Chimonanthus nitens Oliv. Leaf Flavonoids Exert Anti-Inflammatory Effects Through TLR4 Receptor Affecting NF-κB/MAPK Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of COLFs
2.3. Cell Culture
2.4. Cell Viability
2.5. Inflammation Stimulation
2.6. Determination of NO and Inflammatory Factors
2.7. RNA-Seq Analysis
2.8. RT-qPCR Analysis
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Cell Cytotoxicity of COLFs
3.2. COLFs Inhibited the Secretion of NO and Inflammatory Factors

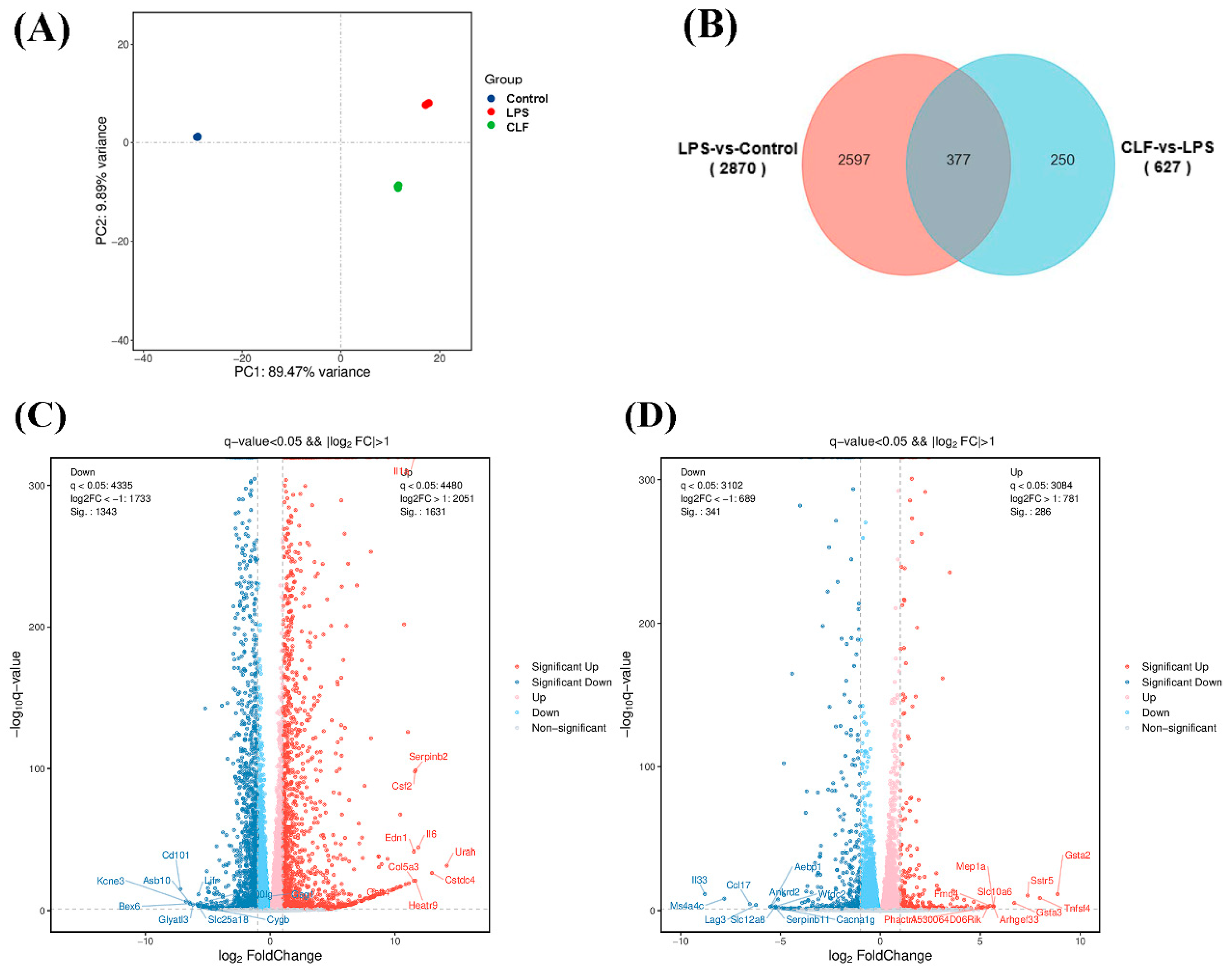
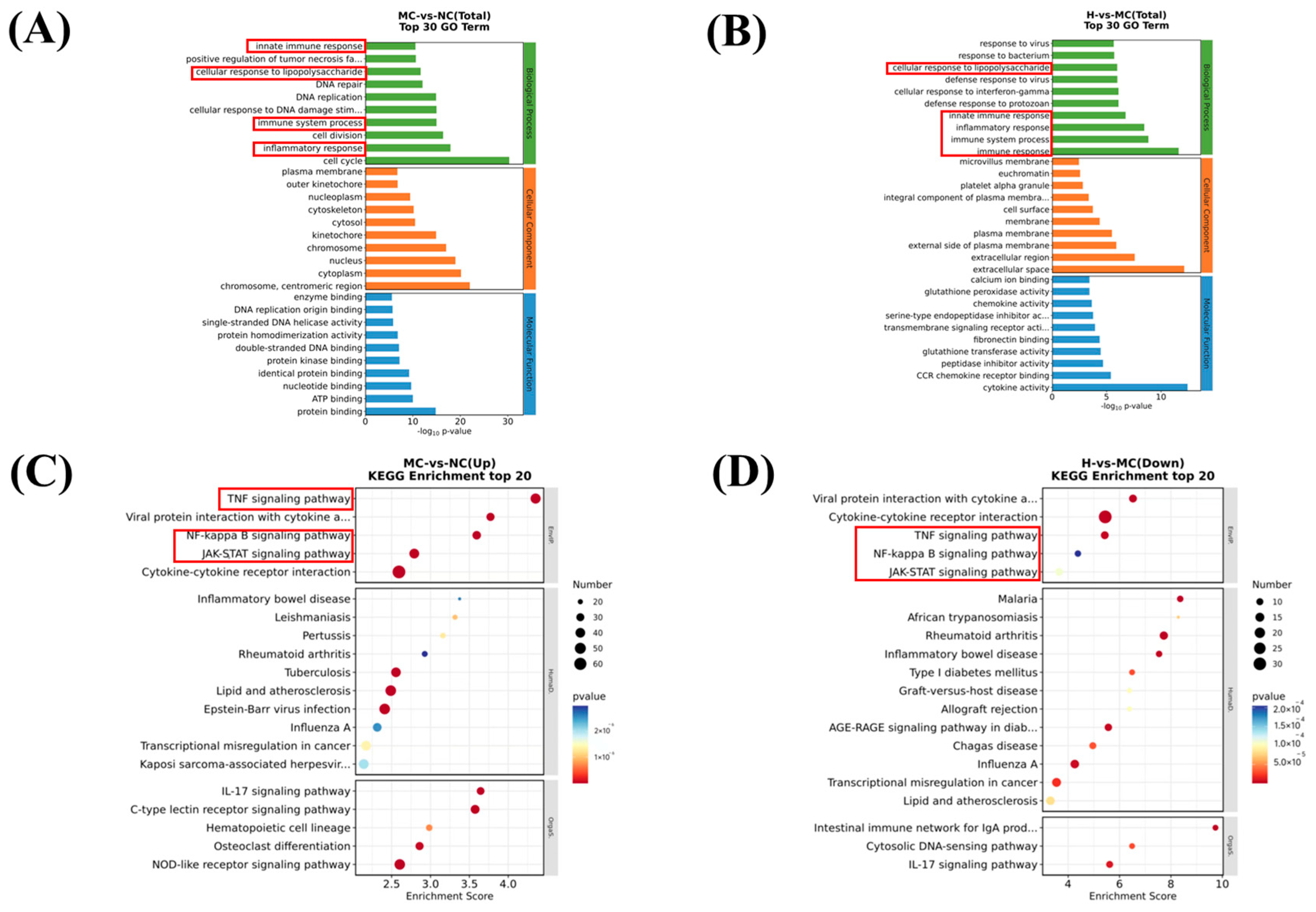
3.3. Differentially Expressed Genes
3.4. Mechanistic Exploration of LPS-Induced Macrophages
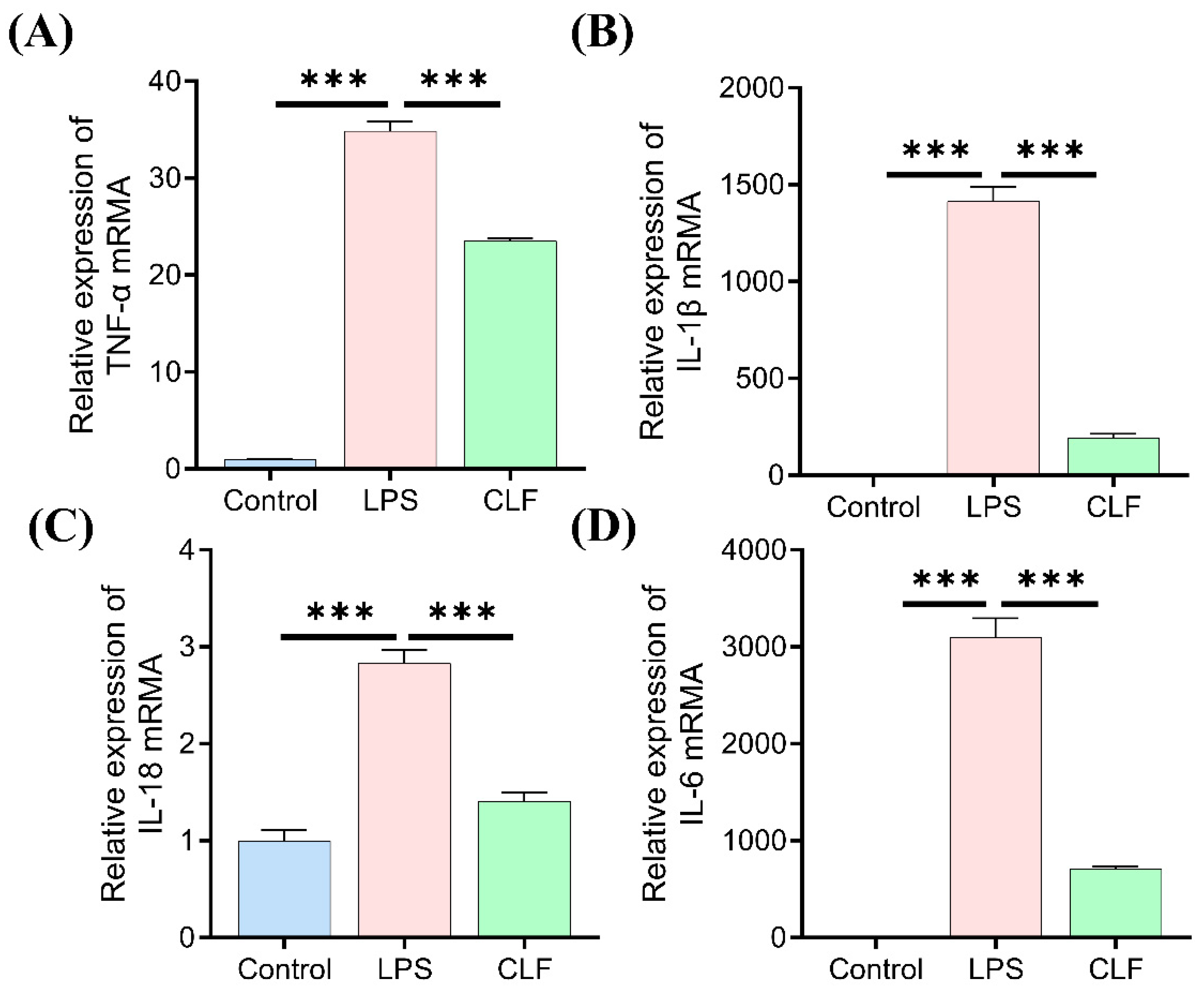
3.5. COLFs Regulated Gene Expression of Cytokines
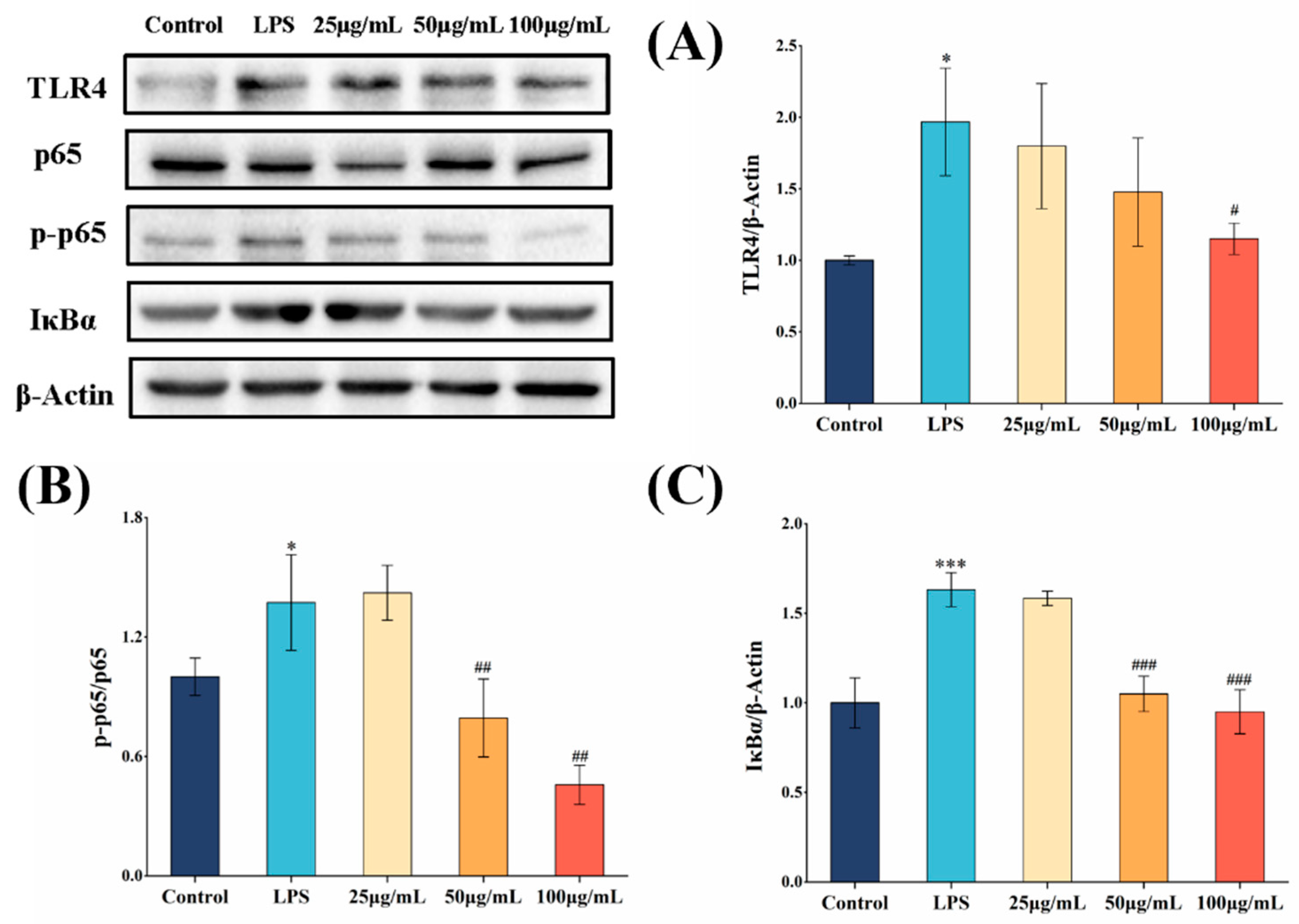
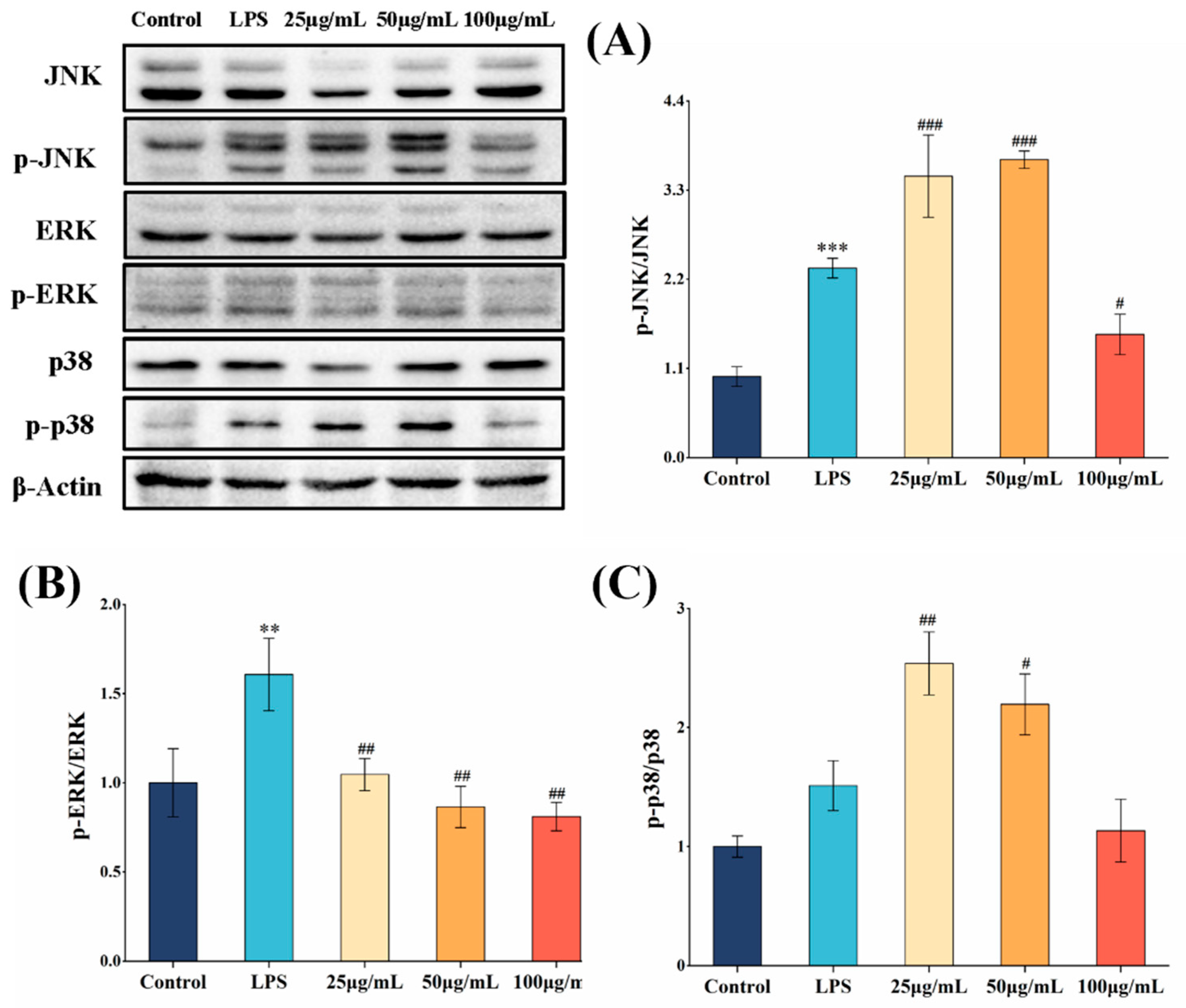
3.6. COLFs Exerted Anti-Inflammatory Activities via NF-κB and MAPK Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274–281. [Google Scholar] [CrossRef]
- Ratnayake, W.M.K.M.; Suresh, T.S.; Abeysekera, A.M.; Salim, N.; Chandrika, U.G. Acute anti-inflammatory and anti-nociceptive activities of crude extracts, alkaloid fraction and evolitrine from Acronychia pedunculata leaves. J. Ethnopharmacol. 2019, 238, 111827. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Polyphenol content of green pea (Pisum sativum L.) Hull under in vitro digestion and effects of digestive products on anti-inflammatory activity and intestinal barrier in the Caco-2/Raw264.7 cculture model. J. Agric. Food Chem. 2022, 70, 3477–3488. [Google Scholar] [CrossRef] [PubMed]
- Kiel, C.; Yus, E.; Serrano, L. Engineering Signal Transduction Pathways. Cell 2010, 140, 33–47. [Google Scholar] [CrossRef]
- Zhou, N.; Yao, Y.; Wu, N.; Du, H.; Xu, M.; Zhao, Y.; Tu, Y. VF-4 and DR-8 derived from salted egg white inhibit inflammatory activity via NF-κB/PI3K-Akt/MAPK signal transduction pathways in HT-29 cells induced by TNF-α. Mol. Nutr. Food Res. 2021, 66, e2100682. [Google Scholar] [CrossRef]
- Geissmann, F.; Manz, M.G.; Jung, S.; Sieweke, M.H.; Merad, M.; Ley, K. Development of monocytes, macrophages, and dendritic cells. Science 2010, 327, 656–661. [Google Scholar] [CrossRef]
- Xie, C.; Li, X.; Zhu, J.; Wu, J.; Geng, S.; Zhong, C. Magnesium isoglycyrrhizinate suppresses LPS-induced inflammation and oxidative stress through inhibiting NF-κB and MAPK pathways in RAW264.7 cells. Bioorganic Med. Chem. 2019, 27, 516–524. [Google Scholar] [CrossRef]
- Fujita, T.; Zysman, M.; Elgrabli, D.; Murayama, T.; Haruta, M.; Lanone, S.; Ishida, T.; Boczkowski, J. Anti-inflammatory effect of gold nanoparticles supported on metal oxides. Sci. Rep. 2021, 11, 23129. [Google Scholar] [CrossRef]
- Loscalzo, J. The identification of nitric oxide as endothelium-derived relaxing factor. Circ. Res. 2013, 113, 100–103. [Google Scholar] [CrossRef]
- Xian, Y.-F.; Li, Y.-C.; Ip, S.-P.; Lin, Z.-X.; Lai, X.-P.; Su, Z.-R. Anti-inflammatory effect of patchouli alcohol isolated from Pogostemonis Herba in LPS-stimulated RAW264.7 macrophages. Exp. Ther. Med. 2011, 2, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yan, X.; Xia, J.; Zhao, J.; Ma, M.; Yu, P.; Gong, D.; Zeng, Z. Assessment of the effect of ethanol extracts from Cinnamomum camphora seed kernel on intestinal inflammation using simulated gastrointestinal digestion and a Caco-2/RAW264.7 co-culture system. Food Funct. 2021, 12, 9197–9210. [Google Scholar] [CrossRef]
- Wang, N.; Liu, X.; Li, J.; Zhang, Q.; Li, X.; An, Q.; Ye, X.; Zhao, Z.; Cai, L.; Han, Y.; et al. Antibacterial mechanism of the synergistic combination between streptomycin and alcohol extracts from the Chimonanthus salicifolius S. Y. Hu. leaves. J. Ethnopharmacol. 2020, 250, 112467. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Cao, L.; Xiong, J.; Xia, G.; Hu, J.-F. Constituents from Chimonanthus praecox (wintersweet). Phytochem. Lett. 2011, 4, 271–274. [Google Scholar] [CrossRef]
- Liu, Z.; Xi, J.; Schröder, S.; Wang, W.; Xie, T.; Wang, Z.; Bao, S.; Fei, J. Chimonanthus nitens var. salicifolius aqueous extract protects against 5-fluorouracil induced gastrointestinal mucositis in a mouse model. Evid.-Based Complement. Altern. Med. 2013, 2013, 789263. [Google Scholar] [CrossRef]
- Chen, H.; Ouyang, K.; Jiang, Y.; Yang, Z.; Hu, W.; Xiong, L.; Wang, N.; Liu, X.; Wang, W. Constituent analysis of the ethanol extracts of Chimonanthus nitens Oliv. leaves and their inhibitory effect on α-glucosidase activity. Int. J. Biol. Macromol. 2017, 98, 829–836. [Google Scholar] [CrossRef]
- Wang, N.; Chen, H.; Xiong, L.; Liu, X.; Li, X.; An, Q.; Ye, X.; Wang, W. Phytochemical profile of ethanolic extracts of Chimonanthus salicifolius S. Y. Hu. leaves and its antimicrobial and antibiotic-mediating activity. Ind. Crops Prod. 2018, 125, 328–334. [Google Scholar] [CrossRef]
- Bach, H.; Sun, Q.; Zhu, J.; Cao, F.; Chen, F. Anti-inflammatory properties of extracts from Chimonanthus nitens Oliv. leaf. PLoS ONE 2017, 12, e0181094. [Google Scholar]
- He, J.; Zhang, Y.; Ouyang, K.; Chen, L.; Meng, W.; Zhang, Y.; Wang, W. Extraction, chemical composition, and protective effect of essential oil from Chimonanthus nitens Oliv. leaves on dextran sodium sulfate-induced colitis in mice. Oxidative Med. Cell. Longev. 2022, 2022, 9701938. [Google Scholar] [CrossRef]
- Meng, W.; Zhao, Z.; Chen, L.; Lin, S.; Zhang, Y.; He, J.; Ouyang, K.; Wang, W. Total flavonoids from Chimonanthus nitens Oliv. leaves ameliorate HFD-induced NAFLD by regulating the gut–liver axis in mice. Foods 2022, 11, 2169. [Google Scholar] [CrossRef]
- Meng, W.; Lin, S.; Ouyang, K.; Chen, L.; Zhang, Y.; Wang, W. Screening and inhibition mechanism of xanthine oxidase inhibitors in ethanolic extracts of Chimonanthus salicifolius Hu leaves. Chem. Biodivers. 2023, 20, e202200480. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lu, Q.; Cui, L.; Zong, M.; Guo, Y.; Liu, L.; Pan, D.; Wu, Z. The fatty acid profiles of mixed fermented milk and its anti-inflammation properties in an LPS-induced RAW264.7 cell model. Food Funct. 2022, 13, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, L.; Li, J.; Lin, L.; Zheng, G. A flavonoid-rich Smilax china L. extract prevents obesity by upregulating the adiponectin-receptor/AMPK signalling pathway and modulating the gut microbiota in mice. Food Funct. 2021, 12, 5862–5875. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.-S.; Kang, N.; Ranasinghe, P.; Lee, H.-S.; Jeon, Y.-J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Bringe, N.A.; de Mejia, E.G. Peptides in pepsin–pancreatin hydrolysates from commercially available soy products that inhibit lipopolysaccharide-induced inflammation in macrophages. Food Chem. 2014, 152, 423–431. [Google Scholar] [CrossRef]
- Fan, Z.; Jia, W.; Du, A.; Shi, L. Pseudo-targeted metabolomics analysis of the therapeutic effect of phenolics-rich extract from Se-enriched green tea (Camellia sinensis) on LPS-stimulated murine macrophage (RAW264.7). Food Res. Int. 2022, 159, 111666. [Google Scholar] [CrossRef]
- Nagalakshmi, U.; Waern, K.; Snyder, M. RNA-seq: A method for comprehensive transcriptome analysis. Curr. Protoc. Mol. Biol. 2010, 89, 4–11. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Chen, L.; Chen, S. The natural compound puerarin alleviates inflammation and apoptosis in experimental cell and rat preeclampsia models. Int. Immunopharmacol. 2021, 99, 108001. [Google Scholar] [CrossRef]
- Teng, J.; Li, J.; Zhao, Y.; Wang, M. Hesperetin, a dietary flavonoid, inhibits AGEs-induced oxidative stress and inflammation in RAW264.7 cells. J. Funct. Foods 2021, 81, 104480. [Google Scholar] [CrossRef]
- Qin, L.; Chen, S.; Xie, L.; Yu, Q.; Chen, Y.; Shen, M.; Xie, J. Mechanisms of RAW264.7 macrophages immunomodulation mediated by polysaccharide from mung bean skin based on RNA-seq analysis. Food Res. Int. 2022, 154, 111017. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, K.; Wang, S.; Ansari, A.R.; Niu, X.; Yang, W.; Lu, M.; Yang, Z.; ur Rehman, Z.; Zou, W.; et al. Visfatin is a multifaceted molecule that exerts regulation effects on inflammation and apoptosis in RAW264.7 cells and mice immune organs. Front. Immunol. 2022, 13, 1018973. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-Kappa B activation in RAW 264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Hinz, M.; Scheidereit, C. The IκB kinase complex in NF-κB regulation and beyond. EMBO Rep. 2013, 15, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Signaling to NF-κB. Genes Dev. 2004, 18, 2195–2224. [Google Scholar] [CrossRef]
- Han, J.M.; Lee, E.K.; Gong, S.Y.; Sohng, J.K.; Kang, Y.J.; Jung, H.J. Sparassis crispa exerts anti-inflammatory activity via suppression of TLR-mediated NF-κB and MAPK signaling pathways in LPS-induced RAW264.7 macrophage cells. J. Ethnopharmacol. 2019, 231, 10–18. [Google Scholar] [CrossRef]
- Li, Y.-R.; Fu, C.-S.; Yang, W.-J.; Wang, X.-L.; Feng, D.; Wang, X.-N.; Ren, D.-M.; Lou, H.-X.; Shen, T. Investigation of constituents from Cinnamomum camphora (L.) J. Presl and evaluation of their anti-inflammatory properties in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2018, 221, 37–47. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Cheang, W.S. Isoflavones daidzin and daidzein inhibit lipopolysaccharide-induced inflammation in RAW264.7 macrophages. Chin. Med. 2022, 17, 95. [Google Scholar] [CrossRef]
- Li, Y.; Meng, T.; Hao, N.; Tao, H.; Zou, S.; Li, M.; Ming, P.; Ding, H.; Dong, J.; Feng, S.; et al. Immune regulation mechanism of Astragaloside IV on RAW264.7 cells through activating the NF-κB/MAPK signaling pathway. Int. Immunopharmacol. 2017, 49, 38–49. [Google Scholar] [CrossRef]
- Liu, Q.; Xiao, X.-H.; Hu, L.-B.; Jie, H.-Y.; Wang, Y.; Ye, W.-C.; Li, M.-M.; Liu, Z. Anhuienoside C ameliorates collagen-induced arthritis through inhibition of MAPK and NF-κB signaling pathways. Front. Pharmacol. 2017, 8, 299. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Hu, T.; Jiang, J.-G.; Zhao, J.-W.; Zhu, W. Antioxidant and anti-inflammatory effects of polyphenols extracted fromIlex latifoliaThunb. RSC Adv. 2018, 8, 7134–7141. [Google Scholar] [CrossRef]
- Yang, L.; Cao, L.; Li, C.; Li, X.; Wang, J.; Chen, H.; He, J. Hostaflavone A from Hosta plantaginea (Lam.) Asch. blocked NF-κB/iNOS/COX-2/MAPKs/Akt signaling pathways in LPS-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2022, 282, 114605. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Varì, R.; Scazzocchio, B.; Benedetto, R.D.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Dell’istituto Super. Sanita 2007, 43, 394–405. [Google Scholar]
- Huang, S.M.; Wu, C.H.; Yen, G.C. Effects of flavonoids on the expression of the pro-inflammatory response in human monocytes induced by ligation of the receptor for AGEs. Mol. Nutr. Food Res. 2006, 50, 1129–1139. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, J.; Zheng, X.; Song, J.; Yin, L.; Guo, H.; Chen, Q.; Liu, Y.; Ma, Q.; Zhang, H.; et al. Kaempferol attenuates doxorubicin-induced renal tubular injury by inhibiting ROS/ASK1-mediated activation of the MAPK signaling pathway. Biomed. Pharmacother. 2023, 157, 114087. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Takeuchi, S.; Kubota, K.; Kobayashi, Y.; Kozakai, S.; Ukai, I.; Shichiku, A.; Okubo, M.; Numasaki, M.; Kanemitsu, Y.; et al. Lipopolysaccharide (LPS)-binding protein stimulates CD14-dependent Toll-like receptor 4 internalization and LPS-induced TBK1–IKKϵ–IRF3 axis activation. J. Biol. Chem. 2018, 293, 10186–10201. [Google Scholar] [CrossRef]
- Wang, D.-D.; Pan, W.-J.; Mehmood, S.; Cheng, X.-D.; Chen, Y. Polysaccharide isolated from Sarcodon aspratus induces RAW264.7 activity via TLR4-mediated NF-κB and MAPK signaling pathways. Int. J. Biol. Macromol. 2018, 120, 1039–1047. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′ → 3′) | Reverse Primer (5′ → 3′) |
|---|---|---|
| β-actin | CCAGCCTTCCTTCTTGGGTA | CAATGCCTGGGTACATGGTG |
| TNF-α | CTCATGCACCACCATCAAGG | ACCTGACCACTCTCCCTTTG |
| IL-1β | GTTCCCCAACTGGTACATCA | CCATACTTTAGGAAGACACGG |
| IL-6 | ACTTCACAAGTCCGGAGAGG | TGCAAGTGCATCATCGTTGT |
| IL-18 | CTTCTGCAACCTCCAGCATC | GTGAAGTCGGCCAAAGTTGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Meng, W.; Chen, H.; Chen, L.; Lin, S.; Ouyang, K.; Wang, W. Chimonanthus nitens Oliv. Leaf Flavonoids Exert Anti-Inflammatory Effects Through TLR4 Receptor Affecting NF-κB/MAPK Signaling Pathway. Appl. Sci. 2025, 15, 5177. https://doi.org/10.3390/app15095177
Shen L, Meng W, Chen H, Chen L, Lin S, Ouyang K, Wang W. Chimonanthus nitens Oliv. Leaf Flavonoids Exert Anti-Inflammatory Effects Through TLR4 Receptor Affecting NF-κB/MAPK Signaling Pathway. Applied Sciences. 2025; 15(9):5177. https://doi.org/10.3390/app15095177
Chicago/Turabian StyleShen, Lirong, Wenya Meng, Hui Chen, Lingli Chen, Suyun Lin, Kehui Ouyang, and Wenjun Wang. 2025. "Chimonanthus nitens Oliv. Leaf Flavonoids Exert Anti-Inflammatory Effects Through TLR4 Receptor Affecting NF-κB/MAPK Signaling Pathway" Applied Sciences 15, no. 9: 5177. https://doi.org/10.3390/app15095177
APA StyleShen, L., Meng, W., Chen, H., Chen, L., Lin, S., Ouyang, K., & Wang, W. (2025). Chimonanthus nitens Oliv. Leaf Flavonoids Exert Anti-Inflammatory Effects Through TLR4 Receptor Affecting NF-κB/MAPK Signaling Pathway. Applied Sciences, 15(9), 5177. https://doi.org/10.3390/app15095177








