Featured Application
This study explores the potential of natural plant extracts as antimicrobial agents for the biological control of wash water pathogens in the agri-food industry. The findings support the development of eco-friendly alternatives to chlorine-based disinfection, contributing to safer and more sustainable food processing practices.
Abstract
Water used in cleaning processes within the agri-food industry can be a vector for post-harvest contaminants, thus contributing to cross-contamination. The contamination risk is increased when water is not replaced between batches or when disinfection protocols are insufficient. Given the increasing focus in recent years on the potential of natural, non-invasive plant extracts to combat a variety of pathogens, including multidrug-resistant bacteria, environmental strains, and clinical isolates, this study aimed to evaluate the antibacterial activity of selected water-ethanol plant extracts against six opportunistic pathogens isolated from wash water in the agri-food industry, along with chromatographic analyses of the selected extracts. Plant extracts were obtained from the fruits, leaves, shoots, roots, and bark of 13 species. Antibacterial activity was assessed using the well diffusion method. The results indicated that antimicrobial activity was exhibited by six extracts: Tilia cordata Mill., Camellia sinensis, Quercus robur L., Betula pendula Roth, Rubus idaeus L., and Salix alba L. The extracts showed strain-dependent antimicrobial activity, with C. sinensis and R. idaeus up to 4.0 mm and 8.0 mm inhibition zones, respectively. P. aeruginosa and E. faecalis were the most susceptible strains, demonstrating the largest inhibition zones. In contrast, P. vulgaris and K. oxytoca were more resistant. The efficacy of the most active extracts can be linked to the presence of phytochemicals identified via GC-MS, including epicatechin, shikimic acid, quinic acid, gallic acid, and caffeine. These metabolites are known to interfere with bacterial cell structures and metabolic pathways. These studies may serve as a preliminary step toward the development of non-invasive water treatment methods for wash water.
1. Introduction
One of the major challenges facing the modern world is the balanced use of water resources [1]. The increasing impacts of climate change, manifested through water scarcity, droughts, and extreme flooding, affect the security of crop and livestock production, human health, and socioeconomic well-being [2,3]. The reuse of water within the framework of a circular economy is a critical component of strategies to reduce water consumption, thereby promoting more efficient management of the resources [4]. In this context, agriculture, as the most water-intensive sector, is responsible for approximately 70% of global freshwater consumption [5,6]. The fresh-cut fruits and vegetables processing sector is a major consumer of water, particularly during the initial stages of production. Water consumption and wastewater generation in the production of fresh-cut products typically range from 2 to 11 m3 of water per ton of product and from 11 to 23 m3 of wastewater per ton, respectively. This means that the production of one ton of fresh-cut fruits or vegetables requires several to over ten cubic meters of clean water, while the volume of wastewater generated can be up to twice as high. Such significant water use and wastewater generation are mainly associated with intensive washing and rinsing processes, which are essential for maintaining hygiene and ensuring the quality of products intended for direct consumption [7,8,9]. These processes primarily aim to remove organic matter residues and soil particles and reduce microbiological contamination of raw material [10].
From a microbiological risk perspective, the raw material can become contaminated at both the pre-harvest and post-harvest stages [11]. Wash water may act as a carrier of post-harvest contamination, contributing to cross-contamination, especially when water is not replaced between batches. Reusing the same water multiple times allows microbiological contaminants from one batch of fruits or vegetables to transfer to subsequent batches, thereby increasing the risk of pathogen propagation [12,13]. From a processing perspective, microorganisms that affect the quality of the final product are particularly undesirable. These include filamentous fungi, yeasts, and saprotrophic bacteria, which contribute to raw material spoilage and shorten its shelf life, leading to premature product deterioration [14]. The second group comprises pathogens that pose a risk to public health, including the ESKAPE pathogens—Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.—as well as Salmonella spp., Listeria monocytogenes, Clostridium spp., Escherichia coli, and coliforms [15,16,17,18] (Figure 1).
Figure 1.
Diagram presenting the potential main risks associated with contamination of the wash water.
Considering the rational use of water within the principles of the circular economy, the optimal solution would be the reuse of rinse water following proper treatment. This approach minimizes wastewater generation in facilities within the agri-food sector [8]. A variety of methods are employed to purify rinse water, including physicochemical processes such as sedimentation, flocculation, and filtration using sand, silica, and carbon, as well as advanced techniques like nano-ultrafiltration [19,20]. Pathogen inactivation is typically achieved through traditional methods involving chlorine compounds, which, although highly effective, can have adverse effects due to the formation of harmful by-products [21]. Consequently, there has been a growing body of research focused on the use of natural, non-invasive plant extracts against various strains, including multidrug-resistant bacteria, virulent strains, and clinical isolates [22,23,24]. However, to the best of the authors’ knowledge, a significant research gap exists regarding the use of plant extracts against environmental strains isolated from wash water in the agri-food sector.
The research hypothesis suggests that extracts from various plant materials are effective against pathogens isolated from wash water in the agri-food industry. It further posits that combining bark extracts, which contain heavy, non-volatile organic compounds, with extracts rich in volatile organic compounds (from leaves, shoots, flowers, and fruits) may result in an increased effect, thereby enhancing antibacterial activity against the tested bacteria.
The aim of the study was to evaluate the antibacterial activity of selected plant extracts against opportunistic pathogens derived from rinse water in the agri-food industry. The second phase of the research involved determining the qualitative composition of the antibacterial extracts, which are characterized by a high content of volatile compounds (from extracts of leaves, fruits, flowers, and shoots), using gas chromatography-mass spectrometry (GC-MS). This technique was selected due to its high sensitivity and specificity for the identification of volatile and semi-volatile organic compounds, which can affect bacterial cells by disrupting membrane integrity, inhibiting DNA and protein synthesis, suppressing biofilm formation, and interfering with efflux pump activity [25,26,27].
2. Materials and Methods
2.1. Research Material
The selection of the 13 plant species was based on a multi-criteria approach combining ethnobotanical relevance, particularly their traditional use in herbal infusions or food preservation; reported antimicrobial properties in the scientific literature; availability in the commercial or local market; and diversity in phytochemical profiles to ensure a broad representation of chemical groups potentially involved in microbial inhibition. The research material consisted of 13 plant-based raw materials selected based on previous reports (Table 1, Figure 2). These materials included various plant parts, such as leaves (Urtica dioica L.), flowers (Tilia cordata Mill.), bark (Quercus robur L., Betula pendula Roth, Salix alba L., Quercus suber L.), fruits (Rosa canina L.), roots (Taraxacum officinale F.H. Wiggers coll.), shoots (Rubus idaeus L., Pinus sylvestris L.), herbs (Verbena officinalis L.), and waste products—coffee grounds (Coffea spp.) and tea grounds (Camellia sinensis). These plants have not yet been used in the control of opportunistic pathogens isolated from water.

Table 1.
Summary of reports on the antibacterial properties of selected plant materials.

Figure 2.
Plant materials selected for the preparation of extracts used in this study (Order: 1—Coffea L., 2—Camellia sinensis, 3—Urtica dioica L., 4—Tilia cordata Mill., 5—Quercus robur L., 6—Rosa canina L., 7—Betula pendula Roth, 8—Taraxacum officinale F.H. Wiggers coll., 9—Quercus suber L., 10—Rubus idaeus L., 11—Salix alba L., 12—Verbena officinalis, 13—Pinus sylvestris L.).
The study on antibacterial activity utilized six isolates of opportunistic bacteria obtained from the collection of the Department of Microbiology and Plant Ecology (Bydgoszcz University of Science and Technology, Bydgoszcz, Poland): P. aeruginosa, E. faecalis, K. oxytoca, K. pneumoniae, S. marcescens, and P. vulgaris. Detailed descriptions of these isolates were provided in previous research conducted by the department [71].
2.2. Preparation of Extracts
Seventy percent water-ethanol extracts were prepared by measuring 25 g of cleaned and dried plant material (pre-dried for 12 h at 30 °C). The material was then ground into a fine powder and soaked in 250 mL of solvent. The extracts were agitated at 150 rpm for 24 h and subsequently filtered using laboratory filter paper. The filtered extracts (150 mL) were concentrated three-fold (50 mL) using a Laborota 4000 efficient rotary evaporator (Heidolph, Schwabach, Germany) at 90 rpm and 65 °C. The extracts were sterilized using syringe filters with a pore size of 0.22 µm and a diameter of 25 mm to ensure sterility.
Extracts exhibiting antibacterial activity, as assessed by the well-diffusion method, were further processed via spray drying at an air intake temperature of 180 °C and an air outflow temperature of 91.9 °C, using a Labspray SD-18A spray dryer (Changsha, China). The percentage recovery of the dried extract was estimated by using the formula:
where C is the mass (g) of the dried extract, and D is the mass (g) of the powdered plant material.
2.3. The Well Difusion Method
The 24-h bacterial cultures were grown on TSA (Tryptic Soy Agar, Merck, Darmstadt, Germany) slants at 36 ± 0.5 °C. After incubation, 1 mL of sterile saline solution (0.9% NaCl) was added to each test tube. The bacterial culture was then collected from the surface of the medium using a sterile loop. The final bacterial inoculum density was determined spectrophotometrically (DEN-1 McFarland Densitometer, Biosan, Riga, Latvia) and adjusted to 1.0–2.0 × 108 CFU/mL, which corresponds to the standard cell density used in antimicrobial susceptibility testing. The resulting suspension was transferred into cooled, still-liquid Mueller-Hinton agar (Merck) and gently mixed. The inoculated medium was then poured into sterile Petri dishes and allowed to solidify. After the agar solidified, six wells (8 mm in diameter) were made on each plate using sterile cork borers. In the final step, 100 μL of the test extracts were added to each well, and the plates were incubated for 24 h at 36 ± 0.5 °C. After incubation, the inhibition zones were measured, excluding the well diameter.
Based on the analysis, extracts with antibacterial properties were identified and subsequently subjected to tests assessing their combined effect. These tests involved combining selected extracts in pairs according to a defined matrix and applying 100 μL of the resulting mixture to the designated wells in the well-diffusion method [72]. The result was presented as an average of two repetitions.
2.4. Gas Chromatography-Mass Spectrometry Analysis
The GC/MS analysis was performed as follows: 10 mg of the tested plant extract was dissolved in 1 mL of 99.8% anhydrous pyridine, and 100 µL of N,O-bis(trimethylsilyl)-trifluoroacetamide (BSTFA) was then added. The mixture was heated at 60 °C for 30 min to allow derivatization. The resulting silylated samples were then analyzed using an Agilent 7890A gas chromatograph coupled with an Agilent 5975C mass selective detector (Agilent, Santa Clara, CA, USA). A 1 µL sample injection was performed using an Agilent 7693A autosampler. Analyte separation was achieved on an HP-5MS fused silica column (30 m × 0.25 mm × 0.25 µm film thickness) with a helium flow rate of 1 mL/min. The injector was operated in split mode (1:10), with the temperature set to 300 °C. The initial column temperature was 50 °C, which was increased to 325 °C at a rate of 3 °C/min and held for 10 min. The ion source temperature was set to 230 °C, and the quadrupole temperature was maintained at 150 °C. Electron ionization mass spectrometry (EIMS) was performed at an ionization energy of 70 eV. Detection was carried out in full-scan mode, with a mass range of 41 to 800 m/z. After data integration, the relative contributions of each compound to the total ion current (% TIC) were calculated. Retention indices for the analytes were determined using the retention times of alkanes as standards. The mass spectra and calculated retention indices were used for compound identification. Mass spectral identification was facilitated by an automated GC-MS data processing system provided by the National Institute of Standards and Technology (NIST) [73], using data from the “Identification of Biologically and Environmentally Significant Organic Compounds Mass Spectra and Retention Indices Library of Trimethylsilyl Derivatives”. The retention indices of the identified compounds were compared with reference values.
3. Results
3.1. Evaluation of Extract Activity
The extracts exhibited antimicrobial activity with varying effectiveness depending on the tested strain (Table 2). For the purpose of this study, inhibition zones were classified as follows: strong activity (≥7 mm), moderate (2–6 mm), minimal (1 mm), and no activity (0 mm). Based on this classification, C. sinensis and R. idaeus demonstrated the largest activity, with maximum inhibition zones reaching 8.0 mm for P. aeruginosa and E. faecalis and 8.0 mm for P. aeruginosa, respectively. Extracts from Q. robur, B. pendula, T. cordata, and S. alba displayed moderate antimicrobial activity, with inhibition zones ranging from 2.0 mm to 6.0 mm. No antimicrobial activity was observed for the extracts from Coffea L., R. canina, T. officinale, Q. suber, V. officinalis, and P. sylvestris, with inhibition zones of 0.0 mm for all tested strains. The U. dioica extract exhibited only minimal activity against P. aeruginosa, with an inhibition zone of 1.0 mm.

Table 2.
Inhibition zone diameter (mm) of tested plant extracts in the well diffusion method.
The susceptibility analysis indicated that P. aeruginosa and E. faecalis were more susceptible to the tested extracts, whereas P. vulgaris and K. oxytoca exhibited higher resistance.
For further studies on the assessment of combined effects, extracts from the following raw materials were selected: C. sinensis, T. cordata, Q. robur, B. pendula, R. idaeus, and S. alba. Combinations of plant extracts were prepared in a 1:1 ratio and tested at the same total volume (100 μL) as single extracts. The percentage increase in antimicrobial activity of the combined extracts was calculated relative to the most active single extract in each pair. This approach assumes that any observed enhancement beyond the highest individual activity may indicate a potential synergistic or additive effect between the extracts. This method provides a conservative estimate of enhanced antimicrobial activity, as it compares the mixture directly with the stronger of the two components. To achieve this, an experimental setup was prepared in which the extracts were combined according to the proposed matrix (Table 3).

Table 3.
Matrix of extract combinations for interaction testing.
The recovery yield of dried extracts obtained after spray drying varied among the plant species, with Camellia sinensis exhibiting the highest recovery (10.59%), followed by Rubus idaeus (7.86%) and Tilia cordata (5.34%) (Table 4).

Table 4.
Recovery percentage of dried plant extracts after spray drying.
Accordingly, 100 μL of each extract applied in the well-diffusion assay contained 8.9 mg of dry extract for Tilia cordata, 13.1 mg for Rubus idaeus, 17.7 mg for Camellia sinensis, 2.6 mg for Quercus robur, 2.6 mg for Salix alba, and 1.8 mg for Betula pendula.
The effect of the combination of Q. robur × B. pendula against the P. vulgaris strain, resulting in a 125% increase in the inhibition zone, was observed. Also, a 50% increase for the combination of B. pendula × S. alba against S. marcescens and T. cordata × R. idaeus against P. aeruginosa was noted. An intermediate effect (33% increase) for the combination of T. cordata × B. pendula against K. oxytoca and Q. robur × R. idaeus against K. pneumoniae was recorded. The lowest effect (8%) was observed for the combination of Q. robur × R. idaeus against E. faecalis. The remaining combinations—did not result in an increased diameter of inhibition zones compared to the individual extracts. This lack of enhanced activity may stem from limited compatibility between active compounds or the absence of additive or synergistic effects in these specific pairings (Figure 3, Table 5).
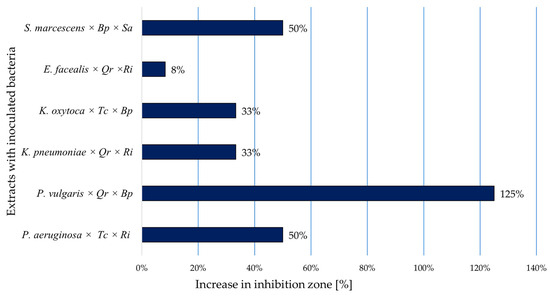
Figure 3.
Increase in diameter of inhibition zones, following application of a mixture of two extracts [%] (Abbreviations: Tc—Tilia cordata Mill.; Qr—Quercus robur L.; Bp—Betula pendula; Ri—Rubus idaeus; Sa—Salix alba L.).

Table 5.
Summary of results for combinations of extracts exhibiting growth inhibition of the bacteria tested.
3.2. GC-MS Analysis of the Extracts
Chromatographic analysis using gas chromatography-mass spectrometry revealed multiple peaks in the tested samples, with retention times of 203, 223, and 239 for tea, linden, and raspberry extracts, respectively (Figure 4). Detailed data for the individual extracts, including the compounds, retention times, experimental retention indices, literature retention indices, and total ion chromatogram (TIC) (%) for the linden, raspberry, and tea extracts, are provided in the Supplementary Materials (S1).
Figure 4.
Comparison of GC-MS chromatographic profiles obtained for the tested extracts.
Among the analyzed chemical groups, amino acids and their derivatives had the highest proportion in tea (23.3%) and the lowest in raspberries (19.6%). Organic acids occurred in similar proportions in all plants, with a slight predominance in lime (27.5%). Sugars and their derivatives dominated raspberries (28.6%), while in tea they accounted for 25.0%. Similarly, in the case of alcohols and polyols, the extract from raspberries showed a 14.3% share, while in tea it was 11.7%. Aromatic and phenolic compounds were present in the highest proportion in tea (7.5%) and the lowest in raspberries (5.4%) (Figure 5).
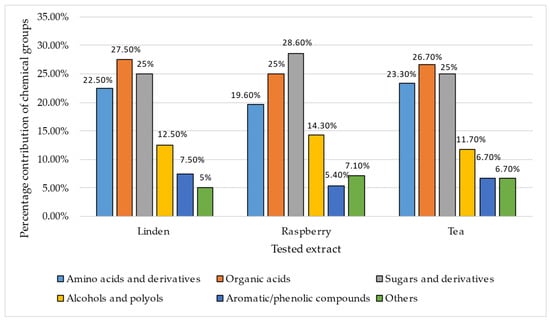
Figure 5.
Distribution of main chemical groups in linden, raspberry, and tea extracts [%].
In the linden extract, the most abundant compound was shikimic acid, constituting 8.81% of TIC, followed by fructose (8.16%) and glucose (7.92% for α-D-glucopyranose and 6.20% for β-D-glucopyranose). Other major compounds included quinic acid (6.74%) and inositol (3.37%). Malic acid accounted for 4.46% TIC, while methyl glucofuranoside contributed 4.42% TIC. The remaining identified compounds each constituted less than 4% TIC. Additionally, p-Coumaric acid (0.76%) and epicatechin (1.34%) were also detected, both of which are known for their bioactivity.
The raspberry shoots extract was characterized by a high content of sugars, including glucose (a combination of β-D-glucopyranose and α-D-glucopyranose, totaling 21.74%), β-fructofuranose (10.08%), and fructose (6.06%). Malic acid constituted 6.00% of TIC, while myo-inositol accounted for 4.45%. Additionally, quinic acid (1.73%) and gallic acid (0.56%) were present, both contributing to the antimicrobial potential of the extract.
The tea extract contained glucose (α-D-glucopyranose—5.13%, β-D-glucopyranose—4.50%) as one of the dominant sugars, along with sucrose (7.44% TIC). Among the organic acids, quinic acid (6.33%) and shikimic acid (1.52%) were present. The extract also contained notable amounts of epicatechin (3.46%) and epicatechin gallate (1.50%), both known for their antibacterial properties. Additionally, caffeine (1.60%) was detected, which has been reported to exhibit antimicrobial activity against certain bacterial strains. The compounds identified in the analyzed spectra, each contributing more than 1% of the total ion current (TIC), are summarized in Table 6.

Table 6.
Summary of identified compounds (>1% TIC) in analyzed spectra.
4. Discussion
In our study, we focused on evaluating the antibacterial activity of plant extracts obtained from various parts of plants, including bark, flowers, leaves, and shoots, against bacteria isolated from water used in the food processing industry. Our findings demonstrated antibacterial activity against opportunistic pathogens, which could contribute to the development of alternative methods for combating bacteria in this context. Research on the effects of various plant extracts on bacteria has been of significant interest to researchers worldwide for many years [74,75,76,77,78,79]. However, to the best of the authors’ knowledge, there is a gap in the research concerning the testing of bacteria isolated from water used in the food processing industry. The second part of the study aligns with numerous scientific reports describing the combined antibacterial effects of various plant parts. However, much of the existing research focuses on extracts from different parts of a single plant, similar extracts from different plant materials, or their interactions with antibiotics [80,81,82,83,84]. In contrast, our study explores combinations of extracts obtained both from different plant parts and different species. This mixed approach may offer a broader spectrum of bioactive compounds and diverse mechanisms of action, potentially enhancing antibacterial effects. While the selection of plant materials was informed by ethnobotanical and antimicrobial literature, the novelty of our study lies in the application of interspecies and inter-part combinations. Furthermore, it is noteworthy that natural substances could serve as an alternative to traditional chemical agents, whose production is energy-intensive and contributes to an increased carbon footprint, negatively impacting the environment [85]. In our study, the 13 plant species were selected based on their traditional use in herbal infusions or food preservation, documented antimicrobial potential, commercial availability, and phytochemical diversity to ensure a broad representation of compounds possibly involved in microbial inhibition.
The medicinal properties of Tilia L. flowers have been utilized for centuries in traditional folk medicine. The most commonly used raw material is dried linden flowers, which are employed in the form of infusions and herbal teas to alleviate symptoms such as colds, sore throats, and coughs [86]. Studies conducted by Pavlović et al. (2020) demonstrated that T. cordata extract exhibited the highest antimicrobial activity against most of the tested pathogens. Notably, some of the strains used in that study were isolated from clinical samples, including urine cultures, vaginal swabs, and oral swabs [40]. Although the antimicrobial properties of linden flower extracts have been confirmed, the current literature lacks studies evaluating their effects on a broader spectrum of pathogens. In contrast, similar research on honey has demonstrated antibacterial activity against reference strains (ATCC®) of P. aeruginosa, E. coli, and S. aureus [87]. Our study did not reveal any effect of the linden flower extract on E. faecalis, which may be attributed to the high environmental adaptability and unique cell wall structure of enterococci [88]. On the other hand, the study by Atanasova et al. did not confirm the antimicrobial activity of the tested linden flower extract against B. cereus, Citrobacter diversus, S. aureus, Pseudomonas spp., and Candida spp. One notable difference compared to our study is the use of a CO2 extract from flowers with bracts. Additionally, a different species, T. tomentosa Moench, was used in their tests [89]. Nevertheless, our study confirmed the antibacterial activity against the other five environmental strains tested. As noted by Oniszczuk et al., the main components of linden flowers include flavonoids (such as quercetin glycosides, kaempferol, and tiliroside), phenolic acids, essential oils, phytosterols, organic acids, tannins, mucilage, and vitamins [90]. GC-MS analysis revealed a high content of shikimic acid, which may have contributed to the inhibition of bacterial growth due to its known biological activity and antibacterial properties [91]. Similarly, malic acid (4.46% TIC) may have contributed to the interaction with pathogens. Research by Adamczak et al. demonstrated the impact of malic acid on E. faecalis, E. coli, and P. aeruginosa, with a minimum inhibitory concentration ranging from 500 to 1000 μg/mL [92]. The tested extract also revealed the presence of p-coumaric acid (0.76%) and epicatechin (1.34%), both of which have demonstrated established antimicrobial activity [93,94].
The shoots, leaves, and buds of raspberries are less studied compared to the commonly consumed fruits, which are rich in vitamins, minerals, organic acids, pectins, sugars, and fiber [95]. Nevertheless, traditional medicine utilizes infusions of young raspberry shoots to treat flu-like infections [96]. Studies on the composition of methanol extracts from raspberry shoots, conducted by Krauze-Baranowska et al., indicate the presence of various phenolic compounds, including ellagic acid and other polyphenols. Inhibitory effects were also observed against Bacillus subtilis, Clostridium sporogenes, Staphylococcus epidermidis, Neisseria meningitidis, Moraxella catarrhalis, and Helicobacter pylori [61]. Our studies also confirm the presence of antibacterial activity in raspberry extracts, with the strongest effect observed against P. aeruginosa and the weakest against S. marcescens. The previously mentioned ellagic and gallic acids were also identified in the tested raspberry shoot extract, at 0.38% and 0.56% TIC, respectively. These polyphenols exhibit antibacterial properties, as demonstrated in studies conducted on animal models [97]. The presence of quinic acid (1.73% TIC in our study) may also promote the antibacterial effect, particularly against Klebsiella spp. and P. aeruginosa [98].
The use of tea waste, specifically tea leaves, to produce extracts with confirmed antimicrobial properties aligns with sustainable resource management and zero-waste policies. The health benefits of green tea are well-documented and have been utilized for centuries in traditional medicinal practices [99]. Our studies demonstrated the antimicrobial activity of green tea extract on all tested pathogens, with the strongest activity against P. aeruginosa and E. faecalis. Research by Sharma et al. also highlighted the efficacy of tea extracts on a broad spectrum of bacteria, including skin pathogens (or pathobionts) such as S. epidermidis, Micrococcus luteus, Brevibacterium linens, P. fluorescens, and B. subtilis [100]. Studies have also confirmed that tea extracts exhibit antibacterial activity against resistant and multidrug-resistant strains, highlighting their potential for future use [101]. One of the key bioactive compounds in green tea extracts is epigallocatechin gallate (EGCG), a predominant catechin known for its strong antibacterial properties, accounting for 1.15% of the total ion current (TIC) in our study. Previous research has also demonstrated that the bioactive compounds in green tea extracts exhibit antimicrobial activity against E. coli and bacteria from the Salmonella genus [102].
Tree bark is a rich source of substances used in the treatment of diseases and the direct combat of pathogens [103]. Studies on tree bark extracts traditionally used by First Nations in North America have demonstrated that 86% of the extracts exhibited activity against methicillin-sensitive S. aureus, 71% against Bacillus subtilis, and 79% against Mycobacterium phlei [104]. Our study demonstrated the effectiveness of extracts from birch, oak, and willow bark against six distinct opportunistic pathogens. This finding is consistent with existing literature, which reports that bark extracts are effective against a variety of bacteria, including Escherichia coli and Pseudomonas aeruginosa [105,106]. The antimicrobial properties of oak bark extracts are primarily attributed to the presence of phenolic compounds, such as gallic and ellagic acids, which exhibit both bacteriostatic and bactericidal activity [43,107]. Our studies did not confirm antibacterial activity for Quercus suber bark, contrary to the findings of Gonçalves et al., who demonstrated antibacterial activity against S. aureus and E. coli [108]. In our studies, we utilized expanded cork, a material produced by exposing cork to high temperatures (typically around 180–200 °C) during its manufacturing process. This thermal treatment could have significantly altered the chemical composition of the cork bark, potentially leading to the degradation or volatilization of thermolabile bioactive compounds responsible for the antimicrobial properties. As for the antibacterial effect of B. pendula extract, our findings are in line with the existing literature. Chemical compounds in birch bark, such as betulin and other triterpenes, have demonstrated antibacterial properties and may inhibit the growth of various bacterial strains [109]. Polyphenols found across the Betula L. genus include catechin, salidroside, rhododendrin, and platyphylloside, which are known for their antioxidant, anti-inflammatory, and antimicrobial properties [110].
The interaction extends beyond bacteria; extracts from birch bark have also shown antiviral and antifungal activity against various pathogenic strains [51]. The presence of salicin is a significant factor contributing to the widespread investigation and utilization of Salix species extracts in therapeutic applications. Alicin, along with other phenolic compounds such as procyanidins, can inhibit bacterial growth through various mechanisms, including antioxidant activity [111]. However, other bioactive compounds present in willow bark may also play a significant role in its antibacterial activity. Research by Piątczak et al. has identified quercetin glycosides, monomeric, dimeric, and trimeric flavan-3-ol derivatives, including B-type procyanidins, as well as cavoylquinone pseudodepsides, all of which may contribute to the observed antimicrobial effects [63].
Our study also revealed that the combination of plant extracts exhibiting antimicrobial activity in the initial screening phase can lead to enhanced inhibition of pathogen growth. All extract mixtures were prepared in a 1:1 (w/w) ratio, and the same total volume (100 μL) was applied in each assay. This means that the individual extracts were present at half the concentration used in the single-extract tests. Therefore, any observed increase in antimicrobial activity in the combinations, despite the lower concentration of each extract, may indicate an increased effect. Notably, five of the six extract mixtures that demonstrated increased antibacterial effects contained at least one bark extract. A similar observation was made in the study by Jeong et al., where the combination of extracts from Solanum torvum and Uncaria gambir significantly augmented their antibacterial activity against methicillin-resistant S. aureus strains [112]. Similarly, Ncube et al., in their research, highlight that utilizing the synergistic effects of plant extracts may have potential therapeutic applications [113]. Individual extracts within the mixture may target distinct cellular mechanisms of the pathogen. For instance, one extract could disrupt the integrity of the bacterial cell membrane, thus facilitating the penetration of a second extract, which could act on other key metabolic processes, such as protein synthesis or DNA replication. Alternatively, one of the extracts may inhibit the pathogen’s resistance mechanisms, such as efflux pumps, thus enhancing the effectiveness of the second component within the mixture [114,115].
Several factors may explain the lack of confirmed antimicrobial activity for the seven extracts despite existing literature reports. The primary factors include the plant species, the type of material used, and the storage conditions of the raw material [116]. The method of extract preparation is also crucial. As noted by Sánchez et al., methanolic extracts consistently exhibited higher antimicrobial activity compared to ethanolic or aqueous extracts. They attributed this difference to the solvent’s ability to dissolve a greater number of bioactive compounds [117]. The selection of strains is equally important, as their origin can significantly influence the variation in response to the applied extracts. The limitations of our study include the use of a single extraction method (ethanol:water), which may not have captured the full spectrum of bioactive compounds. Additionally, the thermal processing of extracts at 65 °C could have led to the degradation of heat-sensitive compounds, potentially reducing their antibacterial activity. Furthermore, the study focused solely on antibacterial activity without testing other bioactivities, and solvent control was not implemented in the study. However, these limitations should be considered in the context of this being a pilot study, which aimed to provide preliminary insights into the antimicrobial potential of plant extracts. The agar-well diffusion method was selected for this study as it provides a practical and reproducible approach for the preliminary screening of antimicrobial activity of plant extracts. This technique allows for the direct application of a defined volume of extract into wells, facilitating better diffusion into the medium. The study was limited to the well-diffusion method; however, complementary techniques such as MIC, MBC, or other quantitative assays could enhance the evaluation of antimicrobial efficacy in future research. Additionally, the use of reference strains would provide a clearer understanding of the differences in the effects of the extracts on environmental strains.
Our study confirmed the research hypothesis, which postulated that the application of extracts from various plant materials is effective against pathogens isolated from rinse water in the agri-food industry. Furthermore, it was established that combining bark extracts with those rich in volatile organic compounds (derived from leaves, shoots, flowers, and fruits) results in an increased effect, enhancing the antibacterial activity against the examined bacterial strains.
The presence of various secondary metabolites in the plant species analyzed in this study has been reported in the literature. Although these compounds have been previously identified, their antimicrobial potential against opportunistic pathogens isolated from water has not been extensively investigated.
5. Conclusions
The study demonstrated that plant extracts exhibit differential antimicrobial activity. C. sinensis and R. idaeus showed the highest efficacy, particularly against P. aeruginosa and E. faecalis, while Q. robur, B. pendula, T. cordata, and S. alba exhibited lower effects. No antimicrobial activity was observed for Coffea L., R. canina, T. officinale, Q. suber, V. officinalis, and P. sylvestris. GC-MS analysis identified various bioactive compounds in the most effective extracts, including epicatechin, shikimic acid, quinic acid, gallic acid, and caffeine, which are known for their antimicrobial properties. The presence of these compounds may explain the observed inhibitory effects and supports further investigation into their mechanisms of action. From an economic perspective, the tested plant materials are locally available and low-cost, which makes them promising candidates for sustainable and accessible antimicrobial applications in water treatment systems. In conclusion, this study highlights the potential of plant-derived extracts as natural antimicrobial agents against strains isolated from wash water in the agri-food industry. In a broader context, this study investigated the potential of natural plant extracts for application in non-invasive water treatment approaches relevant to the agri-food sector. The research was exploratory in nature and represents a preliminary phase toward the development of a practical and scalable solution. The broader project constitutes a research continuum that will ultimately lead to the design and evaluation of a pilot-scale filtration bed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15095199/s1.
Author Contributions
Conceptualization, P.K. and B.B.-B.; methodology, P.K., B.B.-B. and M.S.; validation, P.K.; formal analysis, P.K. and M.S.; investigation, P.K.; resources P.K and B.B-B.; data curation, P.K.; writing—original draft preparation, P.K.; writing—review and editing, P.K. and B.B-B.; visualization, P.K. and M.S.; supervision, B.B.-B.; project administration, B.B-B.; funding acquisition, P.K and B.B-B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by funds from the Ministry of Science and Higher Education of the Republic of Poland, No: DWD/5/0207/2021. The APC was funded by Bydgoszcz University of Science and Technology, as part of the “Działania Naukowe Młodych” program, grant number: DNM/2/24.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data supporting the findings of this article are included within the manuscript. Raw data, as well as any additional materials, are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salehi, M. Global Water Shortage and Potable Water Safety; Today’s Concern and Tomorrow’s Crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of Emerging Implications of Climate Change on Food Production Systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Carvalho, M.; Martins, S. Sustainable Water Management: Understanding the Socioeconomic and Cultural Dimensions. Sustainability 2023, 15, 13074. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Oral, H.V.; Calheiros, C.S.C.; Krzeminski, P.; Güçlü, S.; Pereira, S.A.; Surmacz-Górska, J.; Plaza, E.; Samaras, P.; Binder, P.M.; et al. Current Challenges and Future Perspectives for the Full Circular Economy of Water in European Countries. J. Environ. Manag. 2023, 345, 118627. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water Scarcity in Agriculture: An Overview of Causes, Impacts and Approaches for Reducing the Risks. Heliyon 2023, 9. [Google Scholar] [CrossRef]
- Nasiri, A.R.; Kerachian, R.; Mashhadi, M.; Shahangian, S.A.; Zobeidi, T. Extending the Theory of Planned Behavior to Predict the Behavior of Farmers in Choosing Low-Water-Intensive Medicinal Plants. J. Environ. Manag. 2024, 369, 122333. [Google Scholar] [CrossRef]
- Manzocco, L.; Ignat, A.; Anese, M.; Bot, F.; Calligaris, S.; Valoppi, F.; Nicoli, M.C. Efficient Management of the Water Resource in the Fresh-Cut Industry: Current Status and Perspectives. Trends Food Sci. Technol. 2015, 46, 286–294. [Google Scholar] [CrossRef]
- Lehto, M.; Sipilä, I.; Alakukku, L.; Kymäläinen, H.-R. Water Consumption and Wastewaters in Fresh-Cut Vegetable Production. Agric. Food Sci. 2014, 23, 246–256. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential Alternative Disinfection Methods for Organic Fresh-Cut Industry for Minimizing Water Consumption and Environmental Impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Raffo, A.; Paoletti, F. Fresh-Cut Vegetables Processing: Environmental Sustainability and Food Safety Issues in a Comprehensive Perspective. Front. Sustain. Food Syst. 2022, 5, 681459. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-Cut Product Sanitation and Wash Water Disinfection: Problems and Solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ingram, D.T.; Khurana, K. 7—Preventing Cross-Contamination during Produce Wash Operations. In Global Safety of Fresh Produce; Hoorfar, J., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2014; pp. 103–111. ISBN 978-1-78242-018-7. [Google Scholar]
- Jensen, D.A.; Friedrich, L.M.; Harris, L.J.; Danyluk, M.D.; Schaffner, D.W. Cross Contamination of Escherichia coli O157:H7 between Lettuce and Wash Water during Home-Scale Washing. Food Microbiol. 2015, 46, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Klištincová, N.; Pin, L.; Puškárová, A.; Giannino, D.; Bučková, M.; Lambreva, M.D.; Manfredini, A.; Canfora, L.; Pangallo, D.; Pinzari, F. From Farm to Fork: Fungal and Bacterial Contaminants and Their Diagnostics in the Production Steps of Ready-to-Eat Salads. Trends Food Sci. Technol. 2024, 150, 104573. [Google Scholar] [CrossRef]
- Hwang, J.; Ryu, H.-S.; Kim, H.A.; Hyun, M.; Lee, J.Y.; Yi, H.-A. Prognostic Factors of COVID-19 Infection in Elderly Patients: A Multicenter Study. J. Clin. Med. 2020, 9, 3932. [Google Scholar] [CrossRef]
- Bolívar, A.; Saiz-Abajo, M.J.; García-Gimeno, R.M.; Petri-Ortega, E.; Díez-Leturia, M.; González, D.; Vitas, A.I.; Pérez-Rodríguez, F. Cross Contamination of Escherichia coli O157:H7 in Fresh-Cut Leafy Vegetables: Derivation of a Food Safety Objective and Other Risk Management Metrics. Food Control 2023, 147, 109599. [Google Scholar] [CrossRef]
- Fan, X.; Gurtler, J.B.; Mattheis, J.P. Possible Sources of Listeria monocytogenes Contamination of Fresh-Cut Apples and Antimicrobial Interventions During Antibrowning Treatments: A Review. J. Food Prot. 2023, 86, 100100. [Google Scholar] [CrossRef]
- Oyenuga, N.; Cobo-Díaz, J.F.; Alvarez-Ordóñez, A.; Alexa, E.-A. Overview of Antimicrobial Resistant ESKAPEE Pathogens in Food Sources and Their Implications from a One Health Perspective. Microorganisms 2024, 12, 2084. [Google Scholar] [CrossRef]
- Garnier, C.; Guiga, W.; Lameloise, M.-L.; Fargues, C. Water Reuse in the Food Processing Industries: A Review on Pressure-Driven Membrane Processes as Reconditioning Treatments. J. Food Eng. 2023, 344, 111397. [Google Scholar] [CrossRef]
- Kanarek, P.; Breza-Boruta, B.; Poćwiardowski, W.; Szulc, J. Sustainable Water Use in a Fruit Processing Plant: Evaluation of Microbiological and Physicochemical Properties of Wash Water after Application of a Modular Water Recovery System. Sustainability 2024, 16, 2181. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Allende, A.; Gil, M.I. Recent Progress on the Management of the Industrial Washing of Fresh Produce with a Focus on Microbiological Risks. Curr. Opin. Food Sci. 2021, 38, 46–51. [Google Scholar] [CrossRef]
- Mane, M.B.; Bhandari, V.M.; Balapure, K.; Ranade, V.V. Destroying Antimicrobial Resistant Bacteria (AMR) and Difficult, Opportunistic Pathogen Using Cavitation and Natural Oils/Plant Extract. Ultrason. Sonochem. 2020, 69, 105272. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The Antimicrobial Capacity of Cistus salviifolius and Punica granatum Plant Extracts against Clinical Pathogens Is Related to Their Polyphenolic Composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Essghaier, B.; Ben Khedher, G.; Hannachi, H.; Dridi, R.; Zid, M.F.; Chaffei, C. Green Synthesis of Silver Nanoparticles Using Mixed Leaves Aqueous Extract of Wild Olive and Pistachio: Characterization, Antioxidant, Antimicrobial and Effect on Virulence Factors of Candida. Arch. Microbiol. 2022, 204, 203. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas Chromatography Mass Spectrometry–Based Metabolite Profiling in Plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.; Kubo, A.; Nihei, K.; Ogura, T. Antibacterial Activity of Coriander Volatile Compounds against Salmonella Choleraesuis. J. Agric. Food Chem. 2004, 52, 3329–3332. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid. Based Complement. Alternat. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.A.P.; Farah, A.; Silva, D.A.M.; Nunan, E.A.; Glória, M.B.A. Antibacterial Activity of Coffee Extracts and Selected Coffee Chemical Compounds against Enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Bouhlal, F.; Aqil, Y.; Chamkhi, I.; Belmaghraoui, W.; Labjar, N.; Hajjaji, S.E.; Benabdellah, G.A.; Aurag, J.; Lotfi, E.M.; Mahi, M.E. GC-MS Analysis, Phenolic Compounds Quantification, Antioxidant, and Antibacterial Activities of the Hydro-Alcoholic Extract of Spent Coffee Grounds. J. Biol. Act. Prod. Nat. 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Meletis, C.D. Coffee–Functional Food and Medicinal Herb. Altern. Complement. Ther. 2006, 12, 7–13. [Google Scholar] [CrossRef]
- Weyesa, G.; Tilahun, R. Documentation of Traditional Knowledge on “Coffee” (Coffea arabica) in Jimma, Ilubabor and Wollega Zones. Eur. J. Biophys. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Kim, S.; Ruengwilysup, C.; Fung, D.Y.C. Antibacterial Effect of Water-Soluble Tea Extracts on Foodborne Pathogens in Laboratory Medium and in a Food Model. J. Food Prot. 2004, 67, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Li, H.; Qiu, Z.; Yu, Y. Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts. Foods 2022, 11, 620. [Google Scholar] [CrossRef]
- Boroughani, M.; Tahmasbi, Z.; Heidari, M.M.; Johari, M.; Hashempur, M.H.; Heydari, M. Potential Therapeutic Effects of Green Tea (Camellia sinensis) in Eye Diseases, a Review. Heliyon 2024, 10, e28829. [Google Scholar] [CrossRef] [PubMed]
- Kaewkod, T.; Songkhakul, W.; Tragoolpua, Y. Inhibitory Effects of Tea Leaf and Medicinal Plant Extracts on Enteric Pathogenic Bacteria Growth, Oxidation and Epithelial Cell Adhesion. Pharmacogn. Res. 2021, 14, 71–81. [Google Scholar] [CrossRef]
- Alimoddin, M.; Jayakumari, S.; Fatima, B.; Hasan, N.; Ali, S.; Sami, F.; Ali, M.S.; Nair, R.S.; Ansari, M.T. Pharmacological Applications of Urtica dioica: A Comprehensive Review of Its Traditional Use and Modern Scientific Evidence. J. Herb. Med. 2024, 48, 100935. [Google Scholar] [CrossRef]
- Harrison, F.; Furner-Pardoe, J.; Connelly, E. An Assessment of the Evidence for Antibacterial Activity of Stinging Nettle (Urtica dioica) Extracts. Access Microbiol. 2022, 4, 000336. [Google Scholar] [CrossRef]
- Du, X.-F.; Xiao, M.; Liang, H.-Y.; Sun, Z.; Jiang, Y.-H.; Chen, G.-Y.; Meng, X.-Y.; Zou, G.-L.; Zhang, L.; Liu, Y.-L.; et al. An Improved MLVF Method and Its Comparison with Traditional MLVF, Spa Typing, MLST/SCCmec and PFGE for the Typing of Methicillin-Resistant Staphylococcus Aureus. Int. J. Mol. Sci. 2014, 15, 725–742. [Google Scholar] [CrossRef]
- Motamedi, H.; Seyyednejad, S.M.; Bakhtiari, A.; Vafaei, M. Introducing Urtica dioica, A Native Plant of Khuzestan, As an Antibacterial Medicinal Plant. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e15904. [Google Scholar] [CrossRef]
- Pavlović, T.; Dimkić, I.; Andrić, S.; Milojković-Opsenica, D.; Stanković, S.; Janaćković, P.; Gavrilović, M.; Ristivojević, P. Linden Tea from Serbia—An Insight into the Phenolic Profile, Radical Scavenging and Antimicrobial Activities. Ind. Crops Prod. 2020, 154, 112639. [Google Scholar] [CrossRef]
- Cittan, M.; Altuntaş, E.; Çelik, A. Evaluation of Antioxidant Capacities and Phenolic Profiles in Tilia cordata Fruit Extracts: A Comparative Study to Determine the Efficiency of Traditional Hot Water Infusion Method. Ind. Crops Prod. 2018, 122, 553–558. [Google Scholar] [CrossRef]
- Valencia-Avilés, E.; Martínez-Flores, H.-E.; García-Pérez, M.; Meléndez-Herrera, E.; García-Pérez, M.-E. Investigation of the Antibacterial Activity and Subacute Toxicity of a Quercus Crassifolia Polyphenolic Bark Extract for Its Potential Use in Functional Foods. J. Food Sci. 2019, 84, 1692–1702. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Ciurea, C.N.; Mare, A.D.; Man, A.; Nisca, A.; Nicolescu, A.; Mocan, A.; Babotă, M.; Coman, N.-A.; Tanase, C. Quercus Robur Older Bark—A Source of Polyphenolic Extracts with Biological Activities. Appl. Sci. 2022, 12, 11738. [Google Scholar] [CrossRef]
- Häsler Gunnarsdottir, S.; Sommerauer, L.; Schnabel, T.; Oostingh, G.J.; Schuster, A. Antioxidative and Antimicrobial Evaluation of Bark Extracts from Common European Trees in Light of Dermal Applications. Antibiotics 2023, 12, 130. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The Polyphenolic Profile of Oak (Quercus) Species: A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.Ł.; Kačániová, M. Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, IPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 1888. [Google Scholar] [CrossRef] [PubMed]
- Miljković, V.M.; Nikolić, L.; Mrmošanin, J.; Gajić, I.; Mihajilov-Krstev, T.; Zvezdanović, J.; Miljković, M. Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia. Int. J. Mol. Sci. 2024, 25, 2518. [Google Scholar] [CrossRef]
- Khazaei, M.; Khazaei, M.R.; Pazhouhi, M. An Overview of Therapeutic Potentials of Rosa canina—A Traditionally Valuable Herb. In Vitro. WCRJ 2020, 7, e1580. [Google Scholar]
- Masnavi, E.; Hassanzadeh, S.; Karimi, K.; Malekzadeh, J.; Khoramrooz, S.S. Antibacterial Activities of Hydroalcoholic Extract of Rosa canina L. against Hospital Acquired Infections. J. Clin. Care Ski. 2024, 5, 157–163. [Google Scholar]
- Azman, N.A.M.; Skowyra, M.; Muhammad, K.; Gallego, M.G.; Almajano, M.P. Evaluation of the Antioxidant Activity of Betula pendula Leaves Extract and Its Effects on Model Foods. Pharm. Biol. 2017, 55, 912–919. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal Plants of the Genus Betula—Traditional Uses and a Phytochemical–Pharmacological Review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and Identification of Compounds from Bioactive Extracts of Taraxacum officinale Weber Ex F. H. Wigg. (Dandelion) as a Potential Source of Antibacterial Agents. Evid. Based Complement. Alternat. Med. 2018, 2018, 2706417. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Antibacterial Activity of Taraxacum officinale against Foodborne Pathogens. Pak. J. Zool. 2022, 54, 1–8. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and Related Species—An Ethnopharmacological Review and Its Potential as a Commercial Medicinal Plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Fernandes, I.; Cruz, L.; Mateus, N.; Cabral, M.; de Freitas, V. Antioxidant and Biological Properties of Bioactive Phenolic Compounds from Quercus suber L. J. Agric. Food Chem. 2009, 57, 11154–11160. [Google Scholar] [CrossRef]
- Silva, S.; Machado, M.; Coelho, M.; Costa, E.M.; Pintado, M. Insights into the Antimicrobial Potential of Acorn Extracts (Quercus ilex and Quercus suber). Appl. Sci. 2023, 13, 6820. [Google Scholar] [CrossRef]
- Haytham, B.; Brahmi, F.; Zaidi, K.; Mehiou, A.; Dib, I.; Berraaouan, A.; Touzani, R.; Ziyyat, A.; Chaachouay, N.; Jan, H.A.; et al. Quercus coccifera L. Quercus faginea Lam. Quercus ilex L. Quercus rotundifolia Lam. Quercus infectoria G. Olivier Quercus suber L. Fagaceae. In Ethnobotany of Northern Africa and Levant; Bussmann, R.W., Elachouri, M., Kikvidze, Z., Eds.; Springer: Cham, Switzerland, 2024; pp. 1707–1727. ISBN 978-3-031-43105-0. [Google Scholar]
- Oliveira, B.D.; Rodrigues, A.C.; Cardoso, B.M.I.; Ramos, A.L.C.C.; Bertoldi, M.C.; Taylor, J.G.; da Cunha, L.R.; Pinto, U.M. Antioxidant, Antimicrobial and Anti-Quorum Sensing Activities of Rubus rosaefolius Phenolic Extract. Ind. Crops Prod. 2016, 84, 59–66. [Google Scholar] [CrossRef]
- Zhang, C.; Cock, I.E. Anti-Microbial Activity of Rubus idaeus L. Leaf Extracts in Combination with Antibiotics against Bacterial Triggers of Selected Autoimmune Diseases. Pharmacogn. Commun. 2023, 13, 176–186. [Google Scholar] [CrossRef]
- Mehiou, A.; Dib, I.; Berraaouane, A.; Ziyyat, A.; Elachouri, M.; Bussmann, R.W. Rubus Idaeus L., Rubus Ulmifolius Schott, Rubus Vulgaris Weihe & Nees Rosaceae. In Ethnobotany of Northern Africa and Levant; Bussmann, R.W., Elachouri, M., Kikvidze, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–15. ISBN 978-3-031-13933-8. [Google Scholar]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical Composition and Biological Activity of Rubus Idaeus Shoots—A Traditional Herbal Remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef]
- Javed, B.; Nawaz, K.; Munazir, M. Phytochemical Analysis and Antibacterial Activity of Tannins Extracted from Salix Alba L. Against Different Gram-Positive and Gram-Negative Bacterial Strains. Iran. J. Sci. Technol. Trans. Sci. 2020, 44, 1303–1314. [Google Scholar] [CrossRef]
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef]
- Shara, M.; Stohs, S.J. Efficacy and Safety of White Willow Bark (Salix alba) Extracts. Phytother. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Ahmed Qasim, K.; Ashraf, C.M.; Maab, H. Verbena Officinalis a Herb with Promising Broad Spectrum Antimicrobial Potential. Cogent Chem. 2017, 3, 1363342. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Dominiak, J.; Luczkiewicz, M.; Ekiert, H. Verbena Officinalis (Common Vervain)—A Review on the Investigations of This Medicinally Important Plant Species. Planta Med. 2020, 86, 1241–1257. [Google Scholar] [CrossRef]
- Dziedziński, M.; Kobus-Cisowska, J.; Powałowska, D.S.; Szablewska, K.S.; Baranowska, M. Polyphenols Composition, Antioxidant and Antimicrobial Properties of Pinus sylvestris L. Shoots Extracts Depending on Different Drying Methods. Emir. J. Food Agric. 2020, 229, 229–237. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Rubens, J.; Kibilds, J.; Jansons, M.; Piginka-Vjaceslavova, I.; Barene, I.; Daberte, I.; Liepa, L.; Malniece, A.; Rubens, A.; Starkute, V.; et al. Application of Baltic Pine (Pinus sylvestris) Needle Extract as a Gut Microbiota-Modulating Feed Supplement for Domestic Chickens (Gallus gallus). Plants 2023, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Papp, N.; Purger, D.; Czigle, S.; Czégényi, D.; Stranczinger, S.; Tóth, M.; Dénes, T.; Kocsis, M.; Takácsi-Nagy, A.; Filep, R. The Importance of Pine Species in the Ethnomedicine of Transylvania (Romania). Plants 2022, 11, 2331. [Google Scholar] [CrossRef]
- Kanarek, P.; Breza-Boruta, B.; Bogiel, T. In the Depths of Wash Water: Isolation of Opportunistic Bacteria from Fresh-Cut Processing Plants. Pathogens 2024, 13, 768. [Google Scholar] [CrossRef]
- Mir, I.H.; Sharma, M.K.; Singh, A. Antimicrobial activity of some medicinal plant extracts against multidrug resistant bacteria. J. Adv. Sci. Res. 2021, 12, 42–47. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A Chemical Data Resource on the Internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- Lucas, E.H.; Pearson, K. Preparation of Crude Plant Extracts and Their Assay for Presence of Antibacterial Substances. Food Res. 1948, 13, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial Activity of Pomegranate Peel Extracts as Affected by Cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef]
- Santos, D.K.D.d.N.; de Almeida, V.S.; de Araujo, D.R.C.; Harand, W.; Soares, A.K.d.A.; Moreira, L.R.; de Lorena, V.M.B.; Magalhães, L.P.M.; Ximenes, R.M.; de Sena, K.X.d.F.R.; et al. Evaluation of Cytotoxic, Immunomodulatory and Antibacterial Activities of Aqueous Extract from Leaves of Conocarpus erectus Linnaeus (Combretaceae). J. Pharm. Pharmacol. 2018, 70, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Nocedo-Mena, D.; Garza-González, E.; González-Ferrara, M.; Del Rayo Camacho-Corona, M. Antibacterial Activity of Cissus Incisa Extracts against Multidrug- Resistant Bacteria. Curr. Top. Med. Chem. 2020, 20, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Dell’Anno, M.; Liu, Y.; Rossi, L.; Aksmann, A.; Pogorzelski, G.; Jóźwik, A. Assessment of the Antibacterial and Antioxidant Activities of Seaweed-Derived Extracts. Sci. Rep. 2024, 14, 21044. [Google Scholar] [CrossRef]
- Strompfová, V.; Štempelová, L.; Wolaschka, T. Antibacterial Activity of Plant-Derived Compounds and Cream Formulations against Canine Skin Bacteria. Vet. Res. Commun. 2024, 48, 1459–1470. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Majeed, M.; Hakeem, K.R.; Rehman, R.U. Synergistic Effect of Plant Extract Coupled Silver Nanoparticles in Various Therapeutic Applications- Present Insights and Bottlenecks. Chemosphere 2022, 288, 132527. [Google Scholar] [CrossRef]
- Donkor, M.N.; Donkor, A.-M.; Mosobil, R. Combination Therapy: Synergism among Three Plant Extracts against Selected Pathogens. BMC Res. Notes 2023, 16, 83. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, T.-J. Synergistic Growth Inhibition of Herbal Plant Extract Combinations against Candida albicans. J. Korean Wood Sci. Technol. 2023, 51, 145–156. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dutta, H. Use of Nature-Derived Antimicrobial Substances as Safe Disinfectants and Preservatives in Food Processing Industries: A Review. J. Food Process. Preserv. 2022, 46, e15999. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Ren, Q.; Shen, Y.-B. Comprehensive Review of Tilia L.: Phytochemical Profiles, Edible Value, Therapeutic Potentials, and Ecological Significance. Food Med. Homol. 2025, 2, 9420035. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Lukash, O.; Yakovenko, O.; Opryshko, M.; Maryniuk, M.; Gyrenko, O.; Buyun, L. In Vitro Antibacterial Efficacy of Different Natural Linden Honeys against Some Gram-Positive and Gram-Negative Strains. Agrobiodivers. Improv. Nutr. Health Life Qual. 2023, 7. [Google Scholar] [CrossRef]
- Gaca, A.O.; Lemos, J.A. Adaptation to Adversity: The Intermingling of Stress Tolerance and Pathogenesis in Enterococci. Microbiol. Mol. Biol. Rev. 2019, 83, e00008-19. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, T.; Gochev, V.; Stoyanova, A.; Girova, T.; Djurdjev, I. Chemical Composition and Antimicrobial Activity of Linden (Tilia tomentosa Moench.) CO2 Extract. Plovdiv Univ., Paisii Hilendarski” Bulg. Sci. Pap. 2008, 36, 91. [Google Scholar]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Olech, M.; Nowak, R.; Wojtunik, K.; Klimek, M.; Krawczyk, W.; Hajnos, M. Extruded Corn Gruels Containing Linden Flowers: Quantitation of Phenolic Compounds and Selected Quality Characteristics. Open Chem. 2015, 13, 000010151520150138. [Google Scholar] [CrossRef]
- Hou, L.; Ye, M.; Wang, X.; Zhu, Y.; Sun, X.; Gu, R.; Chen, L.; Fang, B. Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus aureus. Molecules 2024, 29, 1528. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. P-Coumaric Acid Kills Bacteria through Dual Damage Mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Kurahachi, M.; Nonaka, A.; Miyamoto, T. A Study of the Antibacterial Mechanism of Catechins: Isolation and Identification of Escherichia coli Cell Surface Proteins That Interact with Epigallocatechin gallate. Food Control 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules 2021, 26, 327. [Google Scholar] [CrossRef]
- Hummer, K.E. Rubus Pharmacology: Antiquity to the Present. HortScience 2010, 45, 1587–1591. [Google Scholar] [CrossRef]
- Cota, D.; Patil, D. Antibacterial Potential of Ellagic Acid and Gallic Acid against IBD Bacterial Isolates and Cytotoxicity against Colorectal Cancer. Nat. Prod. Res. 2023, 37, 1998–2002. [Google Scholar] [CrossRef]
- Ercan, L.; Doğru, M. Antioxidant and Antimicrobial Capacity of Quinic Acid. Bitlis Eren Üniversitesi Fen Bilim. Derg. 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Hart, R.; Abd_Allah, E.F.; Hashem, A.; Alsayed, M.F.; Ijaz, F.; Ali, N.; Shah, M.; et al. Herbal Teas and Drinks: Folk Medicine of the Manoor Valley, Lesser Himalaya, Pakistan. Plants 2019, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, S.; Sarethy, I.P.; Dang, S.; Gabrani, R. Green Tea Extract: Possible Mechanism and Antibacterial Activity on Skin Pathogens. Food Chem. 2012, 135, 672–675. [Google Scholar] [CrossRef]
- Radji, M.; Agustama, R.A.; Elya, B.; Tjampakasari, C.R. Antimicrobial Activity of Green Tea Extract against Isolates of Methicillin–Resistant Staphylococcus Aureus and Multi–Drug Resistant Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2013, 3, 663–667. [Google Scholar] [CrossRef]
- Hope, O.; Bright, I.E.; Alagbonsi, A.I. GC-MS Biocomponents Characterization and Antibacterial Potency of Ethanolic Crude Extracts of Camellia sinensis. SAGE Open Med. 2022, 10, 20503121221116860. [Google Scholar] [CrossRef]
- Ucella-Filho, J.G.M.; Freire, A.d.S.M.; Carréra, J.C.; Lucas, F.M.F.; Zucolotto, S.M.; Dias Júnior, A.F.; Mori, F.A. Tannin-Rich Bark Extract of Plants as a Source of Antimicrobial Bioactive Compounds: A Bibliometric Analysis. S. Afr. J. Bot. 2022, 150, 1038–1050. [Google Scholar] [CrossRef]
- Omar, S.; Lemonnier, B.; Jones, N.; Ficker, C.; Smith, M.L.; Neema, C.; Towers, G.H.N.; Goel, K.; Arnason, J.T. Antimicrobial Activity of Extracts of Eastern North American Hardwood Trees and Relation to Traditional Medicine. J. Ethnopharmacol. 2000, 73, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Elansary, O.H.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, A.M.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus Spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Šukele, R.; Skadiņš, I.; Koka, R.; Bandere, D. Antibacterial Effects of Oak Bark (Quercus robur) and Heather Herb (Calluna vulgaris L.) Extracts against the Causative Bacteria of Bovine Mastitis. Vet. World 2022, 15, 2315–2322. [Google Scholar] [CrossRef]
- Tanase, C.; Nicolescu, A.; Nisca, A.; Ștefănescu, R.; Babotă, M.; Mare, A.D.; Ciurea, C.N.; Man, A. Biological Activity of Bark Extracts from Northern Red Oak (Quercus rubra L.): An Antioxidant, Antimicrobial and Enzymatic Inhibitory Evaluation. Plants 2022, 11, 2357. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.; Correia, P.; Silva, S.P.; Almeida-Aguiar, C. Evaluation of Antimicrobial Properties of Cork. FEMS Microbiol. Lett. 2016, 363, fnv231. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, D.; St-Pierre, A.; Bourdeau, N.; Bley, J.; Lajeunesse, A.; Desgagné-Penix, I. Antimicrobial Activity and Chemical Composition of White Birch (Betula papyrifera Marshall) Bark Extracts. MicrobiologyOpen 2020, 9, e00944. [Google Scholar] [CrossRef]
- Royer, M.; Houde, R.; Viano, Y.; Stevanovic, T. Non-Wood Forest Products Based on Extractives—A New Opportunity for the Canadian Forest Industry Part 1: Hardwood Forest Species. J. Food Res. 2012, 1, 8. [Google Scholar] [CrossRef]
- Safta, D.A.; Ielciu, I.; Șuștic, R.; Hanganu, D.; Niculae, M.; Cenariu, M.; Pall, E.; Moldovan, M.L.; Achim, M.; Bogdan, C.; et al. Chemical Profile and Biological Effects of an Herbal Mixture for the Development of an Oil-in-Water Cream. Plants 2023, 12, 248. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Jung, I.-G.; Yum, S.-H.; Hwang, Y.-J. In Vitro Synergistic Inhibitory Effects of Plant Extract Combinations on Bacterial Growth of Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 1491. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. In Vitro Antimicrobial Synergism within Plant Extract Combinations from Three South African Medicinal Bulbs. J. Ethnopharmacol. 2012, 139, 81–89. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, L.; Martins, A.P.; Correia, H. Raw Materials: The Importance of Quality and Safety. A Review. Flavour Fragr. J. 2010, 25, 253–271. [Google Scholar] [CrossRef]
- Sánchez, E.; García, S.; Heredia, N. Extracts of Edible and Medicinal Plants Damage Membranes of Vibrio Cholerae. Appl. Environ. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).