The Effect of Trifluoroethanol and Glycerol on the Thermal Properties of Collagen Using Optical Displacement-Enhanced Heterodyne Polarimeter
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Setup
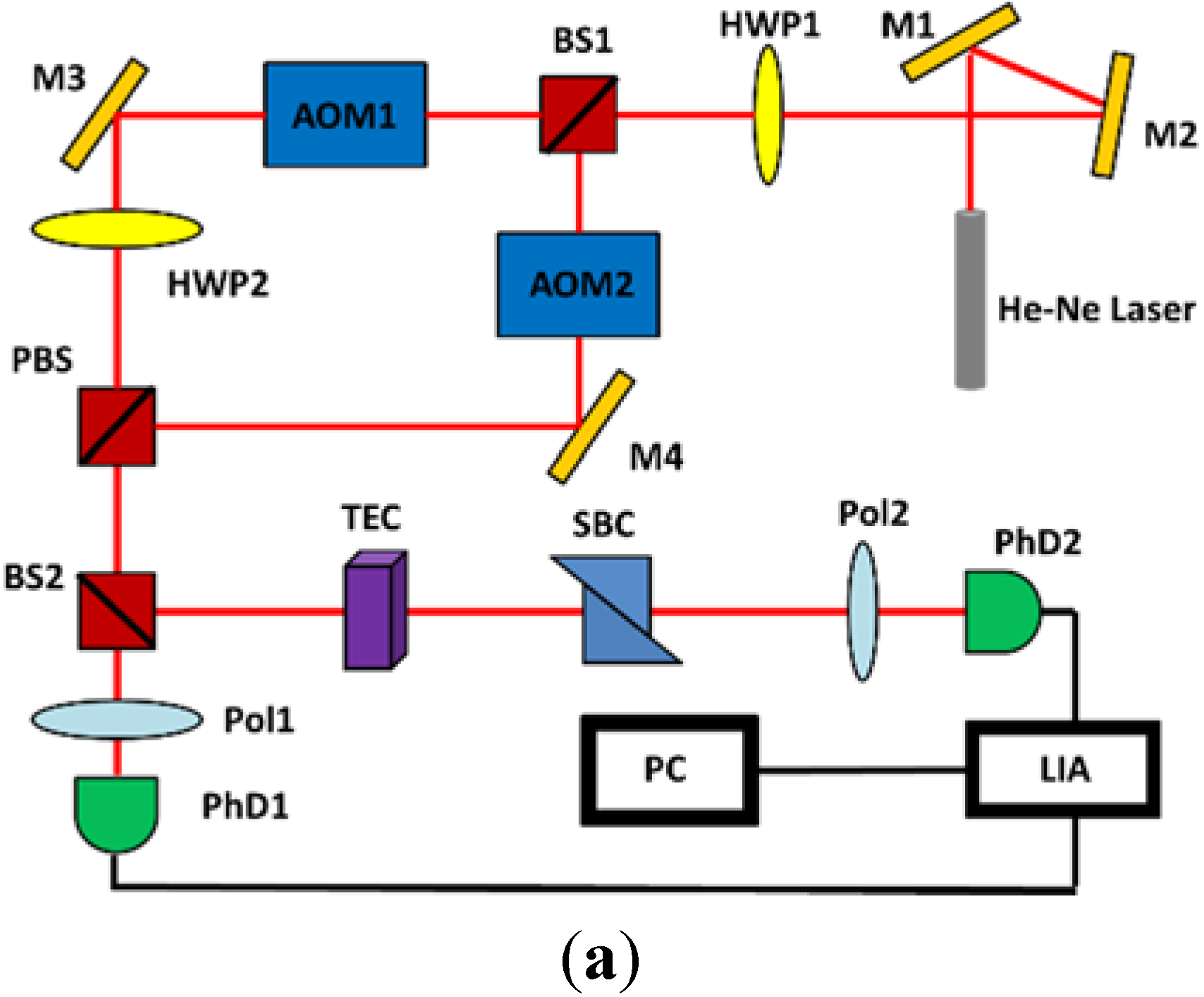
2.2. Sample Preparation and Measurement of Thermal Denaturation
3. Results and Discussion
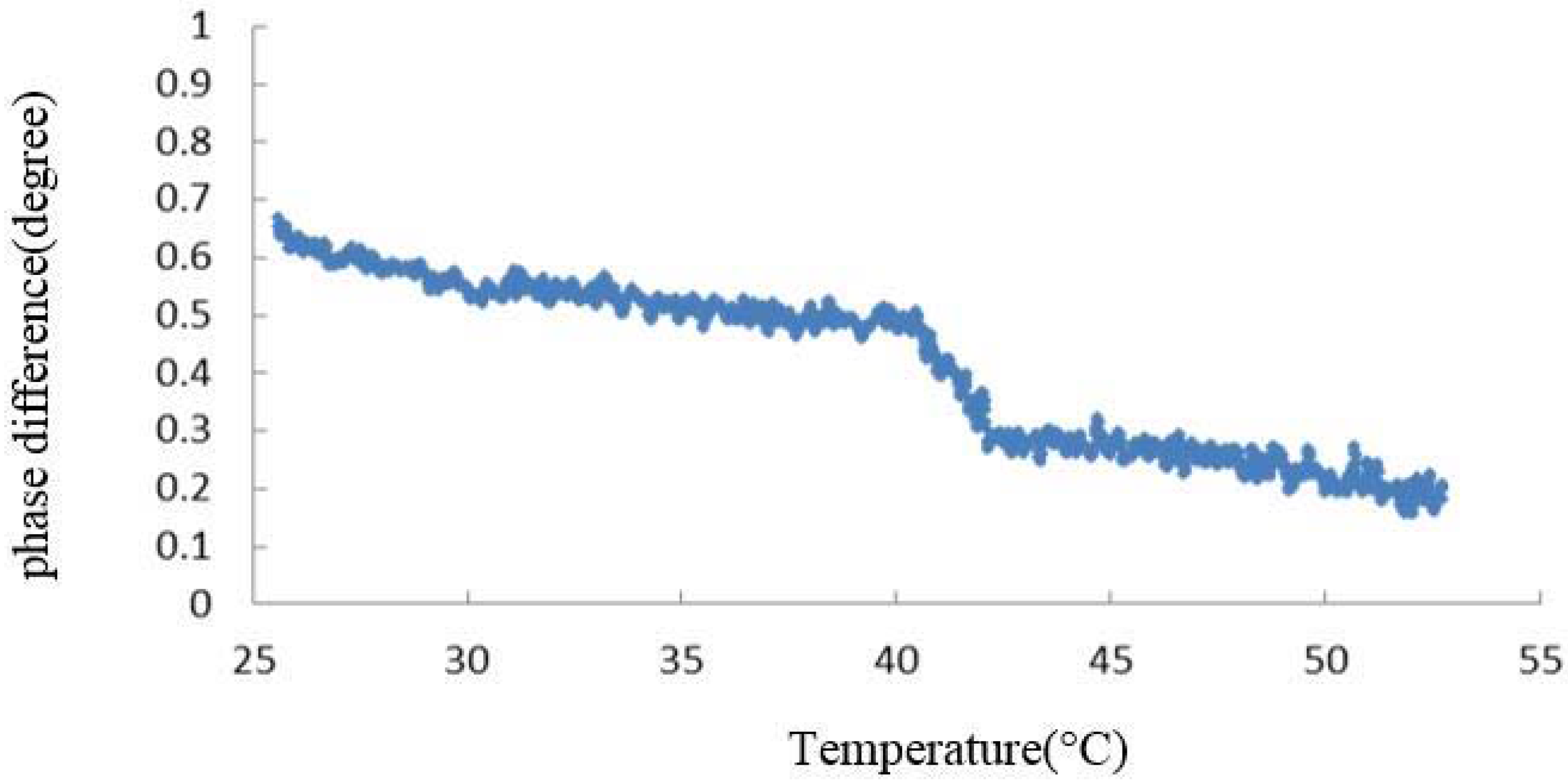
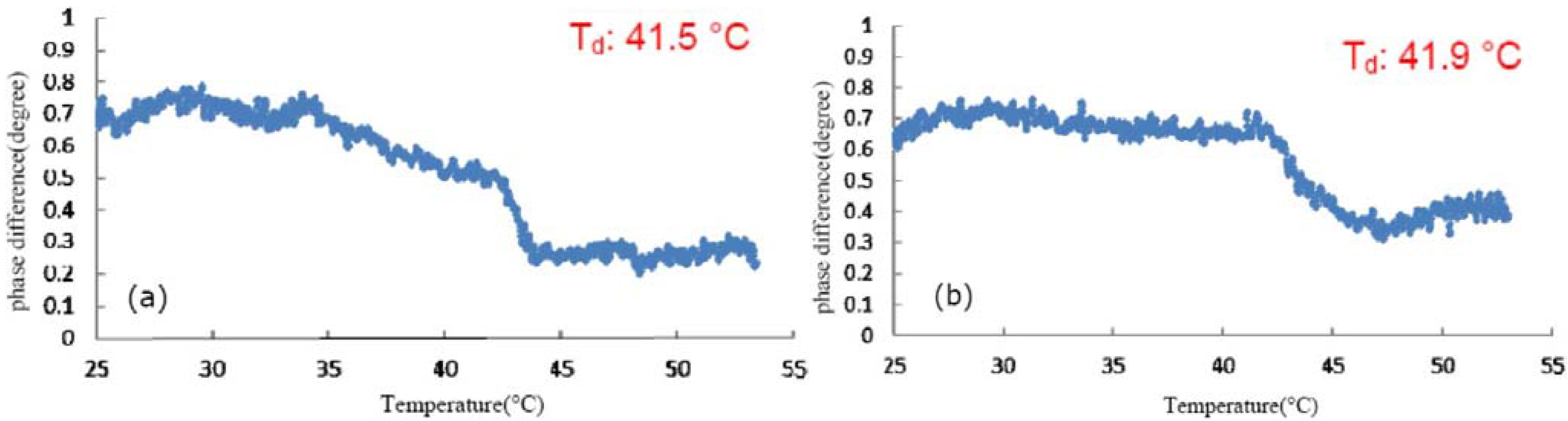
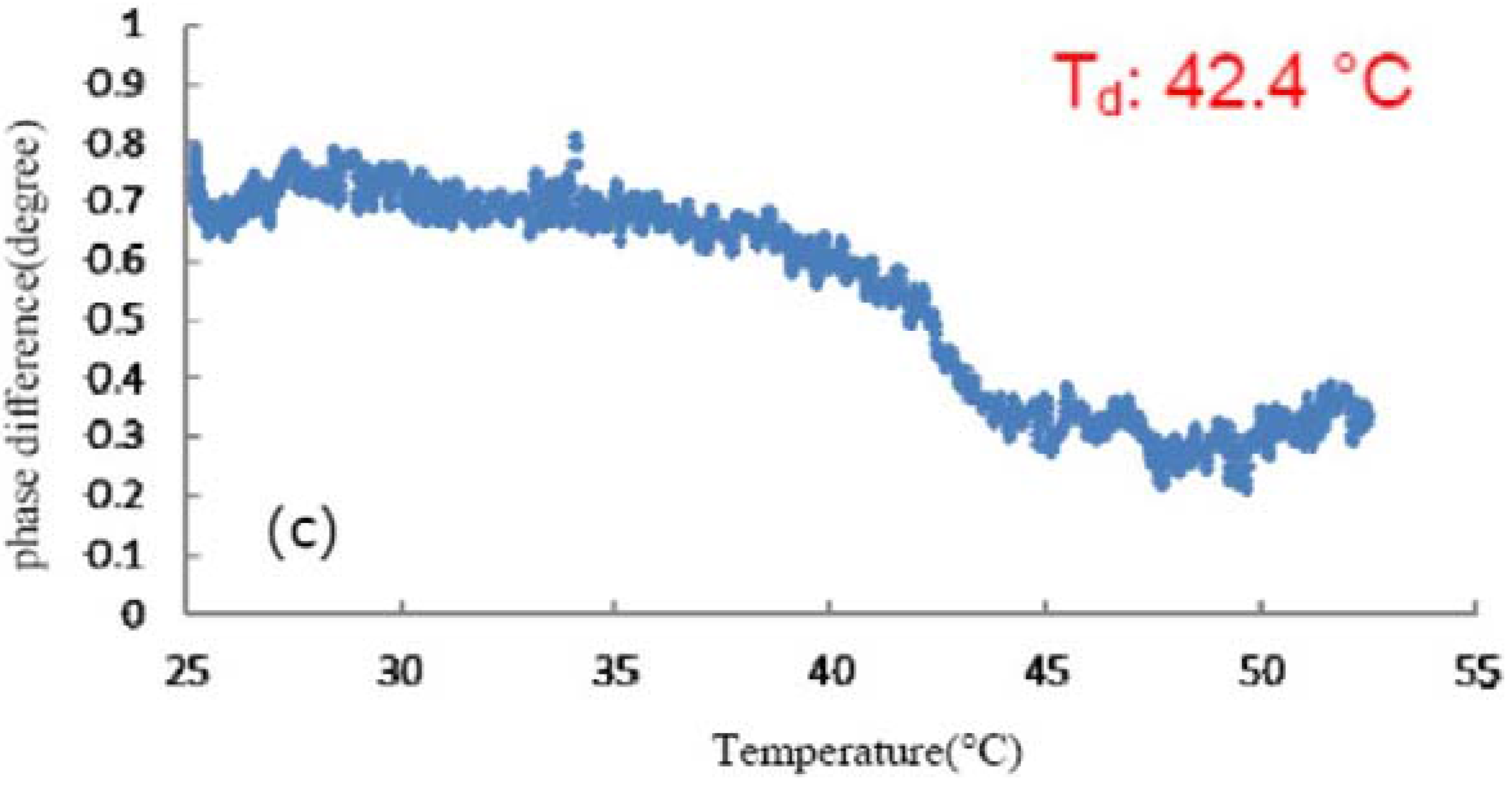
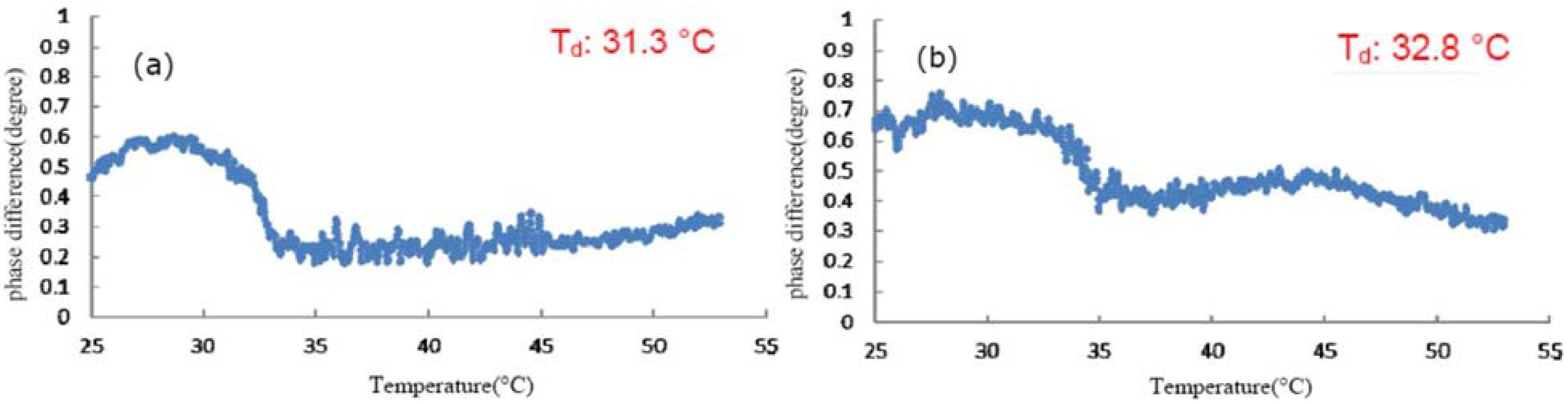
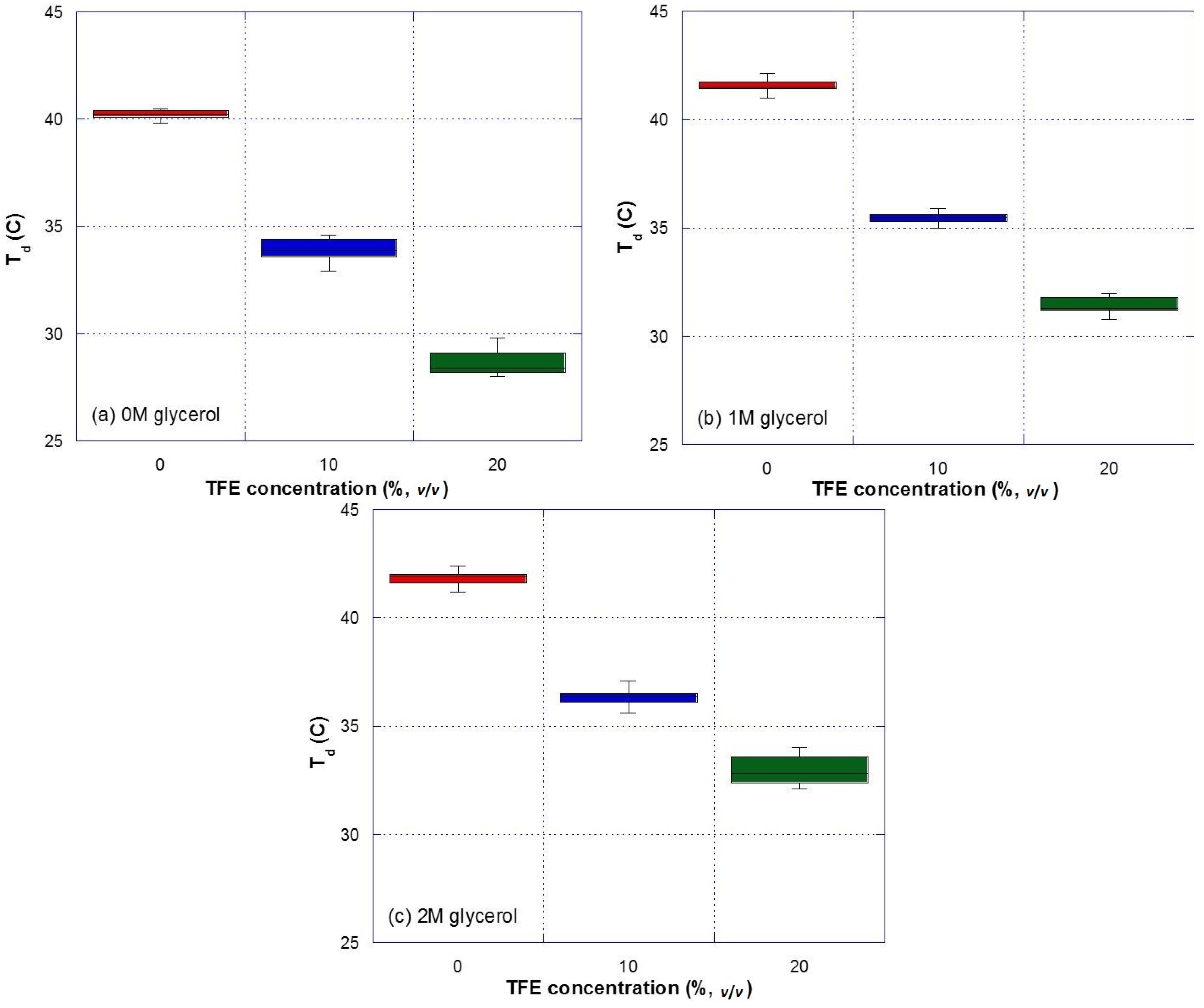
3.1. Effect of TFE on the Thermal Denaturation of Collagen
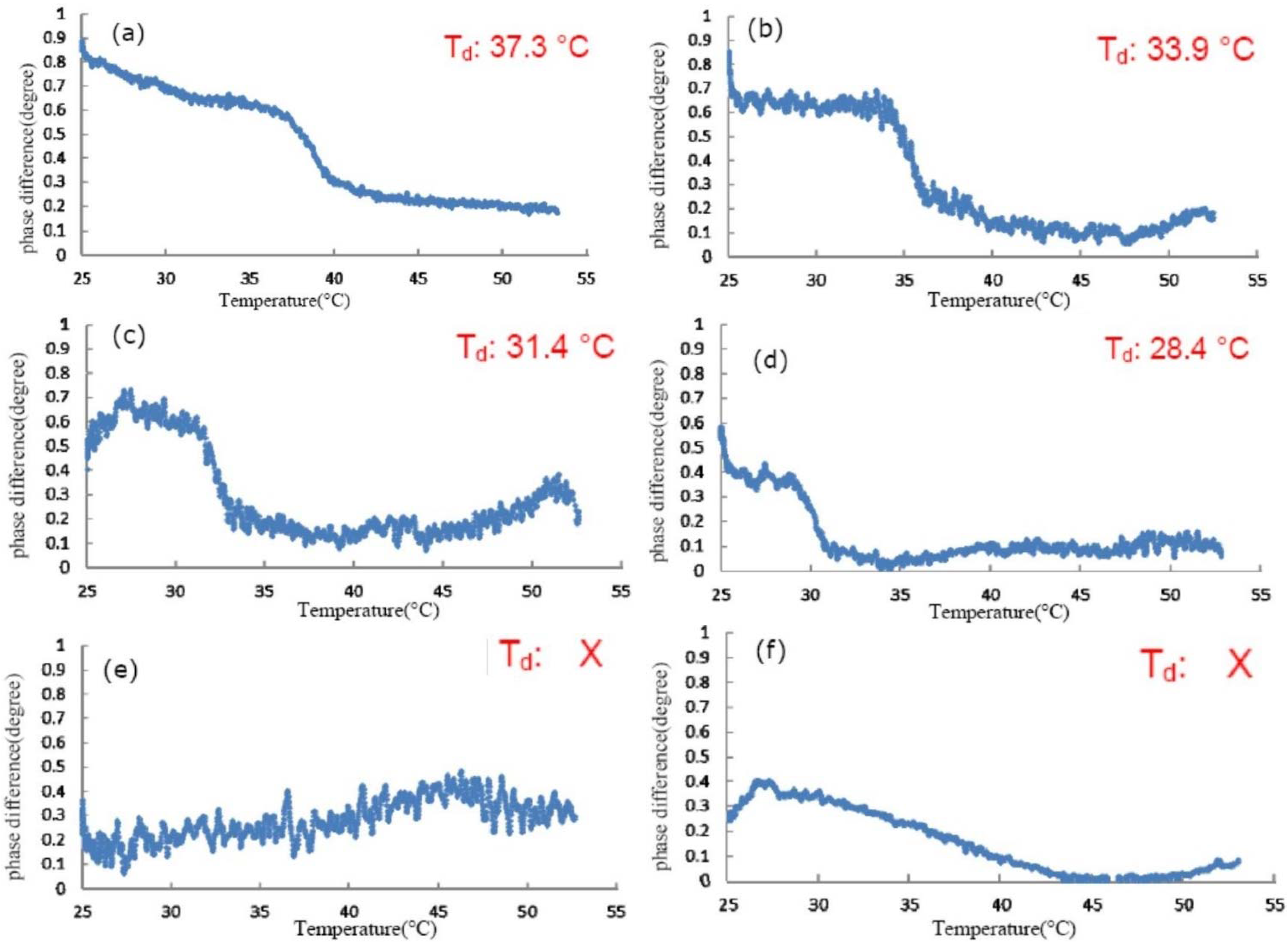
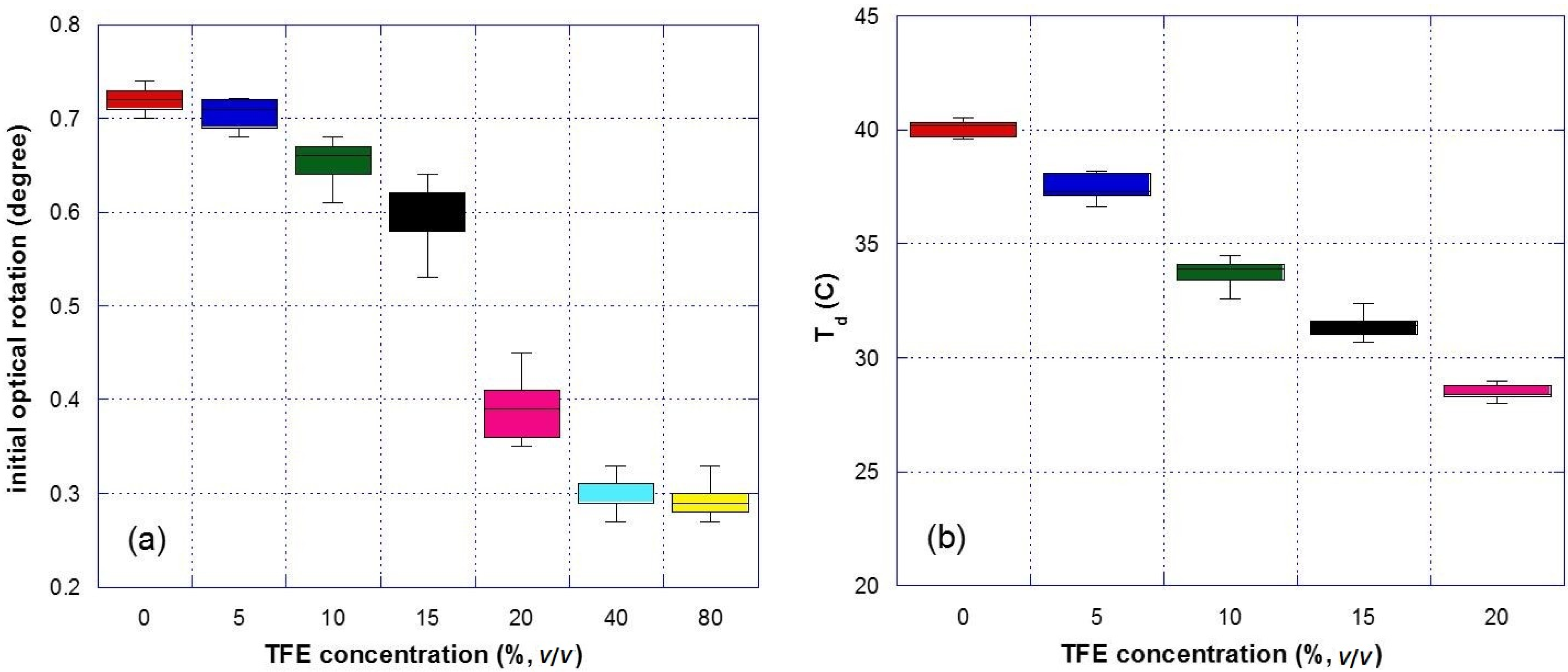
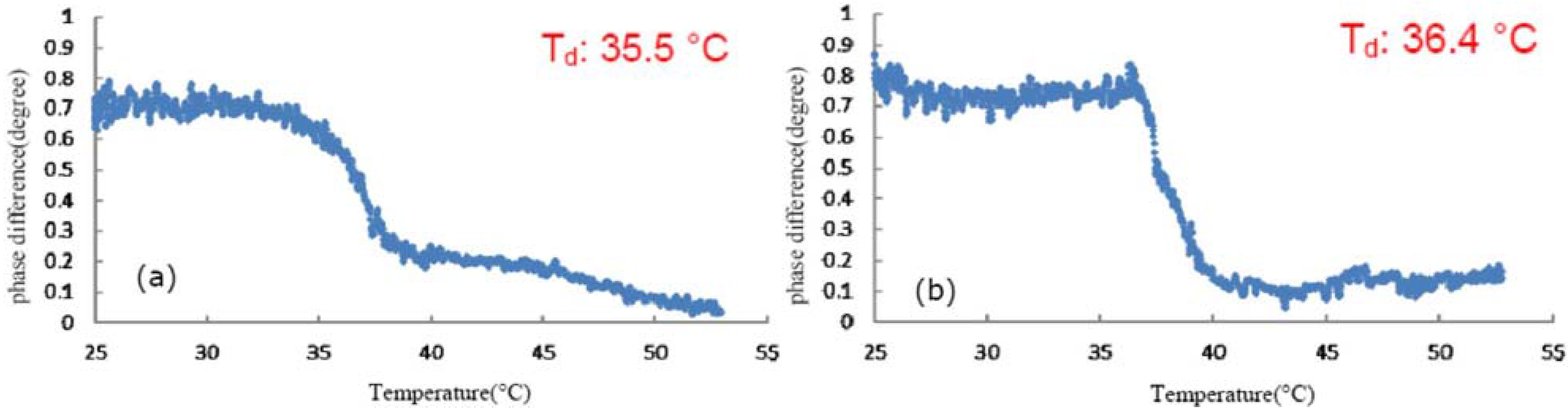
3.2. Effect of Glycerol on the Thermal Denaturation of Collagen and TFE-Mixed Collagen
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhong, S.; Teo, W.E.; Zhu, X.; Beuerman, R.; Ramakrishna, S.; Yung, L.Y.L. Formation of collagen-glycosaminoglycan blended nanofibrous scaffolds and their biological properties. Biomacromolecules 2005, 6, 2998–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.P.; Teo, W.E.; Zhu, X.; Beuerman, R.; Ramakrishna, S.; Yung, L.Y.L. Development of a novel collagen-GAG nanobibrous scaffold via electrospinning. Mater. Sci. Eng. C 2007, 27, 262–266. [Google Scholar] [CrossRef]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.Y.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres-Just an expansive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, G.; Reddy, S.M.M.; Natarajan, V.; Madhan, B. 2,2,2-Trifluoroethanol disrupts the triple helical structure and self-association of type I collagen. Int. J. Biol. Macromol. 2013, 54, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Yeh, A.T.; Choi, B.; Nelson, J.S.; Tromberg, B.J. Reversible dissociation of collagen in tissues. J. Invest. Dermatol. 2003, 121, 1332–1335. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.R.; Russell, A.E.; Hart, G.J. The effects of glycols on the renaturation of soluble collagen. Biochem. J. 1971, 125, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Penkova, R.; Goshev, I.; Gorinstein, S.; Nedkov, P. Stabilizing effect of glycerol on collagen type I isolated from different species. Food Chem. 1999, 66, 483–487. [Google Scholar] [CrossRef]
- Miles, C.A.; Avery, N.C.; Rodin, V.V.; Bailey, A.J. The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J. Mol. Biol. 2005, 346, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Thermal denaturation of UV-irradiated wet rat tail tendon collagen. Int. J. Biol. Macromol. 2005, 35, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Na, G.C. Interaction of calf skin collagen with glycerol: linked function analysis. Biochemistry 1986, 25, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Shoulders, M.D.; Rains, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C. Noninvasive blood glucose monitoring. Diabetes Care 1997, 20, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.M.; Tsai, Y.C. Angular displacement-enhanced heterodyne polarimeter for the measurement of optically active media. Sens. Actuators B 2006, 120, 324–328. [Google Scholar] [CrossRef]
- Cote, G.L.; Fox, M.D.; Northrop, R.B. Noninvasive optical polarimetric glucose sensing using a true phase measurement technique. IEEE Trans. Biomed. Eng. 1992, 39, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.; Huang, Y.C.; Chang, M. Precise optical activity measurement of quartz plate by using a true phase-sensitive technique. Appl. Opt. 1997, 36, 3604–3609. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.M.; Huang, Y.C.; Chang, J.G.; Chang, M.; Chou, C. A true sensitive optical heterodyne polarimeter for glucose concentration measurement. Opt. Commun. 1997, 141, 314–321. [Google Scholar] [CrossRef]
- Williams, B.R.; Gelman, R.A.; Poppke, D.C.; Piez, K.A. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J. Biol. Chem. 1978, 253, 6578–6585. [Google Scholar] [PubMed]
- Rajan, R.; Balaram, P. A model for the interaction of trifluoroethanol with peptides and proteins. Int. J. Peptide Protein Res. 1996, 48, 328–336. [Google Scholar] [CrossRef]
- Bodkin, M.J.; Goodfellow, J.M. Hydrophobic salvation in aqueous trifluoroethanol solution. Biopolymers 1996, 39, 43–50. [Google Scholar] [CrossRef]
- Kotch, F.W.; Raines, R.T. Self-assembly of synthetic collagen triple helices. Proc. Natl. Acad. Sci. USA 2006, 103, 3028–3033. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-M.; Chen, H.-H.; Tseng, K.-H.; Chen, H.-W. The Effect of Trifluoroethanol and Glycerol on the Thermal Properties of Collagen Using Optical Displacement-Enhanced Heterodyne Polarimeter. Appl. Sci. 2015, 5, 1184-1195. https://doi.org/10.3390/app5041184
Wu C-M, Chen H-H, Tseng K-H, Chen H-W. The Effect of Trifluoroethanol and Glycerol on the Thermal Properties of Collagen Using Optical Displacement-Enhanced Heterodyne Polarimeter. Applied Sciences. 2015; 5(4):1184-1195. https://doi.org/10.3390/app5041184
Chicago/Turabian StyleWu, Chien-Ming, Horn-Haw Chen, Kai-Han Tseng, and Hung-Wei Chen. 2015. "The Effect of Trifluoroethanol and Glycerol on the Thermal Properties of Collagen Using Optical Displacement-Enhanced Heterodyne Polarimeter" Applied Sciences 5, no. 4: 1184-1195. https://doi.org/10.3390/app5041184
APA StyleWu, C.-M., Chen, H.-H., Tseng, K.-H., & Chen, H.-W. (2015). The Effect of Trifluoroethanol and Glycerol on the Thermal Properties of Collagen Using Optical Displacement-Enhanced Heterodyne Polarimeter. Applied Sciences, 5(4), 1184-1195. https://doi.org/10.3390/app5041184




