole derivatives containing pyridine moiety. Int. J. Mol. Sci. 2014, 15, 8075–8090. [Google Scholar] [CrossRef] [PubMed]
- Kochikyan, T.V.; Samvelyan, M.A.; Arutyunyan, V.S.; Avetisyan, A.A.; Tamazyan, R.A.; Aivazyan, A.G. Synthesis of 1,2,4-Triazole-3-Thiols and Their S-Substituted Derivatives. Rus. J. Org. Chem. 2010, 46, 551–555. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhai, Z.W.; Xu, X.Y.; Yang, M.Y.; Sun, Z.H.; Weng, J.Q.; Tan, C.X.; Chen, J. Facile and efficient synthesis and herbicidal activity determination of novel 1,2,4-triazolo[4,3-a]pyridin-3(2H)-one derivatives via microwave irradiation. Bioorganic Med. Chem. Lett. 2015, 25, 5524–5528. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.A.; Harakeh, S.; Sakkaf, K.A.; Denetiu, I. Progress in microwave-aided chemical synthesis. Aus. J. Chem. 2012, 65, 1647–1654. [Google Scholar] [CrossRef]
- Mu, J.-X.; Yang, M.-Y.; Sun, Z.-H.; Tan, C.-X.; Weng, J.-Q.; Wu, H.-K.; Liu, X.-H. Synthesis, Crystal Structure and DFT Studies of 8-chloro-3-((3-chlorobenzyl)thio)-[1,2,4]triazolo[4,3-a]pyridine. Crystals 2015, 5, 491–500. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Varma, R.S. Greener and Sustainable Approaches to the Synthesis of Pharmaceutically Active Heterocycles. Curr. Opin. Drug Discov. Dev. 2007, 10, 723–737. [Google Scholar]
- Polshettiwar, V.; Varma, R.S. Greener and Expeditious Synthesis of Bio-Active Heterocycles using Microwave Irradiation. Pure Appl. Chem. 2008, 80, 777–790. [Google Scholar] [CrossRef]
- Zhai, Z.W.; Yang, M.Y.; Sun, Z.H.; Liu, X.H.; Weng, J.Q.; Tan, C.X. Facile and efficient synthesis of novel 1,2,3-thiadiazole derivatives using microwave irradiation. J. Chem. Res. 2015, 39, 340–342. [Google Scholar] [CrossRef]
- Yang, M.Y.; Zhao, W.; Sun, Z.H.; Tan, C.X.; Weng, J.Q.; Liu, X.H. Synthesis and Biological Activity of Acylthiourea Derivatives Contain 1,2,3-Thiadiazole and 1,3,4-Thiadiazole. Lett. Drug Des. Discov. 2015, 12, 314–318. [Google Scholar] [CrossRef]
- Tan, C.X.; Weng, J.Q.; Liu, Z.X.; Liu, X.H.; Zhao, W.G. Synthesis, crystal structure and fungicidal activity of a novel 1,2,3-thiadiazole compound. Phosphorus Sulfur Silicon Relat. Elem. 2012, 187, 990–996. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, L.-J.; Shi, Y.-X.; Wu, H.-K.; Sun, Z.-H.; Liu, X.-H.; Li, B.-J.; Zhang, Y.-G. Microwave-Assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-Triazole Moiety. Appl. Sci. 2015, 5, 1211-1220. https://doi.org/10.3390/app5041211
Min L-J, Shi Y-X, Wu H-K, Sun Z-H, Liu X-H, Li B-J, Zhang Y-G. Microwave-Assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-Triazole Moiety. Applied Sciences. 2015; 5(4):1211-1220. https://doi.org/10.3390/app5041211
Chicago/Turabian StyleMin, Li-Jing, Yan-Xia Shi, Hong-Ke Wu, Zhao-Hui Sun, Xing-Hai Liu, Bao-Ju Li, and Yong-Gang Zhang. 2015. "Microwave-Assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-Triazole Moiety" Applied Sciences 5, no. 4: 1211-1220. https://doi.org/10.3390/app5041211
APA StyleMin, L.-J., Shi, Y.-X., Wu, H.-K., Sun, Z.-H., Liu, X.-H., Li, B.-J., & Zhang, Y.-G. (2015). Microwave-Assisted Synthesis and Antifungal Activity of Some Novel Thioethers Containing 1,2,4-Triazole Moiety. Applied Sciences, 5(4), 1211-1220. https://doi.org/10.3390/app5041211





