A Portable Optical Sensor for Microplastic Detection: Development and Calibration
Abstract
1. Introduction
2. Materials and Methods
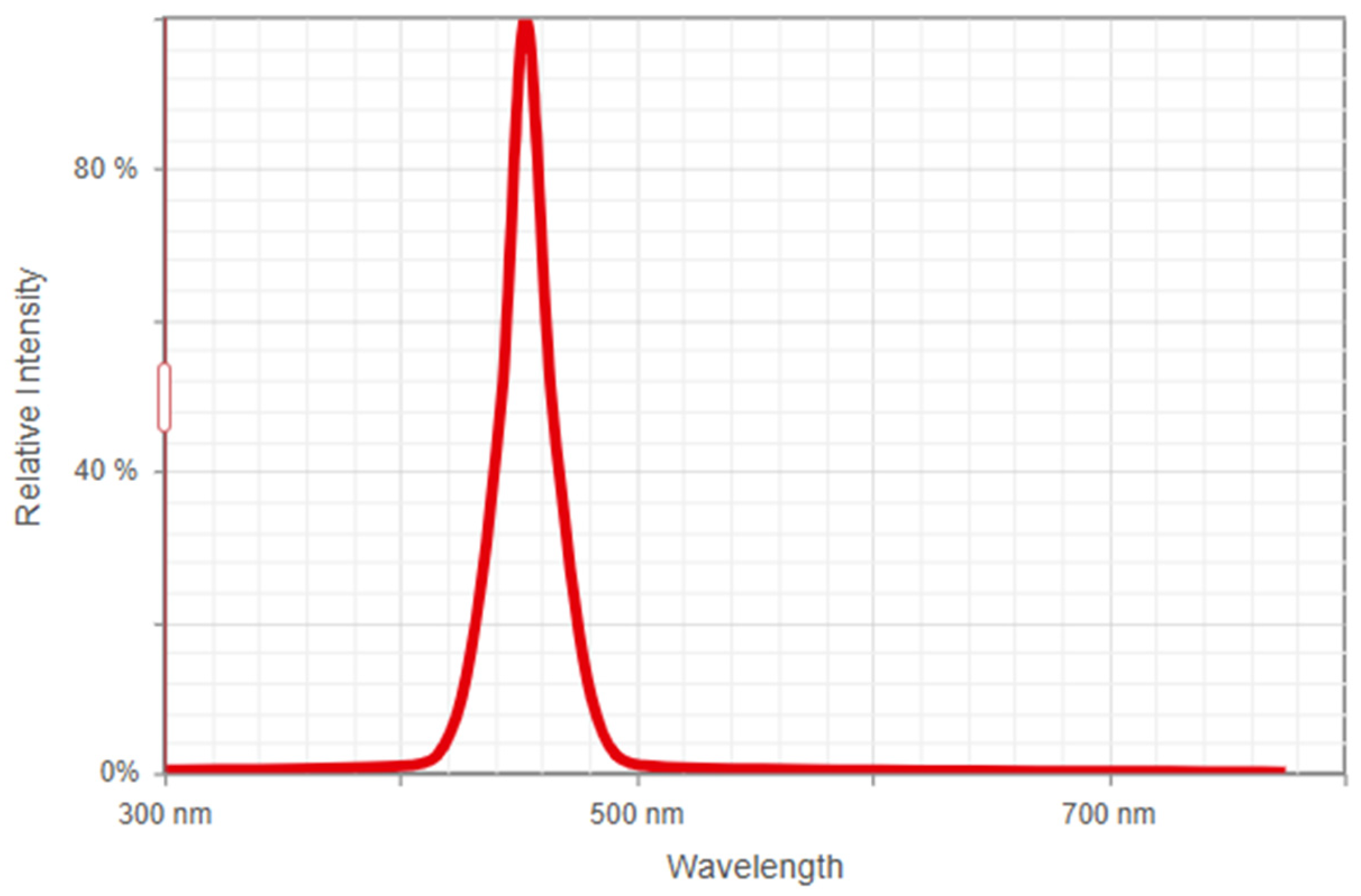
2.1. Wavelength Selection
- Select a light source that emits a range of wavelengths suitable for interacting with the microplastics to be detected. Factors such as power, stability, divergence, and cost must be considered for effective interaction with microplastics;
- Design an optical system to focus and direct a light beam to the area of interest, allowing collection and analysis of the optical signal reflected or transmitted by microplastics.
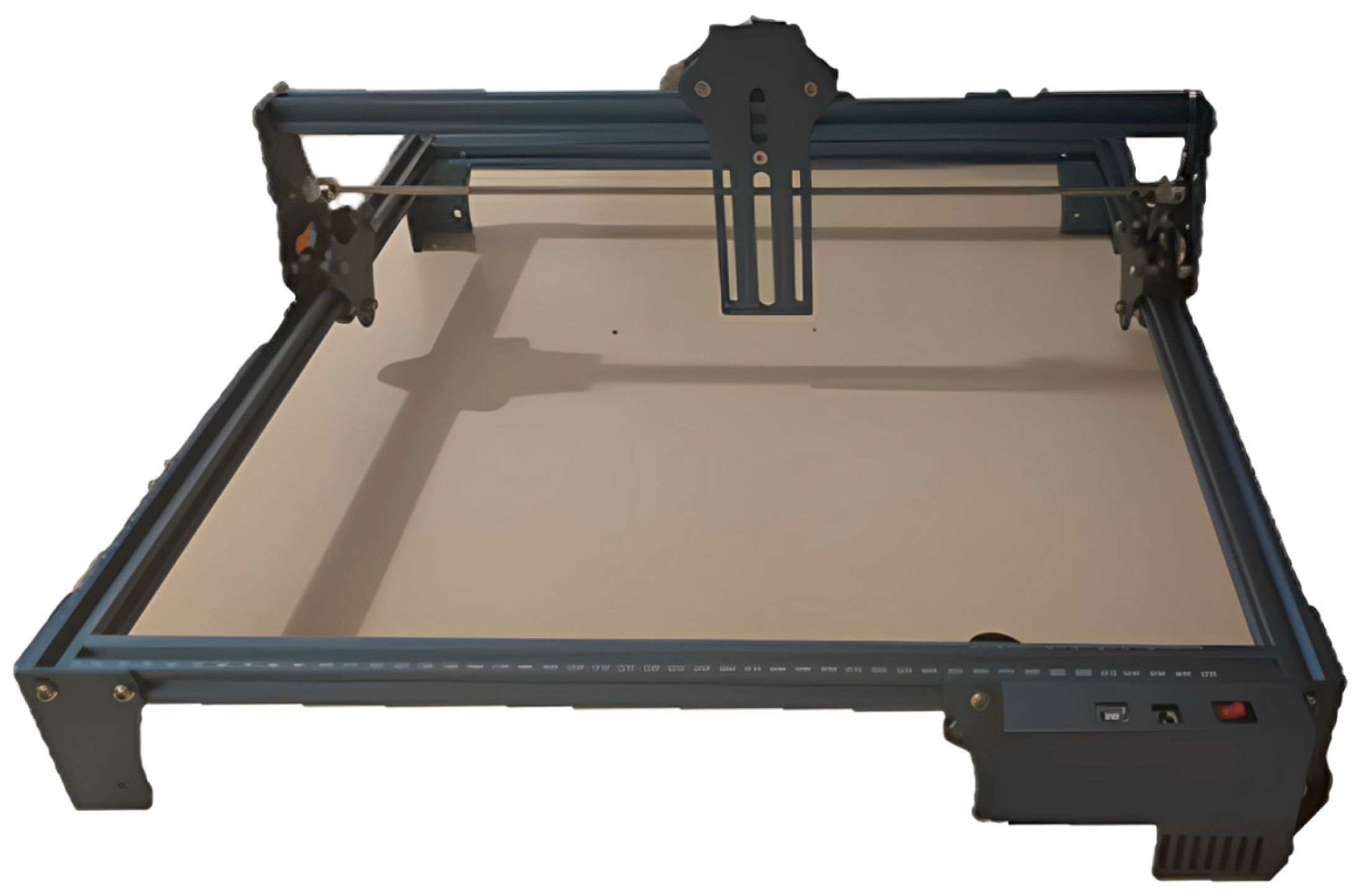
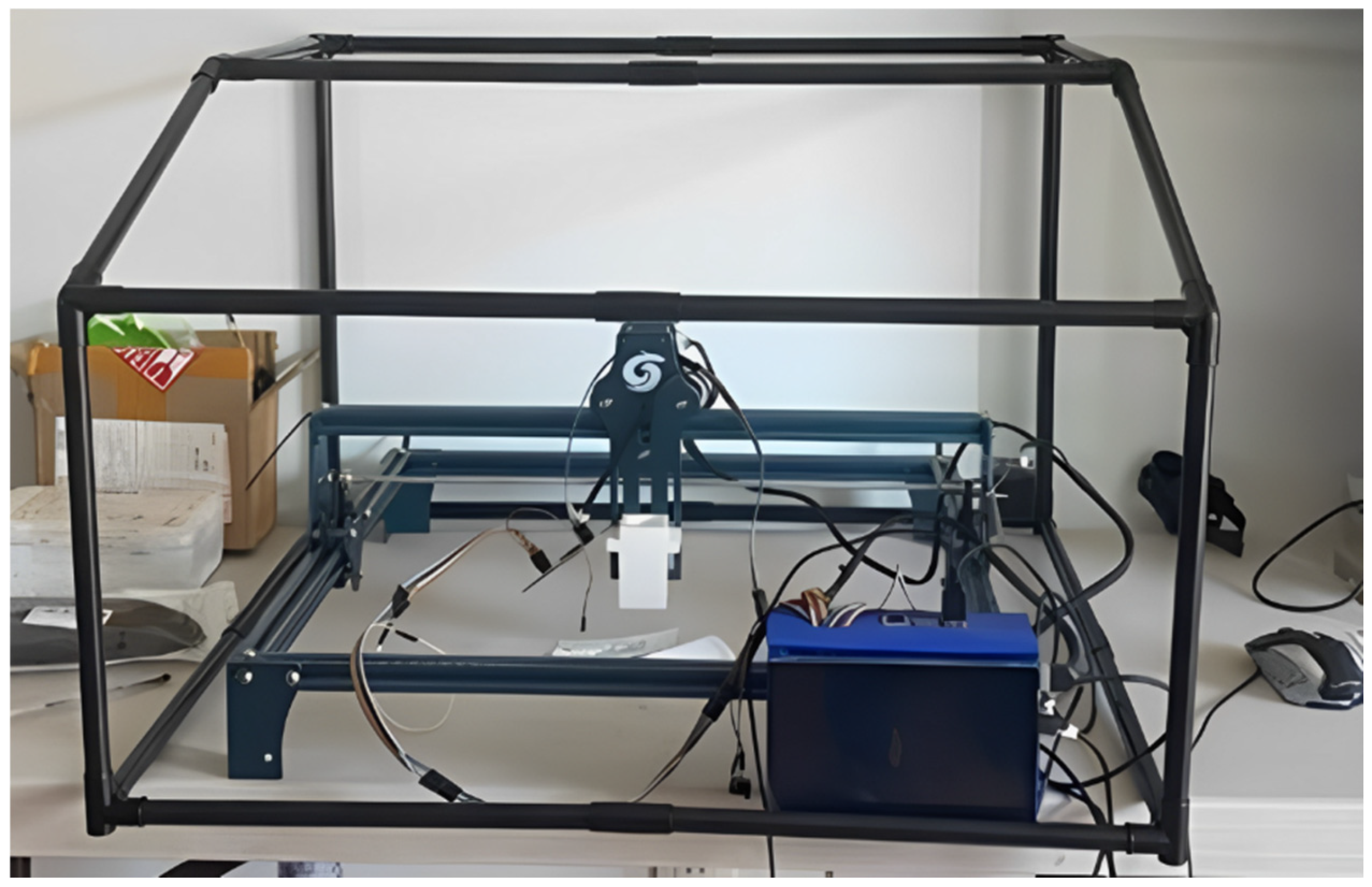
2.2. Four-Bar Structure
2.3. Raspberry Pi 4 (8GB RAM) and A4988 Driver
- The A4988’s step pin was connected to a Raspberry Pi’s GPIO pin to control the stepping;
- The A4988’s direction pin was connected to a Raspberry Pi’s GPIO pin to manage the rotation direction;
- The A4988’s MS1, MS2, and MS3 pins were attached to the Raspberry Pi’s GPIO pins to select the micro-stepping mode, if necessary;
- The A4988’s VMOT pin was connected to an external power supply, which should be between 8 V and 35 V, to power the motor;
- The ground pin of the A4988 was connected to the ground on the Raspberry Pi;
- The A4988’s 1A, 1B, 2A, and 2B pins were connected to the motor’s corresponding coils (NEMA17 motor wires).
- Adjust the current limit using the potentiometer () on the A4988;
- Measure using a voltmeter and set it according to your motor’s specifications.
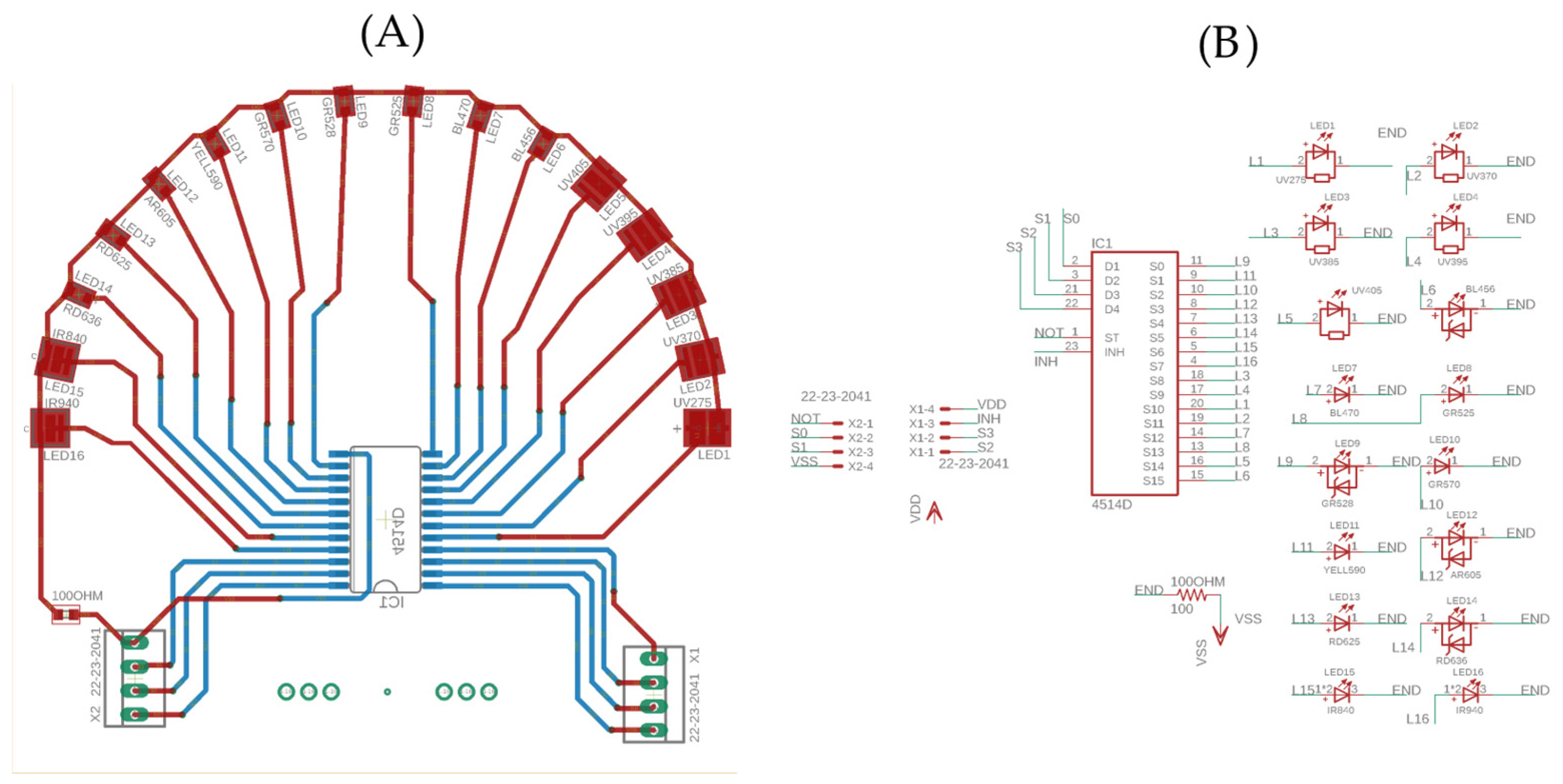
2.4. Printed Circuit Board (PCB) for Wavelength Control
- Multiplexer: the CD4114D multiplexer facilitates switching between different wavelengths;
- Analog-to-digital converter (ADC) circuit: the MCP3202 ensures high-resolution signal transduction with its 12-bit bandwidth rate for accurate measurements.
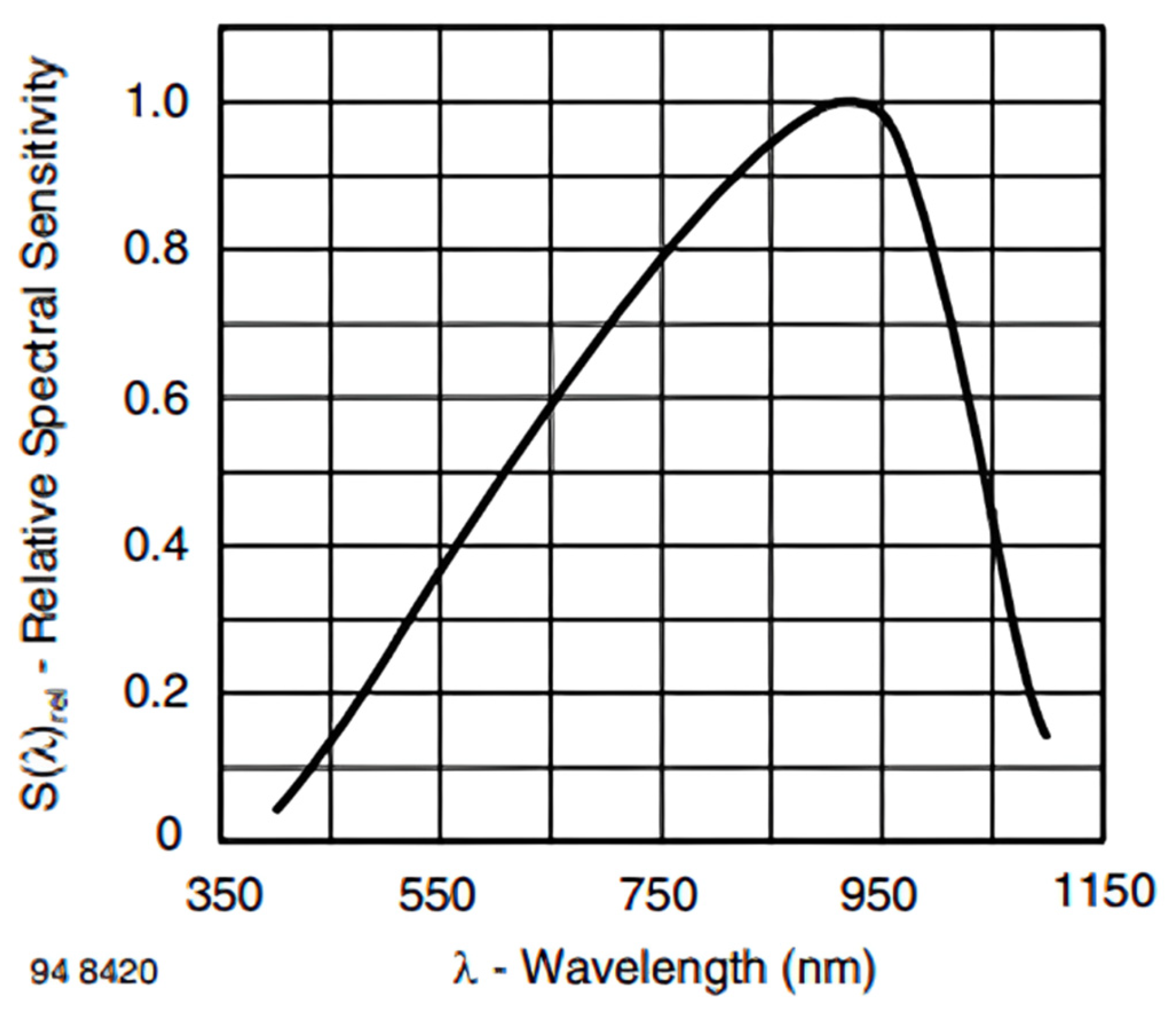
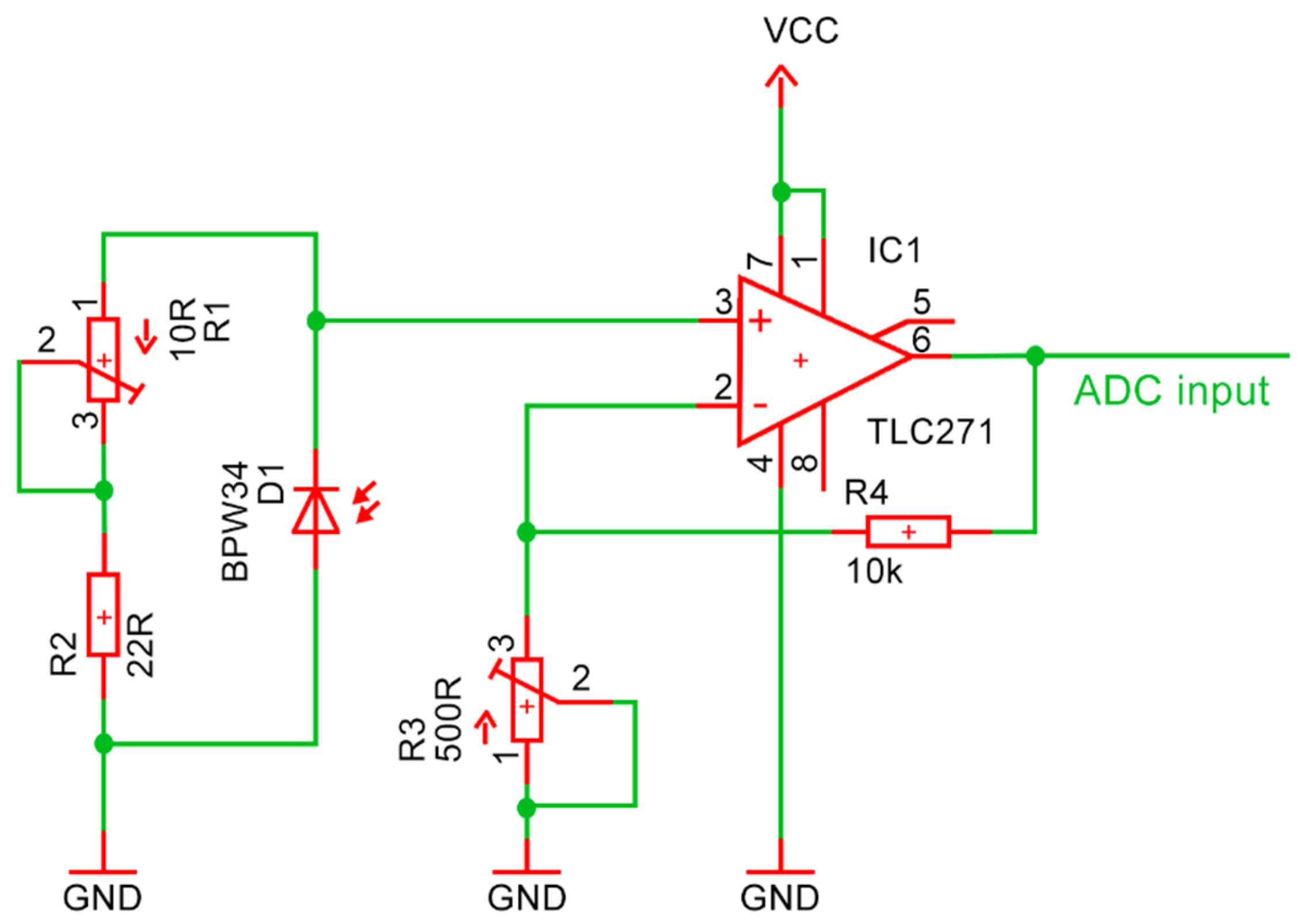
2.5. Detector BP34W
- is the output current generated by the photodiode;
- is the responsivity of the photodiode at the wavelength of the incident light (units: amperes per watt);
- is the optical power of the transmitted light detected by the photodiode (units: watts).
- : amplifier gain, representing the ratio between the output signal and the input signal;
- : feedback resistance connected between the output and the negative input terminal of the operational amplifier;
- : resistance connected between the negative input terminal and the ground.
- Definition: user interface design focuses on anticipating what users need to do;
- Purpose: UI software bridges the gap between users and the underlying functionality of a system;
- Importance:
- o
- Usability: a well-designed UI enhances usability, making it easier for users to achieve their goals efficiently;
- o
- Productivity: intuitive interfaces reduce learning curves and increase productivity;
- o
- Aesthetics: a visually appealing UI creates a positive impression and encourages user engagement;
- o
- Consistency: the UI comprises several screens and components to ensure a cohesive experience;
- o
- Accessibility: thoughtful UI design accommodates diverse user needs, including those with disabilities;
- Methods and techniques:
- o
- Interaction design: defining how users interact with elements (such as buttons, forms, and menus) and ensuring smooth transitions;
- o
- Visual design: designing the look and feel, including colors, typography, and layout;
- o
- Information architecture: organizing content in a logical manner for easy navigation;
- o
- User research: understanding user needs, preferences, and pain points;
- o
- Prototyping: creating interactive mockups to evaluate and iterate on design concepts;
- o
3. Results and Discussion
- Personal protective equipment (PPE): wear clean lab coats, gloves, and safety goggles;
- Disinfect work surfaces: wipe down all work surfaces, including benches, tables, and equipment, with a disinfectant solution (e.g., 70% isopropyl alcohol or a bleach solution);
- Microplastic-handling tools: clean tools (tweezers, scissors, etc.) by rinsing them with distilled water and wiping them with a lint-free cloth soaked in a disinfectant solution;
- Control samples: Use control samples or blanks to verify the absence of external contamination. Handle these samples with care to avoid introducing contaminants.
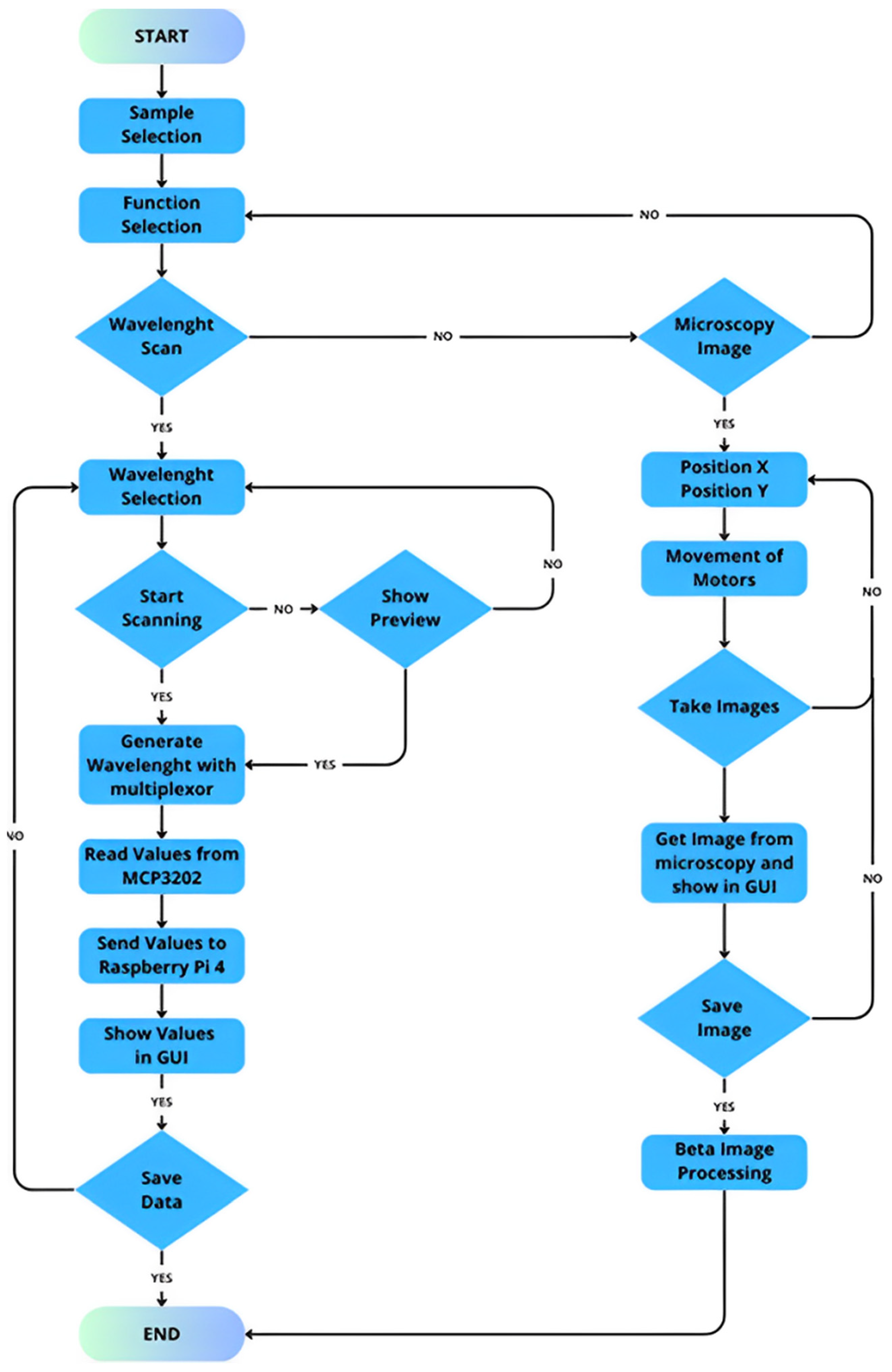
3.1. Operation and Data Acquisition
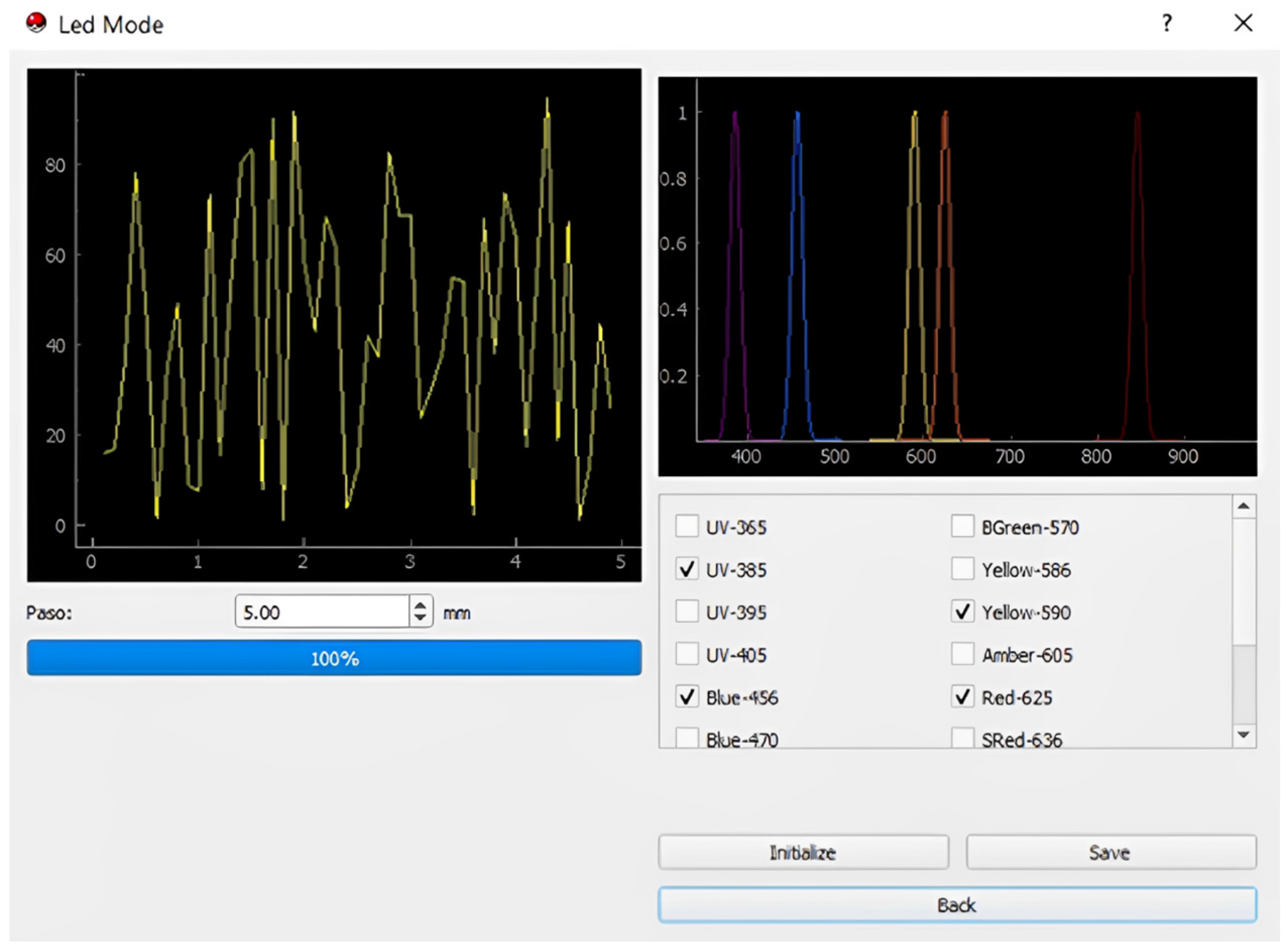
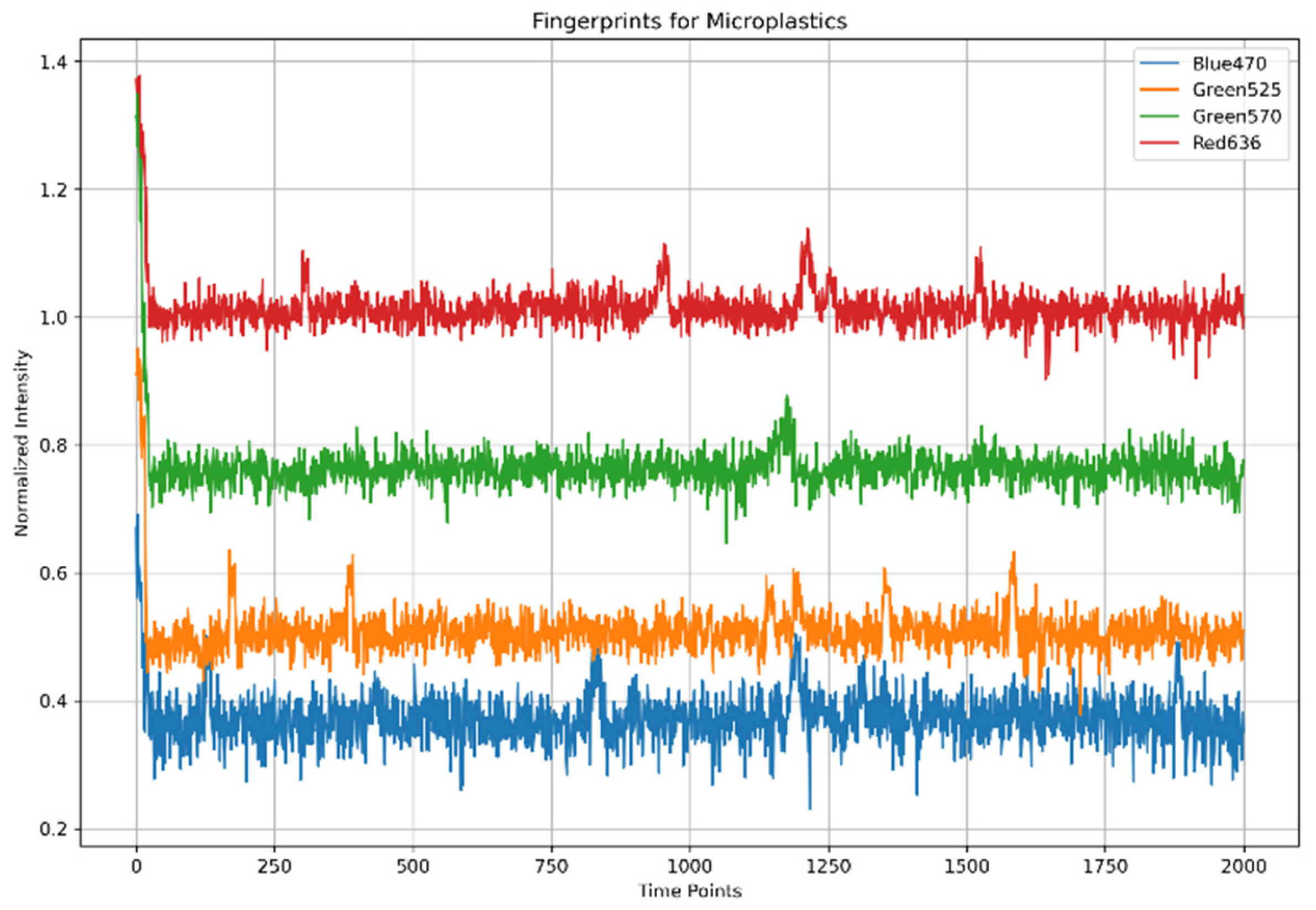
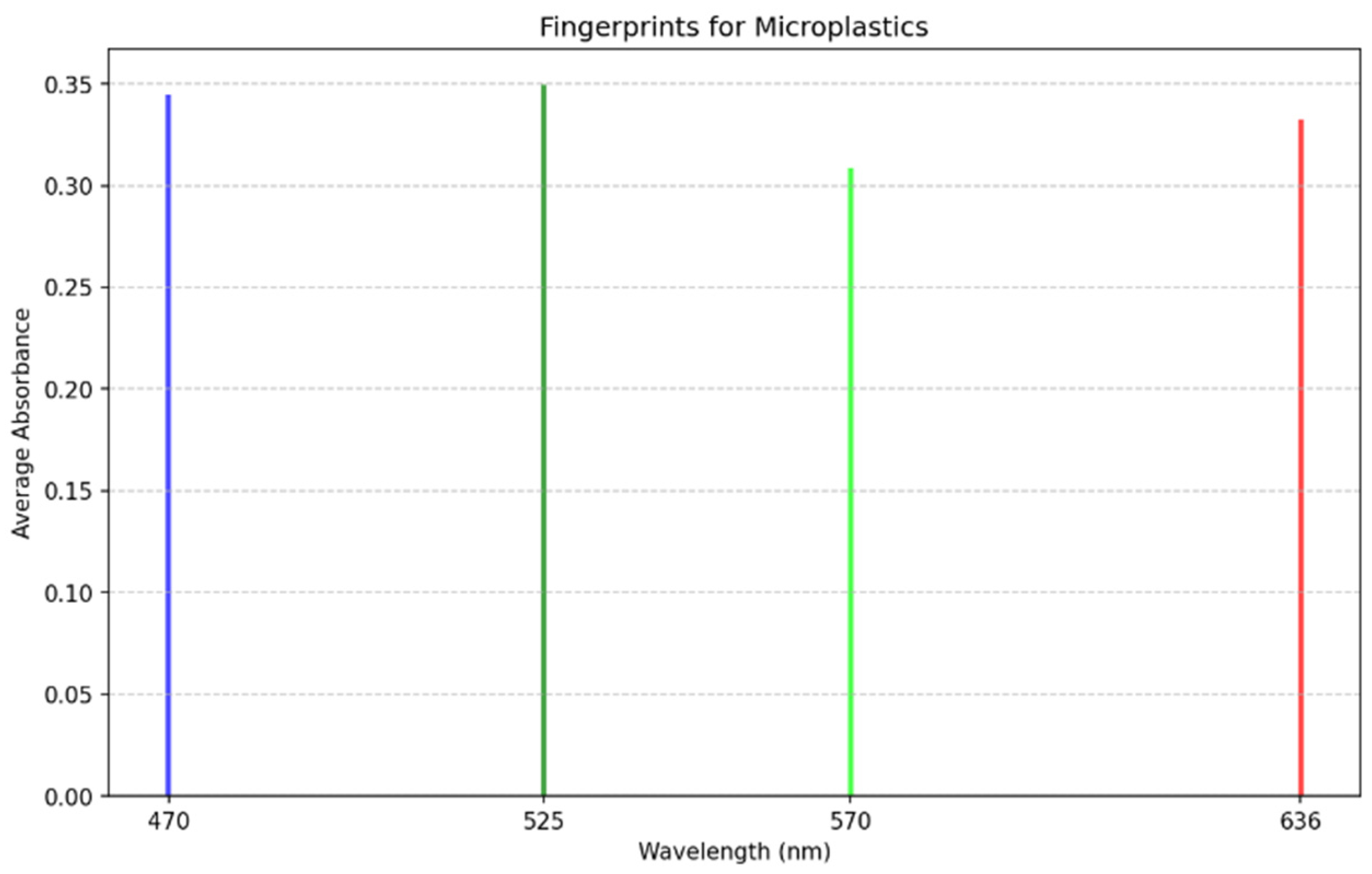
- The process commences with the selection of wavelengths to scan the sample using the multiplexer, ensuring precise and targeted analysis of the material;
- Then, the four-bar structure performs a systematic scan across the filter containing microplastics, ensuring comprehensive coverage of the sample area;
- During the scan, the photodiode captures the transmitted light from the microplastics. The absorbance intensity varies depending on the material’s optical properties;
- The photodiode generates an analog signal, which we route through an analog-to-digital converter (ADC) circuit that digitizes it for further processing;
- The Raspberry Pi processes the digitized data, identifying peaks in the signal that correspond to specific wavelengths. These peaks provide critical information about the sample’s optical response;
- Finally, the intensity of the detected signals determines the identification of the microplastics. The system utilizes these intensity patterns to differentiate microplastics from other materials in the sample.
3.2. Operation in an Uncontrolled Environment
3.3. Operation in a Controlled Environment
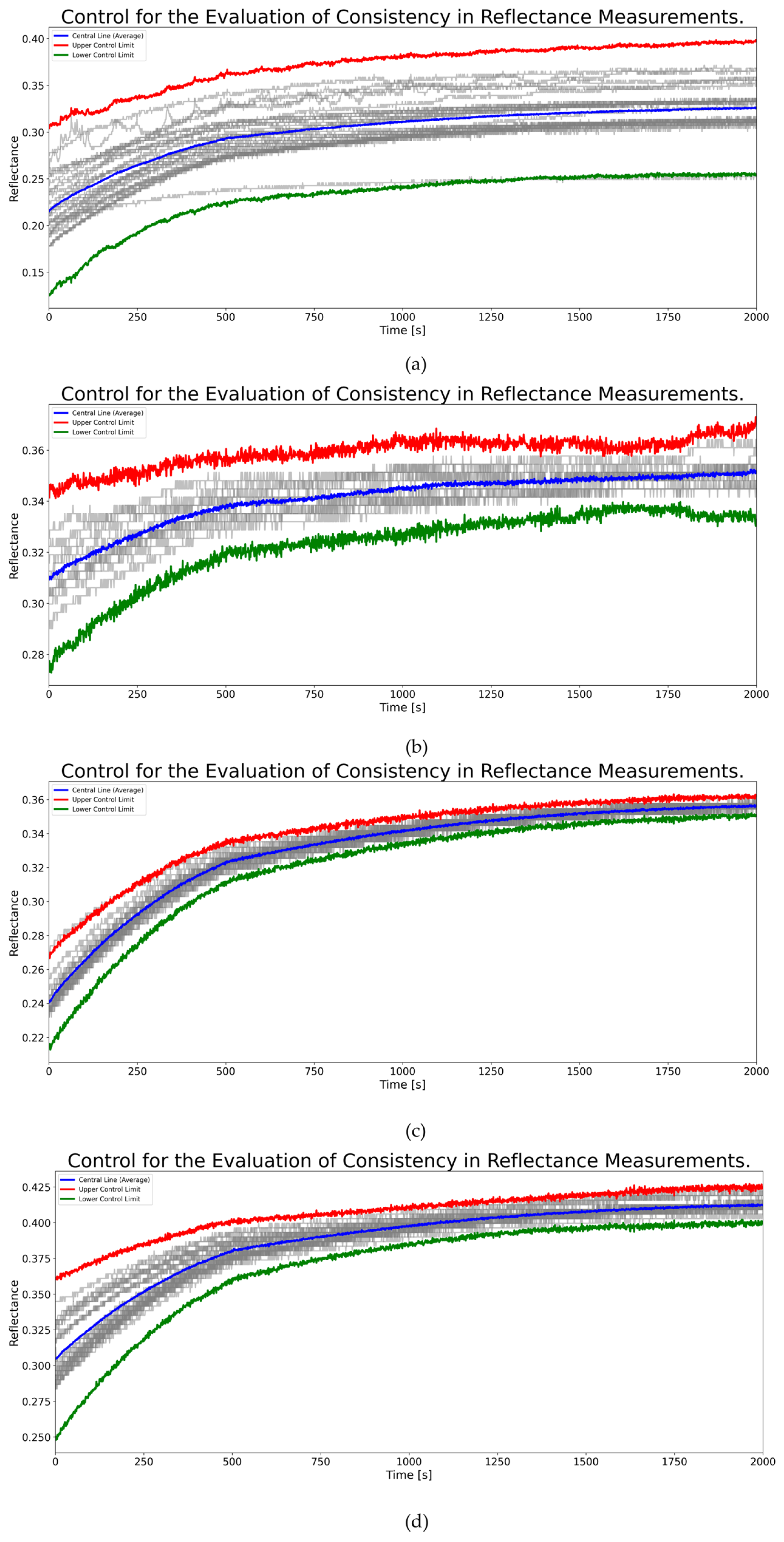
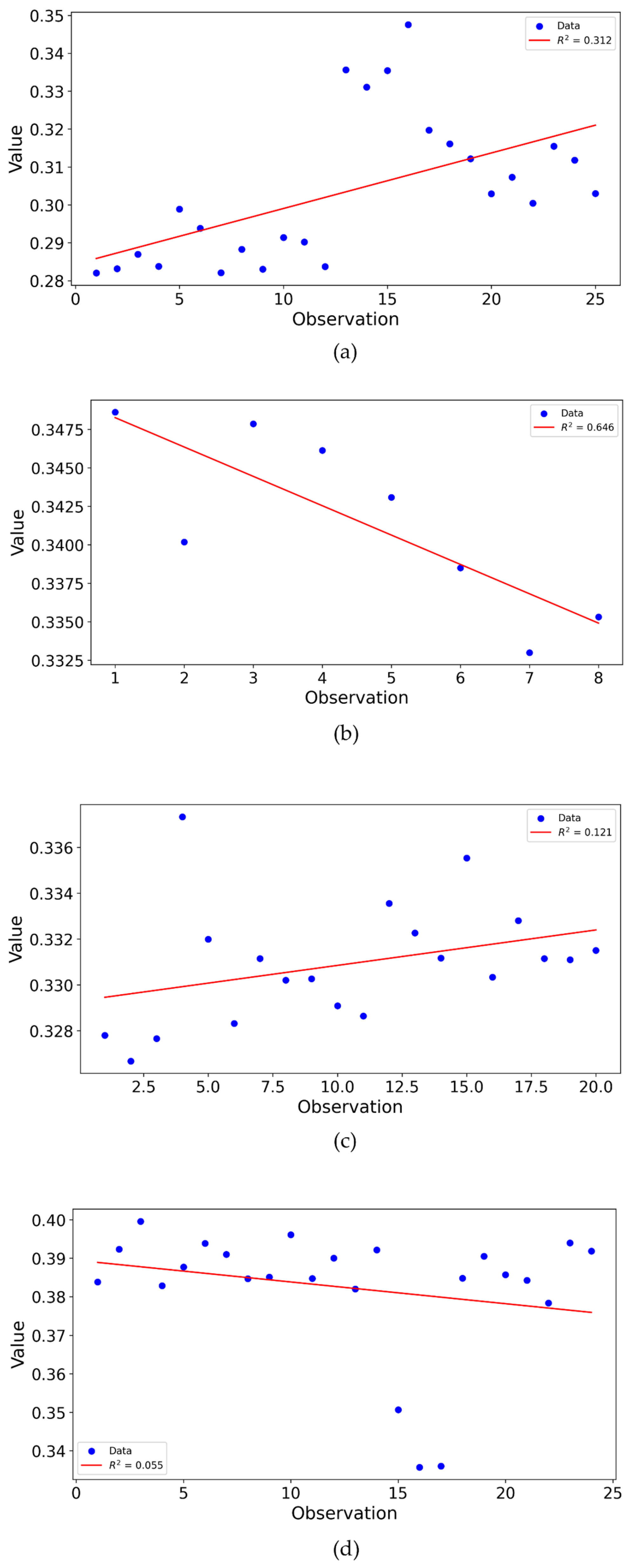
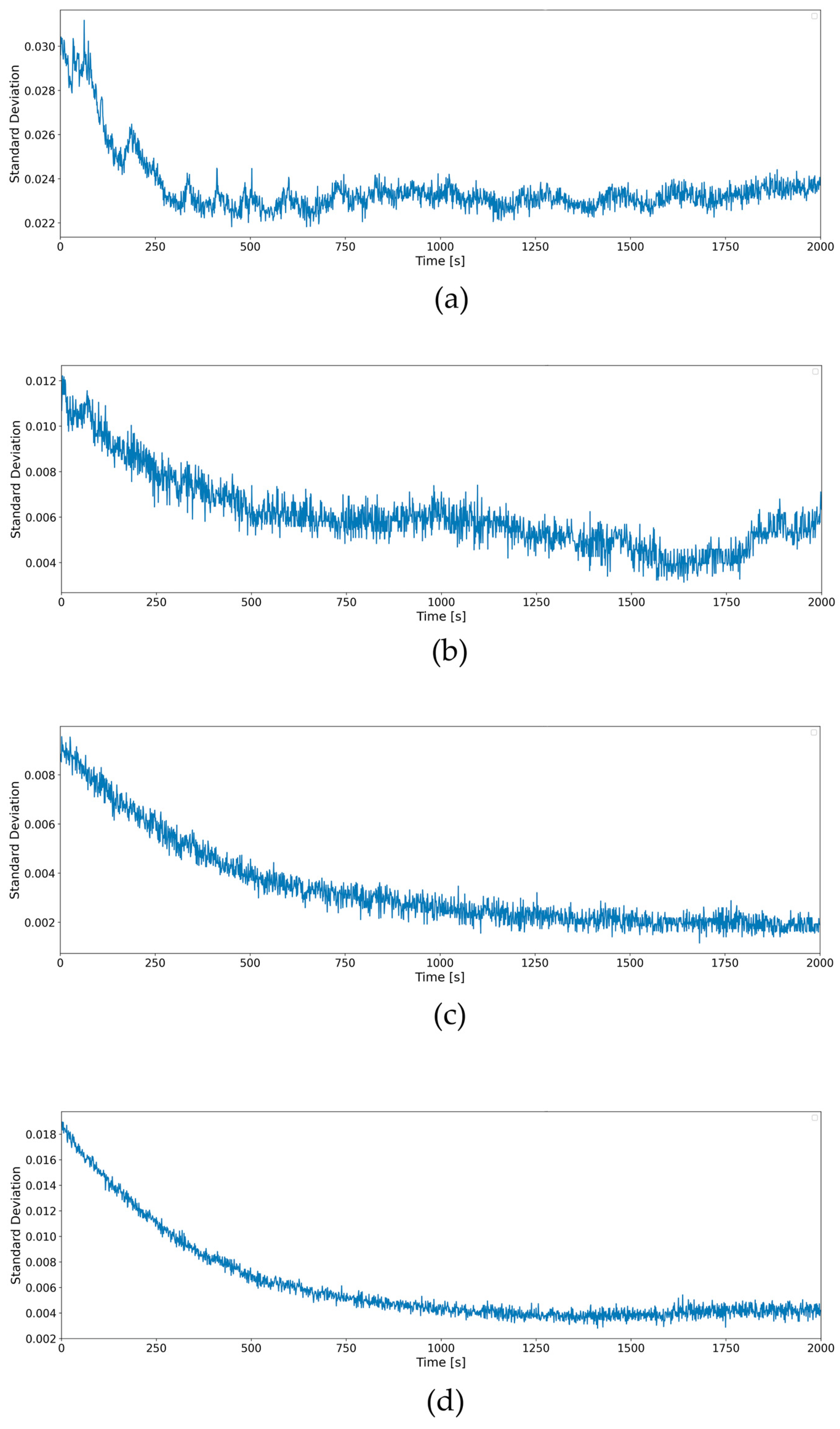
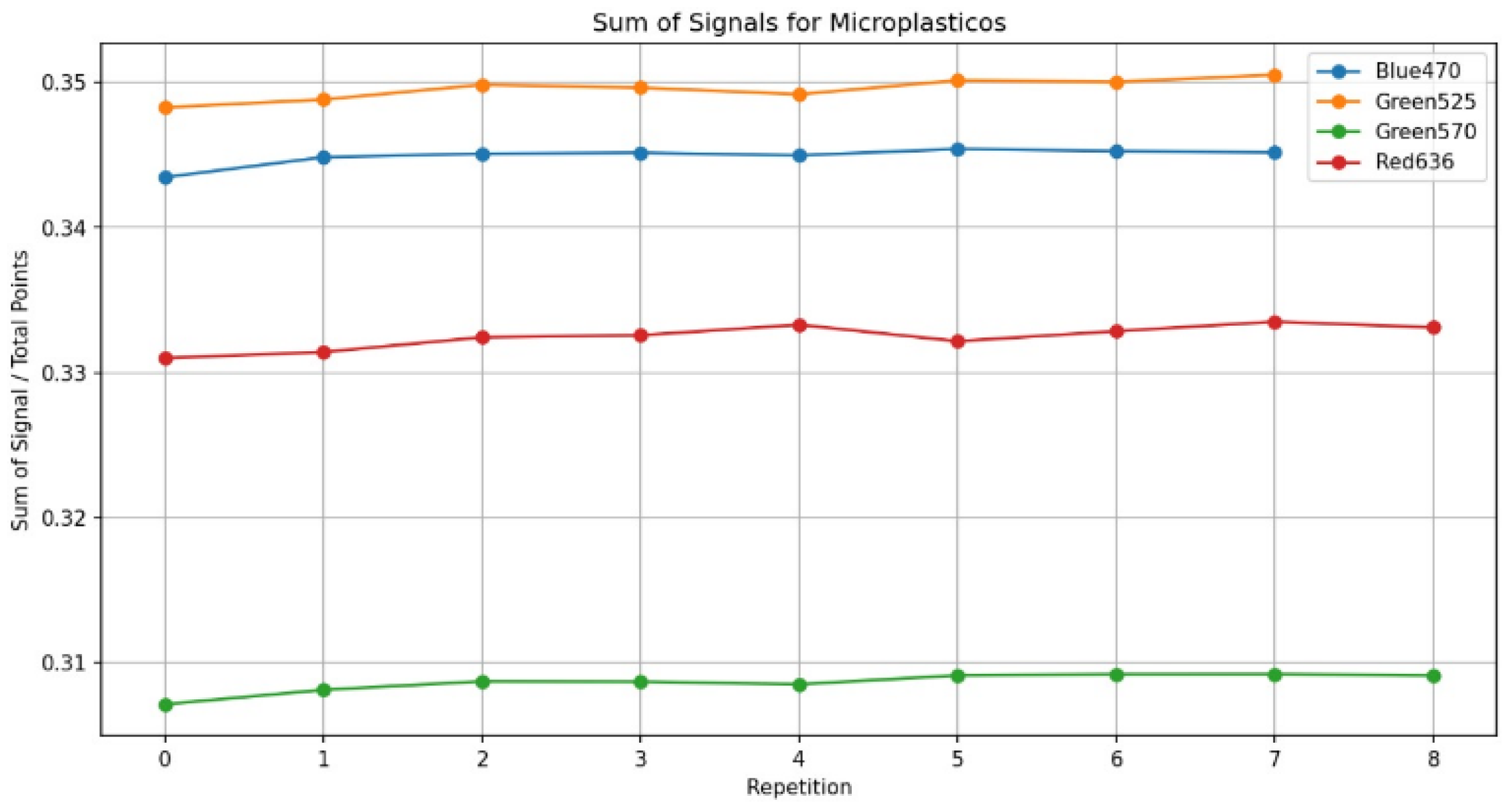
3.4. Reproducibility and Statistical Analysis
3.5. Functional Variance Analysis of Reflectance Measurements with Different Laser Configurations
3.6. Beta Test of Absorption in Real Life Environment
3.7. Microscopy and Image Processing
- Gray filter application: we apply a gray filter to the initial images;
- Gaussian filter application: we use a Gaussian filter to smooth the images;
- Binarization: we binarize the images to distinguish microplastics from the background;
- Morphological operations: we perform closing and opening operations to refine shapes and remove noise;
- Dilation and erosion: before skeletonization, we apply dilation followed by erosion to remove smaller objects from the image;
- Edge detection: We detect edges by subtracting the binarized image after applying a closing operation. This process is efficient and less computationally expensive, making it suitable for processing on a Raspberry Pi;
- Skeletonization: We apply skeletonization to all objects in the image to accurately analyze their structure;
- Chain code application: after skeletonization, we apply chain code to count the number of objects within the image;
- Morphology determination: we use polynomial approximation and fractal dimension analysis to determine the morphology of each object within the image, which makes it easier to classify them.
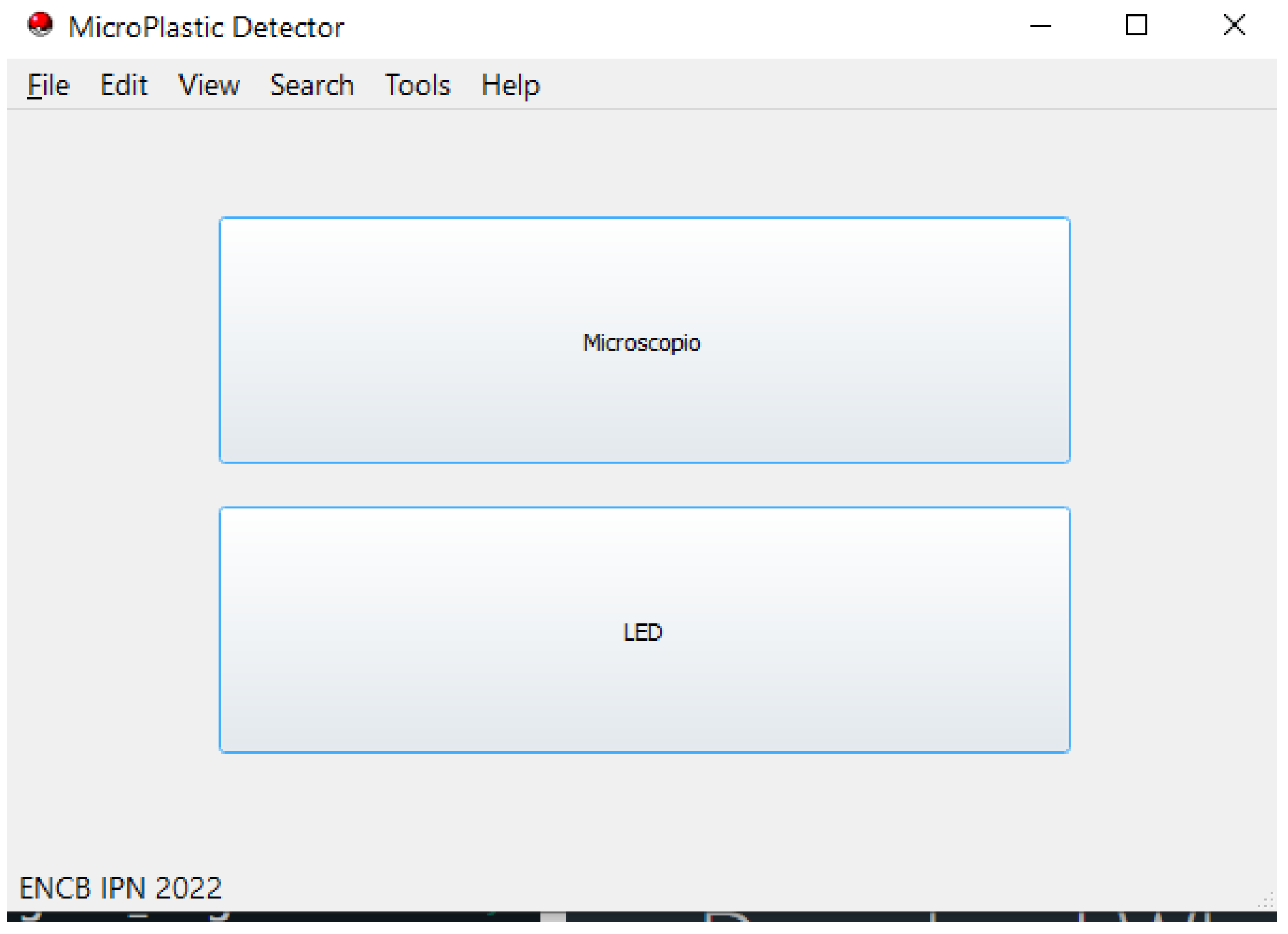
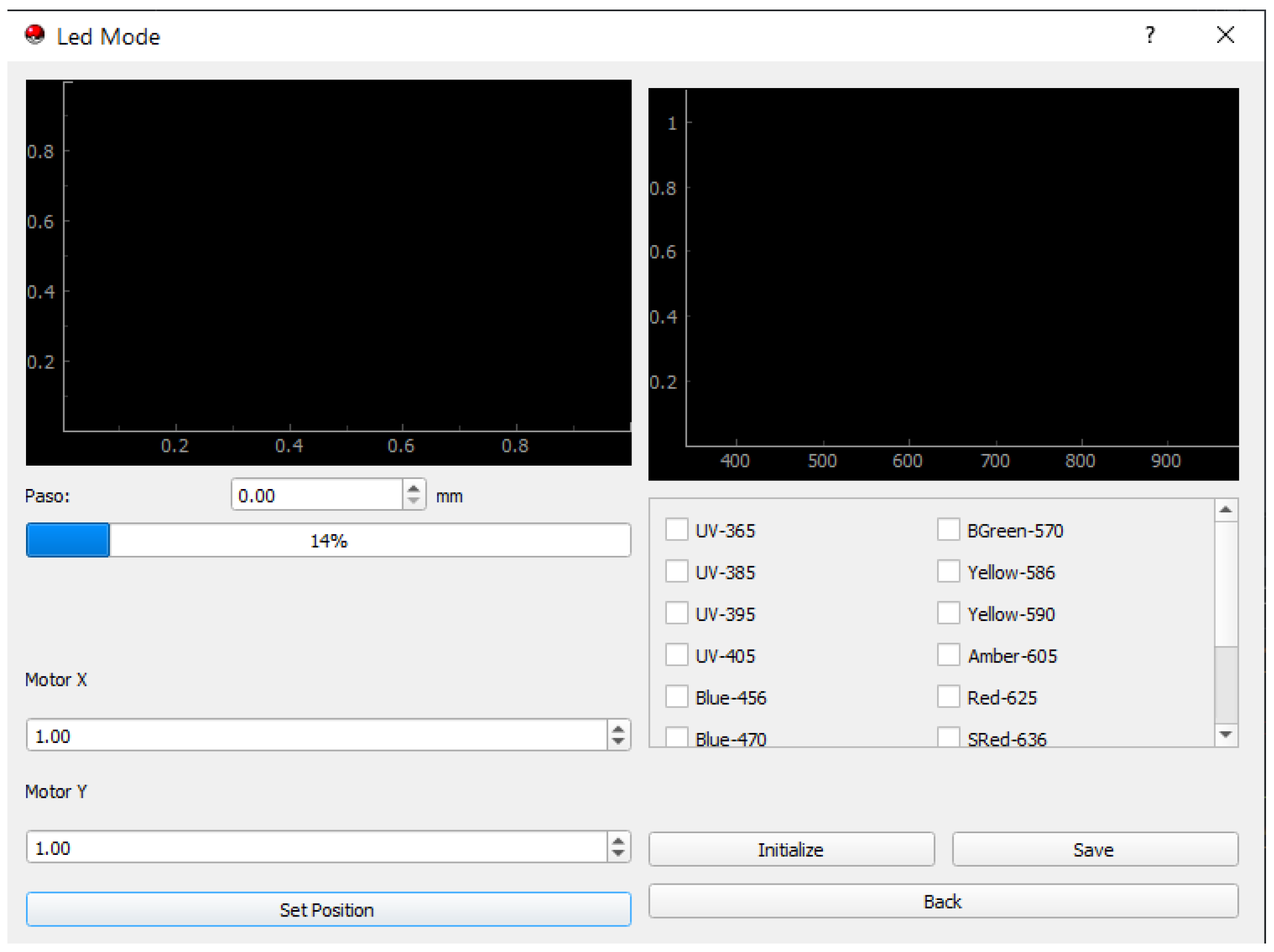
3.8. GUI Usability and Impact on User Experience:
3.9. Operation Limits
- Surface scanning and sample analysis: The sensor scans the surface, up to 20 cm2 per run, allowing it to analyze a large sample area efficiently. The system can process up to three samples per run, thanks to the three custom-made sample holders and the laser holder’s specific design in a C-shaped form;
- Luminosity and environmental conditions: One limitation found during testing is the effect of ambient light. Bright environments can cause detection errors, so a black cloth is needed to reduce light interference during the measurements. The sample should be placed 1.2 to 2 cm away to ensure accurate reading;
- Specificity: Digital image processing (DIP) techniques improve the sensor’s ability to differentiate microplastics from other particles by analyzing their morphological characteristics. This issue is further explored in another published study [28]. This precision is important for reliable environmental monitoring and avoids mistakes in detecting microplastics.
- Sensitivity: sensitivity (S) was determined as the slope of the calibration curve, representing the change in signal intensity per unit concentration of microplastics:
- Limit of Detection (LOD): the LOD is defined as three times the standard deviation of the blank sample () divided by the slope (S) of the calibration curve:
3.10. Comparison with Conventional Techniques
4. Conclusions
- Integrated approach: This study presents a novel sensor prototype that combines precise mechanical design, electronic control, and optical sensing to effectively detect microplastics. The necessity of integrating diverse technological approaches to confront this pressing environmental concern is underscored by the interdisciplinary nature of the study;
- Controlled testing and future applications: To ensure accuracy and reproducibility, the prototype was developed and tested in a controlled environment using commercial PLA microplastics. In addition, future applications may include the deployment of the prototype in real-world environments, such as rivers, oceans, or mountains, for on-site detection. This approach should eliminate the need for subsequent visits to collect additional samples if no microplastics are found. As a result, it would help conserve resources and save time;
- Advanced detection mechanism: The detection is conducted by directing a light source through the samples and measuring the intensity in volts. This method is a reliable way to identify microplastics through absorbance (“experimental absorbance” or “attenuance”) tests.
- Validation and sensitivity: We measured the prototype’s sensitivity and detection limits with absorbance and attenuation equations. We tested the system with commercial PLA microplastics to ensure accuracy. The prototype cannot measure particles as small as FTIR or Raman, but it identifies microplastics in the visible and near-infrared spectrum through light attenuation. It has been validated by comparison with other methods and can process multiple samples efficiently, making it a useful tool for environmental analysis;
- Technological innovation: The system uses LEDs with specific wavelengths and a Raspberry Pi 4 to control them. It also includes stepper motors for precise movement, making the setup efficient and accurate. The incorporation of filters serves to eliminate external noise and ensure the accuracy of the measurements. A graphical user interface (GUI) facilitates operations through two modes: microscopy and LED-based detection;
- Portable and versatile design: The prototype’s modular and portable design allows it to be used in both laboratory and field applications. This flexible approach overcomes the current limitations of traditional detection methods, such as FTIR and Raman spectroscopy, which are often expensive and less flexible.
Future Research and Expansion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| RMS | Raman Spectroscopy |
| FTIR | Fourier-Transform Infrared |
| ABS | Acrylonitrile Butadiene Styrene |
| PLA | Polylactic Acid |
| PET | Polyethylene Terephthalate |
| CNC | Computer Numerical Control |
| LED | Light-Emitting Diode |
| GUI | Graphical User Interface |
| UI | User Interface |
| LED | Light Emitting Diode |
| PCB | Printed Circuit Board |
| DIP | Digital Image Processing |
| NEMA | National Electrical Manufacturers Association |
| GPIO | General-Purpose Input/Output |
| ADC | Analog-to-Digital Converter |
| OP-AMP | Operational Amplifier |
| DEMUX | Demultiplexer |
| UV | Ultraviolet |
| FDA | Functional Data Analysis |
| FANOVA | Functional Analysis of Variance |
References
- Frias, J.P.G.L.; Nash, R. Microplastics: Finding a consensus on the definition. Mar. Pollut. Bull. 2019, 138, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.T.; Su, L.; Zhang, X.; Bennett, G.N.; Silberg, J.J.; Ajo-Franklin, C.M. Real-time bioelectronic sensing of environmental contaminants. Nature 2022, 611, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Lehner, R.; Weder, C.; Petri-Fink, A.; Rothen-Rutishauser, B. Emergence of Nanoplastic in the Environment and Possible Impact on Human Health. Environ. Sci. Technol. 2019, 53, 1748–1765. [Google Scholar] [CrossRef]
- Pham, D.T.; Kim, J.; Lee, S.-H.; Kim, J.; Kim, D.; Hong, S.; Jung, J.; Kwon, J.-H. Analysis of microplastics in various foods and assessment of aggregate human exposure via food consumption in korea. Environ. Pollut. 2023, 322, 121153. [Google Scholar] [CrossRef]
- Synani, K.; Abeliotis, K.; Velonia, K.; Maragkaki, A.; Manios, T.; Lasaridi, K. Environmental Impact and Sustainability of Bioplastic Production from Food Waste. Sustainability 2024, 16, 5529. [Google Scholar] [CrossRef]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Liu, F.-F.; Wang, S.-C.; Zhu, Z.-L.; Liu, G.-Z. Current Progress on Marine Microplastics Pollution Research: A Review on Pollution Occurrence, Detection, and Environmental Effects. Water 2021, 13, 1713. [Google Scholar] [CrossRef]
- Baruah, A.; Sharma, A.; Sharma, S.; Nagraik, R. An insight into different microplastic detection methods. Int. J. Environ. Sci. Technol. 2022, 19, 5721–5730. [Google Scholar] [CrossRef]
- Joshi, A.; Akhtar, N.; Kumar, A. Microplastics Detection Techniques BT-Microplastics Pollution and its Remediation; Kumar, A., Singh, V., Eds.; Springer Nature: Singapore, 2024; pp. 25–53. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, J.; Yao, F.; Zhang, W.; Qi, X.; Gao, X.; Liu, Y.; Wang, J.; Zou, M.; Liang, P. A review of recent progress in the application of Raman spectroscopy and SERS detection of microplastics and derivatives. Microchim. Acta 2023, 190, 465. [Google Scholar] [CrossRef]
- Thomas, D.; Schütze, B.; Heinze, W.M.; Steinmetz, Z. Sample Preparation Techniques for the Analysis of Microplastics in Soil—A Review. Sustainability 2020, 12, 9074. [Google Scholar] [CrossRef]
- Subharthe, S.; Clarke, B. From Collection to Analysis: A Practical Guide to Sample Preparation and Processing of Microplastics. Agilent, 2024. Available online: https://www.agilent.com/cs/library/technicaloverviews/public/te-microplastics-sample-preparation-guide-8700-ldir-5994-7587en-agilent.pdf (accessed on 25 February 2025).
- Vojnović, B.; Mihovilović, P.; Dimitrov, N. Approaches for Sampling and Sample Preparation for Microplastic Analysis in Laundry Effluents. Sustainability 2024, 16, 3401. [Google Scholar] [CrossRef]
- Xu, G.; Cheng, H.; Jones, R.; Feng, Y.; Gong, K.; Li, K.; Fang, X.; Tahir, M.A.; Valev, V.K.; Zhang, L. Surface-Enhanced Raman Spectroscopy Facilitates the Detection of Microplastics <1 μm in the Environment. Environ. Sci. Technol. 2020, 54, 15594–15603. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Cobos, A.G.Z.; Amón, J.; León, E.; Caballero, P. Standardization of FTIR-Based Methodologies for Microplastics Detection in Drinking Water: A Meta-Analysis Indeed and Practical Approach. Water 2024, 16, 3170. [Google Scholar] [CrossRef]
- Willans, M.; Szczecinski, E.; Roocke, C.; Williams, S.; Timalsina, S.; Vongsvivut, J.; McIlwain, J.; Naderi, G.; Linge, K.L.; Hackett, M.J. Development of a rapid detection protocol for microplastics using reflectance-FTIR spectroscopic imaging and multivariate classification. Environ. Sci. Adv. 2023, 2, 663–674. [Google Scholar] [CrossRef]
- Nene, A.; Sadeghzade, S.; Viaroli, S.; Yang, W.; Uchenna, U.P.; Kandwal, A.; Liu, X.; Somani, P.; Galluzzi, M. Recent advances and future technologies in nano-microplastics detection. Environ. Sci. Eur. 2025, 37, 7. [Google Scholar] [CrossRef]
- Carrieri, V.; Varela, Z.; Aboal, J.R.; De Nicola, F.; Fernández, J.A. Suitability of aquatic mosses for biomonitoring micro/meso plastics in freshwater ecosystems. Environ. Sci. Eur. 2022, 34, 72. [Google Scholar] [CrossRef]
- Marcuello, C. Present and future opportunities in the use of atomic force microscopy to address the physico-chemical properties of aquatic ecosystems at the nanoscale level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar] [CrossRef]
- Oßmann, B.E.; Sarau, G.; Schmitt, S.W.; Holtmannspötter, H.; Christiansen, S.H.; Dicke, W. Development of an optimal filter substrate for the identification of small microplastic particles in food by micro-Raman spectroscopy. Anal. Bioanal. Chem. 2017, 409, 4099–4109. [Google Scholar] [CrossRef]
- Taib, N.-A.A.B.; Rahman, R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bin Bakri, M.K.; Bin Julaihi, M.R.M.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Mohammadizadeh, M.; Pourabbas, B.; Mahmoodian, M.; Foroutani, K.; Fallahian, M. Facile and rapid production of conductive flexible films by deposition of polythiophene nanoparticles on transparent poly(ethyleneterephthalate): Electrical and morphological properties. Mater. Sci. Semicond. Process. 2014, 20, 74–83. [Google Scholar] [CrossRef]
- López Galindo, E.; Sarabia, C.H.; Pérez Aguilar, J.A.; Gómez Vieyra, A. Espectrofotometría del PET y PVC basado en Transmitancia. Rev. Tend. Docencia Investig. Química 2016, 2. Available online: https://zaloamati.azc.uam.mx/items/d5d3556b-64fb-4231-914e-b1f5de1cf6cc (accessed on 18 February 2024).
- Martinez-Hernandez, U.; West, G.; Assaf, T. Low-Cost Recognition of Plastic Waste Using Deep Learning and a Multi-Spectral Near-Infrared Sensor. Sensors 2024, 24, 2821. [Google Scholar] [CrossRef]
- Endo, T.; Gemma, A.; Mitsuyoshi, R.; Kodama, H.; Asaka, D.; Kono, M.; Mochizuki, T.; Kojima, H.; Iwamoto, T.; Saito, S. Discussion on effect of material on UV reflection and its disinfection with focus on Japanese Stucco for interior wall. Sci. Rep. 2021, 11, 21840. [Google Scholar] [CrossRef]
- Campos-Lopez, M.; Aguilar-Garay, R.; Bonilla-Martínez, I.B.; O Gomez-Castrejon, J.; A Mendoza-Pérez, J.; A Reyes-Guzmán, M.; Garibay-Febles, V. Advancing Microplastic Detection Technology through Digital Image Processing, Fractal Analysis, and Polynomial Approximation Methods. Microsc. Microanal. 2024, 30 (Suppl. 1), ozae044.195. [Google Scholar] [CrossRef]
- Löder, M.G.J.; Gerdts, G. Methodology Used for the Detection and Identification of Microplastics—A Critical Appraisal BT-Marine Anthropogenic Litter; Bergmann, M., Gutow, L., Klages, M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 201–227. [Google Scholar] [CrossRef]
- Woo, H.; Seo, K.; Choi, Y.; Kim, J.; Tanaka, M.; Lee, K.; Choi, J. Methods of Analyzing Microsized Plastics in the Environment. Appl. Sci. 2021, 11, 10640. [Google Scholar] [CrossRef]
- Würth Elektronik eiSos GmbH & Co. KG. Würth Elektronik. 2024. Available online: https://www.we-online.com/de (accessed on 13 December 2023).
- Asamoah, B.O.; Kanyathare, B.; Roussey, M.; Peiponen, K.-E. A prototype of a portable optical sensor for the detection of transparent and translucent microplastics in freshwater. Chemosphere 2019, 231, 161–167. [Google Scholar] [CrossRef]
- Systemes, D. SolidWorks. SolidWorks Corporation. Available online: https://my.solidworks.com/try-solidworks?mktid=13827&utm_campaign=202007_nam_sw_BINGSWOPT_en_XOP2064_rise_brand_mx_exact&utm_medium=cpc&utm_source=bing&utm_content=search&utm_term=4de94bc1938718e0f04e2de9168f55c0&gclid=4de94bc1938718e0f04e2de9168f55c0&gcl (accessed on 20 January 2024).
- Raspberry Pi Foundation. About Us. Available online: https://www.raspberrypi.com/about/ (accessed on 20 January 2024).
- Autodesk, Inc. Autodesk EAGLE. Available online: http://eagle.autodesk.com/ (accessed on 20 January 2024).
- JLCPCB. Available online: https://jlcpcb.com/ (accessed on 25 January 2024).
- Pini, A. The Basics of Photodiodes and Phototransistors and How to Apply Them. Contributed By DigiKey’s North American Editors. Available online: https://www.digikey.com/en/articles/the-basics-of-photodiodes-and-phototransistors-and-how-to-apply-them (accessed on 20 November 2023).
- VISHAY. BPW34. Vishay Semiconductors. Available online: https://www.vishay.com/docs/81521/bpw34.pdf (accessed on 15 December 2023).
- Slávik, R.; Čekon, M. Correction Factor Estimating of Silicon PIN Photodiode Derived from Outdoor Long-Term Measurement. In Proceedings of the ATF2016 4th Conference on Building Physics and Applied Technology in Architecture and Building Structures, Leuven, Belgium, 15–17 September 2016; Available online: https://www.researchgate.net/publication/311970903_CORRECTION_FACTOR_ESTIMATING_OF_SILICON_PIN_PHOTODIODE_DERIVED_FROM_OUTDOOR_LONGTERM_MEASUREMENT (accessed on 15 December 2023).
- Aponte Suárez, R.A.; Serrato Sosa, J.M.; Rojas Castellanos, M.R. Informe №6: Amplificadores Operacionales. April 2015. Available online: https://www.researchgate.net/publication/327656561_Informe_No6_amplificadores_operacionales (accessed on 16 March 2024).
- Wohlmuth, C.; Correia, N. User Interface for Interactive Scientific Publications: A Design Case Study BT-Digital Libraries for Open Knowledge; Doucet, A., Isaac, A., Golub, K., Aalberg, T., Jatowt, A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 215–223. [Google Scholar]
- Gram, C. A software engineering view of user interface design BT-Engineering for Human-Computer Interaction. In Proceedings of the IFIP TC2/WG2.7 Working Conference on Engineering for Human-Computer Interaction, Yellowstone Park, WY, USA, August 1995; Bass, L.J., Unger, C., Eds.; Springer: Boston, MA, USA, 1996; pp. 293–306. [Google Scholar] [CrossRef]
- Massarelli, C.; Campanale, C.; Uricchio, V. A Handy Open-Source Application Based on Computer Vision and Machine Learning Algorithms to Count and Classify Microplastics. Water 2021, 13, 2104. [Google Scholar] [CrossRef]











| Argument | Name | Type | Description |
|---|---|---|---|
| 1 | Direction | Int | Connects the general-purpose input/output (GPIO) pin that sets the direction of rotation. |
| 2 | Step_Pin | Int | Step-pin Int connects to the GPIO pin that sets the number of steps. |
| 3 | Mode_Pins | Tuple 3 Int | Mode-pin tuple connects the GPIO that determines the micro-stepping mode. |
| 4 | Motor_type | String | Motor-type string controller type, for this project, the. A4988” will be used. |
| Configuration | Estimated Coefficient | Confidence Interval (95%) | p-Value |
|---|---|---|---|
| Green laser without optics | +0.028 | [0.026, 0.030] | <0.001 |
| Blue laser with optics | +0.047 | [0.045, 0.049] | <0.001 |
| Blue laser without optics | −0.010 | [−0.012, −0.008] | <0.001 |
| Feature | FTIR Spectroscopy | Raman Spectroscopy | Uv-Vis Spectroscopy | Developed Sensor |
|---|---|---|---|---|
| Detection Mechanism | Absorption of infrared light | Inelastic scattering of photons | Absorption of ultraviolet and visible light | Absorption of multiple wavelengths using LEDs and detection through DIP |
| Sensitivity Rate | Detects down to ~20 µm; highly detailed molecular info | Detects as small as 1 µm; exceptional morpho-chemical analysis | Detects changes in light absorption for MPs > 100 µm | Detects changes in light absorption optimized for MPs > 100 µm |
| Selectivity | High, based on molecular vibrations | High, based on molecular vibration | Moderate, based on the electronic transition | Moderate, based on electronic transition and based on morphological characteristics |
| Sample preparation | Requires sample preparation [13] | Requires sample preparation [13] | Minimal sample preparation | Minimal sample preparation |
| Measurement Duration | Several minutes | Several minutes | Few minutes | 20 s per LED. And 2 min for image processing. |
| Data Output | Spectra | Spectra | Absorbance spectra | CSV file with 2000 data values, using absorbance as a function of voltage. |
| Automation | Partial | Partial | Partial | Partial |
| Portability | Limited | Limited | Moderate | High, optimized for field use with simple preparation protocols |
| Cost | High | High | Moderate to High | Low to mid-low |
| Reproducibility | High, but it depends on the sample preparation | High, but it depends on the sample preparation | Moderate to High | Moderate varies with environmental conditions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-López, M.; Aguilar-Garay, R.; González-Rodriguez, D.E.; Mejía-Lopez, V.I.; Gamboa-Lugo, M.M.; Garibay-Febles, V.; Reyes-Guzmán, M.A.; Gordillo-Sol, Á.; Mendoza-Pérez, J.A. A Portable Optical Sensor for Microplastic Detection: Development and Calibration. Appl. Sci. 2025, 15, 4757. https://doi.org/10.3390/app15094757
Campos-López M, Aguilar-Garay R, González-Rodriguez DE, Mejía-Lopez VI, Gamboa-Lugo MM, Garibay-Febles V, Reyes-Guzmán MA, Gordillo-Sol Á, Mendoza-Pérez JA. A Portable Optical Sensor for Microplastic Detection: Development and Calibration. Applied Sciences. 2025; 15(9):4757. https://doi.org/10.3390/app15094757
Chicago/Turabian StyleCampos-López, Maximiliano, Ricardo Aguilar-Garay, Dafne E. González-Rodriguez, Verónica I. Mejía-Lopez, Margoth M. Gamboa-Lugo, Vicente Garibay-Febles, Marco A. Reyes-Guzmán, Álvaro Gordillo-Sol, and Jorge A. Mendoza-Pérez. 2025. "A Portable Optical Sensor for Microplastic Detection: Development and Calibration" Applied Sciences 15, no. 9: 4757. https://doi.org/10.3390/app15094757
APA StyleCampos-López, M., Aguilar-Garay, R., González-Rodriguez, D. E., Mejía-Lopez, V. I., Gamboa-Lugo, M. M., Garibay-Febles, V., Reyes-Guzmán, M. A., Gordillo-Sol, Á., & Mendoza-Pérez, J. A. (2025). A Portable Optical Sensor for Microplastic Detection: Development and Calibration. Applied Sciences, 15(9), 4757. https://doi.org/10.3390/app15094757






