Host–Guest Complex of β-Cyclodextrin and Disulfide Form of 4-Aminothiophenol
Abstract
:1. Introduction
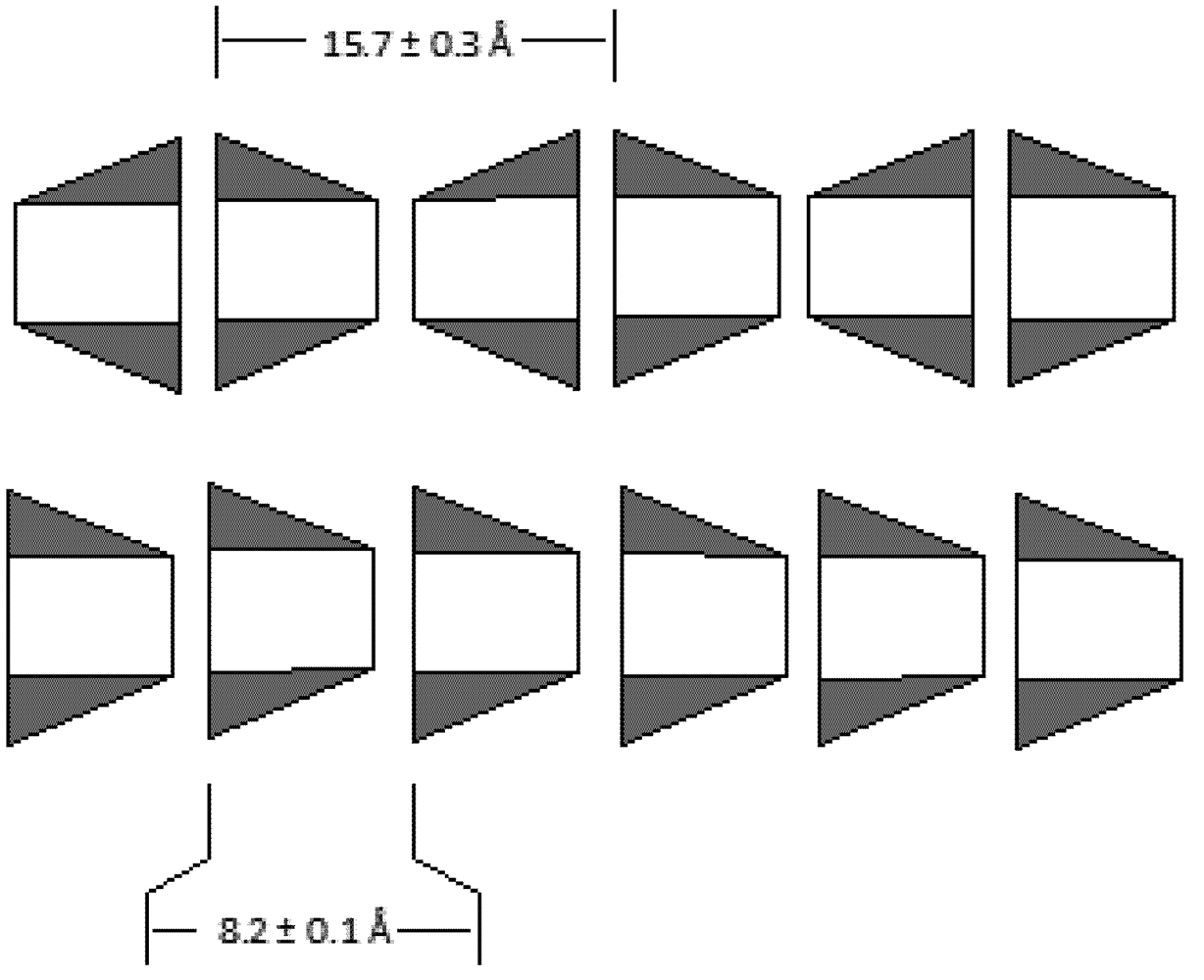
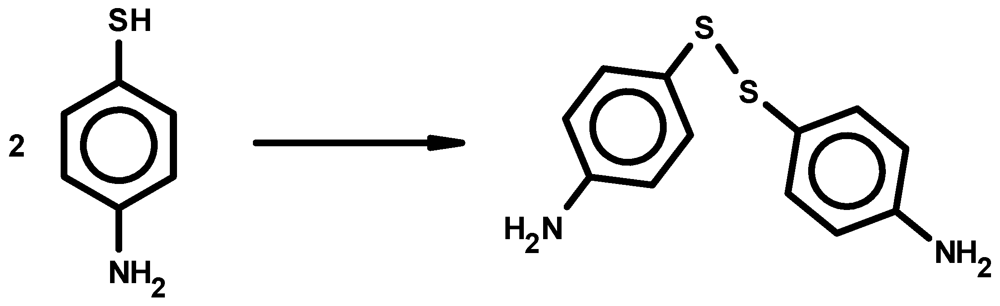
2. Experimental Section
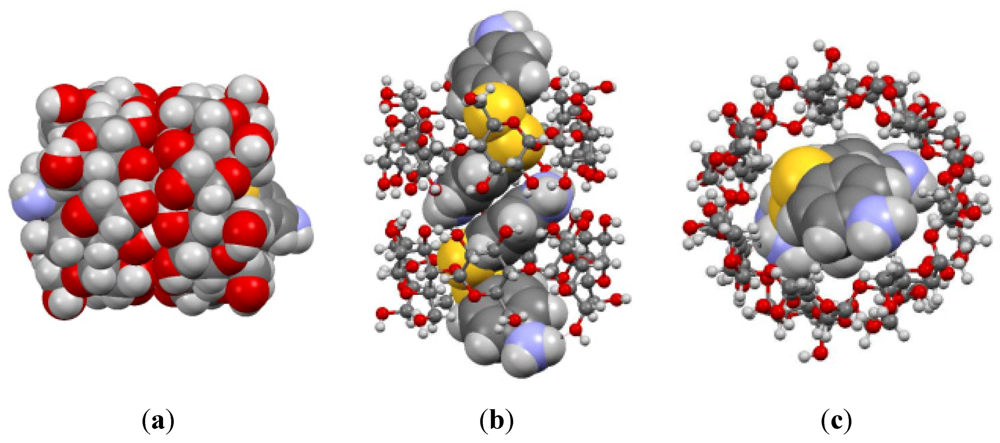
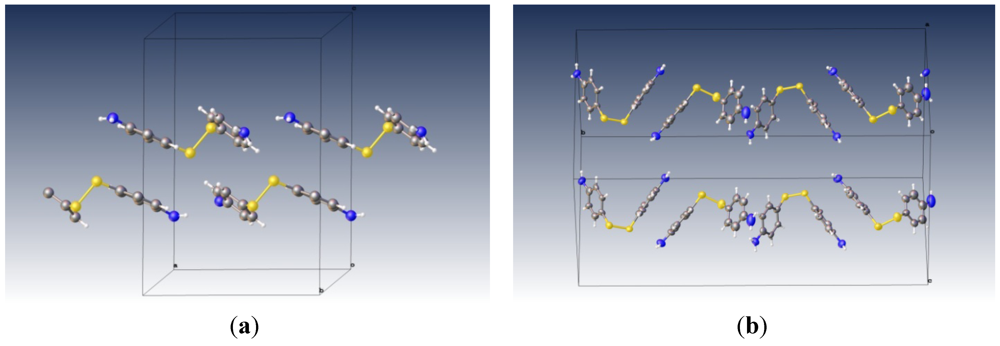
3. Results and Discussion
| Parameter | Crystal Data |
|---|---|
| CCDC deposition number | 879687 |
| Empirical formula | C54 H84 N2 O46.75 S2 |
| Formula weight | 1,573.35 |
| Temperature | 100 (±2) K |
| Wavelength | 1.54187 Å |
| Crystal system | Monoclinic |
| Space group | P21 |
| Unit cell dimensions | a = 14.8541 (±3) Å α = 90° |
| b = 32.3999 (±6) Å β = 103.750(7)° | |
| c = 15.7645 (±11) Å γ = 90° | |
| Volume | 7369.6(±6) Å3 |
| Z | 4 |
| Density (calculated) | 1.418 Mg/m3 |
| Absorption coefficient | 1.596 mm−1 |
| F(000) | 3312 |
| Crystal size | 0.09 × 0.07 × 0.05 mm3 |
| Theta range for data collection | 2.73 to 68.23° |
| Index ranges | −17 ≤ h ≤ 17, −38 ≤ k ≤ 38, −18 ≤ l ≤ 14 |
| Reflections collected | 72735 |
| Independent reflections | 25731 (R(int) = 0.0906) |
| Completeness to theta = 68.23° | 99.7% |
| Max. and min. transmission | 0.9245 and 0.8697 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 25731/1243/1908 |
| Goodness-of-fit on F2 | 1.035 |
| Final R indices (I > 2θ(I)) | R1 = 0.1128, wR2 = 0.2285 |
| R indices (all data) | R1 = 0.2441, wR2 = 0.3136 |
| Absolute structure parameter | 0.01 (±4) |
| Extinction coefficient | 0.00061 (±7) |
| Largest diff. peak and hole | 0.964 and −0.458 e·Å−3 |
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Mavridis, I.M.; Hadjoudis, E.; Tsoucaris, G. The crystal structure of the inclusion complex of cyclomaltoheptaose (β-cyclodextrin) with 3,3-dimethylbutylamine. Carbohyd. Res. 1991, 220, 11–21. [Google Scholar] [CrossRef]
- Makedonopoulou, S.; Mavridis, I.M. Structure of the inclusion complex of β-cyclodextrin with 1,12-dodecanedioic acid using synchrotron radiation data; a detailed dimeric β-cyclodextrin structure. Acta Crystrallogr. B 2000, B56, 322–331. [Google Scholar] [CrossRef]
- Frampton, M.J.; Anderson, H.L. Insulated molecular wires. Angew. Chem. Int. Ed. 2007, 46, 1028–1064. [Google Scholar] [CrossRef]
- Schlenk, H.; Sand, D.M. The association of α- and β-cyclodextrins with organic acids. J. Am. Chem. Soc. 1961, 83, 2312–2320. [Google Scholar] [CrossRef]
- Gopalan, A.I.; Lee, K.-P.; Manesh, K.M.; Santhosh, P.; Kim, J.H. Gold nanoparticles dispersed into poly(aminothiophenol) as a novel electrocatalyst-Fabrication of modified electrode and evaluation of electrocatalytic activities for dioxygen reduction. J. Mol. Catal. A 2006, 256, 335–345. [Google Scholar] [CrossRef]
- Udachin, K.A.; Ripmeester, J.A. A novel mode of inclusion for pyrene in β-cyclodextrin compounds: The crystal structures of β-cyclodextrin with cyclohexanol and pyrene, and with n-octanol and pyrene. J. Am. Chem. Soc. 1998, 120, 1080–1081. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L. Facile synthesis and one-dimensional assembly of cyclodextrin-capped gold nanoparticles and their applications in catalysis and surface-enhanced Raman scattering. J. Phys. Chem. C 2009, 113, 13636–13642. [Google Scholar] [CrossRef]
- Nelles, G.; Weisser, M.; Back, R.; Wohlfart, P.; Wenz, G.; Mittler-neher, S. Controlled orientation of cyclodextrin derivatives immobilized on gold surfaces. J. Am. Chem. Soc. 1996, 118, 5039–5046. [Google Scholar] [CrossRef]
- Park, J.S.; Wilson, J.N.; Hardcastle, K.I.; Bunz, U.H.F.; Srinivasarao, M. Reduced fluorescence quenching of cyclodextrin-acetylene dye rotaxanes. J. Am. Chem. Soc. 2006, 128, 7714–7715. [Google Scholar]
- Buston, J.E.H.; Young, J.R.; Anderson, H.L. Rotaxane-encapsulated cyanine dyes: Enhanced fluorescence efficiency and photostability. Chem. Commun. 2000, 905–906. [Google Scholar]
- Matsuzawa, Y.; Tamura, S.-I.; Matsuzawa, N.; Ata, M. Light stability of a β-cyclodextrin inclusion complex of a cyanine dye. J. Chem. Soc. Faraday Trans. 1994, 90, 3517–3520. [Google Scholar] [CrossRef]
- Buston, J.E.H.; Marken, F.; Anderson, H.L. Enhanced chemical reversibility of redox processes in cyanine dye rotaxanes. Chem. Commun. 2001, 1046–1047. [Google Scholar]
- Stanier, C.A.; O’Connell, M.J.; Clegg, W.; Anderson, H.L. Synthesis of fluorescent stilbene and tolan rotaxanes by Suzuki coupling. Chem. Commun. 2001, 493–494. [Google Scholar]
- Terao, J.; Tang, A.; Michels, J.J.; Krivokapic, A.; Anderson, H.L. Synthesis of poly(para-phenylenevinylene) rotaxanes by aqueous Suzuki coupling. Chem. Commun. 2004, 56–57. [Google Scholar]
- Cacialli, F.; Wilson, J.S.; Michels, J.J.; Daniel, C.; Silva, C.; Friend, R.H.; Sevrin, N.; Samori, P.; Rabe, J.P.; O’Connell, M.J.; et al. Cyclodextrin-threaded conjugated polyrotaxanes as insulated molecular wires with reduced interstrand interactions. Nat. Mater. 2002, 1, 160–164. [Google Scholar] [CrossRef]
- Breslow, R.; Zhang, B. Cholesterol recognition and binding by cyclodextrin dimers. J. Am. Chem. Soc. 1996, 118, 8495–8496. [Google Scholar] [CrossRef]
- Vittal, J.J.; Anjali, K.S. Sinusoidal ribbon waves in the crystal structure of bis(4-aminophenyl) disulfide. Cryst. Eng. 1998, 1, 147–152. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cappadona, T.A.; Daniels, L.M.; Siddiquee, T.A. Host–Guest Complex of β-Cyclodextrin and Disulfide Form of 4-Aminothiophenol. Appl. Sci. 2012, 2, 773-779. https://doi.org/10.3390/app2040773
Cappadona TA, Daniels LM, Siddiquee TA. Host–Guest Complex of β-Cyclodextrin and Disulfide Form of 4-Aminothiophenol. Applied Sciences. 2012; 2(4):773-779. https://doi.org/10.3390/app2040773
Chicago/Turabian StyleCappadona, Taylor A., Lee M. Daniels, and Tasneem A. Siddiquee. 2012. "Host–Guest Complex of β-Cyclodextrin and Disulfide Form of 4-Aminothiophenol" Applied Sciences 2, no. 4: 773-779. https://doi.org/10.3390/app2040773
APA StyleCappadona, T. A., Daniels, L. M., & Siddiquee, T. A. (2012). Host–Guest Complex of β-Cyclodextrin and Disulfide Form of 4-Aminothiophenol. Applied Sciences, 2(4), 773-779. https://doi.org/10.3390/app2040773




