Antimicrobial Activity and Phytochemical Profiling of Natural Plant Extracts for Biological Control of Wash Water in the Agri-Food Industry
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Preparation of Extracts
2.3. The Well Difusion Method
2.4. Gas Chromatography-Mass Spectrometry Analysis
3. Results
3.1. Evaluation of Extract Activity
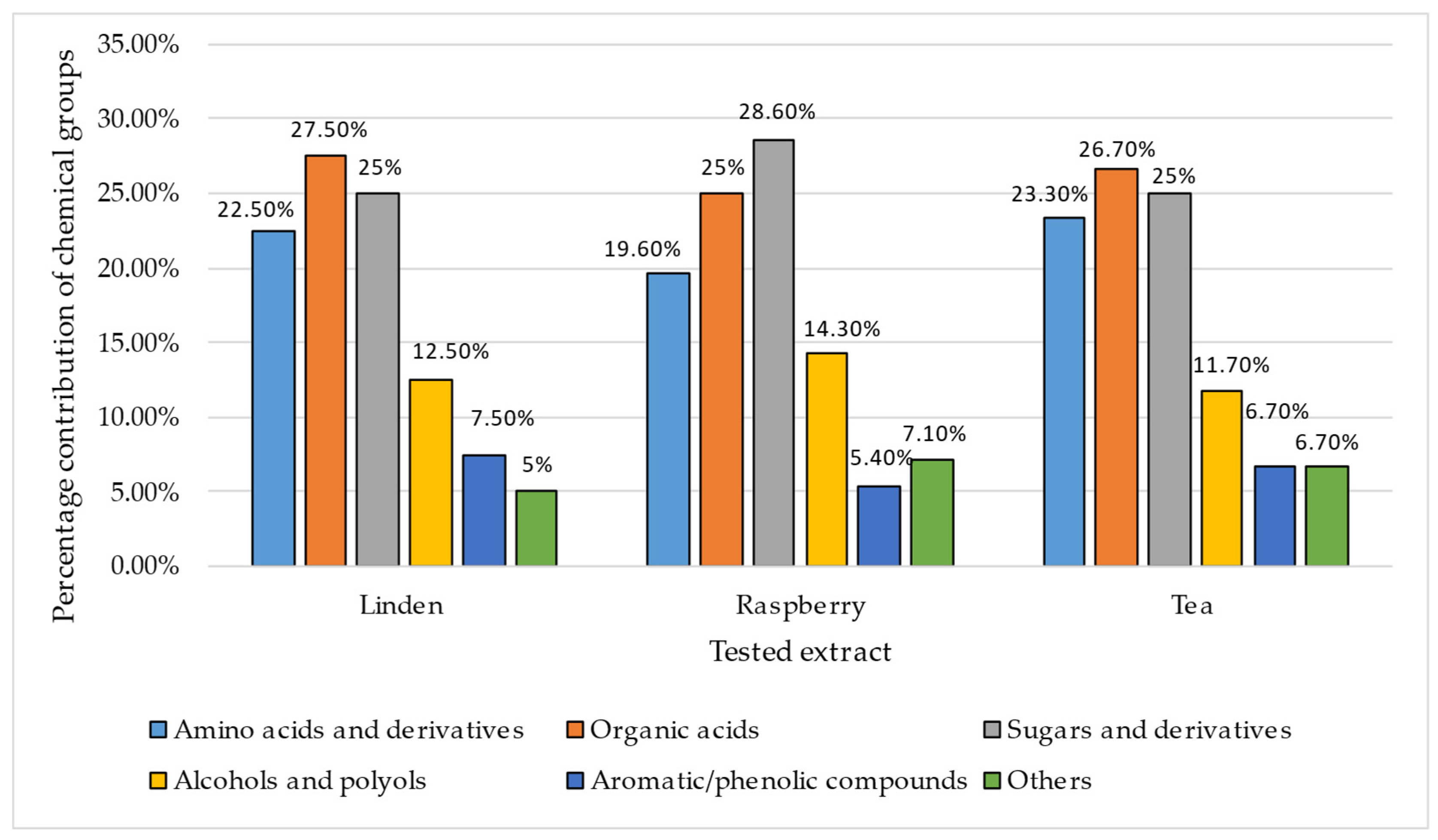
3.2. GC-MS Analysis of the Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salehi, M. Global Water Shortage and Potable Water Safety; Today’s Concern and Tomorrow’s Crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Mejuto, J.C.; Simal-Gandara, J. Mitigation of Emerging Implications of Climate Change on Food Production Systems. Food Res. Int. 2020, 134, 109256. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Carvalho, M.; Martins, S. Sustainable Water Management: Understanding the Socioeconomic and Cultural Dimensions. Sustainability 2023, 15, 13074. [Google Scholar] [CrossRef]
- Koseoglu-Imer, D.Y.; Oral, H.V.; Calheiros, C.S.C.; Krzeminski, P.; Güçlü, S.; Pereira, S.A.; Surmacz-Górska, J.; Plaza, E.; Samaras, P.; Binder, P.M.; et al. Current Challenges and Future Perspectives for the Full Circular Economy of Water in European Countries. J. Environ. Manag. 2023, 345, 118627. [Google Scholar] [CrossRef] [PubMed]
- Ingrao, C.; Strippoli, R.; Lagioia, G.; Huisingh, D. Water Scarcity in Agriculture: An Overview of Causes, Impacts and Approaches for Reducing the Risks. Heliyon 2023, 9. [Google Scholar] [CrossRef]
- Nasiri, A.R.; Kerachian, R.; Mashhadi, M.; Shahangian, S.A.; Zobeidi, T. Extending the Theory of Planned Behavior to Predict the Behavior of Farmers in Choosing Low-Water-Intensive Medicinal Plants. J. Environ. Manag. 2024, 369, 122333. [Google Scholar] [CrossRef]
- Manzocco, L.; Ignat, A.; Anese, M.; Bot, F.; Calligaris, S.; Valoppi, F.; Nicoli, M.C. Efficient Management of the Water Resource in the Fresh-Cut Industry: Current Status and Perspectives. Trends Food Sci. Technol. 2015, 46, 286–294. [Google Scholar] [CrossRef]
- Lehto, M.; Sipilä, I.; Alakukku, L.; Kymäläinen, H.-R. Water Consumption and Wastewaters in Fresh-Cut Vegetable Production. Agric. Food Sci. 2014, 23, 246–256. [Google Scholar] [CrossRef]
- Ölmez, H.; Kretzschmar, U. Potential Alternative Disinfection Methods for Organic Fresh-Cut Industry for Minimizing Water Consumption and Environmental Impact. LWT Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Raffo, A.; Paoletti, F. Fresh-Cut Vegetables Processing: Environmental Sustainability and Food Safety Issues in a Comprehensive Perspective. Front. Sustain. Food Syst. 2022, 5, 681459. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-Cut Product Sanitation and Wash Water Disinfection: Problems and Solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ingram, D.T.; Khurana, K. 7—Preventing Cross-Contamination during Produce Wash Operations. In Global Safety of Fresh Produce; Hoorfar, J., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2014; pp. 103–111. ISBN 978-1-78242-018-7. [Google Scholar]
- Jensen, D.A.; Friedrich, L.M.; Harris, L.J.; Danyluk, M.D.; Schaffner, D.W. Cross Contamination of Escherichia coli O157:H7 between Lettuce and Wash Water during Home-Scale Washing. Food Microbiol. 2015, 46, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Klištincová, N.; Pin, L.; Puškárová, A.; Giannino, D.; Bučková, M.; Lambreva, M.D.; Manfredini, A.; Canfora, L.; Pangallo, D.; Pinzari, F. From Farm to Fork: Fungal and Bacterial Contaminants and Their Diagnostics in the Production Steps of Ready-to-Eat Salads. Trends Food Sci. Technol. 2024, 150, 104573. [Google Scholar] [CrossRef]
- Hwang, J.; Ryu, H.-S.; Kim, H.A.; Hyun, M.; Lee, J.Y.; Yi, H.-A. Prognostic Factors of COVID-19 Infection in Elderly Patients: A Multicenter Study. J. Clin. Med. 2020, 9, 3932. [Google Scholar] [CrossRef]
- Bolívar, A.; Saiz-Abajo, M.J.; García-Gimeno, R.M.; Petri-Ortega, E.; Díez-Leturia, M.; González, D.; Vitas, A.I.; Pérez-Rodríguez, F. Cross Contamination of Escherichia coli O157:H7 in Fresh-Cut Leafy Vegetables: Derivation of a Food Safety Objective and Other Risk Management Metrics. Food Control 2023, 147, 109599. [Google Scholar] [CrossRef]
- Fan, X.; Gurtler, J.B.; Mattheis, J.P. Possible Sources of Listeria monocytogenes Contamination of Fresh-Cut Apples and Antimicrobial Interventions During Antibrowning Treatments: A Review. J. Food Prot. 2023, 86, 100100. [Google Scholar] [CrossRef]
- Oyenuga, N.; Cobo-Díaz, J.F.; Alvarez-Ordóñez, A.; Alexa, E.-A. Overview of Antimicrobial Resistant ESKAPEE Pathogens in Food Sources and Their Implications from a One Health Perspective. Microorganisms 2024, 12, 2084. [Google Scholar] [CrossRef]
- Garnier, C.; Guiga, W.; Lameloise, M.-L.; Fargues, C. Water Reuse in the Food Processing Industries: A Review on Pressure-Driven Membrane Processes as Reconditioning Treatments. J. Food Eng. 2023, 344, 111397. [Google Scholar] [CrossRef]
- Kanarek, P.; Breza-Boruta, B.; Poćwiardowski, W.; Szulc, J. Sustainable Water Use in a Fruit Processing Plant: Evaluation of Microbiological and Physicochemical Properties of Wash Water after Application of a Modular Water Recovery System. Sustainability 2024, 16, 2181. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Allende, A.; Gil, M.I. Recent Progress on the Management of the Industrial Washing of Fresh Produce with a Focus on Microbiological Risks. Curr. Opin. Food Sci. 2021, 38, 46–51. [Google Scholar] [CrossRef]
- Mane, M.B.; Bhandari, V.M.; Balapure, K.; Ranade, V.V. Destroying Antimicrobial Resistant Bacteria (AMR) and Difficult, Opportunistic Pathogen Using Cavitation and Natural Oils/Plant Extract. Ultrason. Sonochem. 2020, 69, 105272. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The Antimicrobial Capacity of Cistus salviifolius and Punica granatum Plant Extracts against Clinical Pathogens Is Related to Their Polyphenolic Composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Essghaier, B.; Ben Khedher, G.; Hannachi, H.; Dridi, R.; Zid, M.F.; Chaffei, C. Green Synthesis of Silver Nanoparticles Using Mixed Leaves Aqueous Extract of Wild Olive and Pistachio: Characterization, Antioxidant, Antimicrobial and Effect on Virulence Factors of Candida. Arch. Microbiol. 2022, 204, 203. [Google Scholar] [CrossRef]
- Lisec, J.; Schauer, N.; Kopka, J.; Willmitzer, L.; Fernie, A.R. Gas Chromatography Mass Spectrometry–Based Metabolite Profiling in Plants. Nat. Protoc. 2006, 1, 387–396. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.; Kubo, A.; Nihei, K.; Ogura, T. Antibacterial Activity of Coriander Volatile Compounds against Salmonella Choleraesuis. J. Agric. Food Chem. 2004, 52, 3329–3332. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.B.; Fatima, A. Review on the Antibacterial Mechanism of Plant-Derived Compounds against Multidrug-Resistant Bacteria (MDR). Evid. Based Complement. Alternat. Med. 2021, 2021, 3663315. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.A.P.; Farah, A.; Silva, D.A.M.; Nunan, E.A.; Glória, M.B.A. Antibacterial Activity of Coffee Extracts and Selected Coffee Chemical Compounds against Enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef]
- Bouhlal, F.; Aqil, Y.; Chamkhi, I.; Belmaghraoui, W.; Labjar, N.; Hajjaji, S.E.; Benabdellah, G.A.; Aurag, J.; Lotfi, E.M.; Mahi, M.E. GC-MS Analysis, Phenolic Compounds Quantification, Antioxidant, and Antibacterial Activities of the Hydro-Alcoholic Extract of Spent Coffee Grounds. J. Biol. Act. Prod. Nat. 2020, 10, 325–337. [Google Scholar] [CrossRef]
- Meletis, C.D. Coffee–Functional Food and Medicinal Herb. Altern. Complement. Ther. 2006, 12, 7–13. [Google Scholar] [CrossRef]
- Weyesa, G.; Tilahun, R. Documentation of Traditional Knowledge on “Coffee” (Coffea arabica) in Jimma, Ilubabor and Wollega Zones. Eur. J. Biophys. 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Kim, S.; Ruengwilysup, C.; Fung, D.Y.C. Antibacterial Effect of Water-Soluble Tea Extracts on Foodborne Pathogens in Laboratory Medium and in a Food Model. J. Food Prot. 2004, 67, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Li, H.; Qiu, Z.; Yu, Y. Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts. Foods 2022, 11, 620. [Google Scholar] [CrossRef]
- Boroughani, M.; Tahmasbi, Z.; Heidari, M.M.; Johari, M.; Hashempur, M.H.; Heydari, M. Potential Therapeutic Effects of Green Tea (Camellia sinensis) in Eye Diseases, a Review. Heliyon 2024, 10, e28829. [Google Scholar] [CrossRef] [PubMed]
- Kaewkod, T.; Songkhakul, W.; Tragoolpua, Y. Inhibitory Effects of Tea Leaf and Medicinal Plant Extracts on Enteric Pathogenic Bacteria Growth, Oxidation and Epithelial Cell Adhesion. Pharmacogn. Res. 2021, 14, 71–81. [Google Scholar] [CrossRef]
- Alimoddin, M.; Jayakumari, S.; Fatima, B.; Hasan, N.; Ali, S.; Sami, F.; Ali, M.S.; Nair, R.S.; Ansari, M.T. Pharmacological Applications of Urtica dioica: A Comprehensive Review of Its Traditional Use and Modern Scientific Evidence. J. Herb. Med. 2024, 48, 100935. [Google Scholar] [CrossRef]
- Harrison, F.; Furner-Pardoe, J.; Connelly, E. An Assessment of the Evidence for Antibacterial Activity of Stinging Nettle (Urtica dioica) Extracts. Access Microbiol. 2022, 4, 000336. [Google Scholar] [CrossRef]
- Du, X.-F.; Xiao, M.; Liang, H.-Y.; Sun, Z.; Jiang, Y.-H.; Chen, G.-Y.; Meng, X.-Y.; Zou, G.-L.; Zhang, L.; Liu, Y.-L.; et al. An Improved MLVF Method and Its Comparison with Traditional MLVF, Spa Typing, MLST/SCCmec and PFGE for the Typing of Methicillin-Resistant Staphylococcus Aureus. Int. J. Mol. Sci. 2014, 15, 725–742. [Google Scholar] [CrossRef]
- Motamedi, H.; Seyyednejad, S.M.; Bakhtiari, A.; Vafaei, M. Introducing Urtica dioica, A Native Plant of Khuzestan, As an Antibacterial Medicinal Plant. Jundishapur J. Nat. Pharm. Prod. 2014, 9, e15904. [Google Scholar] [CrossRef]
- Pavlović, T.; Dimkić, I.; Andrić, S.; Milojković-Opsenica, D.; Stanković, S.; Janaćković, P.; Gavrilović, M.; Ristivojević, P. Linden Tea from Serbia—An Insight into the Phenolic Profile, Radical Scavenging and Antimicrobial Activities. Ind. Crops Prod. 2020, 154, 112639. [Google Scholar] [CrossRef]
- Cittan, M.; Altuntaş, E.; Çelik, A. Evaluation of Antioxidant Capacities and Phenolic Profiles in Tilia cordata Fruit Extracts: A Comparative Study to Determine the Efficiency of Traditional Hot Water Infusion Method. Ind. Crops Prod. 2018, 122, 553–558. [Google Scholar] [CrossRef]
- Valencia-Avilés, E.; Martínez-Flores, H.-E.; García-Pérez, M.; Meléndez-Herrera, E.; García-Pérez, M.-E. Investigation of the Antibacterial Activity and Subacute Toxicity of a Quercus Crassifolia Polyphenolic Bark Extract for Its Potential Use in Functional Foods. J. Food Sci. 2019, 84, 1692–1702. [Google Scholar] [CrossRef]
- Ștefănescu, R.; Ciurea, C.N.; Mare, A.D.; Man, A.; Nisca, A.; Nicolescu, A.; Mocan, A.; Babotă, M.; Coman, N.-A.; Tanase, C. Quercus Robur Older Bark—A Source of Polyphenolic Extracts with Biological Activities. Appl. Sci. 2022, 12, 11738. [Google Scholar] [CrossRef]
- Häsler Gunnarsdottir, S.; Sommerauer, L.; Schnabel, T.; Oostingh, G.J.; Schuster, A. Antioxidative and Antimicrobial Evaluation of Bark Extracts from Common European Trees in Light of Dermal Applications. Antibiotics 2023, 12, 130. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Renda, G. The Polyphenolic Profile of Oak (Quercus) Species: A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2020, 19, 1379–1426. [Google Scholar] [CrossRef]
- Rovná, K.; Ivanišová, E.; Žiarovská, J.; Ferus, P.; Terentjeva, M.; Kowalczewski, P.Ł.; Kačániová, M. Characterization of Rosa canina Fruits Collected in Urban Areas of Slovakia. Genome Size, IPBS Profiles and Antioxidant and Antimicrobial Activities. Molecules 2020, 25, 1888. [Google Scholar] [CrossRef] [PubMed]
- Miljković, V.M.; Nikolić, L.; Mrmošanin, J.; Gajić, I.; Mihajilov-Krstev, T.; Zvezdanović, J.; Miljković, M. Chemical Profile and Antioxidant and Antimicrobial Activity of Rosa canina L. Dried Fruit Commercially Available in Serbia. Int. J. Mol. Sci. 2024, 25, 2518. [Google Scholar] [CrossRef]
- Khazaei, M.; Khazaei, M.R.; Pazhouhi, M. An Overview of Therapeutic Potentials of Rosa canina—A Traditionally Valuable Herb. In Vitro. WCRJ 2020, 7, e1580. [Google Scholar]
- Masnavi, E.; Hassanzadeh, S.; Karimi, K.; Malekzadeh, J.; Khoramrooz, S.S. Antibacterial Activities of Hydroalcoholic Extract of Rosa canina L. against Hospital Acquired Infections. J. Clin. Care Ski. 2024, 5, 157–163. [Google Scholar]
- Azman, N.A.M.; Skowyra, M.; Muhammad, K.; Gallego, M.G.; Almajano, M.P. Evaluation of the Antioxidant Activity of Betula pendula Leaves Extract and Its Effects on Model Foods. Pharm. Biol. 2017, 55, 912–919. [Google Scholar] [CrossRef]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Medicinal Plants of the Genus Betula—Traditional Uses and a Phytochemical–Pharmacological Review. J. Ethnopharmacol. 2015, 159, 62–83. [Google Scholar] [CrossRef]
- Díaz, K.; Espinoza, L.; Madrid, A.; Pizarro, L.; Chamy, R. Isolation and Identification of Compounds from Bioactive Extracts of Taraxacum officinale Weber Ex F. H. Wigg. (Dandelion) as a Potential Source of Antibacterial Agents. Evid. Based Complement. Alternat. Med. 2018, 2018, 2706417. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Antibacterial Activity of Taraxacum officinale against Foodborne Pathogens. Pak. J. Zool. 2022, 54, 1–8. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and Related Species—An Ethnopharmacological Review and Its Potential as a Commercial Medicinal Plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Fernandes, I.; Cruz, L.; Mateus, N.; Cabral, M.; de Freitas, V. Antioxidant and Biological Properties of Bioactive Phenolic Compounds from Quercus suber L. J. Agric. Food Chem. 2009, 57, 11154–11160. [Google Scholar] [CrossRef]
- Silva, S.; Machado, M.; Coelho, M.; Costa, E.M.; Pintado, M. Insights into the Antimicrobial Potential of Acorn Extracts (Quercus ilex and Quercus suber). Appl. Sci. 2023, 13, 6820. [Google Scholar] [CrossRef]
- Haytham, B.; Brahmi, F.; Zaidi, K.; Mehiou, A.; Dib, I.; Berraaouan, A.; Touzani, R.; Ziyyat, A.; Chaachouay, N.; Jan, H.A.; et al. Quercus coccifera L. Quercus faginea Lam. Quercus ilex L. Quercus rotundifolia Lam. Quercus infectoria G. Olivier Quercus suber L. Fagaceae. In Ethnobotany of Northern Africa and Levant; Bussmann, R.W., Elachouri, M., Kikvidze, Z., Eds.; Springer: Cham, Switzerland, 2024; pp. 1707–1727. ISBN 978-3-031-43105-0. [Google Scholar]
- Oliveira, B.D.; Rodrigues, A.C.; Cardoso, B.M.I.; Ramos, A.L.C.C.; Bertoldi, M.C.; Taylor, J.G.; da Cunha, L.R.; Pinto, U.M. Antioxidant, Antimicrobial and Anti-Quorum Sensing Activities of Rubus rosaefolius Phenolic Extract. Ind. Crops Prod. 2016, 84, 59–66. [Google Scholar] [CrossRef]
- Zhang, C.; Cock, I.E. Anti-Microbial Activity of Rubus idaeus L. Leaf Extracts in Combination with Antibiotics against Bacterial Triggers of Selected Autoimmune Diseases. Pharmacogn. Commun. 2023, 13, 176–186. [Google Scholar] [CrossRef]
- Mehiou, A.; Dib, I.; Berraaouane, A.; Ziyyat, A.; Elachouri, M.; Bussmann, R.W. Rubus Idaeus L., Rubus Ulmifolius Schott, Rubus Vulgaris Weihe & Nees Rosaceae. In Ethnobotany of Northern Africa and Levant; Bussmann, R.W., Elachouri, M., Kikvidze, Z., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–15. ISBN 978-3-031-13933-8. [Google Scholar]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical Composition and Biological Activity of Rubus Idaeus Shoots—A Traditional Herbal Remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef]
- Javed, B.; Nawaz, K.; Munazir, M. Phytochemical Analysis and Antibacterial Activity of Tannins Extracted from Salix Alba L. Against Different Gram-Positive and Gram-Negative Bacterial Strains. Iran. J. Sci. Technol. Trans. Sci. 2020, 44, 1303–1314. [Google Scholar] [CrossRef]
- Piątczak, E.; Dybowska, M.; Płuciennik, E.; Kośla, K.; Kolniak-Ostek, J.; Kalinowska-Lis, U. Identification and Accumulation of Phenolic Compounds in the Leaves and Bark of Salix alba (L.) and Their Biological Potential. Biomolecules 2020, 10, 1391. [Google Scholar] [CrossRef]
- Shara, M.; Stohs, S.J. Efficacy and Safety of White Willow Bark (Salix alba) Extracts. Phytother. Res. 2015, 29, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Ahmed Qasim, K.; Ashraf, C.M.; Maab, H. Verbena Officinalis a Herb with Promising Broad Spectrum Antimicrobial Potential. Cogent Chem. 2017, 3, 1363342. [Google Scholar] [CrossRef]
- Kubica, P.; Szopa, A.; Dominiak, J.; Luczkiewicz, M.; Ekiert, H. Verbena Officinalis (Common Vervain)—A Review on the Investigations of This Medicinally Important Plant Species. Planta Med. 2020, 86, 1241–1257. [Google Scholar] [CrossRef]
- Dziedziński, M.; Kobus-Cisowska, J.; Powałowska, D.S.; Szablewska, K.S.; Baranowska, M. Polyphenols Composition, Antioxidant and Antimicrobial Properties of Pinus sylvestris L. Shoots Extracts Depending on Different Drying Methods. Emir. J. Food Agric. 2020, 229, 229–237. [Google Scholar] [CrossRef]
- Nisca, A.; Ștefănescu, R.; Stegăruș, D.I.; Mare, A.D.; Farczadi, L.; Tanase, C. Comparative Study Regarding the Chemical Composition and Biological Activity of Pine (Pinus nigra and P. sylvestris) Bark Extracts. Antioxidants 2021, 10, 327. [Google Scholar] [CrossRef]
- Rubens, J.; Kibilds, J.; Jansons, M.; Piginka-Vjaceslavova, I.; Barene, I.; Daberte, I.; Liepa, L.; Malniece, A.; Rubens, A.; Starkute, V.; et al. Application of Baltic Pine (Pinus sylvestris) Needle Extract as a Gut Microbiota-Modulating Feed Supplement for Domestic Chickens (Gallus gallus). Plants 2023, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Papp, N.; Purger, D.; Czigle, S.; Czégényi, D.; Stranczinger, S.; Tóth, M.; Dénes, T.; Kocsis, M.; Takácsi-Nagy, A.; Filep, R. The Importance of Pine Species in the Ethnomedicine of Transylvania (Romania). Plants 2022, 11, 2331. [Google Scholar] [CrossRef]
- Kanarek, P.; Breza-Boruta, B.; Bogiel, T. In the Depths of Wash Water: Isolation of Opportunistic Bacteria from Fresh-Cut Processing Plants. Pathogens 2024, 13, 768. [Google Scholar] [CrossRef]
- Mir, I.H.; Sharma, M.K.; Singh, A. Antimicrobial activity of some medicinal plant extracts against multidrug resistant bacteria. J. Adv. Sci. Res. 2021, 12, 42–47. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. The NIST Chemistry WebBook: A Chemical Data Resource on the Internet. J. Chem. Eng. Data 2001, 46, 1059–1063. [Google Scholar] [CrossRef]
- Lucas, E.H.; Pearson, K. Preparation of Crude Plant Extracts and Their Assay for Presence of Antibacterial Substances. Food Res. 1948, 13, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial Activity of Pomegranate Peel Extracts as Affected by Cultivar. J. Sci. Food Agric. 2017, 97, 802–810. [Google Scholar] [CrossRef]
- Santos, D.K.D.d.N.; de Almeida, V.S.; de Araujo, D.R.C.; Harand, W.; Soares, A.K.d.A.; Moreira, L.R.; de Lorena, V.M.B.; Magalhães, L.P.M.; Ximenes, R.M.; de Sena, K.X.d.F.R.; et al. Evaluation of Cytotoxic, Immunomodulatory and Antibacterial Activities of Aqueous Extract from Leaves of Conocarpus erectus Linnaeus (Combretaceae). J. Pharm. Pharmacol. 2018, 70, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Nocedo-Mena, D.; Garza-González, E.; González-Ferrara, M.; Del Rayo Camacho-Corona, M. Antibacterial Activity of Cissus Incisa Extracts against Multidrug- Resistant Bacteria. Curr. Top. Med. Chem. 2020, 20, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Hejna, M.; Dell’Anno, M.; Liu, Y.; Rossi, L.; Aksmann, A.; Pogorzelski, G.; Jóźwik, A. Assessment of the Antibacterial and Antioxidant Activities of Seaweed-Derived Extracts. Sci. Rep. 2024, 14, 21044. [Google Scholar] [CrossRef]
- Strompfová, V.; Štempelová, L.; Wolaschka, T. Antibacterial Activity of Plant-Derived Compounds and Cream Formulations against Canine Skin Bacteria. Vet. Res. Commun. 2024, 48, 1459–1470. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Majeed, M.; Hakeem, K.R.; Rehman, R.U. Synergistic Effect of Plant Extract Coupled Silver Nanoparticles in Various Therapeutic Applications- Present Insights and Bottlenecks. Chemosphere 2022, 288, 132527. [Google Scholar] [CrossRef]
- Donkor, M.N.; Donkor, A.-M.; Mosobil, R. Combination Therapy: Synergism among Three Plant Extracts against Selected Pathogens. BMC Res. Notes 2023, 16, 83. [Google Scholar] [CrossRef]
- Yoon, J.; Kim, T.-J. Synergistic Growth Inhibition of Herbal Plant Extract Combinations against Candida albicans. J. Korean Wood Sci. Technol. 2023, 51, 145–156. [Google Scholar] [CrossRef]
- Chakraborty, S.; Dutta, H. Use of Nature-Derived Antimicrobial Substances as Safe Disinfectants and Preservatives in Food Processing Industries: A Review. J. Food Process. Preserv. 2022, 46, e15999. [Google Scholar] [CrossRef]
- Zhou, Y.-J.; Ren, Q.; Shen, Y.-B. Comprehensive Review of Tilia L.: Phytochemical Profiles, Edible Value, Therapeutic Potentials, and Ecological Significance. Food Med. Homol. 2025, 2, 9420035. [Google Scholar] [CrossRef]
- Tkachenko, H.; Kurhaluk, N.; Lukash, O.; Yakovenko, O.; Opryshko, M.; Maryniuk, M.; Gyrenko, O.; Buyun, L. In Vitro Antibacterial Efficacy of Different Natural Linden Honeys against Some Gram-Positive and Gram-Negative Strains. Agrobiodivers. Improv. Nutr. Health Life Qual. 2023, 7. [Google Scholar] [CrossRef]
- Gaca, A.O.; Lemos, J.A. Adaptation to Adversity: The Intermingling of Stress Tolerance and Pathogenesis in Enterococci. Microbiol. Mol. Biol. Rev. 2019, 83, e00008-19. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, T.; Gochev, V.; Stoyanova, A.; Girova, T.; Djurdjev, I. Chemical Composition and Antimicrobial Activity of Linden (Tilia tomentosa Moench.) CO2 Extract. Plovdiv Univ., Paisii Hilendarski” Bulg. Sci. Pap. 2008, 36, 91. [Google Scholar]
- Oniszczuk, A.; Wójtowicz, A.; Oniszczuk, T.; Olech, M.; Nowak, R.; Wojtunik, K.; Klimek, M.; Krawczyk, W.; Hajnos, M. Extruded Corn Gruels Containing Linden Flowers: Quantitation of Phenolic Compounds and Selected Quality Characteristics. Open Chem. 2015, 13, 000010151520150138. [Google Scholar] [CrossRef]
- Hou, L.; Ye, M.; Wang, X.; Zhu, Y.; Sun, X.; Gu, R.; Chen, L.; Fang, B. Synergism with Shikimic Acid Restores β-Lactam Antibiotic Activity against Methicillin-Resistant Staphylococcus aureus. Molecules 2024, 29, 1528. [Google Scholar] [CrossRef]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Antibacterial Activity of Some Flavonoids and Organic Acids Widely Distributed in Plants. J. Clin. Med. 2020, 9, 109. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Rao, S.; Sun, J.; Ma, C.; Li, J. P-Coumaric Acid Kills Bacteria through Dual Damage Mechanisms. Food Control 2012, 25, 550–554. [Google Scholar] [CrossRef]
- Nakayama, M.; Shimatani, K.; Ozawa, T.; Shigemune, N.; Tsugukuni, T.; Tomiyama, D.; Kurahachi, M.; Nonaka, A.; Miyamoto, T. A Study of the Antibacterial Mechanism of Catechins: Isolation and Identification of Escherichia coli Cell Surface Proteins That Interact with Epigallocatechin gallate. Food Control 2013, 33, 433–439. [Google Scholar] [CrossRef]
- Krzepiłko, A.; Prażak, R.; Święciło, A. Chemical Composition, Antioxidant and Antimicrobial Activity of Raspberry, Blackberry and Raspberry-Blackberry Hybrid Leaf Buds. Molecules 2021, 26, 327. [Google Scholar] [CrossRef]
- Hummer, K.E. Rubus Pharmacology: Antiquity to the Present. HortScience 2010, 45, 1587–1591. [Google Scholar] [CrossRef]
- Cota, D.; Patil, D. Antibacterial Potential of Ellagic Acid and Gallic Acid against IBD Bacterial Isolates and Cytotoxicity against Colorectal Cancer. Nat. Prod. Res. 2023, 37, 1998–2002. [Google Scholar] [CrossRef]
- Ercan, L.; Doğru, M. Antioxidant and Antimicrobial Capacity of Quinic Acid. Bitlis Eren Üniversitesi Fen Bilim. Derg. 2022, 11, 1018–1025. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Hart, R.; Abd_Allah, E.F.; Hashem, A.; Alsayed, M.F.; Ijaz, F.; Ali, N.; Shah, M.; et al. Herbal Teas and Drinks: Folk Medicine of the Manoor Valley, Lesser Himalaya, Pakistan. Plants 2019, 8, 581. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gupta, S.; Sarethy, I.P.; Dang, S.; Gabrani, R. Green Tea Extract: Possible Mechanism and Antibacterial Activity on Skin Pathogens. Food Chem. 2012, 135, 672–675. [Google Scholar] [CrossRef]
- Radji, M.; Agustama, R.A.; Elya, B.; Tjampakasari, C.R. Antimicrobial Activity of Green Tea Extract against Isolates of Methicillin–Resistant Staphylococcus Aureus and Multi–Drug Resistant Pseudomonas aeruginosa. Asian Pac. J. Trop. Biomed. 2013, 3, 663–667. [Google Scholar] [CrossRef]
- Hope, O.; Bright, I.E.; Alagbonsi, A.I. GC-MS Biocomponents Characterization and Antibacterial Potency of Ethanolic Crude Extracts of Camellia sinensis. SAGE Open Med. 2022, 10, 20503121221116860. [Google Scholar] [CrossRef]
- Ucella-Filho, J.G.M.; Freire, A.d.S.M.; Carréra, J.C.; Lucas, F.M.F.; Zucolotto, S.M.; Dias Júnior, A.F.; Mori, F.A. Tannin-Rich Bark Extract of Plants as a Source of Antimicrobial Bioactive Compounds: A Bibliometric Analysis. S. Afr. J. Bot. 2022, 150, 1038–1050. [Google Scholar] [CrossRef]
- Omar, S.; Lemonnier, B.; Jones, N.; Ficker, C.; Smith, M.L.; Neema, C.; Towers, G.H.N.; Goel, K.; Arnason, J.T. Antimicrobial Activity of Extracts of Eastern North American Hardwood Trees and Relation to Traditional Medicine. J. Ethnopharmacol. 2000, 73, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Elansary, O.H.; Szopa, A.; Kubica, P.; Ekiert, H.; Mattar, A.M.; Al-Yafrasi, M.A.; El-Ansary, D.O.; Zin El-Abedin, T.K.; Yessoufou, K. Polyphenol Profile and Pharmaceutical Potential of Quercus Spp. Bark Extracts. Plants 2019, 8, 486. [Google Scholar] [CrossRef]
- Šukele, R.; Skadiņš, I.; Koka, R.; Bandere, D. Antibacterial Effects of Oak Bark (Quercus robur) and Heather Herb (Calluna vulgaris L.) Extracts against the Causative Bacteria of Bovine Mastitis. Vet. World 2022, 15, 2315–2322. [Google Scholar] [CrossRef]
- Tanase, C.; Nicolescu, A.; Nisca, A.; Ștefănescu, R.; Babotă, M.; Mare, A.D.; Ciurea, C.N.; Man, A. Biological Activity of Bark Extracts from Northern Red Oak (Quercus rubra L.): An Antioxidant, Antimicrobial and Enzymatic Inhibitory Evaluation. Plants 2022, 11, 2357. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.; Correia, P.; Silva, S.P.; Almeida-Aguiar, C. Evaluation of Antimicrobial Properties of Cork. FEMS Microbiol. Lett. 2016, 363, fnv231. [Google Scholar] [CrossRef] [PubMed]
- Blondeau, D.; St-Pierre, A.; Bourdeau, N.; Bley, J.; Lajeunesse, A.; Desgagné-Penix, I. Antimicrobial Activity and Chemical Composition of White Birch (Betula papyrifera Marshall) Bark Extracts. MicrobiologyOpen 2020, 9, e00944. [Google Scholar] [CrossRef]
- Royer, M.; Houde, R.; Viano, Y.; Stevanovic, T. Non-Wood Forest Products Based on Extractives—A New Opportunity for the Canadian Forest Industry Part 1: Hardwood Forest Species. J. Food Res. 2012, 1, 8. [Google Scholar] [CrossRef]
- Safta, D.A.; Ielciu, I.; Șuștic, R.; Hanganu, D.; Niculae, M.; Cenariu, M.; Pall, E.; Moldovan, M.L.; Achim, M.; Bogdan, C.; et al. Chemical Profile and Biological Effects of an Herbal Mixture for the Development of an Oil-in-Water Cream. Plants 2023, 12, 248. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Jung, I.-G.; Yum, S.-H.; Hwang, Y.-J. In Vitro Synergistic Inhibitory Effects of Plant Extract Combinations on Bacterial Growth of Methicillin-Resistant Staphylococcus aureus. Pharmaceuticals 2023, 16, 1491. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. In Vitro Antimicrobial Synergism within Plant Extract Combinations from Three South African Medicinal Bulbs. J. Ethnopharmacol. 2012, 139, 81–89. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 12, 753518. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, L.; Martins, A.P.; Correia, H. Raw Materials: The Importance of Quality and Safety. A Review. Flavour Fragr. J. 2010, 25, 253–271. [Google Scholar] [CrossRef]
- Sánchez, E.; García, S.; Heredia, N. Extracts of Edible and Medicinal Plants Damage Membranes of Vibrio Cholerae. Appl. Environ. Microbiol. 2010, 76, 6888–6894. [Google Scholar] [CrossRef] [PubMed]



| N° | Name | Common Name | Material | Traditional Usage | Examples of In-Vitro Tested Pathogens | References |
|---|---|---|---|---|---|---|
| 1 | Coffea L. | Coffee | grounds | common cold, diarrhea, wound treatment | Salmonella enterica, Streptococcus mutans, K. oxytoca, E. coli | [28,29,30,31] |
| 2 | Camellia sinensis | Tea | grounds | diarrhea, streptococcal pharyngitis, | E. faecalis, S. aureus, E. coli, S. typhimurium, Bacillus cereus, Shigella dysenteriae | [32,33,34,35] |
| 3 | Urtica dioica L. | Common nettle | leaves | skin diseases, urinary disorders, respiratory diseases | Bacillus cereus, S. aureus, Staphylococcus epidermidis, E. coli | [36,37,38,39] |
| 4 | Tilia cordata Mill. | Small-leaved lime | flowers | pneumonia, sore throat, diarrhea | Candida glabrata, S. aureus, Streptococcus pyogenes, Bacillus subtilis | [40,41] |
| 5 | Quercus robur L. | English oak | bark | tonsillitis, wound treatment, diarrhea | E. coli, Streptococcus agalactiae, Streptococcus uberis, Serratia liquefaciens, S. aureus | [42,43,44,45] |
| 6 | Rosa canina L. | Dog rose | fruits | common colds, influenza, diarrhea, cough, urinary tract infections | S. aureus, K. pneumoniae, E. coli | [46,47,48,49] |
| 7 | Betula pendula Roth | Silver birch | bark | urinary tract infections, wound treatment | Enterococcus faecalis, Salmonella typhimurium, Escherichia coli, Bacillus subtilis, Pseudomonas aeruginosa | [44,50,51] |
| 8 | Taraxacum officinale F.H. Wiggers coll. | Dandelion | root | urinary infections | S. aureus, K. pneumoniae, E. coli, Proteus mirabilis | [52,53,54] |
| 9 | Quercus suber L. | Cork oak | expanded bark | diarrhea, gastritis, ulcer, and skin infection | S. aureus, B. cereus, and Listeria monocytogenes | [55,56,57] |
| 10 | Rubus idaeus L. | Red Raspberry | shoots | common cold, fever, and flu-like infections | Proteus mirabilis, A. baylyi, P. aeruginosa | [58,59,60,61] |
| 11 | Salix alba L. | White Willow | bark | flu symptoms including fever, and generalized pain | S. aureus, E. coli | [62,63,64] |
| 12 | Verbena officinalis | Common Vervain | shoots | anti-inflammatory, anti-fungal, common cold | P. aeruginosa, Citrobacter freundii, S. aureus. | [65,66] |
| 13 | Pinus sylvestris L. | Scots Pine | shoots | colds, cough, wound treatment | E. coli, S. aureus, K. pneumoniae, B. subtilis | [67,68,69,70] |
| Tested Bacteria | |||||||
|---|---|---|---|---|---|---|---|
| P. aeruginosa | P. vulgaris | K. pneumoniae | K. oxytoca | E. faecalis | S. marcescens | ||
| Plant Extract | Material | Mean of Inhibition Zone [mm] (After Exclusion of the Well Diameter) | |||||
| Coffea L. | grounds | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Camellia sinensis | grounds | 8.0 | 7.0 | 4.0 | 2.0 | 8.0 | 4.0 |
| Urtica dioica L. | leaves | 1.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Tilia cordata Mill. | flowers | 6.0 | 4.0 | 3.0 | 2.0 | 0.0 | 3.0 |
| Quercus robur L. | flowers | 6.0 | 2.0 | 4.0 | 5.0 | 6.0 | 4.0 |
| Rosa canina L. | fruit | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Betula pendula Roth | bark | 4.0 | 4.0 | 5.0 | 6.0 | 4.0 | 4.0 |
| Taraxacum officinale F.H. Wiggers coll. | root | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Quercus suber L. | expanded bark | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Rubus idaeus L. | shoots | 8.0 | 4.0 | 6.0 | 6.0 | 5.0 | 4.0 |
| Salix alba L. | bark | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 2.0 |
| Verbena officinalis | shoots | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Pinus sylvestris L. | shoots | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| * Postive control: | 37.5 | 36.5 | 38.0 | 30.0 | 22.5 | 41.5 | |
| Cs × Tc | ||||
| Cs × Qr | Tc × Qr | |||
| Cs × Bp | Tc × Bp | Qr × Bp | ||
| Cs × Ri | Tc × Ri | Qr × Ri | Bp × Ri | |
| Cs × Sa | Tc ×Sa | Qr × Sa | Bp × Sa | Sa × Ri |
| Plant Species | Initial Plant Mass (g) | Dried Extract Mass (g) | Recovery (%) |
|---|---|---|---|
| Tilia cordata | 25 | 1.335 | 5.34 |
| Rubus idaeus | 25 | 1.965 | 7.86 |
| Camellia sinensis | 25 | 2.648 | 10.59 |
| Quercus robur | 25 | 0.385 | 1.54 |
| Betula pendula | 25 | 0.265 | 1.06 |
| Salix alba | 25 | 0.390 | 1.56 |
| Combination | P. aeruginosa | P. vulgaris | K. pneumoniae | K. oxytoca | E. faecalis | S. marcescens |
|---|---|---|---|---|---|---|
| Cs × Tc | 8.0 | 7.0 | 4.0 | 2.0 | 8.0 | 4.0 |
| Cs × Qr | 8.0 | 7.0 | 4.0 | 5.0 | 8.0 | 4.0 |
| Tc × Qr | 6.0 | 4.0 | 4.0 | 5.0 | 6.0 | 4.0 |
| Cs × Bp | 8.0 | 7.0 | 5.0 | 6.0 | 8.0 | 4.0 |
| Tc × Bp | 6.0 | 4.0 | 5.0 | 4.0 | 4.0 | 4.0 |
| Qr × Bp | 6.0 | 4.5 | 5.0 | 6.0 | 6.0 | 4.0 |
| Cs × Ri | 12.0 | 7.0 | 6.0 | 6.0 | 8.0 | 4.0 |
| Tc × Ri | 8.0 | 4.0 | 6.0 | 6.0 | 5.0 | 4.0 |
| Qr × Ri | 8.0 | 4.0 | 4.0 | 6.0 | 6.5 | 4.0 |
| Bp × Ri | 8.0 | 4.0 | 6.0 | 6.0 | 5.0 | 4.0 |
| Cs × Sa | 8.0 | 7.0 | 4.0 | 4.0 | 8.0 | 4.0 |
| Tc × Sa | 6.0 | 4.0 | 4.0 | 4.0 | 4.0 | 3.0 |
| Qr × Sa | 6.0 | 4.0 | 4.0 | 5.0 | 6.0 | 4.0 |
| Bp × Sa | 4.0 | 4.0 | 5.0 | 6.0 | 4.0 | 6.0 |
| Sa × Ri | 8.0 | 4.0 | 6.0 | 6.0 | 5.0 | 4.0 |
| Compound | TIC (%) | Compound | TIC (%) | Compound | TIC (%) | |||
|---|---|---|---|---|---|---|---|---|
| Linden (SIL) | Shikimic acid, tetra-TMS | 8.81 | Tea (SIL) | Sucrose, octa-TMS | 7.44 | Raspberry (SIL) | β-D-Glucopyranose, penta-TMS | 11.26 |
| β-Fructofuranose, penta-TMS | 8.16 | Carbohydrate, TMS | 7.31 | α-D-Glucopyranose, penta-TMS | 10.48 | |||
| α-D-Glucopyranose, penta-TMS | 7.92 | Quinic acid, penta-TMS | 6.33 | β-Fructofuranose, penta-TMS | 10.08 | |||
| Quinic acid, penta-TMS | 6.74 | α-D-Glucopyranose, penta-TMS | 5.13 | α-Fructofuranose, penta-TMS | 6.06 | |||
| β-D-Glucopyranose, penta-TMS | 6.20 | Carbohydrate, TMS | 4.89 | Malic acid, tri-TMS | 6.00 | |||
| Malic acid, tri-TMS | 4.46 | NN | 4.87 | myo-Inositol, hexa-TMS | 4.45 | |||
| Methyl glucofuranoside, tetra-TMS, isomer 2 | 4.42 | β-D-Glucopyranose, penta-TMS | 4.50 | β-Glucofuranose, penta-TMS | 2.96 | |||
| deoxy-Inositol, penta-TMS | 3.37 | Methyl glucofuranoside, tetra-TMS, isomer 1 | 4.14 | Carbohydrate, TMS | 2.61 | |||
| Glucopyranose, penta-TMS | 3.22 | NN | 3.61 | β-Mannopyranose, penta-TMS | 2.35 | |||
| Methyl glucofuranoside, tetra-TMS, isomer 1 | 3.10 | Epicatechin, penta-TMS | 3.46 | Methyl glucofuranoside, tetra-TMS, isomer 2 | 2.27 | |||
| Carbohydrate, TMS | 3.02 | β-Fructofuranose, penta-TMS | 3.04 | deoxy-Inositol, penta-TMS | 2.16 | |||
| Carbohydrate, TMS | 2.76 | β-Mannopyranose, penta TMS | 2.82 | Glycerol, tri-TMS | 2.08 | |||
| β-Mannopyranose, penta-TMS | 2.66 | NN | 2.00 | NN | 2.02 | |||
| Sucrose, octa-TMS | 2.27 | Carbohydrate, TMS | 1.77 | 2,3,4-Trihydroxybutyric acid, isomer 2, tetra-TMS | 1.96 | |||
| Carbohydrate, TMS | 2.01 | Malic acid, tri-TMS | 1.75 | Gluconic acid, δ-lactone, tetra-TMS | 1.73 | |||
| β-Glucofuranose, penta-TMS | 2.00 | Glucopyranose, penta-TMS | 1.73 | Quinic acid, penta-TMS | 1.73 | |||
| 2,3,4-Trihydroxybutyric acid, isomer 2, tetra-TMS | 1.81 | Caffeine | 1.60 | Carbohydrate, TMS | 1.70 | |||
| myo-Inositol, hexa-TMS | 1.59 | Methyl glucofuranoside, tetra-TMS, isomer 2 | 1.55 | Phosphotic acid, tri-TMS | 1.62 | |||
| Carbohydrate, TMS | 1.57 | Shikimic acid, tetra-TMS | 1.52 | Glucopyranose, penta-TMS | 1.38 | |||
| Scyllo-Inositol, penta-TMS | 1.53 | Epicatechin gallate, hepta-TMS | 1.50 | Carbohydrate, TMS | 1.37 | |||
| Glycerol, tri-TMS | 1.44 | Carbohydrate, TMS | 1.40 | Methyl glucofuranoside, tetra-TMS, isomer 1 | 1.25 | |||
| Phosphotic acid, tri-TMS | 1.42 | Raffinose, TMS | 1.39 | <1% TIC | ||||
| Epicatechin, penta-TMS | 1.34 | Carbohydrate, TMS | 1.27 | |||||
| Carbohydrate, TMS | 1.33 | Aspartic acid, tri-TMS | 1.21 | |||||
| Gluconic acid, hexa-TMS | 1.30 | Carbohydrate, TMS | 1.16 | |||||
| Gluconic acid, δ-lactone, tetra-TMS | 1.02 | Carbohydrate, TMS | 1.06 | |||||
| Carbohydrate, TMS | 1.01 | - | - | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanarek, P.; Breza-Boruta, B.; Stocki, M. Antimicrobial Activity and Phytochemical Profiling of Natural Plant Extracts for Biological Control of Wash Water in the Agri-Food Industry. Appl. Sci. 2025, 15, 5199. https://doi.org/10.3390/app15095199
Kanarek P, Breza-Boruta B, Stocki M. Antimicrobial Activity and Phytochemical Profiling of Natural Plant Extracts for Biological Control of Wash Water in the Agri-Food Industry. Applied Sciences. 2025; 15(9):5199. https://doi.org/10.3390/app15095199
Chicago/Turabian StyleKanarek, Piotr, Barbara Breza-Boruta, and Marcin Stocki. 2025. "Antimicrobial Activity and Phytochemical Profiling of Natural Plant Extracts for Biological Control of Wash Water in the Agri-Food Industry" Applied Sciences 15, no. 9: 5199. https://doi.org/10.3390/app15095199
APA StyleKanarek, P., Breza-Boruta, B., & Stocki, M. (2025). Antimicrobial Activity and Phytochemical Profiling of Natural Plant Extracts for Biological Control of Wash Water in the Agri-Food Industry. Applied Sciences, 15(9), 5199. https://doi.org/10.3390/app15095199






