The Preparation and Application of Dendrimer Modified CdTe/CdS Near Infrared Quantum Dots for Brain Cancer Cells Imaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CdTe/CdS Core/Shell QDs
2.3. Structural and Optical Characterizations
2.4. Cell Viability and Proliferation Assays
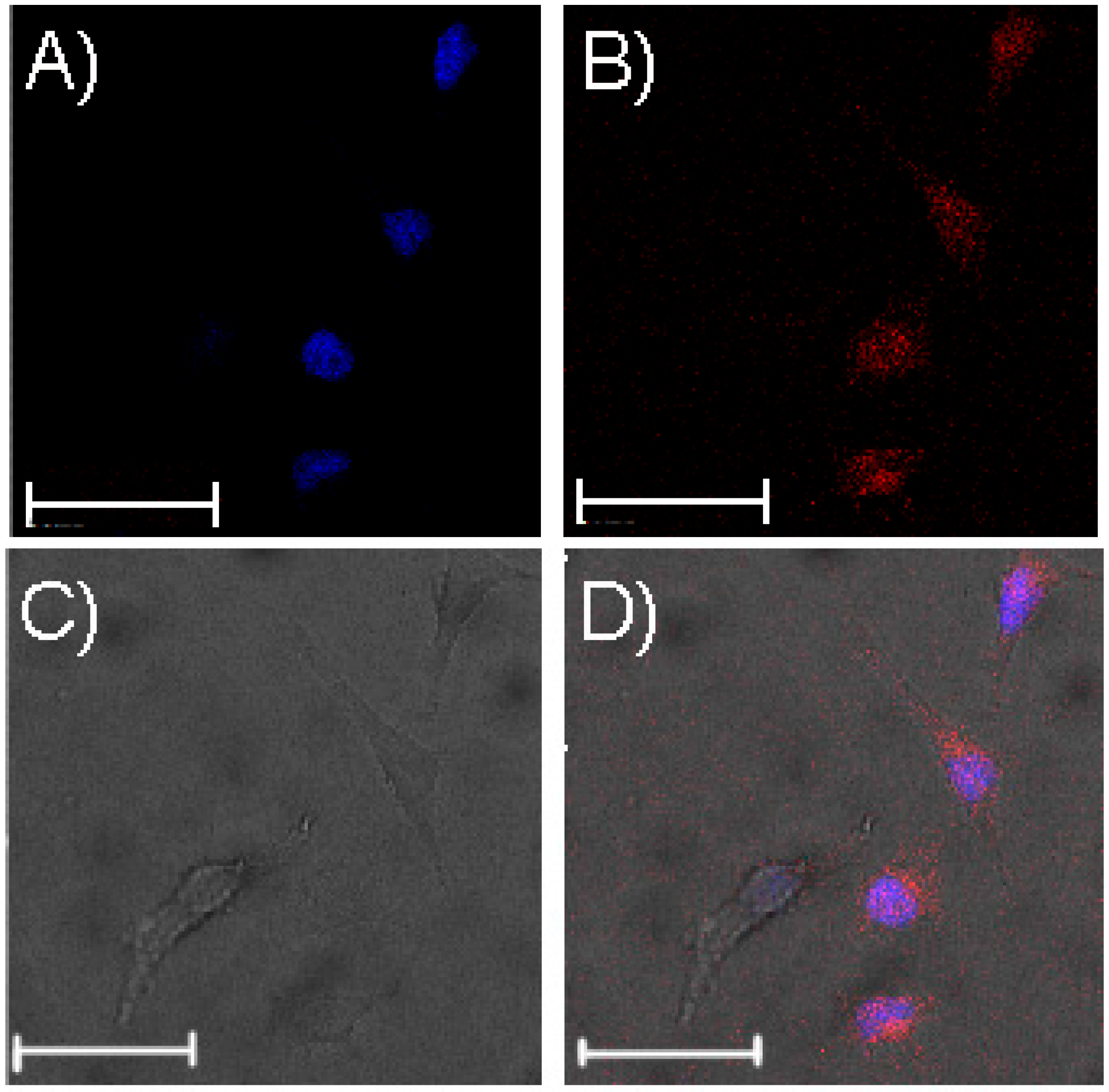
2.5. Cell Imaging
3. Results and Discussion
3.1. Characterization of Dendrimer Modified CdTe/CdS QDs
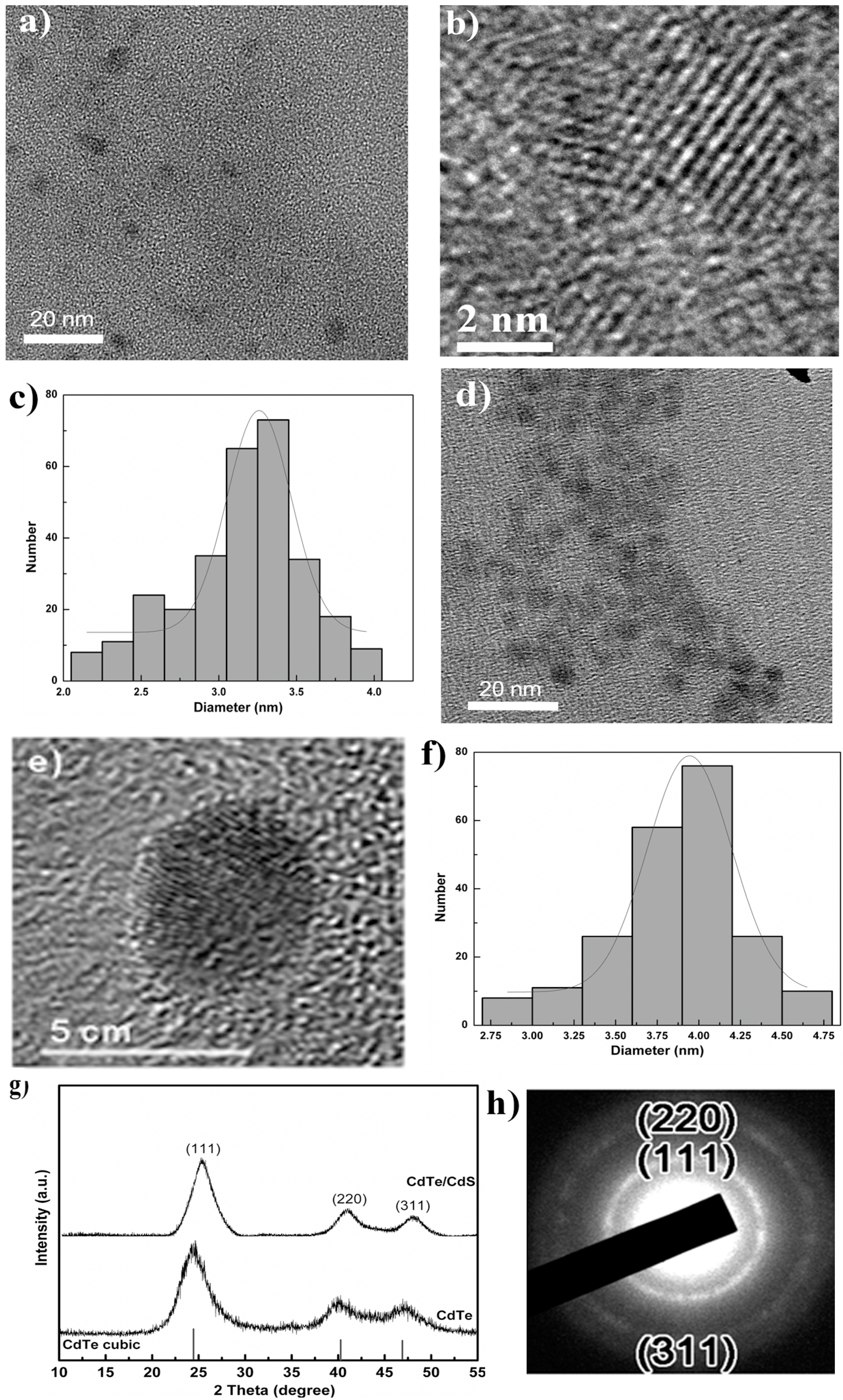
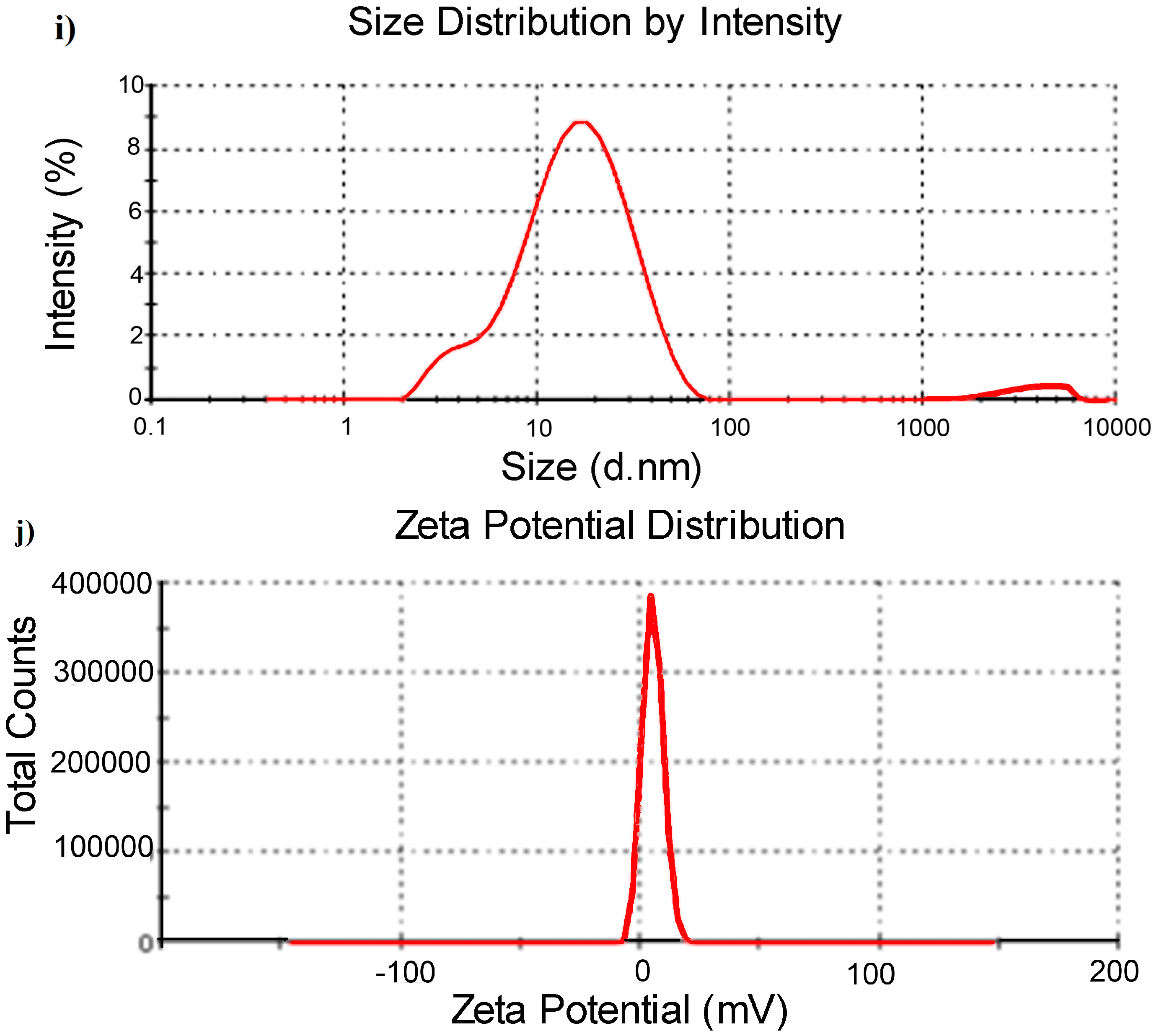
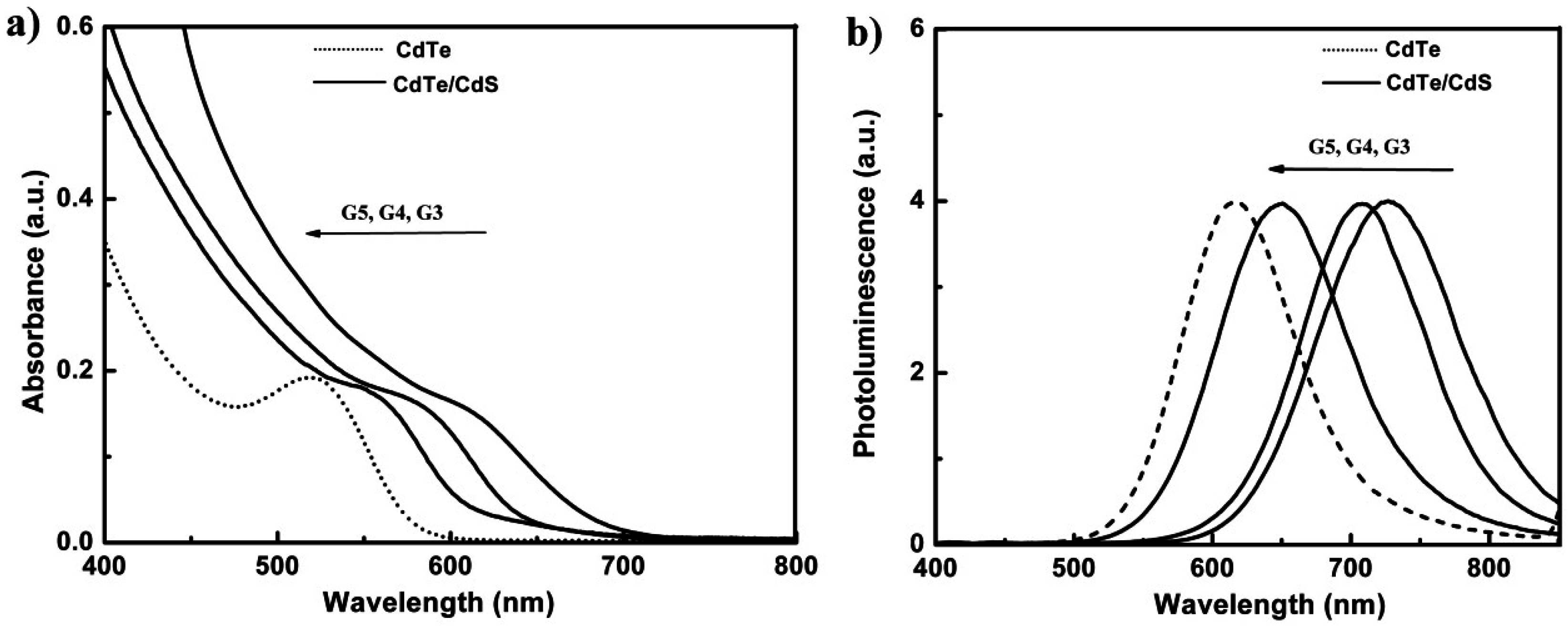
3.2. The Stability of Dendrimer Modified CdTe/CdS QDs
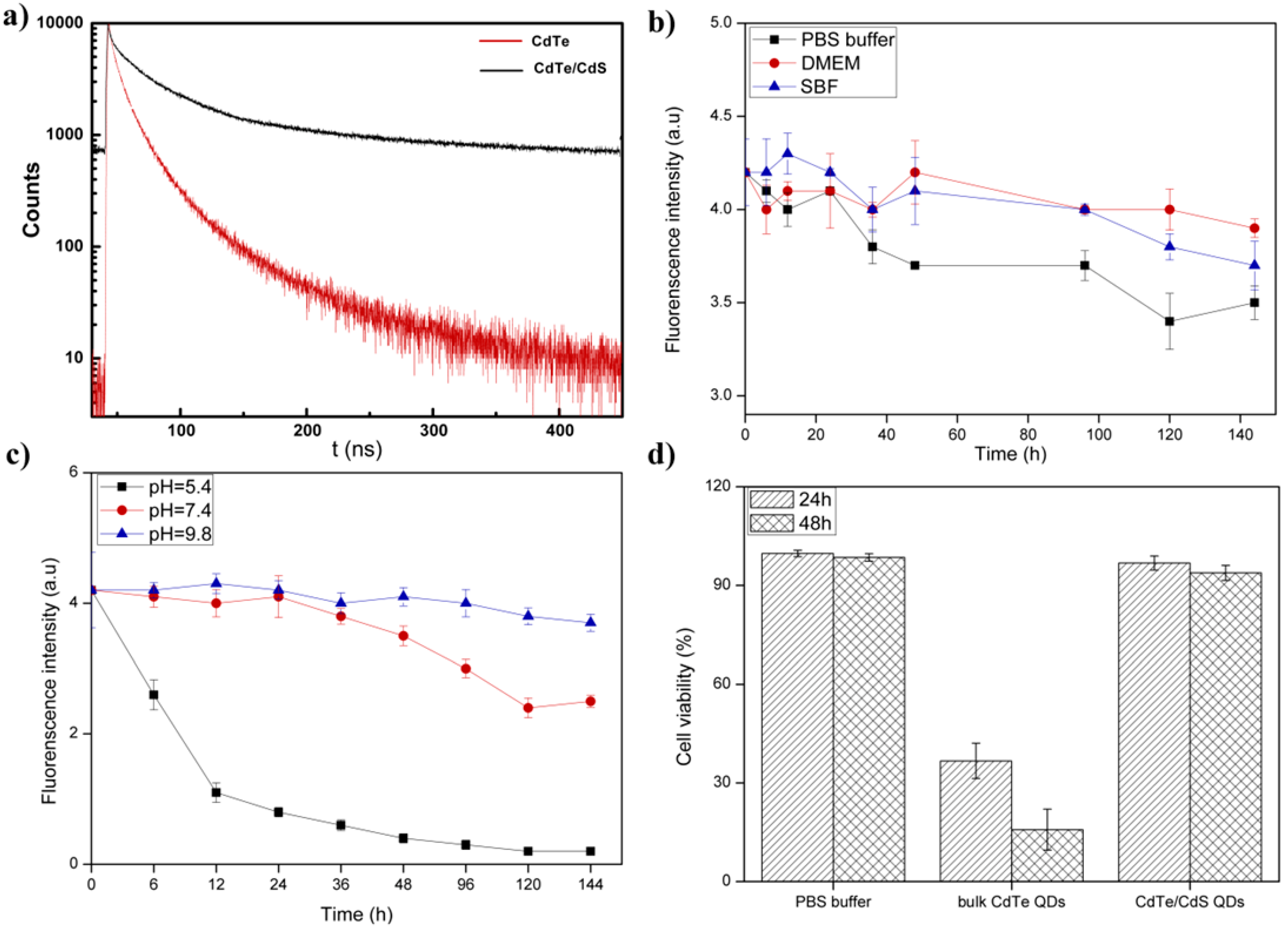
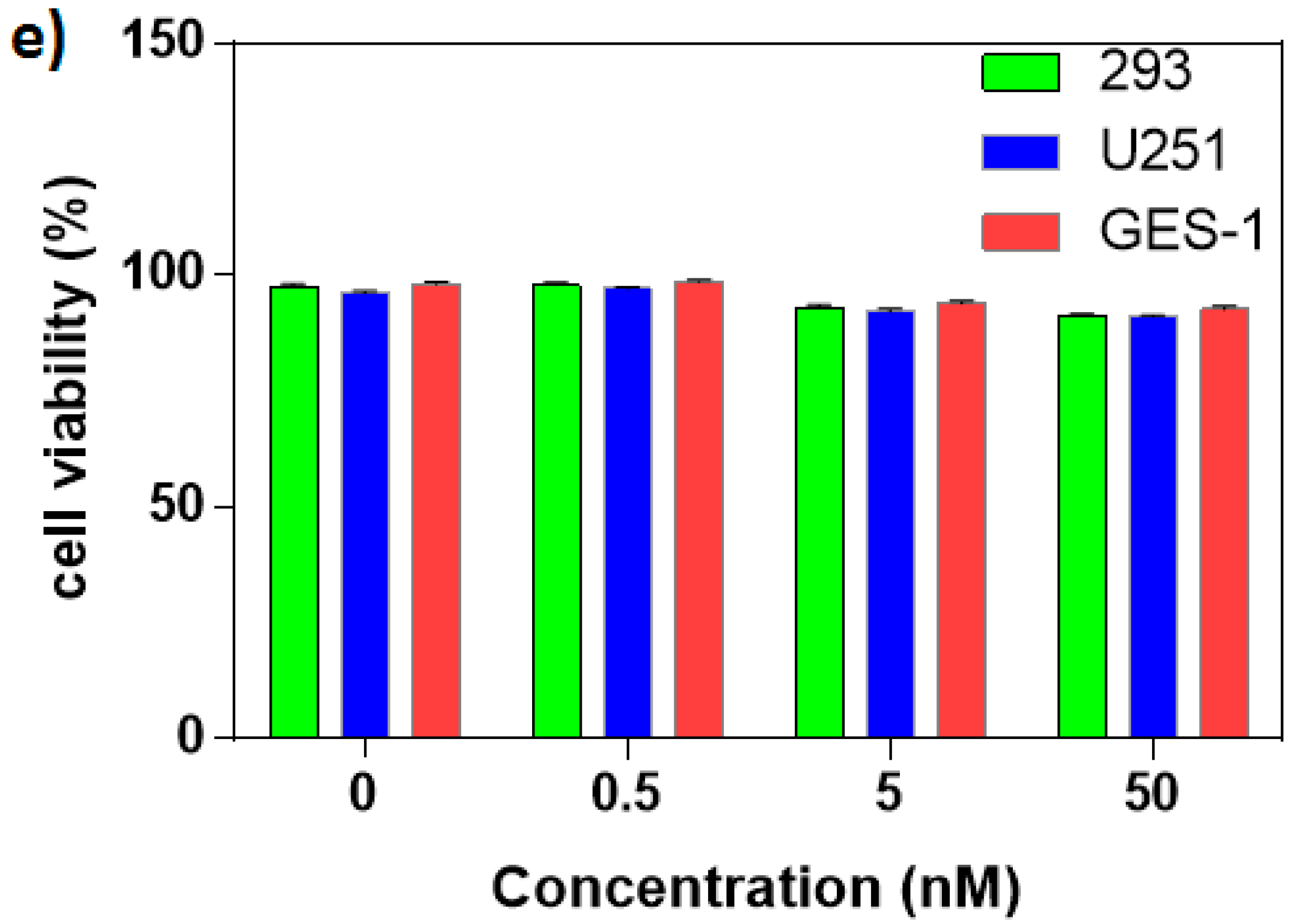
3.3. Cell Imaging
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Young, G.S.; Stauss, J.; Mukundan, S. Advanced Imaging of Adult Brain Tumors with MRI and PET. In Neuro-Oncology: Blue Books of Neurology Series; Saunders: Philadelphia, PA, USA, 2010; pp. 71–78. [Google Scholar]
- Hoshi, Y. Functional Near-Infrared Optical Imaging: Utility and Limitations in Human Brain Mapping. Psychophysiology 2003, 40, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.F.; Mahmood, U.; King, R.S.; Weissleder, R.; Josephson, L. A Multimodal Nanoparticle for Preoperative Magnetic Resonance Imaging and Intraoperative Optical Brain Tumor Delineation. Cancer Res. 2003, 63, 8122–8125. [Google Scholar] [PubMed]
- Koo, Y.-E.L.; Reddy, G.R.; Bhojani, M.; Schneider, R.; Philbert, M.A.; Rehemtulla, A.; Ross, B.D.; Kopelman, R. Brain cancer diagnosis and therapy with nanoplatforms. Adv. Drug Deliv. Rev. 2006, 58, 1556–1577. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; He, Z.; Chan, W.; Choi, M.M. Synthesis and characterization of high-quality water-soluble near-infrared-emitting CdTe/CdS quantum dots capped by N-acetyl-l-cysteine via hydrothermal method. J. Phys. Chem. C 2009, 113, 1293–1300. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Xu, J.; Ji, J.; Zhang, J.; Hu, Y.; Gu, Y. Non-invasive near infrared fluorescence imaging of CdHgTe quantum dots in mouse model. J. Fluores. 2008, 18, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Guo, J.; Yang, W.; Wang, C.; He, J.; Chen, J. Synthesis of high-quality near-infrared-emitting CdTeS alloyed quantum dots via the hydrothermal method. Nanotechnology 2007, 18, 485–611. [Google Scholar] [CrossRef]
- Liang, G.-X.; Gu, M.-M.; Zhang, J.-R.; Zhu, J.-J. Preparation and bioapplication of high-quality, water-soluble, biocompatible, and near-infrared-emitting CdSeTe alloyed quantum dots. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Cong, R.-M.; Luo, Y.-J.; Li, G.-P.; Tan, H.-M. In-situ Synthesis of CdS-ZnS Core-shell Structure Quantum Dots Inside PAMAM Dendrimer Templates. Chem. J. Chin. Univ. 2006, 27, 793–796. [Google Scholar]
- Yamamoto, D.; Koshiyama, T.; Watanabe, S.; Miyahara, M.T. Synthesis and photoluminescence characterization of dendrimer-encapsulated CdS quantum dots. Colloids Surf. A Physicochem. Eng. Asp. 2012, 411, 12–17. [Google Scholar] [CrossRef]
- Zhiming, L.; Peng, H.; Rong, H.; Xiaomin, Z.; Chenchen, B.; Qiushi, R.; Daxiang, C. Aptamer-conjugated dendrimer-modified quantum dots for glioblastoma cells imaging. J. Phys. Conf. Ser. 2009, 188, 012032. [Google Scholar]
- Yu, K.; Zaman, B.; Romanova, S.; Wang, D.-S.; Ripmeester, J.A. Sequential synthesis of type II colloidal CdTe/CdSe core-shell nanocrystals. Small 2005, 1, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-Y.; Wang, S.-R.; Yang, C.-H. Synthesis and characterization of CdTe/CdS and CdTe/CdSe core/shell type-II quantum dots in a noncoordinating solvent. Nanotechnology 2007, 18, 345602–345606. [Google Scholar] [CrossRef]
- Campos, B.B.; Algarra, M.; Alonso, B.; Casado, C.M.; Esteves da Silva, J.C.G. Mercury(ii) sensing based on the quenching of fluorescence of CdS-dendrimer nanocomposites. Analyst 2009, 134, 2447–2452. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, Q.; Zhao, Z.; Sui, H.; Chen, J.; Xie, X.; Wen, F. The Preparation and Application of Dendrimer Modified CdTe/CdS Near Infrared Quantum Dots for Brain Cancer Cells Imaging. Appl. Sci. 2015, 5, 1076-1085. https://doi.org/10.3390/app5041076
Bai Q, Zhao Z, Sui H, Chen J, Xie X, Wen F. The Preparation and Application of Dendrimer Modified CdTe/CdS Near Infrared Quantum Dots for Brain Cancer Cells Imaging. Applied Sciences. 2015; 5(4):1076-1085. https://doi.org/10.3390/app5041076
Chicago/Turabian StyleBai, Qingke, Zhenguo Zhao, Haijing Sui, Juan Chen, Xiuhai Xie, and Feng Wen. 2015. "The Preparation and Application of Dendrimer Modified CdTe/CdS Near Infrared Quantum Dots for Brain Cancer Cells Imaging" Applied Sciences 5, no. 4: 1076-1085. https://doi.org/10.3390/app5041076
APA StyleBai, Q., Zhao, Z., Sui, H., Chen, J., Xie, X., & Wen, F. (2015). The Preparation and Application of Dendrimer Modified CdTe/CdS Near Infrared Quantum Dots for Brain Cancer Cells Imaging. Applied Sciences, 5(4), 1076-1085. https://doi.org/10.3390/app5041076




