Feasibility Study for Determination of Trace Iron in Red Sandstone via O-Phenanthroline Spectrophotometry
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
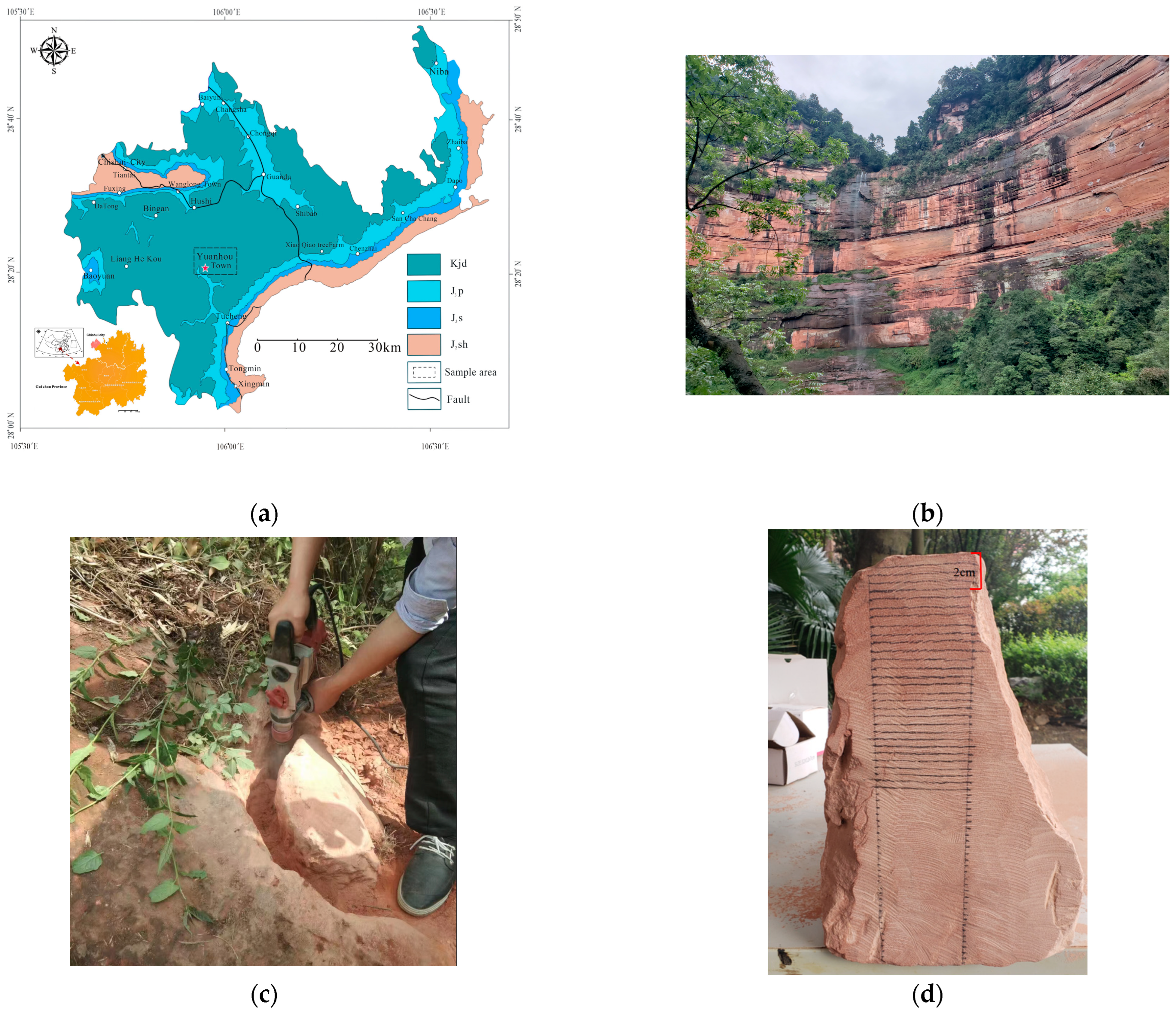
2.1.1. Samples
2.1.2. Reagents
- A 25 mg/L Fe2+ standard solution was prepared by dissolving 0.702 g of ferrous ammonium sulfate in 50 mL of 50% sulfuric acid and diluting the solution to 100 mL. An aliquot of 25 mL was then transferred to a 100 mL volumetric flask and diluted to volume.
- A 3.6 mol/L dilute sulfuric acid solution was obtained by mixing one part concentrated sulfuric acid with 4 parts deionized water and then cooling the mixture to room temperature.
- A 0.5% o-phenanthroline solution (chromogenic agent) was prepared by dissolving 0.5 g of o-phenanthroline (Tianjin Comio Chemical Reagent Co., Ltd., Tianjin, China) in 40 mL of deionized water. Three to five drops of concentrated hydrochloric acid were added to facilitate dissolution, after which the solution was diluted to 100 mL for immediate use. The reaction between o-phenanthroline and Fe2+ formed a stable, orange Fe2+–Phen complex within a pH range of 2–9.
- A 10% hydroxylamine hydrochloride solution (reducing agent) was prepared by dissolving 10 g of hydroxylamine hydrochloride (Tianjin Comio Chemical Reagent Co., Ltd., Tianjin, China) in 40 mL of deionized water and diluting it to 100 mL. This reagent reduced all the Fe3+ to Fe2+ in solution.
- A 40% acetic acid-ammonium acetate buffer solution (buffer solution) was prepared by dissolving 40 g of ammonium acetate (Chengdu Jinshan Chemical Reagent Co., Ltd., Chengdu, China) in a volumetric flask and then adding 50 mL of glacial acetic acid (Tianjin Fuyu Fine Chemical Co., Ltd., Tianjin, China) before diluting to 100 mL. This buffer solution maintained a stable pH for color development of the Fe2+–Phen complex.
- High-purity hydrofluoric acid (Shanghai Wokai Biotechnology Co., Ltd., Shanghai, China), a highly reactive reagent toward siliceous materials, was used to dissolve the quartz in the red sandstone and to mask the presence of Fe3+.
2.2. Methods
2.2.1. Equipment
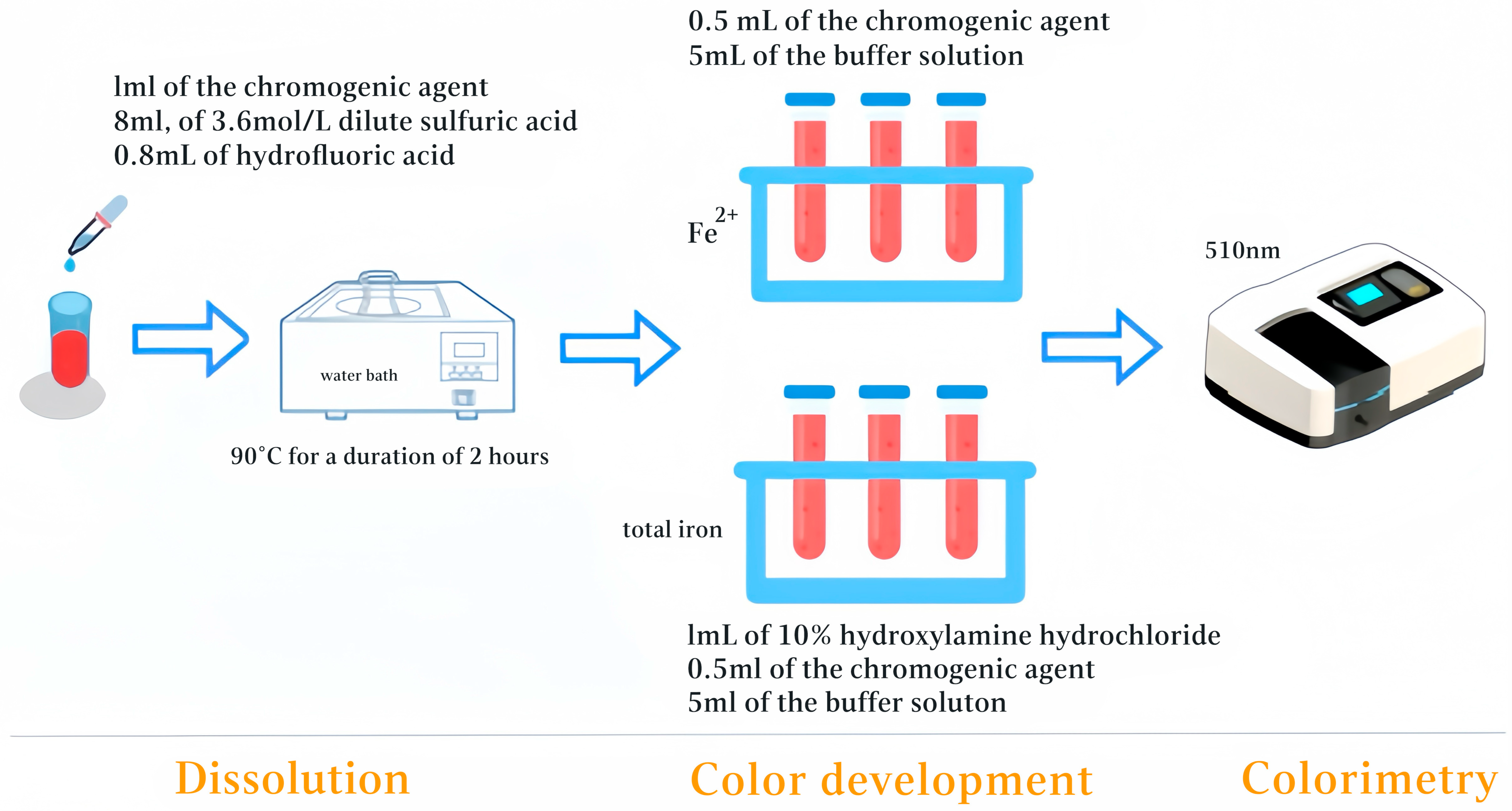
2.2.2. Dissolution
2.2.3. Color Development
2.2.4. Colorimetry
2.3. Standard Curve
3. Results and Discussion
3.1. Optimization of Experimental Conditions and Reagent Usage
3.1.1. Sample
3.1.2. Dissolution Optimization
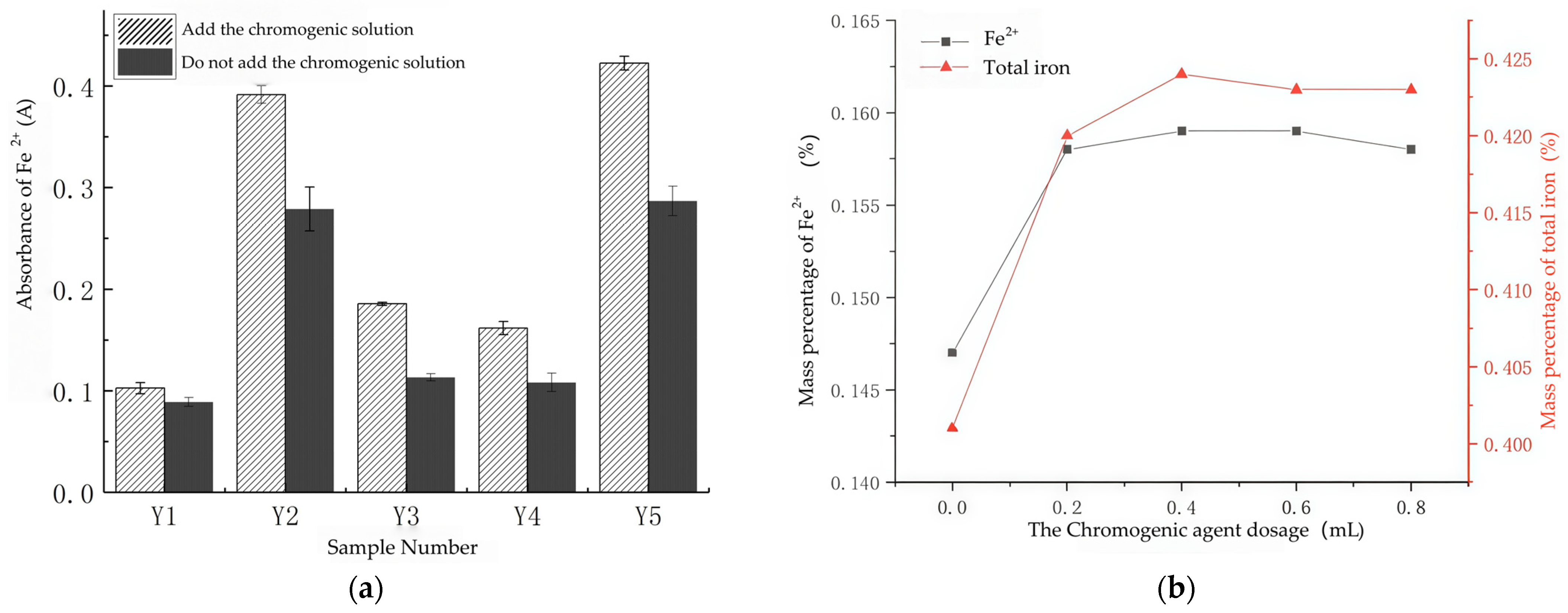
3.1.3. O-Phenanthroline Concentration
3.1.4. Hydroxylamine Hydrochloride Concentration
3.1.5. Buffer Solution Concentrations
3.1.6. Chromogenic Time and Temperature
3.2. Validation of Method Effectiveness
3.2.1. Detection Limit
- Photometric Method
- Integrated Concentration Method
3.2.2. Precision
3.2.3. Pike Recovery
3.2.4. Comparative Analysis
- X-ray Fluorescence Spectrometry
- Potassium Dichromate Volumetric
3.3. Applicability and Geological Significance
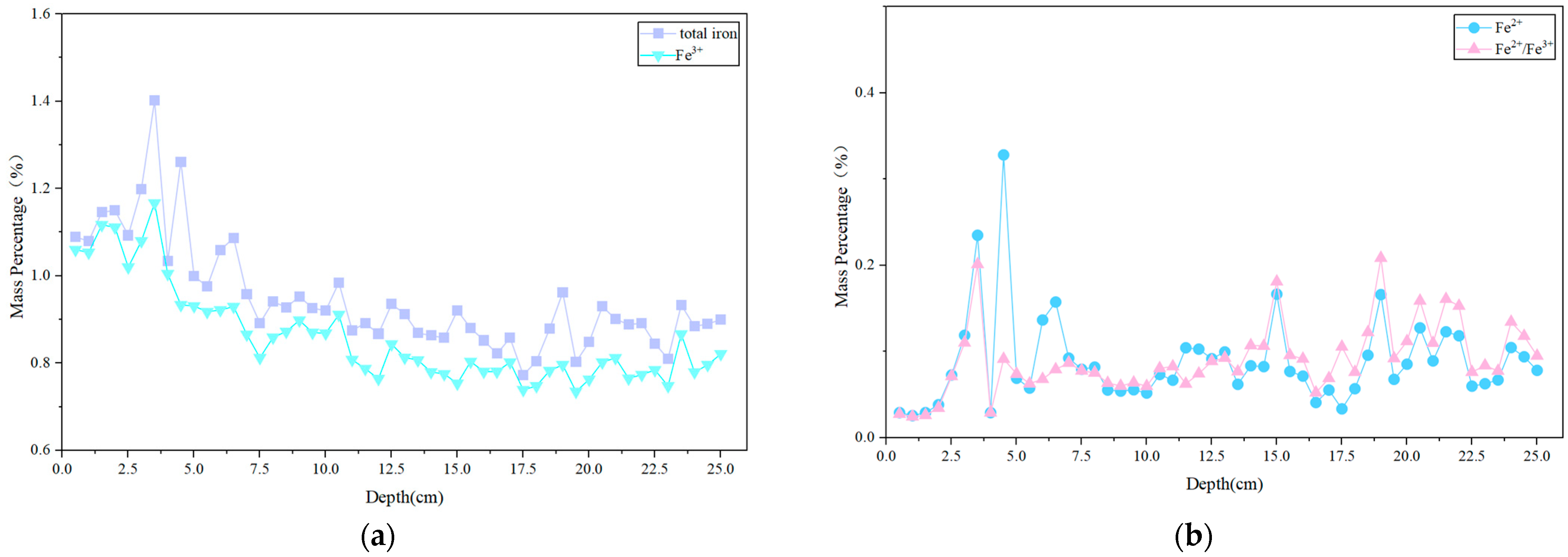
3.3.1. Trace Iron
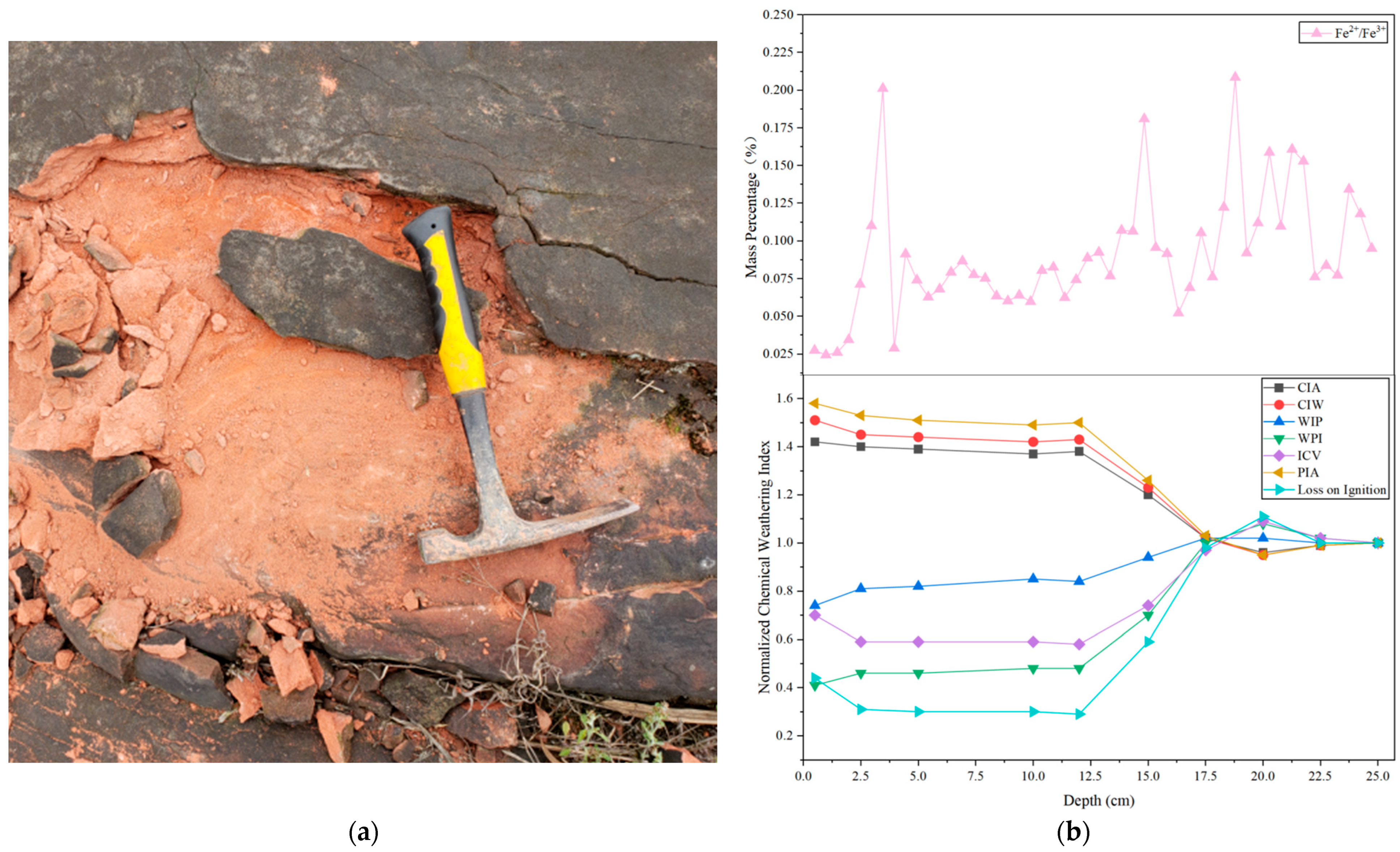
3.3.2. Chemical Weathering Indices
3.3.3. Geochemical Significance Analysis
4. Conclusions
- A chemical analysis method for determining trace Fe2+ and total iron in red sandstone via o-phenanthroline spectrophotometry was established. This method has a low detection limit, high precision, and excellent recovery rates, confirming its reliability and practicality for trace Fe2+ and total iron determination.
- The addition of an appropriate amount of o-phenanthroline prior to sample dissolution effectively prevents Fe2+ oxidation. Simultaneously, F− from hydrofluoric acid acts as a masking agent for coexisting Fe3+, eliminating its interference. This approach enables multitarget analysis within a single solution and effectively simplifies the experimental process, reducing the need for multiple reagents in iron determination.
- The application of o-phenanthroline spectrophotometry to red sandstone samples from the Chishui Danxia landform area revealed Fe2+ contents ranging from 0.01% to 0.10% and total iron contents ranging from 0.70% to 1.50%. The calculated Fe3+ content ranged from 0.70% to 1.50%.
- In the red sandstone of the study area, the vertical distribution of the Fe2+/Fe3+ ratio tends to be consistent with the macro weathering zones (0–5 cm, 5–15 cm, and >15 cm) indicated by traditional weathering indices such as the CIA, CIW, WPI, and ICV. This suggests that, under similar geological and biogeochemical conditions, the Fe2+/Fe3+ ratio has the potential to characterize weathering zones along vertical depth and could serve as a simple auxiliary indicator for quickly identifying and delineating the relative weathering zones of red sandstone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, H.; Zheng, D.; Xu, G.; Dong, X.; Liu, W.; Zou, Y.; Wang, H. Research on the evolution mechanisms and prevention countermeasures behind a large-scale landslide in complicated-geological-structure red beds. Sci. Rep. 2025, 15, 40294. [Google Scholar] [CrossRef]
- Liu, D.; Ao, T.; Cao, K.; Meng, X. Characterization and degradation of ancient architectural red sandstone in a natural erosion environment. Appl. Sci. 2023, 13, 9159. [Google Scholar] [CrossRef]
- Yu, C.; Zhong, W.; Zhang, X.; Li, T.; Fei, Z. Study on the damage characteristics of red sandstone foundation under rainfall infiltration in the red-bed area of the sichuan basin—Taking zhongjiang county as an example. Buildings 2024, 14, 3406. [Google Scholar] [CrossRef]
- Yan, L.; Peng, H.; Zhang, S.; Zhang, R.; Kašanin-Grubin, M.; Lin, K.; Tu, X. The spatial patterns of red beds and danxia landforms: Implication for the formation factors–China. Sci. Rep. 2019, 9, 1961. [Google Scholar] [CrossRef]
- Xiao, W.; Zhang, D.; Wang, X. Experimental study on progressive failure process and permeability characteristics of red sandstone under seepage pressure. Eng. Geol. 2020, 265, 105406. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, S.; Liu, J.; Liu, Z.; Zhu, D.; Li, Y. Mechanism of damage and deterioration of red sandstone in south China under different pH treatments. Adv. Civ. Eng. 2024, 2024, 3684358. [Google Scholar] [CrossRef]
- Lin, H.; Liu, W.; Zhang, D.; Chen, B.; Zhang, X. Study on the degradation mechanism of mechanical properties of red sandstone under static and dynamic loading after different high temperatures. Sci. Rep. 2025, 15, 11611. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Huang, J.; Si, X.; Wu, W.; Li, S. Failure characteristics and energy properties of red sandstone under uniaxial compression: Water content effect and its application. Bull. Eng. Geol. Environ. 2025, 84, 57. [Google Scholar] [CrossRef]
- Wang, W.; Gan, Z.; Zhang, X.; Li, S.; Xu, Y. Temporal variation in the chemical index of alteration in early cretaceous black shale as a proxy for paleoclimate. J. Geol. 2022, 130, 393–411. [Google Scholar] [CrossRef]
- Kwewouo Janpou, A.; Ngueutchoua, G.; Ekoa Bessa, A.Z.; Armstrong-Altrin, J.S.; Kankeu Kayou, U.R.; Mbella Nguetnga, O.-A.N.N.; Njanko, T.; Bela, V.A.; Tiotsop, M.S.K.; Tankou, J.G. Composition, weathering, and provenance of beach sands adjacent to volcanic rocks in the northern gulf of guinea, SW cameroon. J. Afr. Earth Sci. 2022, 188, 104473. [Google Scholar] [CrossRef]
- Ulusoy, E.; Kadioğlu, Y.K. Petrography and geochemical decomposition parameters of crystalline rocks; demirköy intrusive body (DIB), NW turkey. Bull. Miner. Res. Explor. 2021, 165, 253–265. [Google Scholar] [CrossRef]
- He, W.; Yang, Z.; Du, H.; Hu, J.; Zhang, K.; Hou, W.; Li, H. Micro-mechanisms and implications of continental red beds. Minerals 2022, 12, 934. [Google Scholar] [CrossRef]
- Bábek, O.; Sracek, O.; Všianský, D.; Holá, M. Groundwater red beds in holocene fluvial sediments as a product of iron and manganese redox cycling; morava river, czechia. Sedimentology 2023, 70, 2220–2240. [Google Scholar] [CrossRef]
- Van Houten, F.B. Iron oxides in red beds. Geol. Soc. Am. Bull. 1968, 79, 399. [Google Scholar] [CrossRef]
- Gruber, A.; Müller, R.; Wagner, A.; Colucci, S.; Spasić, M.V.; Leopold, K. Total reflection X-ray fluorescence spectrometry for trace determination of iron and some additional elements in biological samples. Anal. Bioanal. Chem. 2020, 412, 6419–6429. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, Z.; Cheng, Q.; Weindorf, D.C.; Zhang, Z.; Yang, J.; Zhang, X.; Chen, G.; Xie, S. Quantitative analysis of iron and silicon concentrations in iron ore concentrate using portable X-ray fluorescence (XRF). Appl. Spectrosc. 2020, 74, 55–62. [Google Scholar] [CrossRef]
- Revenko, A.G.; Pashkova, G.V. X-ray fluorescence spectrometry: Current status and prospects of development. J. Anal. Chem. 2023, 78, 1452–1468. [Google Scholar] [CrossRef]
- Ostadrahimi, M.; Farrokhpay, S.; Karimnejad, K.; Rahimian, A.; Molavi, M.; Shahkarami, G. A comparison of Fe(III) to Fe(II) reduction methods in iron analysis via titration. Chem. Pap. 2024, 78, 5407–5414. [Google Scholar] [CrossRef]
- Chubarov, V.M.; Amosova, A.A.; Finkelshtein, A.L. X-ray fluorescence determination of ore elements in ferromanganese formations. Inorg. Mater. 2020, 56, 1423–1430. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y. A new 1,10-phenanthroline method for oxalate-extractable iron measurement. Appl. Geochem. 2025, 183, 106354. [Google Scholar] [CrossRef]
- Yang, L.; Yao, G. A modified spectrophotometric method for the determination of ferrous ion during the fenton process. Int. J. Environ. Anal. Chem. 2022, 102, 3194–3206. [Google Scholar] [CrossRef]
- Smith, G.L.; Reutovich, A.A.; Srivastava, A.K.; Reichard, R.E.; Welsh, C.H.; Melman, A.; Bou-Abdallah, F. Complexation of ferrous ions by ferrozine, 2,2′-bipyridine and 1,10-phenanthroline: Implication for the quantification of iron in biological systems. J. Inorg. Biochem. 2021, 220, 111460. [Google Scholar] [CrossRef]
- Tarafder, P.K.; Thakur, R. An optimised 1,10-phenanthroline method for the determination of ferrous and ferric oxides in silicate rocks, soils and minerals. Geostand. Geoanalytical Res. 2013, 37, 155–168. [Google Scholar] [CrossRef]
- Shyla, B.; Bhaskar, C.V.; Nagendrappa, G. Iron(III) oxidized nucleophilic coupling of catechol with o-tolidine/p-toluidine followed by 1,10-phenanthroline as new and sensitivity improved spectrophotometric methods for iron present in chemicals, pharmaceutical, edible green leaves, nuts and lake water samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 86, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, M.; Yasui, H.; Sakurai, H.; Yamaguchi, T.; Fujita, Y. Improved spectrophotometric determination of total iron and iron(III) with o-hydroxyhydroquinonephthalein and their characterization. Yakugaku Zasshi 2011, 131, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Chandrakiran, P.; Kumari, A.S.; Sathwik, M.; Raghava, P. A rapid colorimetric method for the determination of iron in biological samples using o-phenanthroline method. Indo Glob. J. Pharm. Sci. 2018, 8, 76–79. [Google Scholar] [CrossRef]
- LY/T 1262−1999; Measurement of Available Iron in Forest Soil. State Forestry Administration: Beijing, China, 1999.
- HJ/T 345−2007; Determination of Iron in Water Quality o–Phenanthroline Spectrophotometry. Ministry of Ecology and Environment: Beijing, China, 2007.
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis: (IUPAC technical report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Huber, W. Basic calculations about the limit of detection and its optimal determination. Accredit. Qual. Assur. 2003, 8, 213–217. [Google Scholar] [CrossRef]
- Ostra, M.; Ubide, C.; Vidal, M.; Zuriarrain, J. Detection limit estimator for multivariate calibration by an extension of the IUPAC recommendations for univariate methods. Analyst 2008, 133, 532. [Google Scholar] [CrossRef]
- Allegrini, F.; Olivieri, A.C. IUPAC-consistent approach to the limit of detection in partial least-squares calibration. Anal. Chem. 2014, 86, 7858–7866. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Schwager, B.; Stoll, B.; Wilson, S.A.; Haug, G.H.; Andreae, M.O.; Enzweiler, J. Reference Values Following ISO Guidelines for Frequently Requested Rock Reference Materials. Geostand. Geoanalytical Res. 2016, 40, 333–350. [Google Scholar] [CrossRef]
- Alcott, L.J.; Krause, A.J.; Hammarlund, E.U.; Bjerrum, C.J.; Scholz, F.; Xiong, Y.; Hobson, A.J.; Neve, L.; Mills, B.J.W.; März, C.; et al. Development of iron speciation reference materials for palaeoredox analysis. Geostand. Geoanalytical Res. 2020, 44, 581–591. [Google Scholar] [CrossRef]
- Olivares, I.R.B.; Souza, G.B.; Nogueira, A.R.A.; Toledo, G.T.K.; Marcki, D.C. Trends in developments of certified reference materials for chemical analysis—Focus on food, water, soil, and sediment matrices. TrAC Trends Anal. Chem. 2018, 100, 53–64. [Google Scholar] [CrossRef]
- GB/T 673.8–2016; Iron Ores—Determination of Iron(II) Content—Potassium Dichromate Titrimetric Method. China Iron and Steel Association: Beijing, China, 2016.
- Garzanti, E.; Resentini, A. Provenance control on chemical indices of weathering (Taiwan river sands). Sediment. Geol. 2016, 336, 81–95. [Google Scholar] [CrossRef]
- Potysz, A.; Bartz, W.; Zboińska, K.; Schmidt, F.; Lenz, M. Deterioration of sandstones: Insights from experimental weathering in acidic, neutral and biotic solutions with acidithiobacillus thiooxidans. Constr. Build. Mater. 2020, 246, 118474. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, J.; Zhu, R.; Zhu, J.; Chen, Q.; Liang, X.; He, H. Photoreductive dissolution of iron (hydr)oxides and its geochemical significance. ACS Earth Space Chem. 2022, 6, 811–829. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Zhang, R.; Liu, J.; Liu, J.; Dai, Y.; Zhang, C.; Jia, H. Interfacial reaction between organic acids and iron-containing clay minerals: Hydroxyl radical generation and phenolic compounds degradation. Sci. Total Environ. 2021, 783, 147025. [Google Scholar] [CrossRef]
- Fu, H.; Jian, X.; Pan, H. Bias in sediment chemical weathering intensity evaluation: A numerical simulation study. Earth-Sci. Rev. 2023, 246, 104574. [Google Scholar] [CrossRef]
- Torabi-Kaveh, M.; Rizi, F.S.; Tajbakhsh, G.; Khodami, M.; Ménendez, B. The use of chemical and textural indices to predict geotechnical properties of granites with different degrees of weathering. Bull. Eng. Geol. Environ. 2023, 82, 362. [Google Scholar] [CrossRef]
- Pandarinath, K. Application potential of chemical weathering indices in the identification of hydrothermally altered surface volcanic rocks from geothermal fields. Geosci. J. 2022, 26, 415–442. [Google Scholar] [CrossRef]
- Nadłonek, W.; Bojakowska, I. Variability of chemical weathering indices in modern sediments of the vistula and odra rivers (poland). Appl. Ecol. Environ. Res. 2018, 16, 2453–2473. [Google Scholar] [CrossRef]
- Shao, J.; Yang, S.; Li, C. Chemical indices (CIA and WIP) as proxies for integrated chemical weathering in China: Inferences from analysis of fluvial sediments. Sediment. Geol. 2012, 265–266, 110–120. [Google Scholar] [CrossRef]
- Boukoffa, M.; Bouabsa, L.; Lamouri, B.; Fagel, N. Evaluation des indices d’alteration chimique sur un profil kaolinise cas: Tamazert (ne algerie). Courr. Du Savoir Sci. Et Tech. 2017, 23, 95–100. [Google Scholar]
- Bensharada, M.; Telford, R.; Stern, B.; Gaffney, V. Loss on ignition vs. thermogravimetric analysis: A comparative study to determine organic matter and carbonate content in sediments. J. Paleolimnol. 2022, 67, 191–197. [Google Scholar] [CrossRef]
| Measurement Value | Average | Standard Deviation | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Absorbance | 0.075 | 0.074 | 0.076 | 0.074 | 0.076 | 0.075 | 0.076 | 0.073 | 0.075 | 0.074 | 0.0745 | 0.0011 |
| 0.076 | 0.075 | 0.075 | 0.073 | 0.074 | 0.074 | 0.073 | 0.073 | 0.074 | 0.075 | |||
| Concentration (mg/L) | 0.3463 | 0.3414 | 0.3511 | 0.3414 | 0.3511 | 0.3463 | 0.3511 | 0.3366 | 0.3463 | 0.3414 | 0.3438 | 0.0051 |
| 0.3511 | 0.3463 | 0.3463 | 0.3366 | 0.3414 | 0.3414 | 0.3366 | 0.3366 | 0.3414 | 0.3463 | |||
| Sample Number | Surveillance Project | Absorbance | Average Absorbance | Mass Percentage (%) | RSD (%) | |||
|---|---|---|---|---|---|---|---|---|
| W-1 | Fe2+ | 0.838 | 0.850 | 0.847 | 0.867 | 0.8536 | 0.27 | 1.56 |
| 0.856 | 0.832 | 0.857 | 0.882 | |||||
| total iron | 0.595 | 0.578 | 0.614 | 0.60 | 0.5985 | 1.13 | 1.21 | |
| 0.611 | 0.596 | 0.606 | 0.588 | |||||
| W-2 | Fe2+ | 0.126 | 0.137 | 0.130 | 0.145 | 0.1345 | 0.25 | 0.56 |
| 0.134 | 0.137 | 0.133 | 0.134 | |||||
| total iron | 0.407 | 0.418 | 0.410 | 0.417 | 0.4134 | 0.78 | 0.44 | |
| 0.413 | 0.409 | 0.419 | 0.414 | |||||
| Sample Number | Samples (mg) | Fe2+ Standard Solution (mL) | Calculated Value (mg) | Measured Value (mg) | Recovery Rate (%) | Average Value (%) |
|---|---|---|---|---|---|---|
| W-1 | 0 | 0.5 | 0.05 | 0.0488 | 97.63 | 95.18 |
| 1.0 | 0.10 | 0.0951 | 95.14 | |||
| 1.5 | 0.15 | 0.1392 | 92.77 | |||
| 50 | 0.0 | / | 0.1436 | / | 94.74 | |
| 0.5 | 0.1936 | 0.1908 | 94.32 | |||
| 1.0 | 0.2436 | 0.2427 | 99.07 | |||
| 1.5 | 0.2936 | 0.2799 | 90.84 | |||
| W-2 | 0 | 0.5 | 0.01 | 0.0097 | 96.71 | 101.35 |
| 1.0 | 0.02 | 0.0209 | 104.45 | |||
| 1.5 | 0.03 | 0.0309 | 102.89 | |||
| 50 | 0.0 | / | 0.0288 | / | 92.56 | |
| 0.5 | 0.0388 | 0.0384 | 95.94 | |||
| 1.0 | 0.0488 | 0.0468 | 90.21 | |||
| 1.5 | 0.0588 | 0.0563 | 91.54 |
| Depth (cm) | Surveillance Project | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | BaO | CaO | Cr2O3 | TFe2O3 | K2O | MgO | MnO | Na2O | P2O5 | SiO2 | SO3 | SrO | TiO2 | LOI 1000 | |
| % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | |
| 0.5 | 7.07 | 0.03 | 0.18 | <0.01 | 2.58 | 1.44 | 0.57 | 0.02 | 1.62 | 0.01 | 84.37 | 0.02 | 0.01 | 0.64 | 1.91 |
| 2.5 | 7.57 | 0.03 | 0.18 | <0.01 | 1.61 | 1.34 | 0.59 | 0.03 | 2.09 | <0.01 | 84.90 | <0.01 | 0.01 | 0.55 | 1.36 |
| 5.0 | 7.41 | 0.03 | 0.20 | <0.01 | 1.41 | 1.34 | 0.62 | 0.05 | 2.14 | <0.01 | 85.55 | <0.01 | 0.02 | 0.46 | 1.31 |
| 10.0 | 7.38 | 0.04 | 0.21 | <0.01 | 1.32 | 1.37 | 0.64 | 0.04 | 2.24 | 0.03 | 85.07 | <0.01 | 0.01 | 0.42 | 1.32 |
| 12.5 | 7.51 | 0.04 | 0.20 | <0.01 | 1.32 | 1.37 | 0.63 | 0.03 | 2.23 | 0.02 | 85.29 | <0.01 | 0.01 | 0.42 | 1.27 |
| 15.0 | 7.63 | 0.04 | 1.84 | <0.01 | 1.42 | 1.39 | 0.70 | 0.03 | 2.28 | 0.03 | 82.40 | 0.01 | 0.01 | 0.46 | 2.55 |
| 17.5 | 7.46 | 0.03 | 4.12 | <0.01 | 1.41 | 1.35 | 0.69 | 0.03 | 2.19 | 0.03 | 77.78 | 0.01 | 0.02 | 0.50 | 4.27 |
| 20.0 | 7.04 | 0.03 | 4.79 | <0.01 | 1.55 | 1.27 | 0.66 | 0.03 | 2.11 | 0.03 | 77.02 | <0.01 | 0.01 | 0.52 | 4.82 |
| 22.5 | 7.12 | 0.03 | 4.37 | <0.01 | 1.51 | 1.29 | 0.66 | 0.03 | 2.08 | 0.02 | 77.81 | <0.01 | 0.01 | 0.48 | 4.37 |
| 25.0 | 7.13 | 0.04 | 4.26 | <0.01 | 1.40 | 1.32 | 0.66 | 0.03 | 2.08 | 0.02 | 78.62 | 0.01 | 0.01 | 0.44 | 4.35 |
| Sample Number | Potassium Dichromate Volumetric | O-Phenanthroline Spectrophotometry | Absolute Difference (%) | Relative Difference (%) |
|---|---|---|---|---|
| Y-1 | 0.1089% | 0.1082 ± 0.0022% | −0.0007% | −0.64% |
| Y-2 | 0.0544% | 0.0558 ± 0.0011% | +0.0014% | +2.57% |
| Y-3 | 0.1089% | 0.1146 ± 0.0023% | +0.0057% | +5.23% |
| Y-4 | 0.0389% | 0.0435 ± 0.0009% | +0.0046% | +11.83% |
| Y-5 | 0.0467% | 0.0476 ± 0.0009% | +0.0009% | +1.93% |
| Number | Chemical Weathering Index | Calculation Formula |
|---|---|---|
| 1 | Chemical Index of Alteration (CIA) | 100 × Al2O3/(Al2O3 + Na2O + K2O + CaO) |
| 2 | Chemical Index of Weathering (CIW) | 100 × Al2O3/(Al2O3 + Na2O + CaO) |
| 3 | Weathering Potential Index (WPI) | 100 × (Na2O + K2O + CaO)/(Si2O + Al2O3 + Fe2O3 + TiO2 + Na2O + K2O + CaO + MgO) |
| 4 | Modified Weathering Index (MWPI) | 100 × (Na2O + K2O + CaO + MgO)/(Si2O + Al2O3 + Fe2O3 + TiO2 + Na2O + K2O + CaO + MgO) |
| 5 | Index of Compositional Variability (ICV) | (Fe2O3 + TiO2 + CaO + MgO + MnO + Na2O + K2O)/Al2O3 |
| 6 | Plagioclase Index of Alteration (PIA) | 100 × [(Al2O3 − K2O/(Al2O3 + Na2O + CaO − K2O)] |
| 7 | Loss on Ignition | Percentage of mass of H2O+ to total mass of sample |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Wang, D.; Yang, G.; Shi, W.; Wang, Y. Feasibility Study for Determination of Trace Iron in Red Sandstone via O-Phenanthroline Spectrophotometry. Appl. Sci. 2026, 16, 243. https://doi.org/10.3390/app16010243
Wang D, Yang G, Shi W, Wang Y. Feasibility Study for Determination of Trace Iron in Red Sandstone via O-Phenanthroline Spectrophotometry. Applied Sciences. 2026; 16(1):243. https://doi.org/10.3390/app16010243
Chicago/Turabian StyleWang, Dajuan, Genlan Yang, Wenbing Shi, and Yong Wang. 2026. "Feasibility Study for Determination of Trace Iron in Red Sandstone via O-Phenanthroline Spectrophotometry" Applied Sciences 16, no. 1: 243. https://doi.org/10.3390/app16010243
APA StyleWang, D., Yang, G., Shi, W., & Wang, Y. (2026). Feasibility Study for Determination of Trace Iron in Red Sandstone via O-Phenanthroline Spectrophotometry. Applied Sciences, 16(1), 243. https://doi.org/10.3390/app16010243






