In operando Detection of Three-Way Catalyst Aging by a Microwave-Based Method: Initial Studies
Abstract
:1. Introduction
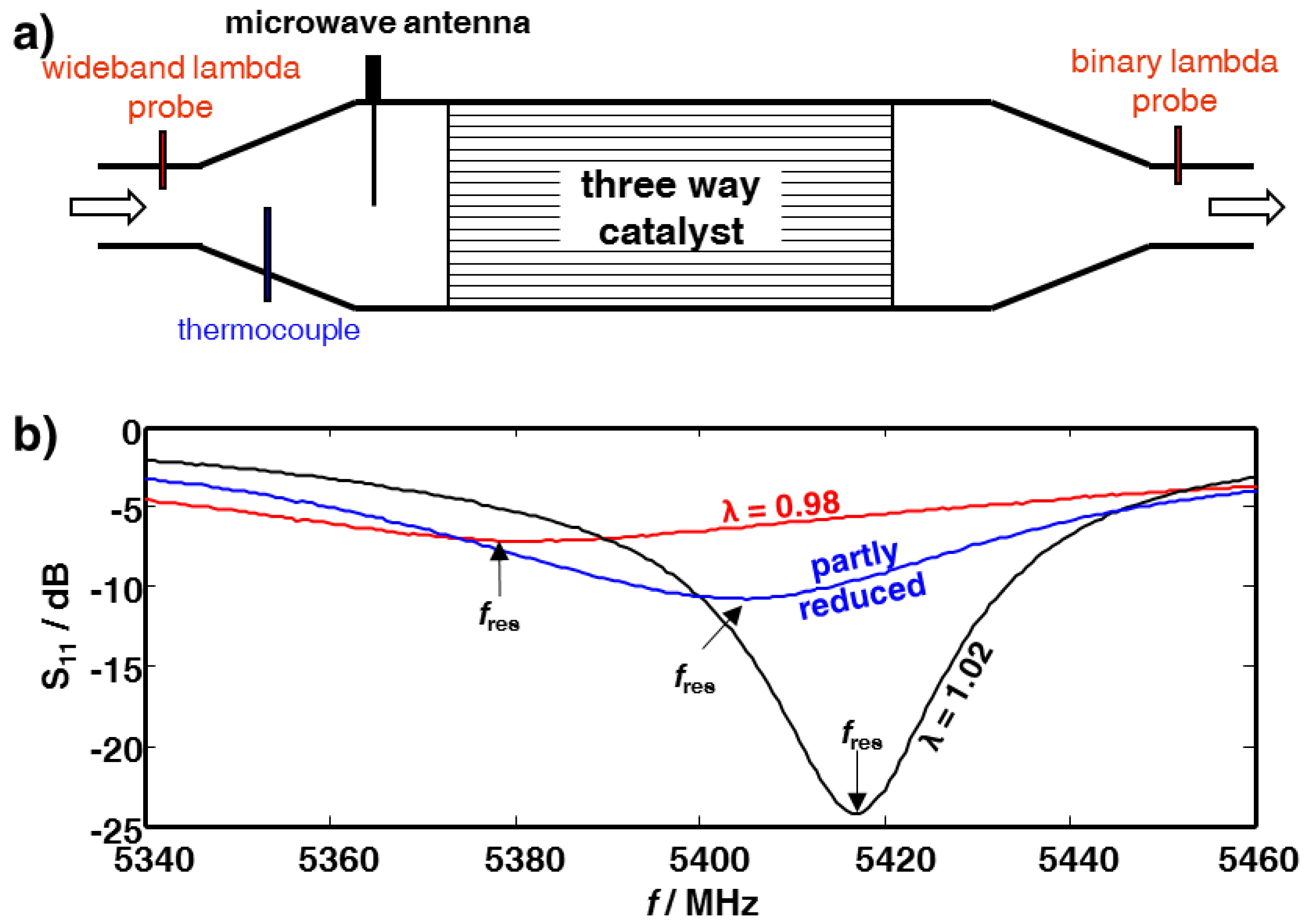
2. Experimental Section
3. Results and Discussion
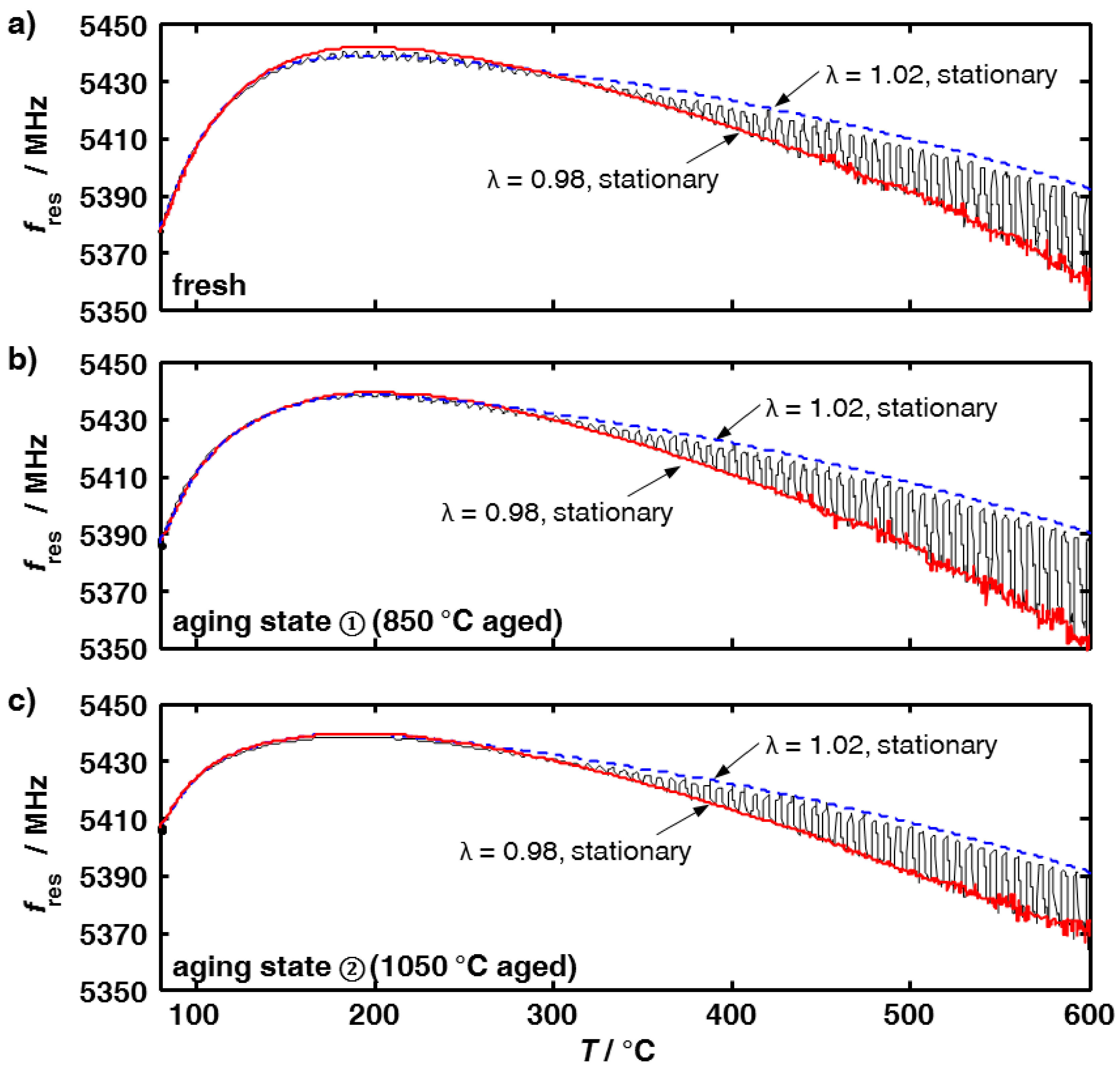
3.1. Aging Dependency of the Resonance Frequencies of Reduced Ceria
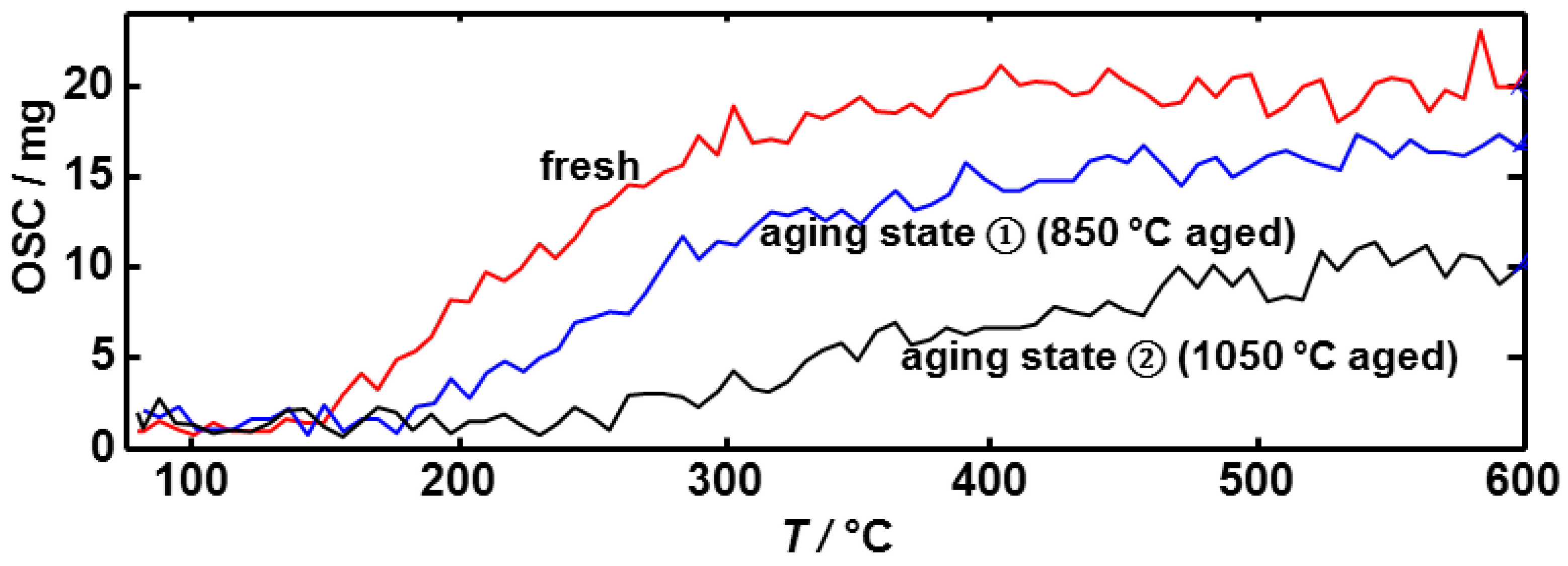
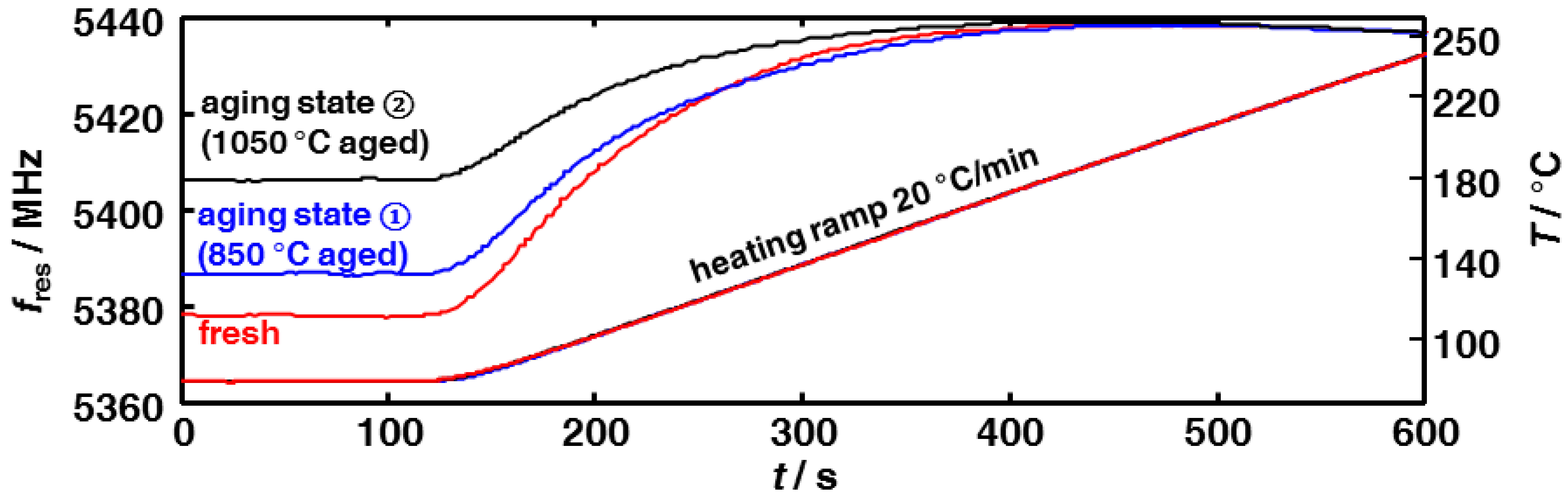
3.2. Microwave-Based Detection of Aging State Dependent Water Adsorption at Low Temperatures
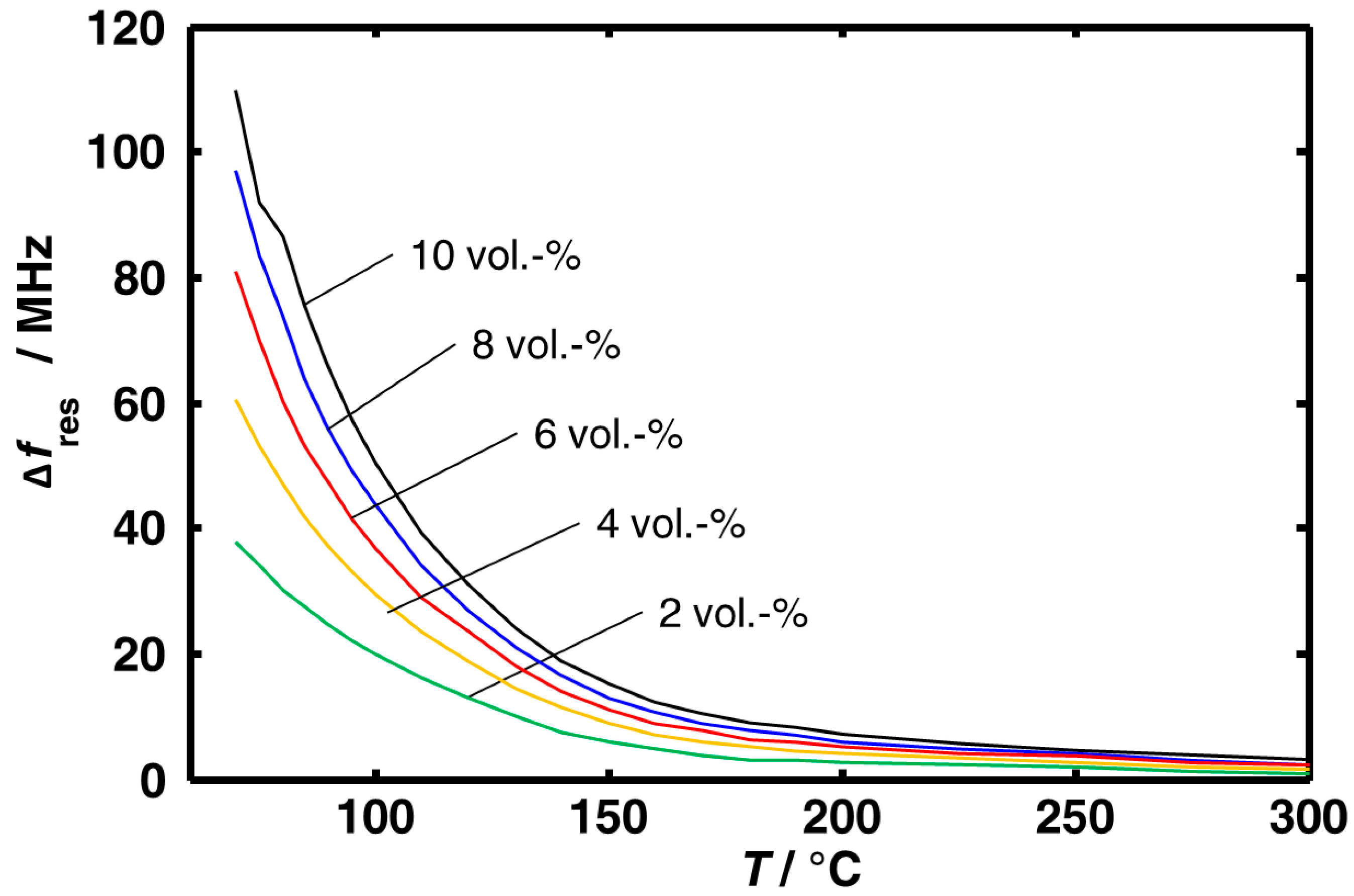
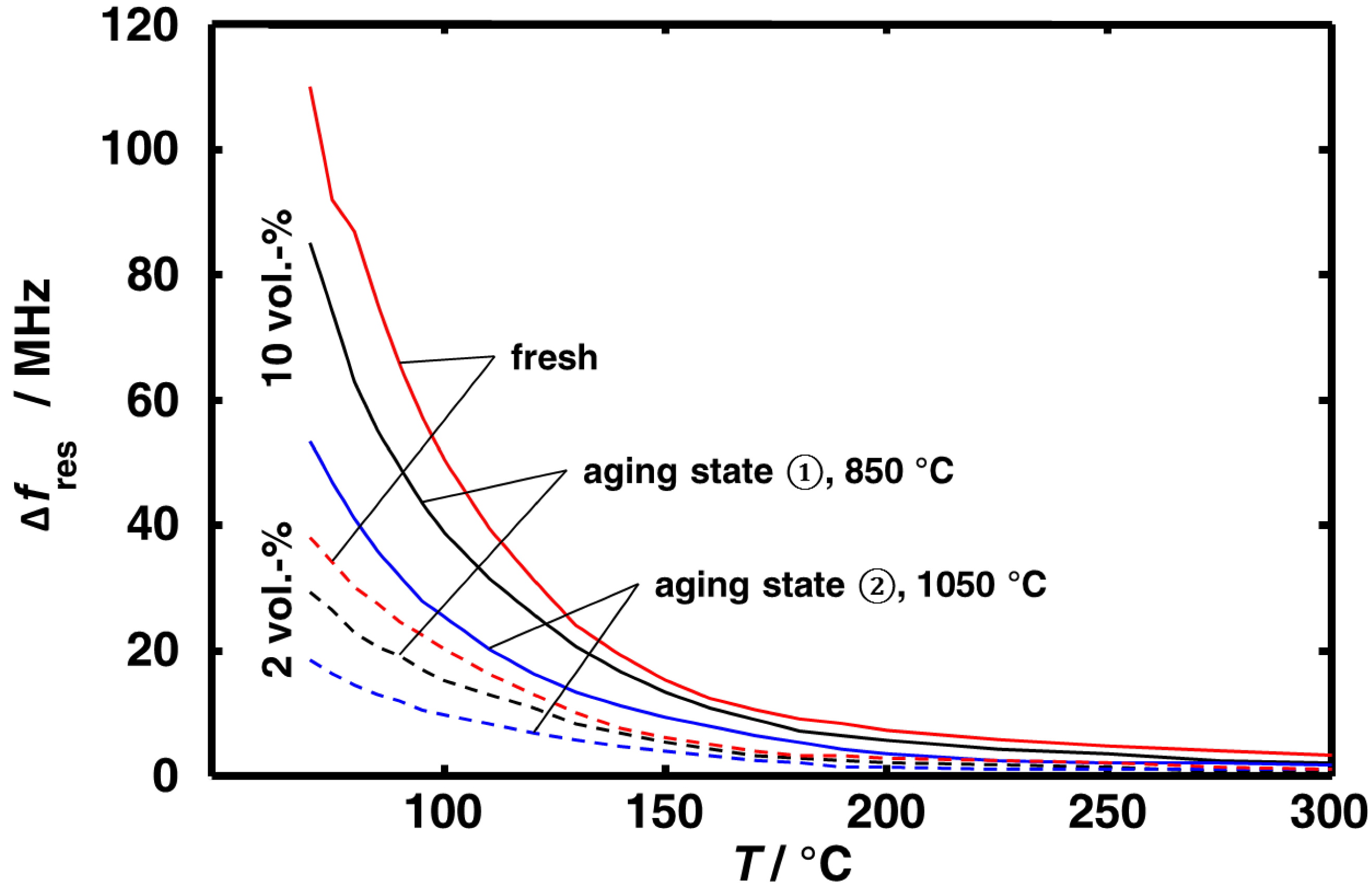
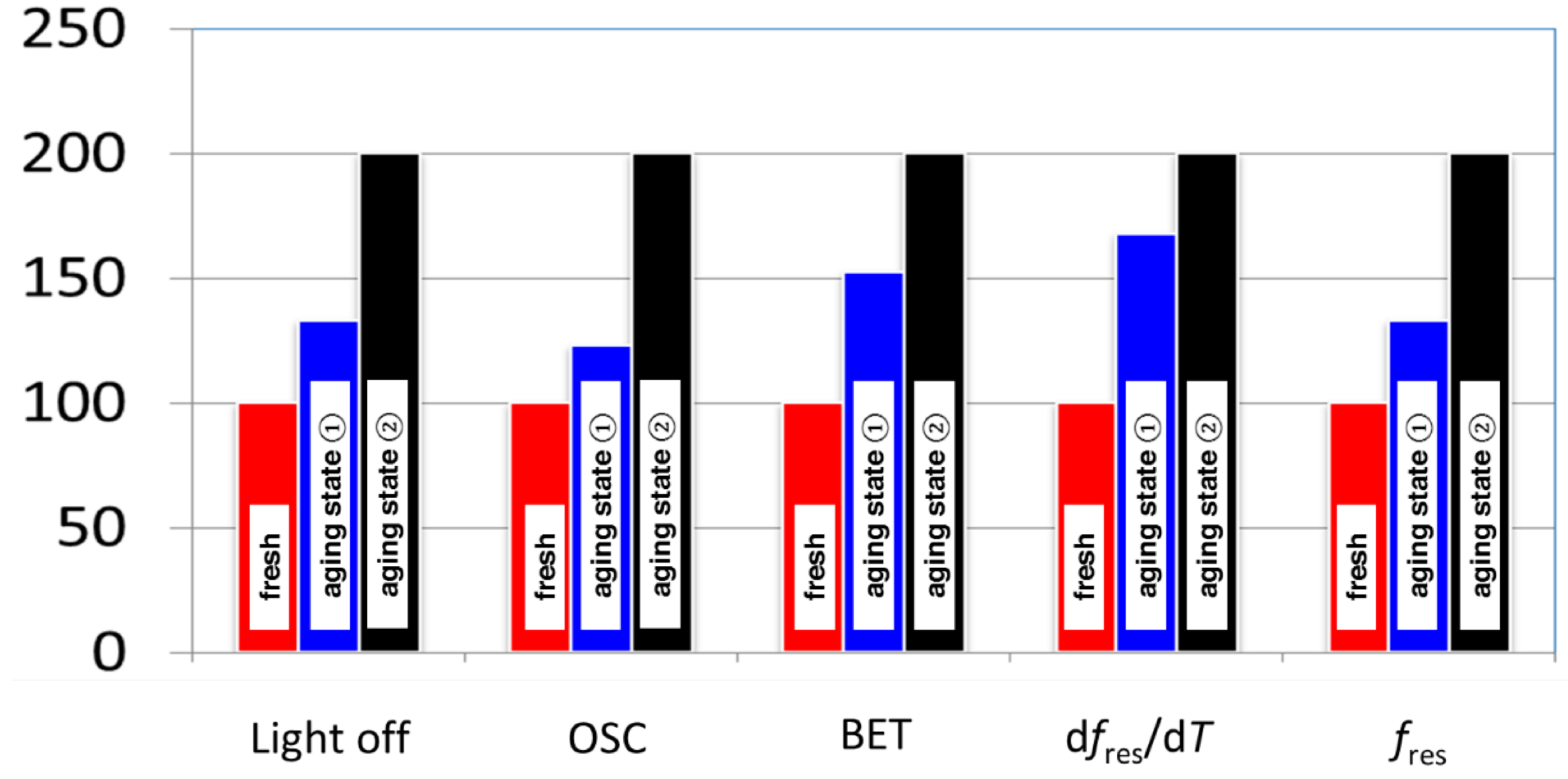
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Johnson, T. Vehicular Emissions in Review. SAE Int. J. Engines 2014, 7, 1207–1227. [Google Scholar] [CrossRef]
- Winkler, M.; Grimm, J.; Lenga, H.; Min, B.H. Gasoline engine combustion development for EU 6c emission legislation. In Internationaler Motorenkongress 2014; Springer: Wiesbaden, Germany, 2014; pp. 193–206. [Google Scholar]
- Deutschmann, O.; Grunwaldt, J.D. Abgasnachbehandlung in mobilen Systemen: Stand der Technik, Herausforderungen und Perspektiven. Chem. Ing. Tech. 2014, 85, 595–617. [Google Scholar] [CrossRef]
- Baunach, T.; Schänzlin, K.; Diehl, L. Sauberes Abgas durch Keramiksensoren. Phys. J. 2006, 5, 33–38. [Google Scholar]
- Riegel, J.; Wiedenmann, H.M.; Neumann, H. Chemical Sensors for Oxygen Detection and Air/Fuel Ratio Control. In Sensors for Automotive Applications; Marek, J., Trah, H.P., Suzuki, Y., Yokomori, I., Eds.; Sensors Applications; Wiley: Weinheim, Germany, 2006; Volume 4, pp. 480–499. [Google Scholar]
- Beulertz, G.; Votsmeier, M.; Moos, R. Effect of propene, propane, and methane on conversion and oxidation state of three-way catalysts: A microwave cavity perturbation study. Appl. Catal. B Environ. 2015, 165, 369–377. [Google Scholar] [CrossRef]
- Möller, R.; Votsmeier, M.; Onder, C.; Guzzella, L.; Gieshoff, J. Is oxygen storage in three-way catalysts an equilibrium controlled process? Appl. Catal. B Environ. 2009, 91, 30–38. [Google Scholar] [CrossRef]
- Beulertz, G.; Fritsch, M.; Fischerauer, G.; Herbst, F.; Gieshoff, J.; Votsmeier, M.; Hagen, G.; Moos, R. Microwave Cavity Perturbation as a Tool for Laboratory in situ Measurement of the Oxidation State of Three Way Catalysts. Top. Catal. 2013, 56, 405–409. [Google Scholar] [CrossRef]
- Beulertz, G.; Votsmeier, M.; Herbst, F.; Moos, R. Replacing the lambda probe by radio frequency-based in operando three-way catalyst oxygen loading detection. In Proceedings of the 14th International Meeting on Chemical Sensors, IMCS 14, Nuremberg, Germany, 20–23 May 2012; pp. 1426–1428.
- Schödel, S.; Moos, R.; Votsmeier, M.; Fischerauer, G. SI-Engine Control with Microwave-Assisted Direct Observation of Oxygen Storage Level in Three-Way Catalysts. IEEE Trans. Control Syst. Technol. 2014, 22, 2346–2353. [Google Scholar] [CrossRef]
- Reiß, S.; Spörl, M.; Hagen, G.; Fischerauer, G.; Moos, R. Combination of wirebound and microwave measurements for in situ characterization of automotive three-way catalysts. IEEE Sens. J. 2011, 11, 434–438. [Google Scholar]
- Moos, R.; Spörl, M.; Hagen, G.; Gollwitzer, A.; Wedemann, M.; Fischerauer, G. TWC: Lambda Control and OBD without Lambda Probe—An Initial Approach; SAE Technical Paper 2008-01-0916; SAE International: Warrendale, PA, USA, April 2008. [Google Scholar] [CrossRef]
- Rauch, D.; Kubinski, D.; Simon, U.; Moos, R. Detection of the ammonia loading of a Cu Chabazite SCR catalyst by a radio frequency-based method. Sens. Actuators B Chem. 2014, 205, 88–93. [Google Scholar] [CrossRef]
- Sappok, A.; Bromberg, L.; Parks, J.; Prikhodko, V. Loading and Regeneration Analysis of a Diesel Particulate Filter with a Radio Frequency-Based Sensor; SAE Technical Paper 2010-01-2126; SAE International: Warrendale, PA, USA, October 2010. [Google Scholar] [CrossRef]
- Moos, R. Microwave-Based Catalyst State Diagnosis—State of the Art and Future Perspectives. SAE Int. J. Engines 2015, 8, 1240–1245. [Google Scholar] [CrossRef]
- Boaro, M.; Trovarelli, A.; Hwang, J.H.; Mason, T.O. Electrical and oxygen storage/release properties of nanocrystalline ceria-zirconia solid solutions. Solid State Ion. 2002, 147, 85–95. [Google Scholar] [CrossRef]
- Pozar, D.M. Microwave Engineering, 4th ed.; John Wiley & Sons: New York, NY, USA, 2012. [Google Scholar]
- Reiß, S. Direkte Zustandssensorik von Automobilabgaskatalysatoren. Ph.D. Thesis, Universität Bayreuth, Bayreuth, Germany, 2012. [Google Scholar]
- Fischerauer, G.; Spörl, M.; Gollwitzer, A.; Wedemann, M.; Moos, R. Catalyst State Observation via the Perturbation of a Microwave Cavity Resonator. Frequenz 2008, 62, 180–184. [Google Scholar] [CrossRef]
- Moos, R.; Wedemann, M.; Spörl, M.; Reiß, S.; Fischerauer, G. Direct catalyst monitoring by electrical means: An overview on promising novel principles. Top. Catal. 2009, 52, 2035–2040. [Google Scholar] [CrossRef]
- Dietrich, M.; Rauch, D.; Porch, A.; Moos, R. A Laboratory Test Setup for in situ Measurements of the Dielectric Properties of Catalyst Powder Samples under Reaction Conditions by Microwave Cavity Perturbation: Set up and Initial Tests. Sensors 2014, 14, 16856–16868. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.B.; Kwon, H.J.; Nam, I.S.; Song, Y.I.; Oh, S.H. Activity Function for Describing Alteration of Three-Way Catalyst Performance over Palladium-Only Three-Way Catalysts by Catalyst Mileage. Ind. Eng. Chem. Res. 2011, 50, 5499–5509. [Google Scholar] [CrossRef]
- Brinkmeier, C. Automotive Three-Way Exhaust Aftertreatment under Transient Conditions—Measurements, Modeling and Simulation. Ph.D. Thesis, University of Stuttgart, Stuttgart, Germany, 2006. [Google Scholar]
- Tuller, H.L.; Nowick, A.S. Defect Structure and Electrical Properties of Nonstoichiometric CeO2 Single Crystals. J. Electrochem. Soc. 1979, 126, 209–217. [Google Scholar] [CrossRef]
- Feulner, M.; Müller, A.; Stöber, R.; Fischerauer, G.; Moos, R. Messungen zum Einfluss von Wasser auf die Beladungserkennung von Dieselpartikelfiltern mit Mikrowellentechnik. In Proceedings of the 11th Dresdner Sensor-Symposium, Dresden, Germany, 9–11 December 2013; Gerlach, G., Schütze, A., Eds.; pp. 239–242. [CrossRef]
- Nahar, R.K.; Khanna, V.K. A study of capacitance and resistance of Al2O3 humidity sensor. Int. J. Electron. 1982, 52, 557–567. [Google Scholar] [CrossRef]
- Ralohs, K.; D’Agostino, C.; Burch, R.; Chansai, S.; Gladden, L.F.; Hardacre, C.; James, S.L.; Mitchell, J.; Taylor, S.F.R. Assessing the surface modifications following the mechanochemical preparation of a Ag/Al2O3 selective catalytic reduction catalyst. Catal. Sci. Technol. 2014, 4, 531–539. [Google Scholar] [CrossRef]
- Thissen, P.; Grundmeier, G.; Wippermann, S.; Schmidt, W.G. Water adsorption on the α-Al2O3 (0001) surface. Phys. Rev. B 2009, 80. [Google Scholar] [CrossRef]
- Molinari, M.; Parker, S.C.; Sayle, D.C.; Islam, M.S. Water Adsorption and Its Effect on the Stability of Low Index Stoichiometric and Reduced Surfaces of Ceria. J. Phys. Chem. C 2012, 116, 7073–7082. [Google Scholar] [CrossRef]
- Xie, W.; Liu, B.; Xiao, S.; Li, H.; Wang, Y.; Cai, D.; Wang, D.; Wang, L.; Liu, Y.; Li, Q.; et al. High performance humidity sensors based on CeO2 nanoparticles. Sens. Actuators B Chem. 2015, 215, 125–132. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beulertz, G.; Votsmeier, M.; Moos, R. In operando Detection of Three-Way Catalyst Aging by a Microwave-Based Method: Initial Studies. Appl. Sci. 2015, 5, 174-186. https://doi.org/10.3390/app5030174
Beulertz G, Votsmeier M, Moos R. In operando Detection of Three-Way Catalyst Aging by a Microwave-Based Method: Initial Studies. Applied Sciences. 2015; 5(3):174-186. https://doi.org/10.3390/app5030174
Chicago/Turabian StyleBeulertz, Gregor, Martin Votsmeier, and Ralf Moos. 2015. "In operando Detection of Three-Way Catalyst Aging by a Microwave-Based Method: Initial Studies" Applied Sciences 5, no. 3: 174-186. https://doi.org/10.3390/app5030174
APA StyleBeulertz, G., Votsmeier, M., & Moos, R. (2015). In operando Detection of Three-Way Catalyst Aging by a Microwave-Based Method: Initial Studies. Applied Sciences, 5(3), 174-186. https://doi.org/10.3390/app5030174





