Overcoming the pH Dependence of Iron-Based Catalysts and Efficient Generation of High-Valent Ferrite by Constructing a Neutral Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Catalysts
2.3. Degradation Experiments
2.4. Characterization
2.5. Analysis Method
3. Results and Discussion
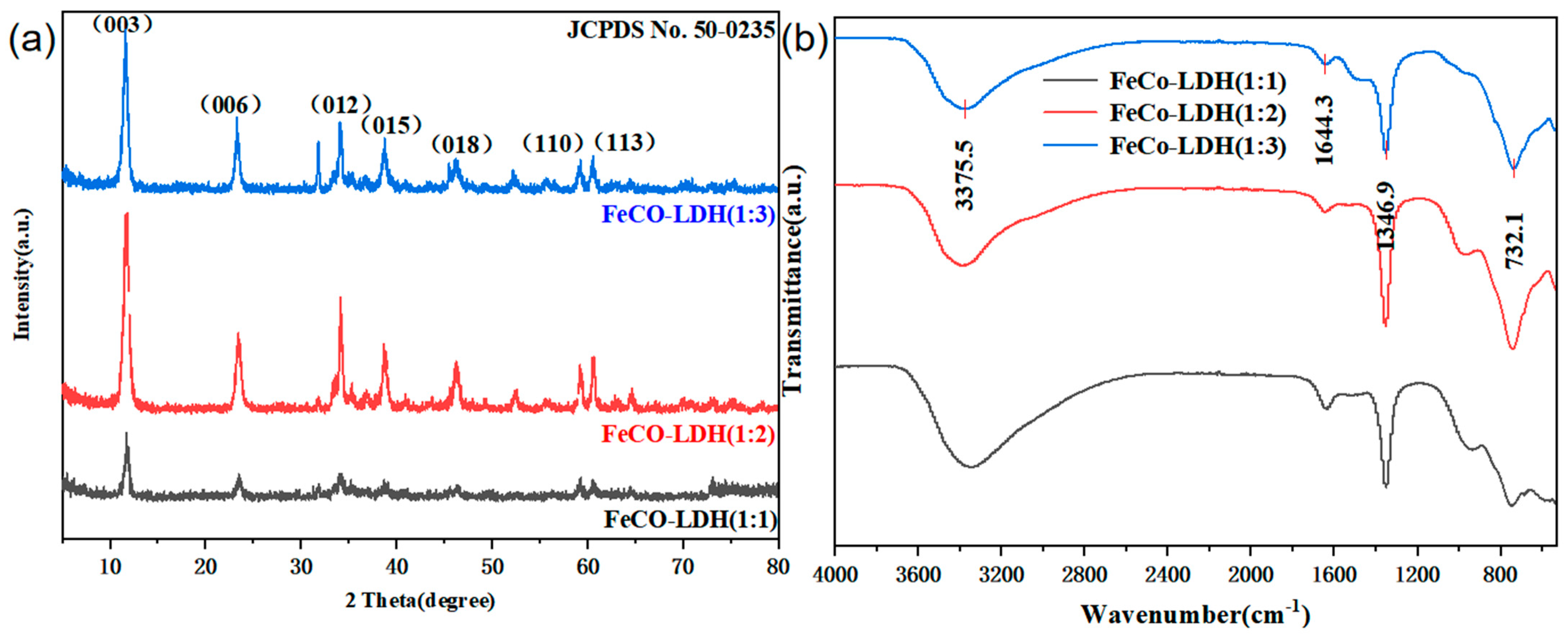
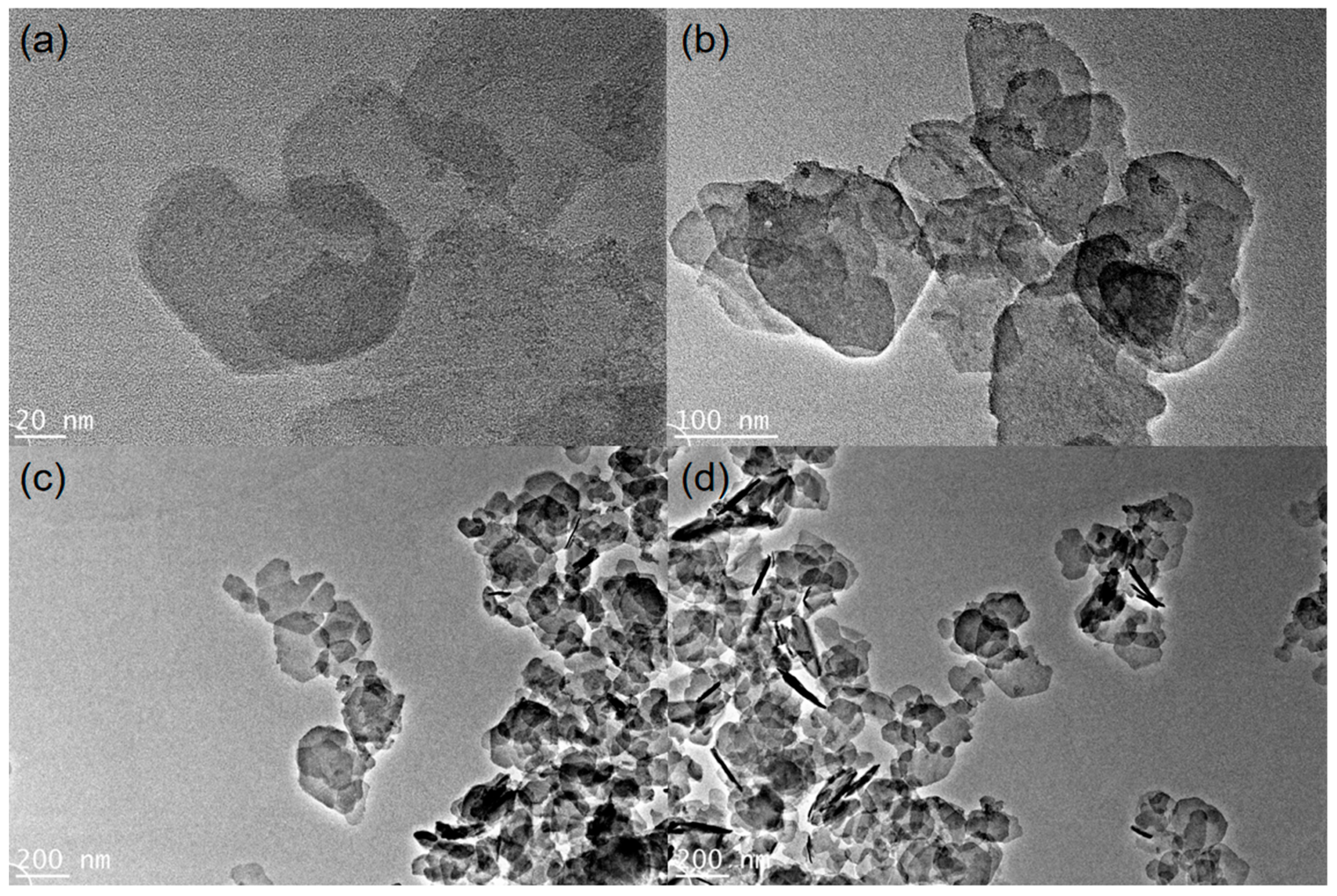
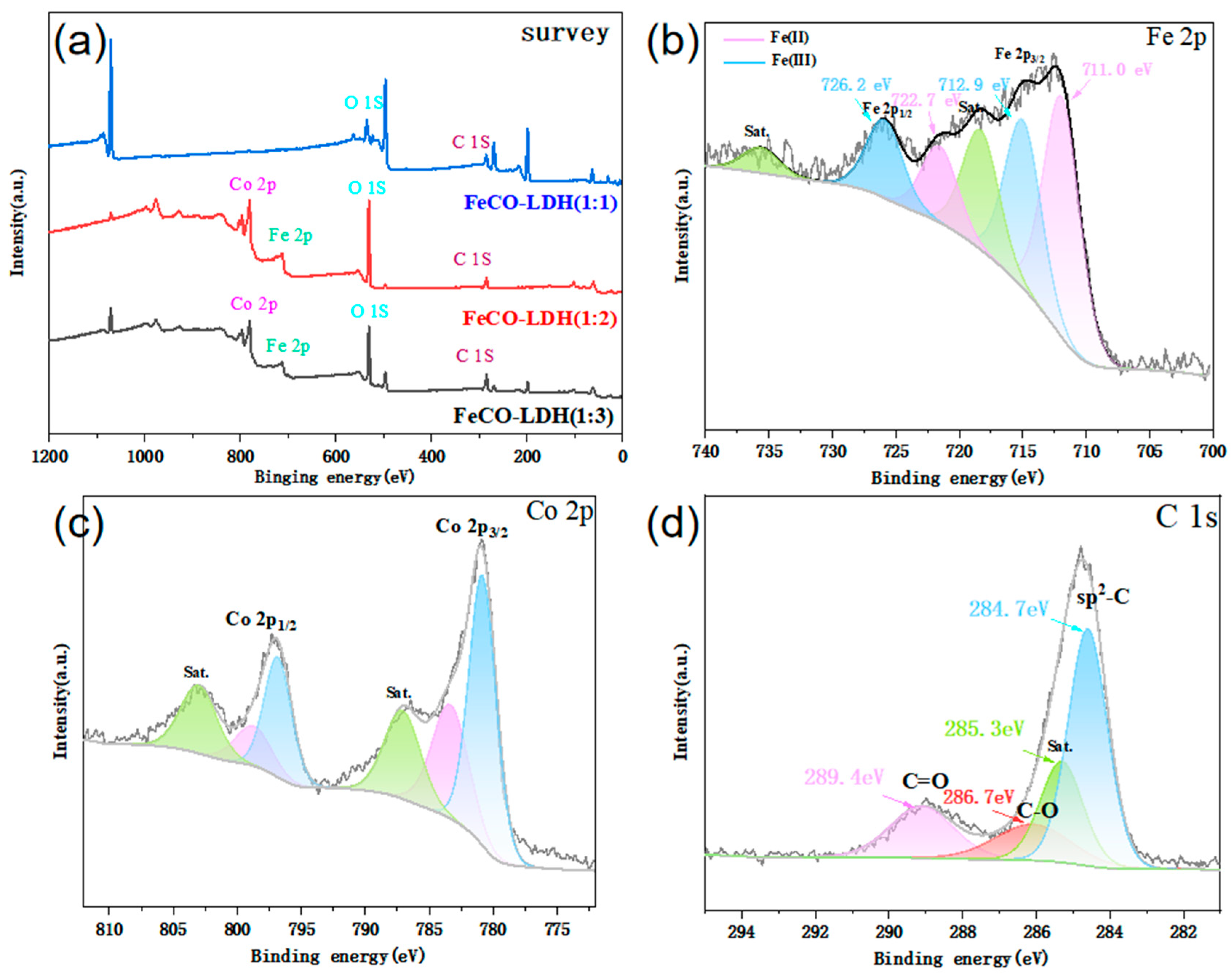
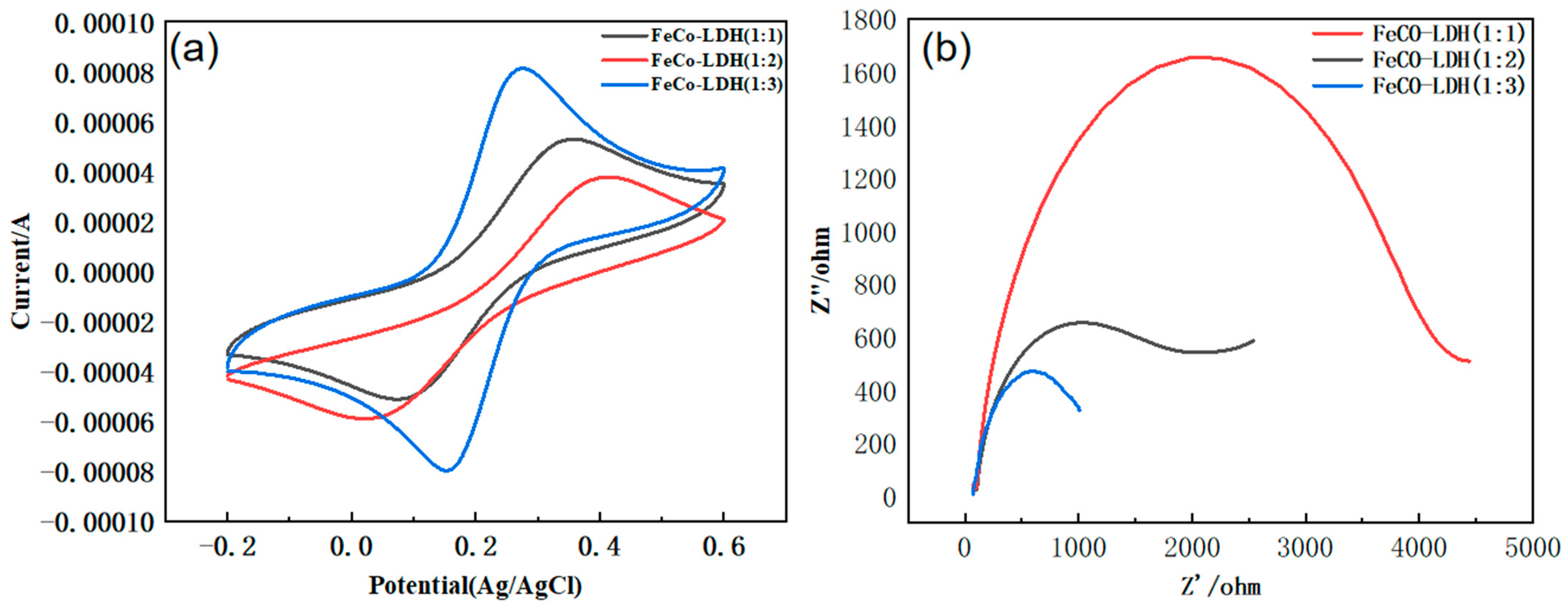
3.1. Characterization of Catalysts
3.2. Effective Activation of PMS with Catalysts for Contaminant Removal
3.3. Reactive Oxygen Species During PMS Activation
3.4. FeCo-LDH System Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, F.N.; Guo, Z.B.; Qiao, X.B.; Fan, J.Y.; Li, W.; Mi, M.; Tao, Z.G.; He, M.C. Large deformation mechanism of thin-layered carbonaceous slate and energy coupling support technology of NPR anchor cable in Minxian Tunnel: A case study. Tunn. Undergr. Space Technol. 2021, 117, 104151. [Google Scholar] [CrossRef]
- Tian, T.; Zhang, J.; Ge, S.; Tian, L. Enhanced gaseous benzene degradation by bimetallic MIL-101(Fe, Cu) activated persulfate system: Efficiency and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2025, 706, 135785. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, Y.; Zheng, S.; Chen, H.; Pei, J.; Yang, Y.; Ji, H.; Han, C. Critical review on the abatement of endocrine disrupting chemicals using synthesized iron-based materials in persulfate systems: Reactive species, reaction mechanisms, and perspectives. Chem. Eng. J. 2025, 511, 161675. [Google Scholar] [CrossRef]
- Kim, S.-Y. Enhancing the degradation of 2,4-dinitrotoluene using zero-valent magnesium in the presence of persulfate and hydrogen peroxide. J. Water Process Eng. 2025, 73, 107485. [Google Scholar]
- Wang, Z.; Li, H.; Ma, W.; Wang, Y.; Cui, P.; Qi, J.; Chen, Z.; Zhu, Z.; Meng, F. Highly efficient electro-catalysis activationof peroxymonosulfate by “used” As/Cr/Mo@FeOOH material for the degradation of metronidazole: Degradation mechanism and toxicity assessment. J. Taiwan Inst. Chem. Eng. 2021, 121, 302–312. [Google Scholar] [CrossRef]
- Patel, A.; Raikar, L.G.; Gandhi, J.; Prakash, H. Degradation of cefixime antibiotic by continuous flow ultraviolet-C persulfate based advanced oxidation process: Fresh and marine water matrices, antibacterial activity removal, and cost analysis. Chem. Eng. J. 2025, 512, 162178. [Google Scholar] [CrossRef]
- Lalas, K.; Panaras, G.; Souliotis, M.; Frontistis, Z. Degradation of micropollutants in water matrices using light activated—Persulfate: A review. J. Environ. Manag. 2025, 373, 123868. [Google Scholar] [CrossRef]
- Bougouizi, N.; Ahmedchekkat, F.; Chiha, M. Heat and iron-activated persulfate for Orange G degradation: Kinetics and synergy effect studies. Desalination Water Treat. 2025, 322, 101090. [Google Scholar] [CrossRef]
- Chen, T.; Liu, R.; Zhang, Y.; Shen, Y.; Yao, L.; Wang, X. Ultrasonic-activated persulfate treatment for enhancing pore volume and permeability of poplar wood. Ind. Crops Prod. 2025, 227, 120786. [Google Scholar] [CrossRef]
- Meng, Z.; Li, C.; Zhao, G.; Yang, Y.; Yan, Y.; Hu, C.; Wang, W. Activation mechanism of persulfate by acidified oil shale semi-coke for catalytic degradation of volatile phenols: The synergistic effect of transition metal and surface organic matter. J. Environ. Chem. Eng. 2025, 13, 115780. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J.; Hong, T.; Peng, X.; Fu, F. Dual heteroatom-doped porous biochar from chitosan/lignosulfonate gels for enhanced removal of tetracycline by persulfate activation: Performance and mechanism. Int. J. Biol. Macromol. 2025, 295, 139690. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.-H.; Pei, D.-S. A review on efficient removal of phthalic acid esters via biochars and transition metals-activated persulfate systems. Chemosphere 2021, 277, 130256. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Qin, X.; Lu, X.; Liu, Z.; Zhao, Y.; Yang, Y.; Zhen, G. Coupling weak magnetic field and zero-valent iron-activated persulfate oxidation process activation for improving dewaterability of waste activated sludge. J. Water Process Eng. 2024, 64, 105632. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical reactions in environmental remediation processes: Uncertainty and challenges. Appl. Catal. B Environ. 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Hu, P.; Long, M. Cobalt-catalyzed sulfate radical-based advanced oxidation: A review on heterogeneous catalysts and applications. Appl. Catal. B Environ. 2016, 181, 103–117. [Google Scholar] [CrossRef]
- Zhang, S.; Yin, Z.; Lu, Z.; Xu, Z.; Hu, C.; Li, F. A tandem dual-pathway non-radical water purification simultaneously induced by single-molecule peroxymonosulfate with a catalyst of coexisting Cu single atoms and Fe3C nanoparticles. Chem. Eng. J. 2024, 500, 157136. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, K.; Zhu, C.; Li, Y.; Yang, N. A novel persulfate activation strategy by double Z-scheme Bi2O3/CuBi2O4/BiOBr heterojunction: Non-radical dominated pathway for levofloxacin degradation. J. Environ. Chem. Eng. 2024, 12, 114139. [Google Scholar] [CrossRef]
- Mazivila, S.J.; Ricardo, I.A.; Leitão, J.M.M.; da Silva, J.C.G.E. A review on advanced oxidation processes: From classical to new perspectives coupled to two- and multi-way calibration strategies to monitor degradation of contaminants in environmental samples. Trends Environ. Anal. Chem. 2019, 24, e00072. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, P.; Hou, H.; Hu, J.; Liu, L.; Wu, L.; Chen, S.; Pan, K.; Liang, S.; Yuan, S.; et al. An iron chlorophyll derivative for enhanced degradation of bisphenol A: New insight into the generation mechanism of high-valent iron oxo species. Chem. Eng. J. 2023, 451, 138688. [Google Scholar] [CrossRef]
- Wang, Z.; Qiu, W.; Pang, S.-Y.; Zhou, Y.; Gao, Y.; Guan, C.; Jiang, J. Further understanding the involvement of Fe(IV) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment. Chem. Eng. J. 2019, 371, 842–847. [Google Scholar] [CrossRef]
- Lin, T.; Zhou, H.; Zhao, L.; Liang, S.; Luo, Y.; He, L.; Shan, S.; Hu, T.; Liu, Z.; Du, W. Direct generation of high-valent iron-oxo species to eliminate oxytetracycline at circumneutral pH via paper mill sludge ash activating peroxymonosulfate. Chem. Eng. J. 2024, 484, 150021. [Google Scholar] [CrossRef]
- Li, Y.; Hu, J.; Bao, Y.; Xiao, Y.; Lin, L.; Li, B.; Li, X.-Y. High-valent iron–oxo species in heterogeneous catalysis: Formation, mechanism, and reactivity for the degradation of various emerging organic contaminants. Chem. Eng. J. 2024, 501, 157490. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Highly effective degradation of selected groups of organic compounds by cavitation based AOPs under basic pH conditions. Ultrason. Sonochemistry 2018, 45, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zhang, Y.; Xiang, W.; Zhou, T.; Wu, X.; Mao, J. Efficient adsorption of Mn(II) by layered double hydroxides intercalated with diethylenetriaminepentaacetic acid and the mechanistic study. J. Environ. Sci. 2019, 85, 56–65. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, Y.; Zhang, T.; He, M.; Wang, Y.; Yang, X.; Yang, Y. Preparation and characterization of lactate-intercalated Co–Fe layered double hydroxides and exfoliated nanosheet film with low infrared emissivity. Appl. Surf. Sci. 2012, 263, 132–138. [Google Scholar] [CrossRef]
- Poudel, M.; Bustani, Z.; Li, X.; Zhu, J. Ring opening of cyclopropylmethylidene with trimethylaluminum: Synthesis of 1-cyclopropylethyl ether as an acid labile protecting group for hydroxyl Protection and carbohydrate synthesis. J. Carbohydr. Chem. 2025, 44, 81–93. [Google Scholar] [CrossRef]
- Kunimatsu, K.; Uchida, H.; Watanabe, M. Interactions between adsorbed hydrogen and water molecules on a polycrystalline Pt electrode studied by in-situ ATR-FTIR spectroscopy. J. Electroanal. Chem. 2024, 954, 118037. [Google Scholar] [CrossRef]
- Kristinaitytė, K.; Dagys, L.; Kausteklis, J.; Klimavicius, V.; Doroshenko, I.; Pogorelov, V.; Valevičienė, N.R.; Balevicius, V. NMR and FTIR studies of clustering of water molecules: From low-temperature matrices to nano-structured materials used in innovative medicine. J. Mol. Liq. 2017, 235, 1–6. [Google Scholar] [CrossRef]
- Yang, Q.; Zhong, Y.; Li, X.; Li, X.; Luo, K.; Wu, X.; Chen, H.; Liu, Y.; Zeng, G. Adsorption-coupled reduction of bromate by Fe(II)–Al(III) layered double hydroxide in fixed-bed column: Experimental and breakthrough curves analysis. J. Ind. Eng. Chem. 2015, 28, 54–59. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, Y.; Shen, J.; Zhang, X.; Li, H.; Xie, S.; Li, Y.; Zhang, Z.C. High-performance overall water electrolysis enabled by a one-step fabricated bifunctional Pt/NiFe LDH catalyst on iron nickel foam. Int. J. Hydrogen Energy 2024, 94, 749–755. [Google Scholar] [CrossRef]
- Guo, P.; Xu, L.; Yu, T.; Zhao, P.; Xu, J.; Shen, B. Transition metal in-situ doped BiOBr: Introducing oxygen vacancies to enhance hydroxyl radical generation for improved photocatalytic degradation of toluene. Sep. Purif. Technol. 2025, 354, 129247. [Google Scholar] [CrossRef]
- Wei, Q.-X.; Zhang, A.-Y.; Yang, Z.; Hu, S.; Wang, D.-J.; Zhang, C.; Liu, C.; Liu, R. Oxygen-exfoliated cobalt-doped C3N4 for superior Fenton-like catalysis: The accessible metal exposure and synergistic pollutant adsorption from three-dimensional layered configuration. J. Environ. Chem. Eng. 2023, 11, 111067. [Google Scholar] [CrossRef]
- Kalita, A.; Talukdar, A.K. Streamlined synthesis of iron and cobalt loaded MCM-48: High-performance heterogeneous catalysts for selective liquid-phase oxidation of toluene to benzaldehyde. Heliyon 2024, 10, e27296. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, H.; Yang, M.; He, X.; Ni, P.; Kang, L.; Liu, Z.-H. Topochemical synthesis of Ni2+–Fe3+ layered double hydroxides with large size. Appl. Clay Sci. 2011, 52, 51–55. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, Z.; Wan, J.; Wang, Y.; Ye, G.; Huang, S.; Zeng, C.; Yi, J. Synthesis of Fe-Nx site-based iron-nitrogen co-doped biochar catalysts for efficient removal of sulfamethoxazole from water by activation of persulfate: Electron transfer mechanism of non-free radical degradation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130174. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, S.; Xu, F.; Wang, Z.; Zhao, C.; Xu, X.; Gao, B.; Li, Q. Revealing the fundamental role of MoO2 in promoting efficient and stable activation of persulfate by iron carbon based catalysts: Efficient Fe2+/Fe3+ cycling to generate reactive species. Water Res. 2022, 225, 119142. [Google Scholar] [CrossRef]
- Sun, H.; Peng, X.; Zhang, S.; Liu, S.; Xiong, Y.; Tian, S.; Fang, J. Activation of peroxymonosulfate by nitrogen-functionalized sludge carbon for efficient degradation of organic pollutants in water. Bioresour. Technol. 2017, 241, 244–251. [Google Scholar] [CrossRef]
- Duan, M.; Qiu, M.; Sun, S.; Guo, X.; Liu, Y.; Zheng, X.; Cao, F.; Kong, Q.; Zhang, J. Intercalating assembly of NiFe LDH nanosheets/CNTs composite as high-performance electrocatalyst for oxygen evolution reaction. Appl. Clay Sci. 2022, 216, 106360. [Google Scholar] [CrossRef]
- Liv, L.; Tosun, S.G. A novel perspective for estimating the passivation and energy storage characteristics in micro-mass coatings using CV, EIS, and EQCM techniques: Ultrahigh supercapacitor behaviors of Bismarck Brown R and Bismarck Brown Y polymer films. J. Energy Storage 2025, 115, 115985. [Google Scholar] [CrossRef]
- Mahato, N.; Singh, S.; Sreekanth, T.V.M.; Yoo, K.; Kim, J. Polycrystalline phases engineered in-situ in polyaniline-graphene composite evoluting an exceptional stability and high charge storage capacity: An EIS investigative approach to evaluate material’s stability. J. Energy Storage 2024, 88, 111464. [Google Scholar] [CrossRef]
- He, J.; Huang, J.; Wang, Z.; Liu, Z.; Chen, Y.; Su, R.; Ni, X.; Li, Y.; Xu, X.; Zhou, W.; et al. The enhanced catalytic degradation of sulfamethoxazole over Fe@nitrogen-doped carbon-supported nanocomposite: Insight into the mechanism. Chem. Eng. J. 2022, 439, 135784. [Google Scholar] [CrossRef]
- Bi, C.-C.; Ke, X.-X.; Chen, X.; Weerasooriya, R.; Hong, Z.-Y.; Wang, L.-C.; Wu, Y.-C. Assembling reduced graphene oxide with sulfur/nitrogen- “hooks” for electrochemical determination of Hg(II). Anal. Chim. Acta 2020, 1100, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Rachid, Y.; El-Asri, A.; El Gaayda, J.; Titchou, F.E.; Errami, M.; Aziz, J.; Yap, P.-S.; Abdelaziz, O.; Bakas, I.; Akbour, R.A. Adsorption of synthetic cationic dyes from water (experimental, DFT and Monte Carlo studies) and treatment of real industrial wastewater using dextrose compound-modified layered double hydroxide. Process Saf. Environ. Prot. 2024, 194, 1605–1624. [Google Scholar] [CrossRef]
- Nazir, M.A.; Bashir, M.A.; Najam, T.; Javed, M.S.; Suleman, S.; Hussain, S.; Kumar, O.P.; Shah, S.S.A.; Rehman, A.U. Combining structurally ordered intermetallic nodes: Kinetic and isothermal studies for removal of malachite green and methyl orange with mechanistic aspects. Microchem. J. 2021, 164, 105973. [Google Scholar] [CrossRef]
- Tian, H.; Peng, S.; Zhao, L.; Chen, Y.; Cui, K. Simultaneous adsorption of Cd(II) and degradation of OTC by activated biochar with ferrate: Efficiency and mechanism. J. Hazard. Mater. 2023, 447, 130711. [Google Scholar] [CrossRef]
- Ghiasi, S.; Mohammadi, T.; Tofighy, M.A. Hybrid nanofiltration thin film hollow fiber membranes with adsorptive supports containing bentonite and LDH nanoclays for boron removal. J. Membr. Sci. 2022, 655, 120576. [Google Scholar] [CrossRef]
- In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J. Environ. Manag. 2020, 267, 110629. [CrossRef]
- GB 25467-2010/XG1-2013; Emission Standard of Pollutants for Copper, Nickel, Cobalt Industry. China Environmental Science Press: Beijing, China, 2010.
- Cheng, C.; Ren, W.; Zhang, H.; Duan, X.; Wang, S. Single-atom iron catalysts for peroxymonosulfate-based advanced oxidation processes: Coordination structure versus reactive species. Chin. J. Catal. 2024, 59, 15–37. [Google Scholar] [CrossRef]
- Wu, X.; Liu, T.; Ni, W.; Yang, H.; Huang, H.; He, S.; Li, C.; Ning, H.; Wu, W.; Zhao, Q.; et al. Engineering controllable oxygen vacancy defects in iron hydroxide oxide immobilized on reduced graphene oxide for boosting visible light-driven photo-Fenton-like oxidation. J. Colloid Interface Sci. 2022, 623, 9–20. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Chen, Y.; Dou, L.; Ren, Y.; Li, N.; Lai, B.; Tan, B. Cobalt doped g-C3N4 activated peroxymonosulfate for organic pollutant degradation: Alterations in cobalt species and reactive oxygen species. Chemosphere 2024, 369, 143763. [Google Scholar] [CrossRef]
- Zhang, H.; Smith, J.R.L.; Shen, F.; Qi, X. ZnFe layered double hydroxide wrapped metal-organic framework derived cobalt-nitrogen-doped carbon material as peroxymonosulfate activator for ciprofloxacin degradation. Chem. Eng. J. 2024, 496, 154191. [Google Scholar] [CrossRef]
- Wang, S.; Deng, Y.; Shao, B.; Zhu, J.; Guan, X. Reinvestigation of the oxidation of organic contaminants by Fe(VI): Kinetics and effects of water matrix constituents. J. Hazard. Mater. 2022, 430, 128421. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Fu, S.; Xu, Z.; Zhou, X.; Li, J.; Zhang, Y.; Lu, Y.; Li, S.; Zhang, T. Unveiling the long-range interaction of sulfur in the second shell of Fe-N4 single-atom sites for highly selective generation of high-valent iron-oxo species in peroxymonosulfate activation. Chem. Eng. J. 2025, 505, 159684. [Google Scholar] [CrossRef]
- Zhou, X.; Yin, R.; Kang, J.; Li, Z.; Pan, Y.; Bai, J.; Li, A.J.; Qiu, R. Atomic cation-vacancy modulated peroxymonosulfate nonradical oxidation of sulfamethoxazole via high-valent iron-oxo species. Appl. Catal. B Environ. 2023, 330, 122640. [Google Scholar] [CrossRef]
- Liu, L.; Wang, A.; Hu, J.; Hou, H.; Liang, S.; Yang, J. Peroxymonosulfate activated by natural porphyrin derivatives for rapid degradation of organic pollutants via singlet oxygen and high-valent iron-oxo species. Chemosphere 2023, 331, 138783. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Wang, X.; Gong, W.; Zhu, J.; Peng, G. New insights into the quantiffcation of Fe(IV) using methyl phenyl sulfoxide (PMSO) as probe in the iron-based heterogeneous catalyst activated persulfate process. Environ. Pollut. 2024, 362, 124924. [Google Scholar] [CrossRef]
- Cao, S.; Yin, Y.; Zheng, S.; Song, C.; Gan, X.; Zhang, W.; Feng, D.; Shang, J.; Cheng, X. Peroxymonosulfate activation by Ni–Co nanoparticles encapsulated in nitrogen-doped carbon derived from Co-doped Ni metal–organic frameworks for efficient enrofloxacin degradation. Chem. Eng. J. 2025, 503, 158281. [Google Scholar] [CrossRef]
- Li, Y.; Yang, T.; Qiu, S.; Lin, W.; Yan, J.; Fan, S.; Zhou, Q. Uniform N-coordinated single-atomic iron sites dispersed in porous carbon framework to activate PMS for efficient BPA degradation via high-valent iron-oxo species. Chem. Eng. J. 2020, 389, 124382. [Google Scholar] [CrossRef]
- Wang, N.; Hsu, C.; Zhu, L.; Tseng, S.; Hsu, J.-P. Influence of metal oxide nanoparticles concentration on their zeta potential. J. Colloid Interface Sci. 2013, 407, 22–28. [Google Scholar] [CrossRef]
- Ulu, F.; Gengec, E.; Kobya, M. Removal of natural organic matter from Lake Terkos by EC process: Studying on removal mechanism by floc size and zeta potential measurement and characterization by HPSEC method. J. Water Process Eng. 2019, 31, 100831. [Google Scholar] [CrossRef]
- Joshua, A.M.; Ooi, E.H.; Chang, W.S.; Lau, E.V. Mechanical and physicochemical characteristics of sub-millimeter and macrobubbles: Insights from AFM analysis, zeta potential measurements and rise velocity. Results Surf. Interfaces 2024, 16, 100262. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, Y.; Yao, L.; Yang, X. Colloid-bound radicals formed in NOM-enhanced Fe(III)/peroxymonosulfate process accelerate the degradation of trace organic contaminants in water. Water Res. 2024, 248, 120880. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Yu, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S. Activation of persulfate by modified drinking water treatment residuals for sulfamethoxazole degradation. Chem. Eng. J. 2018, 353, 490–498. [Google Scholar] [CrossRef]
- Zhi, K.; Liu, D.; Xu, J.; Li, Z.; Li, S.; Luo, L.; Gong, G.; Han, R.; Yin, A.; Guo, L. N-doped graphene loaded with Ru sites as PMS activators for SMX degradation via non-radical pathway: Efficiency, selectivity and mechanism. Chem. Eng. J. 2025, 512, 162230. [Google Scholar] [CrossRef]
- Yang, W.; Dong, H.; Tian, Y.; Tong, Y.; Lu, J.; Liao, L.; Niu, J. Electrochemistry enhanced multi-stage biological contact oxidation treating sulfamethoxazole wastewater: Performance, degradation pathway and microbial community. Sep. Purif. Technol. 2025, 357, 130044. [Google Scholar] [CrossRef]
- Xia, X.; Gao, Q.; Ji, X.; Wan, J.; Wang, Y.; Ma, Y. Novel cobalt-doped CuFe2O4 spinel catalyst for enhanced peroxymonosulfate activation to achieve rapid degradation of tetracycline hydrochloride: Dominant roles of O2- and 1O2 in the oxidation process. J. Water Process Eng. 2025, 69, 106638. [Google Scholar] [CrossRef]
- Tian, M.; Ren, X.; Fu, N.; Chen, Z.; Wei, Y.; Fan, X.; Yang, Z.; An, J. Ethylene glycol-assisted synthesis of Mo/Fe co-doped g-C3N4 composite 8-CNMF12 efficiently activates PMS to degrade phenol. Colloids Surf. A Physicochem. Eng. Asp. 2025, 709, 136134. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, M.; Zhou, X. Reliability analysis of NaN3 as quencher of 1O2 in heterogeneous persulfate catalytic oxidation system. Catal. Commun. 2023, 183, 106774. [Google Scholar] [CrossRef]
- Deng, X.; Yang, H.; Zhao, D.; Guan, J.; Gu, Z.; Zhang, L. Mn-Cu co-doped graphitic carbon nitride accelerates permonosulfate to generate more singlet oxygen in pollutant degradation. J. Water Process Eng. 2024, 64, 105755. [Google Scholar] [CrossRef]
- Su, S.; Guo, W.; Leng, Y.; Yi, C.; Ma, Z. Heterogeneous activation of Oxone by Co(x)Fe(3-x)O4 nanocatalysts for degradation of rhodamine B. J. Hazard. Mater. 2013, 244–245, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liao, C.; Kong, L.; Wu, D.; Liu, Y.; Lee, P.-H.; Shih, K. Facile synthesis of highly reactive and stable Fe-doped g-C3N4 composites for peroxymonosulfate activation: A novel nonradical oxidation process. J. Hazard. Mater. 2018, 354, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; de Vidales, M.J.M.; Gorni, G.; Gómez-Herrero, A.; Fernández-Martínez, F.; Dos santos-García, A.J. Enhanced performance and recyclability for peroxymonosulfate activation via g-C3N4 supported CoFe layer double oxide. Chem. Eng. J. 2022, 444, 136610. [Google Scholar] [CrossRef]
- Yan, H.; Xu, L.; Wei, H.; Li, X. Biotransformation of sulfamethoxazole by newly isolated surfactant-producing strainProteus mirabilis sp. ZXY4: Removal efficiency, pathways, and mechanisms. Bioresour. Technol. 2023, 385, 129422. [Google Scholar] [CrossRef]
- Zhu, Y.-Y.; Liu, Y.; Xu, J.; Ni, B.-J. Three-dimensional excitation-emission matrix (EEM) fluorescence approach to probing the binding interactions of polystyrene microplastics to bisphenol A. J. Hazard. Mater. Adv. 2022, 5, 100046. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Chen, Y.; Airiken, M. Estimation of surface water quality parameters based on hyper-spectral and 3D-EEM fluorescence technologies in the Ebinur Lake Watershed, China. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102895. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, J.; Gao, B.; Sillanpää, M. The effect of autochthonous metal ions and humic substances on landfill leachate ozonation: Reactive oxygen species and 3D fluorescence analysis. Sep. Purif. Technol. 2025, 354, 129433. [Google Scholar] [CrossRef]
- Roy, N.; Roy, S.; Debroy, A.; Mukherjee, A. Chlorella sp. EPS loaded alginate microsphere: A novel bionanomaterial for adsorptive removal of sulfamethoxazole from the aqueous solutions. Environ. Technol. Innov. 2024, 33, 103513. [Google Scholar] [CrossRef]
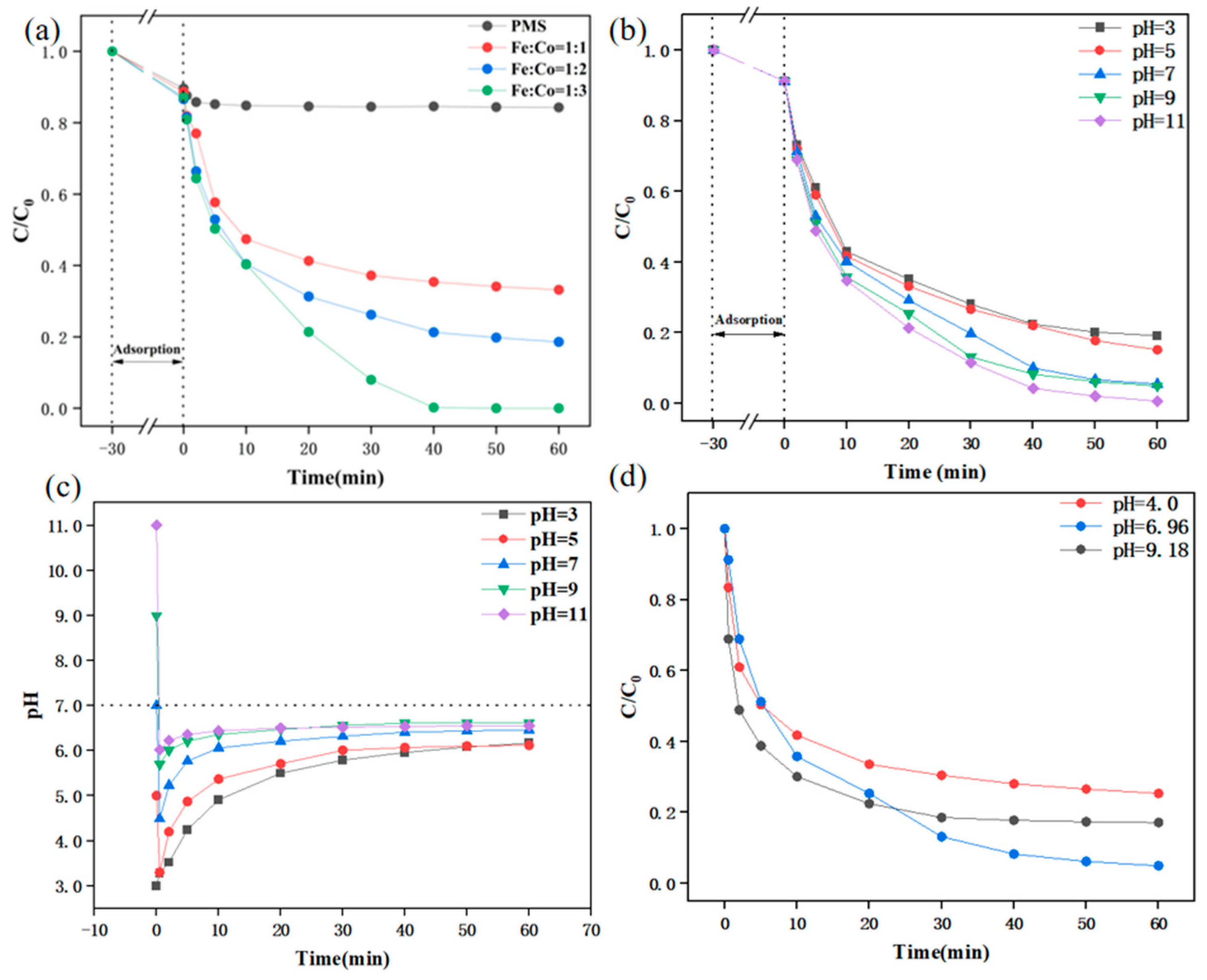
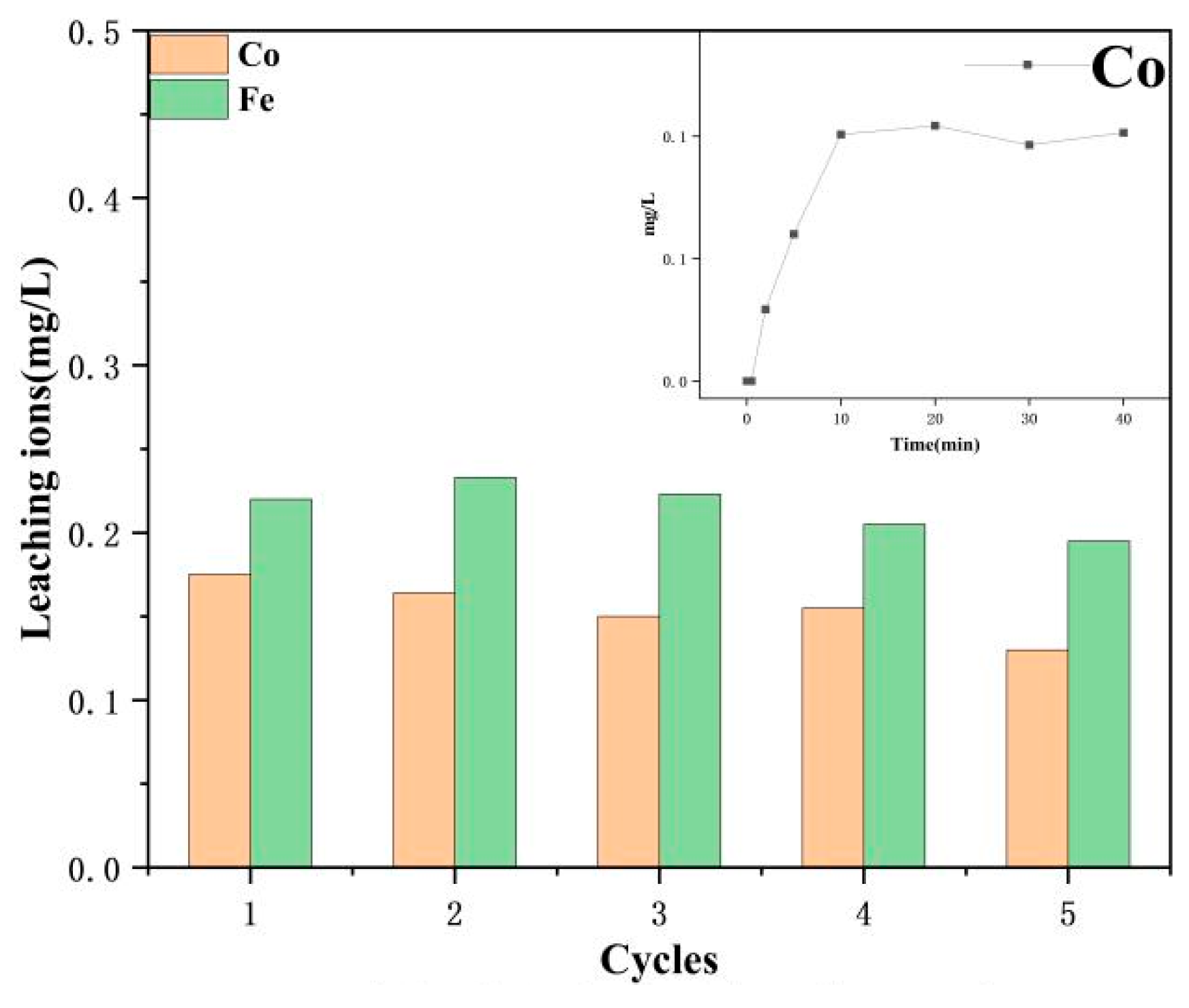
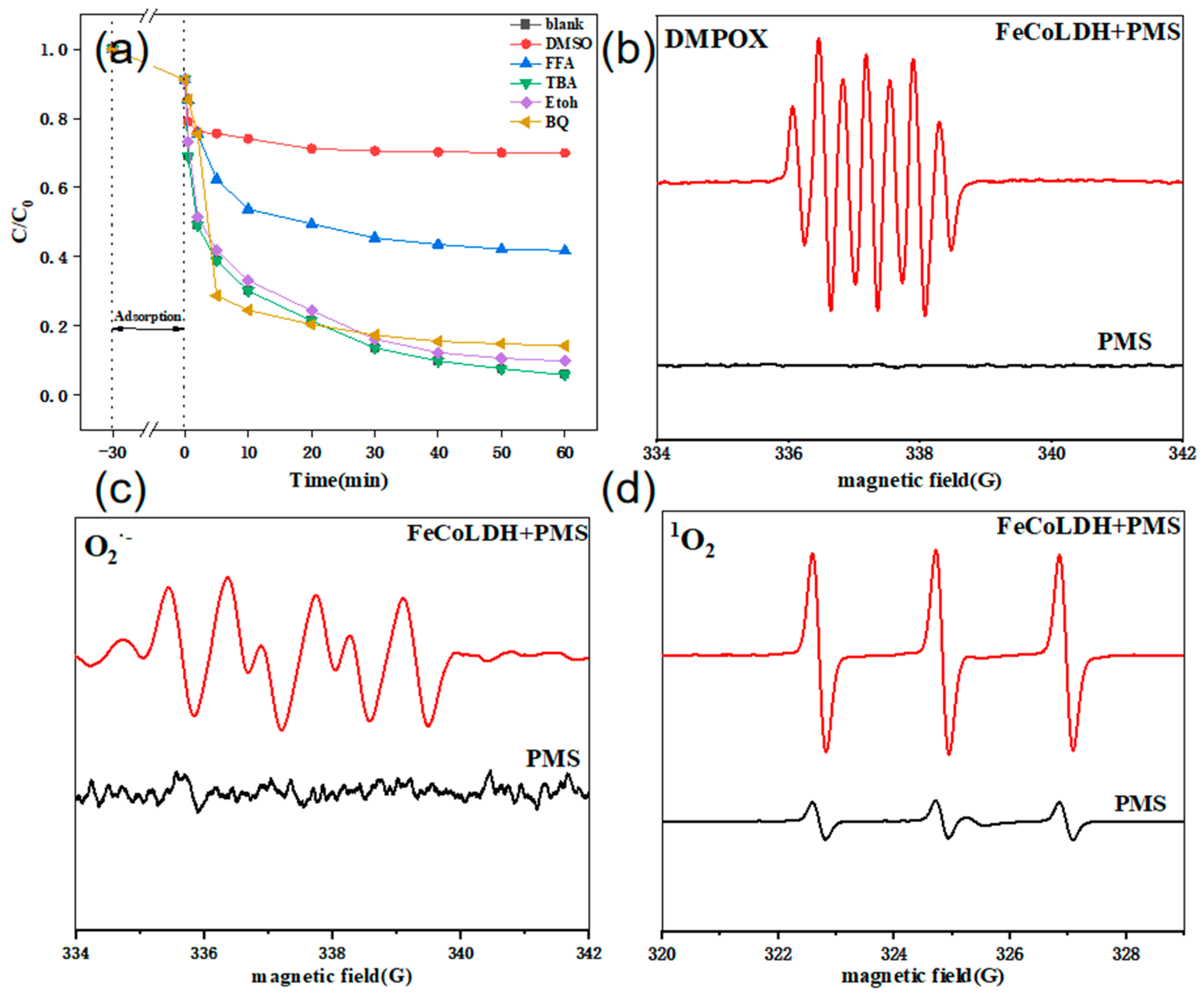
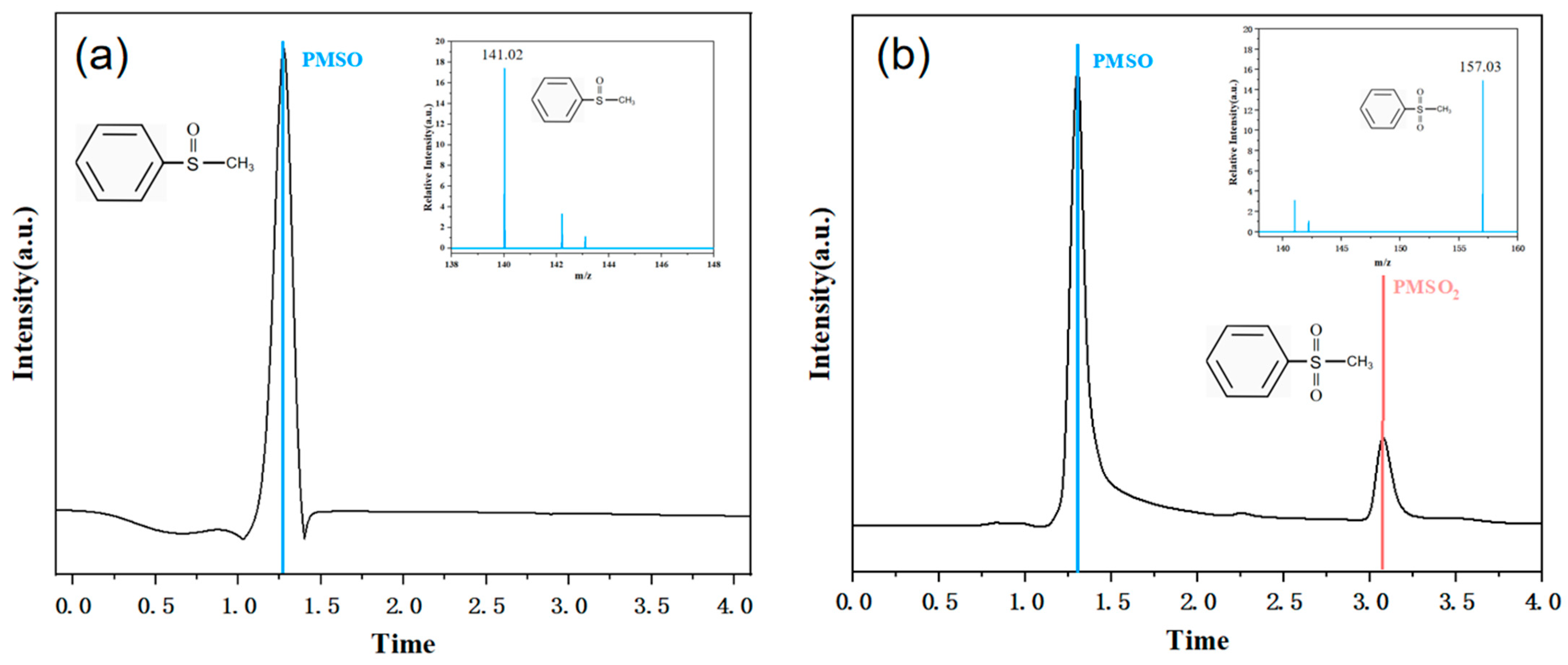
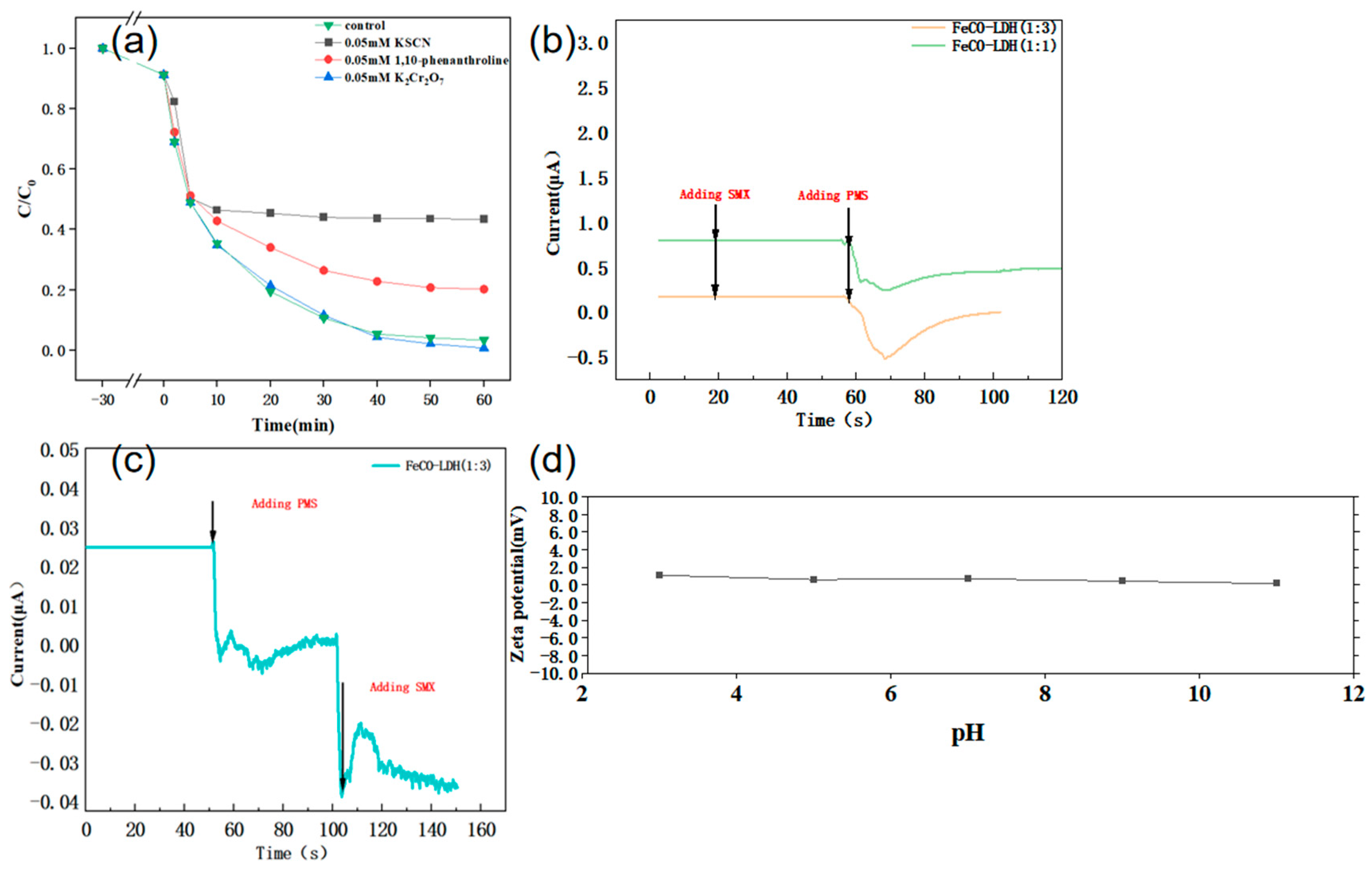
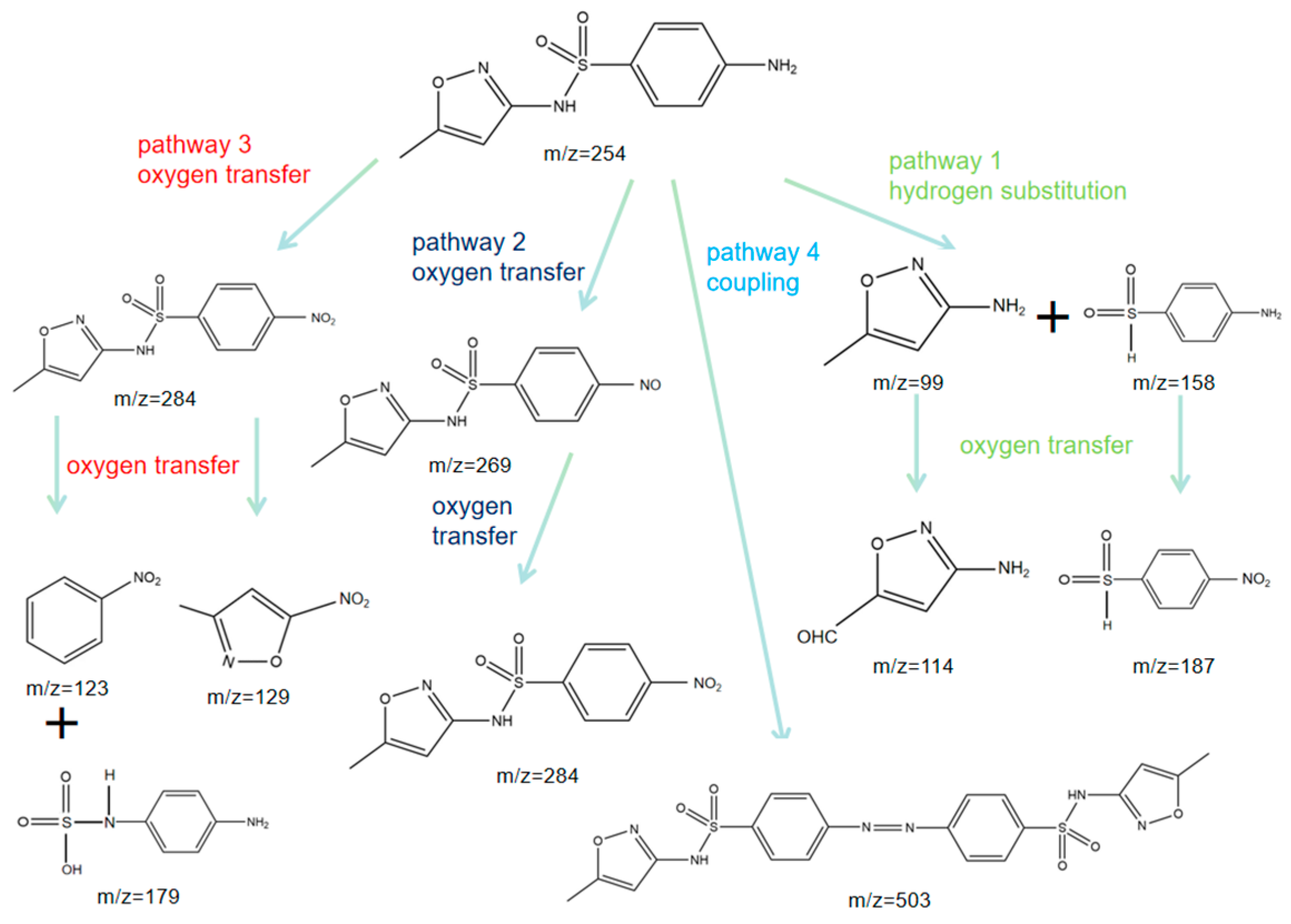
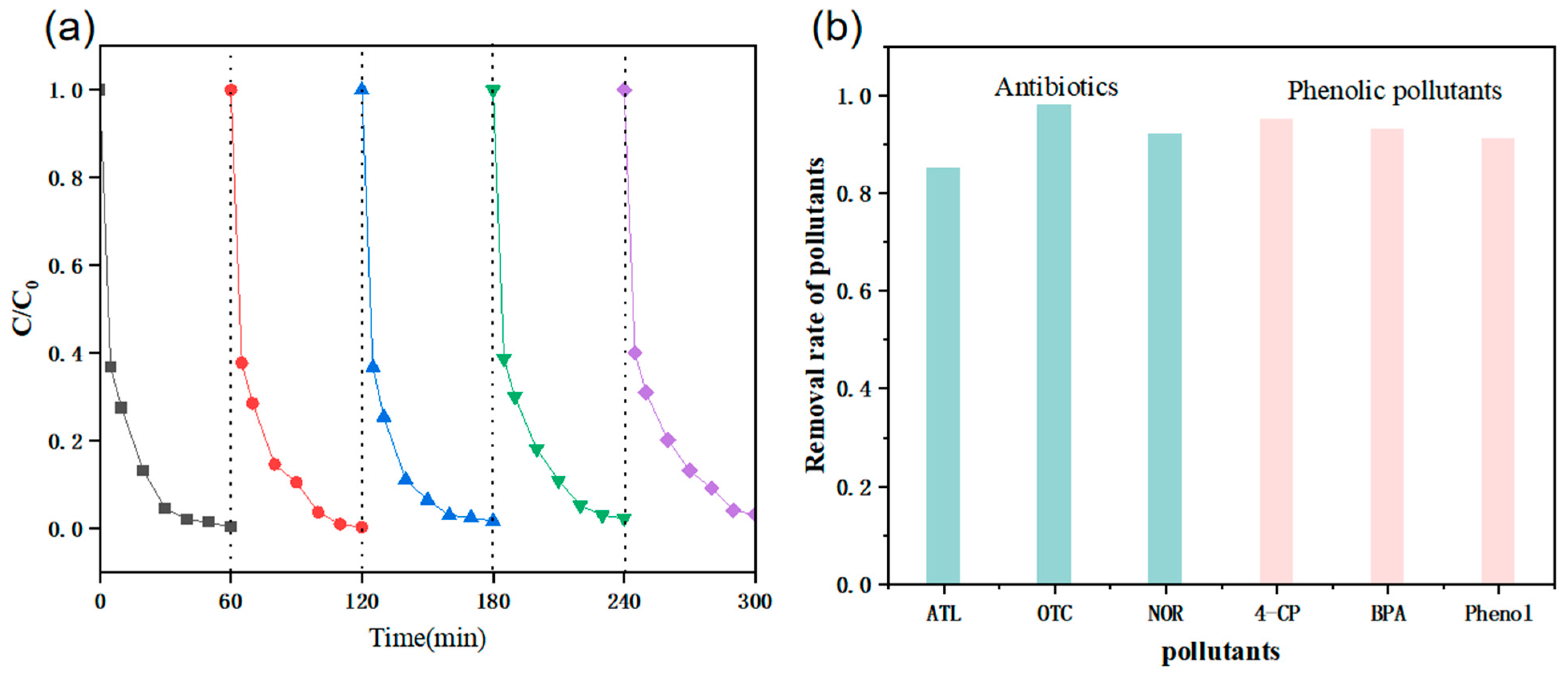
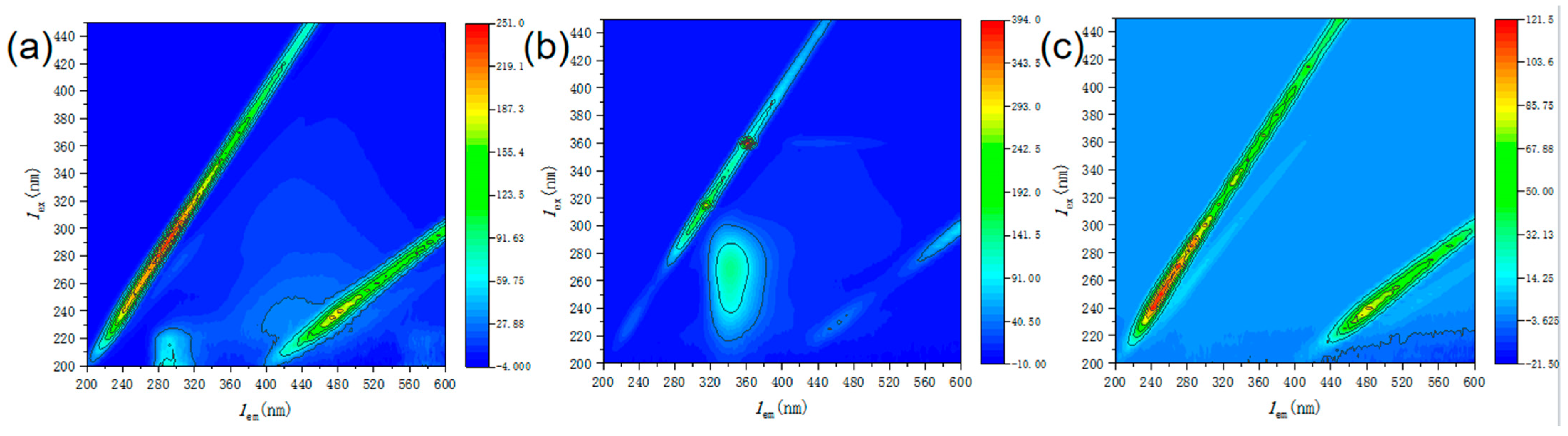
| Experimental Conditions | Catalyst Type | Initial pH | SMX Concentration (mg/L) | PMS Dosing Amount (mmol/L) | Catalyst Dosage (g/L) |
|---|---|---|---|---|---|
| Catalyst type | 3 types of catalysts | 7 | 10 | 0.3 | 0.1 |
| SMX concentration | FeCo-LDH | 7 | 10, 20, 25, 50 | 0.3 | 0.1 |
| PMS dosing amount | FeCo-LDH | 7 | 10 | 0.1, 0.3, 0.5, 1 | 0.1 |
| Catalyst dosage | FeCo-LDH | 7 | 10 | 0.3 | 0.05, 0.1, 0.2, 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Cui, K. Overcoming the pH Dependence of Iron-Based Catalysts and Efficient Generation of High-Valent Ferrite by Constructing a Neutral Microenvironment. Appl. Sci. 2025, 15, 5100. https://doi.org/10.3390/app15095100
Chen J, Cui K. Overcoming the pH Dependence of Iron-Based Catalysts and Efficient Generation of High-Valent Ferrite by Constructing a Neutral Microenvironment. Applied Sciences. 2025; 15(9):5100. https://doi.org/10.3390/app15095100
Chicago/Turabian StyleChen, Jingwei, and Kangping Cui. 2025. "Overcoming the pH Dependence of Iron-Based Catalysts and Efficient Generation of High-Valent Ferrite by Constructing a Neutral Microenvironment" Applied Sciences 15, no. 9: 5100. https://doi.org/10.3390/app15095100
APA StyleChen, J., & Cui, K. (2025). Overcoming the pH Dependence of Iron-Based Catalysts and Efficient Generation of High-Valent Ferrite by Constructing a Neutral Microenvironment. Applied Sciences, 15(9), 5100. https://doi.org/10.3390/app15095100






