Focused Ultrasounds in the Rehabilitation Setting: A Narrative Review
Abstract
1. Introduction
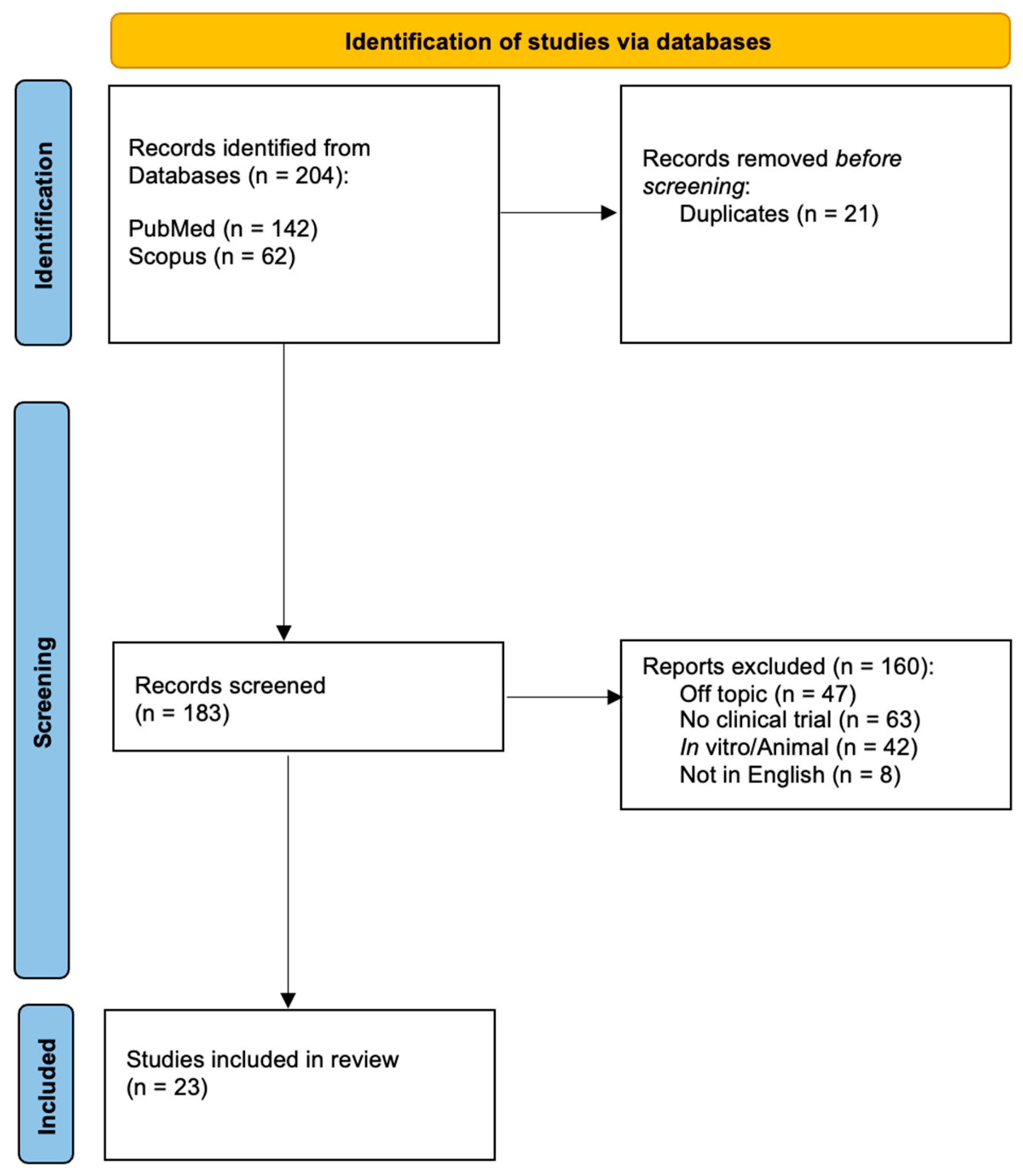
2. Methods
Data Extraction
- General characteristics of the paper: first author, year of publication, and study design.
- Study population characteristics: age, gender, and type of disease.
- Methods: type of FUS, parameters applied, setting, and rehabilitation protocol applied.
- Outcome measures, results, and adverse events.
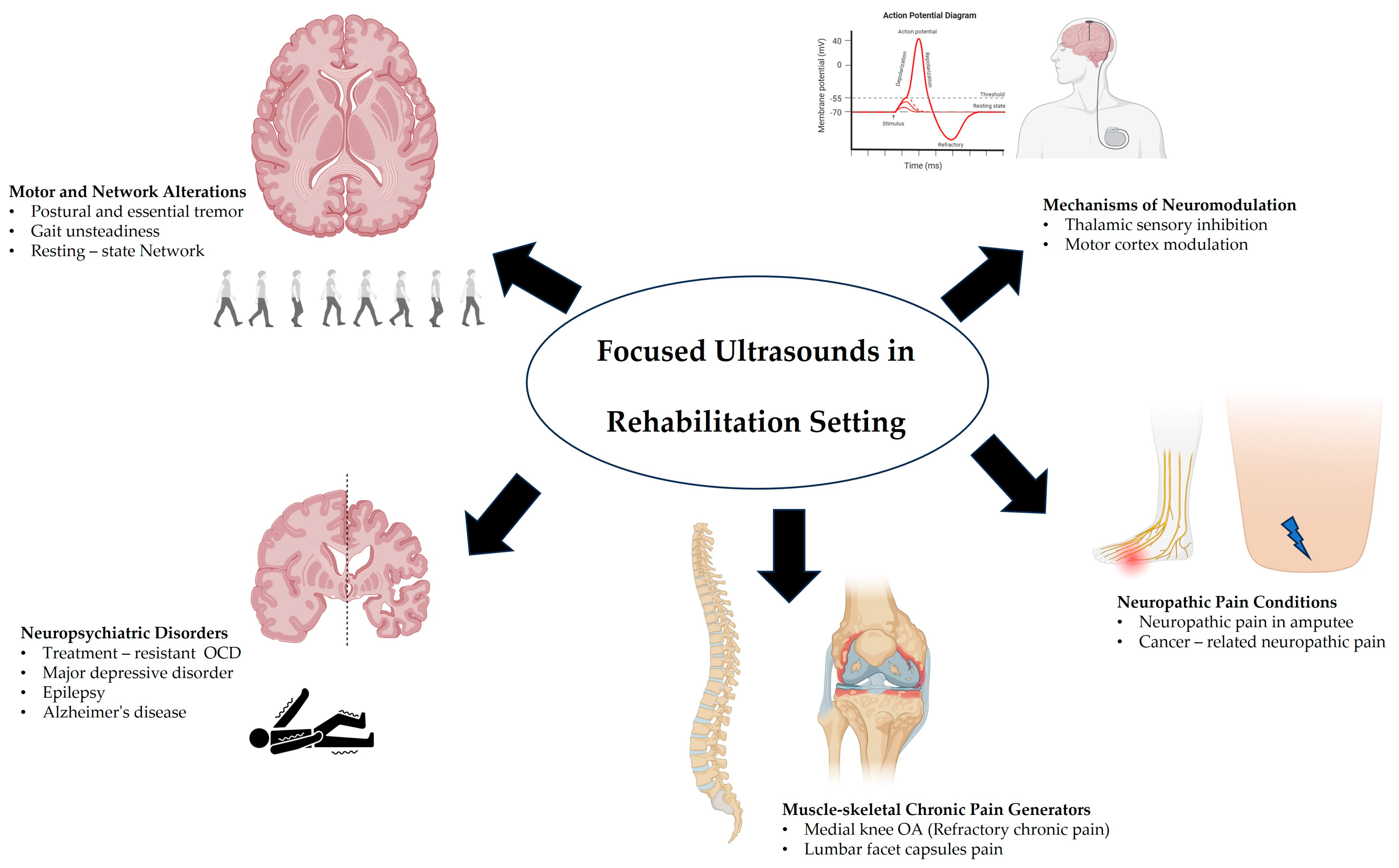
3. Focused Ultrasound Applications in Disabling Conditions
3.1. Movement Disorders
3.2. Psychiatric/Neurological Disorders
3.3. Musculoskeletal Applications
3.4. Neuropathic Pain/Amputation Applications
3.5. Cognitive Functions
3.6. Adverse Events
4. Discussion and Future Directions
4.1. Neurological and Psychiatric Disabilities
4.2. Musculoskeletal Disabilities
4.3. FUS Controversial Aspects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cline, H.E.; Schenck, J.F.; Hynynen, K.; Watkins, R.D.; Souza, S.P.; Jolesz, F.A. MR-Guided Focused Ultrasound Surgery. J. Comput. Assist. Tomogr. 1992, 16, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Rhim, H.; Choi, M.J.; Lim, H.K.; Choi, D. High-Intensity Focused Ultrasound Therapy: An Overview for Radiologists. Korean J. Radiol. 2008, 9, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.; Lockwood, D.; Mason, E.J.; Obusez, E.; Poturalski, M.; Rammo, R.; Nagel, S.J.; Jones, S.E. Clinical Intervention Using Focused Ultrasound (FUS) Stimulation of the Brain in Diverse Neurological Disorders. Front. Neurol. 2022, 13, 880814. [Google Scholar] [CrossRef]
- Aubry, J.-F.; Pauly, K.B.; Moonen, C.; Haar, G.; Ries, M.; Salomir, R.; Sokka, S.; Sekins, K.M.; Shapira, Y.; Ye, F.; et al. The Road to Clinical Use of High-Intensity Focused Ultrasound for Liver Cancer: Technical and Clinical Consensus. J. Ther. Ultrasound 2013, 1, 13. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chung, H.H.; Kang, S.Y.; Park, E.-J.; Park, D.H.; Son, K.; Han, J.K. Portable Ultrasound-Guided High-Intensity Focused Ultrasound with Functions for Safe and Rapid Ablation: Prospective Clinical Trial for Uterine Fibroids—Short-Term and Long-Term Results. Eur. Radiol. 2020, 30, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Bachu, V.S.; Kedda, J.; Suk, I.; Green, J.J.; Tyler, B. High-Intensity Focused Ultrasound: A Review of Mechanisms and Clinical Applications. Ann. Biomed. Eng. 2021, 49, 1975–1991. [Google Scholar] [CrossRef]
- Meng, Y.; Hynynen, K.; Lipsman, N. Applications of Focused Ultrasound in the Brain: From Thermoablation to Drug Delivery. Nat. Rev. Neurol. 2021, 17, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Darrow, D.P. Focused Ultrasound for Neuromodulation. Neurotherapeutics 2019, 16, 88–99. [Google Scholar] [CrossRef]
- Natera-Villalba, E.; Ruiz-Yanzi, M.-A.; Gasca-Salas, C.; Matarazzo, M.; Martínez-Fernández, R. MR-Guided Focused Ultrasound in Movement Disorders and beyond: Lessons Learned and New Frontiers. Park. Relat. Disord. 2024, 122, 106040. [Google Scholar] [CrossRef]
- Henn, M.C.; Smith, H.D.; Lopez Ramos, C.G.; Shafie, B.; Abaricia, J.; Stevens, I.; Rockhill, A.P.; Cleary, D.R.; Raslan, A.M. A Systematic Review of Focused Ultrasound for Psychiatric Disorders: Current Applications, Opportunities, and Challenges. Neurosurg. Focus. 2024, 57, E8. [Google Scholar] [CrossRef]
- Bae, S.; Liu, K.; Pouliopoulos, A.N.; Ji, R.; Jiménez-Gambín, S.; Yousefian, O.; Kline-Schoder, A.R.; Batts, A.J.; Tsitsos, F.N.; Kokossis, D.; et al. Transcranial Blood-Brain Barrier Opening in Alzheimer’s Disease Patients Using a Portable Focused Ultrasound System with Real-Time 2-D Cavitation Mapping. medRxiv 2024, 2023.12.21.23300222. [Google Scholar] [CrossRef]
- di Biase, L.; Falato, E.; Caminiti, M.L.; Pecoraro, P.M.; Narducci, F.; Di Lazzaro, V. Focused Ultrasound (FUS) for Chronic Pain Management: Approved and Potential Applications. Neurol. Res. Int. 2021, 2021, 8438498. [Google Scholar] [CrossRef]
- Abe, K.; Horisawa, S.; Yamaguchi, T.; Hori, H.; Yamada, K.; Kondo, K.; Furukawa, H.; Kamada, H.; Kishima, H.; Oshino, S. Focused Ultrasound Thalamotomy for Refractory Essential Tremor: A Japanese Multicenter Single-Arm Study. Neurosurgery 2021, 88, 751–757. [Google Scholar] [CrossRef]
- Hashida, M.; Maesawa, S.; Kato, S.; Nakatsubo, D.; Tsugawa, T.; Torii, J.; Tanei, T.; Ishizaki, T.; Mutoh, M.; Ito, Y.; et al. Outcomes and Prognostic Factors of Magnetic Resonance-Guided Focused Ultrasound Thalamotomy for Essential Tremor at 2-Year Follow-Up. Neurol. Med. Chir. 2024, 64, 137–146. [Google Scholar] [CrossRef]
- Gopinath, G.; Scantlebury, N.; Sewell, I.J.; Rohringer, C.R.; Sivadas, S.; McSweeney, M.; Boshmaf, S.Z.; Lam, B.; Hamani, C.; Abrahao, A.; et al. Changes in Caregiver Burden Following Unilateral Magnetic Resonance-Guided Focused Ultrasound Thalamotomy for Essential Tremor. Mov. Disord. Clin. Pract. 2024, 11, 905–908. [Google Scholar] [CrossRef]
- Sinai, A.; Nassar, M.; Sprecher, E.; Constantinescu, M.; Zaaroor, M.; Schlesinger, I. Focused Ultrasound Thalamotomy in Tremor Dominant Parkinson’s Disease: Long-Term Results. J. Parkinson’s Dis. 2022, 12, 199–206. [Google Scholar] [CrossRef]
- Tani, N.; Oshino, S.; Hosomi, K.; Hattori, N.; Mihara, M.; Yanagisawa, T.; Khoo, H.M.; Kanemoto, M.; Watanabe, Y.; Mochizuki, H.; et al. Altered Thalamic Connectivity Due to Focused Ultrasound Thalamotomy in Patients with Essential Tremor. World Neurosurg. 2022, 164, e1103–e1110. [Google Scholar] [CrossRef]
- Petersen, J.; McGough, J.; Gopinath, G.; Scantlebury, N.; Tripathi, R.; Brandmeir, C.; Boshmaf, S.Z.; Brandmeir, N.J.; Sewell, I.J.; Konrad, P.E.; et al. Cognitive Outcomes Following Unilateral Magnetic Resonance–Guided Focused Ultrasound Thalamotomy for Essential Tremor: Findings from Two Cohorts. Brain Commun. 2024, 6, fcae293. [Google Scholar] [CrossRef]
- Scantlebury, N.; Rohringer, C.R.; Rabin, J.S.; Yunusova, Y.; Huang, Y.; Jones, R.M.; Meng, Y.; Hamani, C.; McKinlay, S.; Gopinath, G.; et al. Safety of Bilateral Staged Magnetic Resonance-Guided Focused Ultrasound Thalamotomy for Essential Tremor. Mov. Disord. Clin. Pract. 2023, 10, 1559–1561. [Google Scholar] [CrossRef]
- Kato, S.; Maesawa, S.; Bagarinao, E.; Nakatsubo, D.; Tsugawa, T.; Mizuno, S.; Kawabata, K.; Tsuboi, T.; Suzuki, M.; Shibata, M.; et al. Magnetic Resonance–Guided Focused Ultrasound Thalamotomy Restored Distinctive Resting-State Networks in Patients with Essential Tremor. J. Neurosurg. 2023, 138, 306–317. [Google Scholar] [CrossRef]
- Davidson, B.; Hamani, C.; Meng, Y.; Baskaran, A.; Sharma, S.; Abrahao, A.; Richter, M.A.; Levitt, A.; Giacobbe, P.; Lipsman, N.; et al. Examining Cognitive Change in Magnetic Resonance-Guided Focused Ultrasound Capsulotomy for Psychiatric Illness. Transl. Psychiatry 2020, 10, 397. [Google Scholar] [CrossRef]
- Krishna, V.; Mindel, J.; Sammartino, F.; Block, C.; Dwivedi, A.K.; Van Gompel, J.J.; Fountain, N.; Fisher, R. A Phase 1 Open-label Trial Evaluating Focused Ultrasound Unilateral Anterior Thalamotomy for Focal Onset Epilepsy. Epilepsia 2023, 64, 831–842. [Google Scholar] [CrossRef]
- Huang, Z.; Charalambous, C.C.; Chen, M.; Kim, T.; Sokhadze, E.; Song, A.; Jung, S.-H.; Shekhar, S.; Feld, J.A.; Jiang, X.; et al. Low Intensity Focused Ultrasound Stimulation in Stroke: A Phase I Safety & Feasibility Trial. Brain Stimul. 2025, 18, 179–187. [Google Scholar] [CrossRef]
- Meng, Y.; Goubran, M.; Rabin, J.S.; McSweeney, M.; Ottoy, J.; Pople, C.B.; Huang, Y.; Storace, A.; Ozzoude, M.; Bethune, A.; et al. Blood–Brain Barrier Opening of the Default Mode Network in Alzheimer’s Disease with Magnetic Resonance-Guided Focused Ultrasound. Brain 2023, 146, 865–872. [Google Scholar] [CrossRef]
- Kawasaki, M.; Muramatsu, S.; Namba, H.; Izumi, M.; Ikeuchi, M.; Yaogawa, S.; Morio, K.; Ushida, T. Efficacy and Safety of Magnetic Resonance-Guided Focused Ultrasound Treatment for Refractory Chronic Pain of Medial Knee Osteoarthritis. Int. J. Hyperth. 2021, 38, 46–55. [Google Scholar] [CrossRef]
- Tiegs-Heiden, C.A.; Lehman, V.T.; Gorny, K.R.; Boon, A.J.; Hesley, G.K. Improved Treatment Response Following Magnetic Resonance Imaging–Guided Focused Ultrasound for Lumbar Facet Joint Pain. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 109–113. [Google Scholar] [CrossRef]
- Ezeokeke, C.K.; Bobola, M.S.; Selby, M.; Ko, J.H.; Friedly, J.L.; Mourad, P.D. Case Study of an Amputee Regaining Sensation and Muscle Function in a Residual Limb after Peripheral Nerve Stimulation by Intense Focused Ultrasound. Brain Stimul. 2020, 13, 527–529. [Google Scholar] [CrossRef]
- Mourad, P.D.; Friedly, J.L.; McClintic, A.M.; Olmstead, T.A.; Loeser, J.D. Intense Focused Ultrasound Preferentially Stimulates Transected Nerves Within Residual Limbs: Pilot Study. Pain. Med. 2018, 19, 541–549. [Google Scholar] [CrossRef]
- Patel, A.A.; Zhukovsky, M.; Sidharthan, S.; Jotwani, R.; Rakesh, N.; Gulati, A. Preliminary Effects of Low-Intensity Focused Ultrasound Treatment Program for Cancer-Related Neuropathic Pain. Pain. Manag. 2021, 11, 613–621. [Google Scholar] [CrossRef]
- Legon, W.; Ai, L.; Bansal, P.; Mueller, J.K. Neuromodulation with Single-Element Transcranial Focused Ultrasound in Human Thalamus. Hum. Brain Mapp. 2018, 39, 1995–2006. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Bansal, P.; Tyshynsky, R.; Ai, L.; Mueller, J.K. Transcranial Focused Ultrasound Neuromodulation of the Human Primary Motor Cortex. Sci. Rep. 2018, 8, 10007. [Google Scholar] [CrossRef]
- Legon, W.; Adams, S.; Bansal, P.; Patel, P.D.; Hobbs, L.; Ai, L.; Mueller, J.K.; Meekins, G.; Gillick, B.T. A Retrospective Qualitative Report of Symptoms and Safety from Transcranial Focused Ultrasound for Neuromodulation in Humans. Sci. Rep. 2020, 10, 5573. [Google Scholar] [CrossRef]
- Mueller, J.; Legon, W.; Opitz, A.; Sato, T.F.; Tyler, W.J. Transcranial Focused Ultrasound Modulates Intrinsic and Evoked EEG Dynamics. Brain Stimul. 2014, 7, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Park, T.Y.; Jeong, J.H.; Chung, Y.A.; Yeo, S.H.; Kim, H. Application of Subject-Specific Helmets for the Study of Human Visuomotor Behavior Using Transcranial Focused Ultrasound: A Pilot Study. Comput. Methods Programs Biomed. 2022, 226, 107127. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Z.; Zeng, K.; Nankoo, J.; Darmani, G.; Tran, S.; Ding, M.Y.R.; Chen, R. Effects of the Motor Cortical Theta-burst Transcranial-focused Ultrasound Stimulation on the Contralateral Motor Cortex. J. Physiol. 2024, 602, 2931–2943. [Google Scholar] [CrossRef]
- De Bie, R.M.A. Outcome of Unilateral Pallidotomy in Advanced Parkinson’s Disease: Cohort Study of 32 Patients. J. Neurol. Neurosurg. Psychiatry 2001, 71, 375–382. [Google Scholar] [CrossRef]
- Schuurman, P.R.; Bosch, D.A.; Bossuyt, P.M.M.; Bonsel, G.J.; Van Someren, E.J.W.; De Bie, R.M.A.; Merkus, M.P.; Speelman, J.D. A Comparison of Continuous Thalamic Stimulation and Thalamotomy for Suppression of Severe Tremor. N. Engl. J. Med. 2000, 342, 461–468. [Google Scholar] [CrossRef]
- Gendre, T.; Carle, G.; Mesrati, F.; Hubsch, C.; Mauras, T.; Roze, E.; Houot, M.; Degos, B.; Garcin, B. Quality of Life in Functional Movement Disorders Is as Altered as in Organic Movement Disorders. J. Psychosom. Res. 2019, 116, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ruscio, A.M.; Stein, D.J.; Chiu, W.T.; Kessler, R.C. The Epidemiology of Obsessive-Compulsive Disorder in the National Comorbidity Survey Replication. Mol. Psychiatry 2010, 15, 53–63. [Google Scholar] [CrossRef]
- Volpini, M.; Giacobbe, P.; Cosgrove, G.R.; Levitt, A.; Lozano, A.M.; Lipsman, N. The History and Future of Ablative Neurosurgery for Major Depressive Disorder. Ster. Funct. Neurosurg. 2017, 95, 216–228. [Google Scholar] [CrossRef]
- Pepper, J.; Hariz, M.; Zrinzo, L. Deep Brain Stimulation versus Anterior Capsulotomy for Obsessive-Compulsive Disorder: A Review of the Literature. J. Neurosurg. 2015, 122, 1028–1037. [Google Scholar] [CrossRef]
- Subramanian, L.; Bracht, T.; Jenkins, P.; Choppin, S.; Linden, D.E.J.; Phillips, G.; Simpson, B.A. Clinical Improvements Following Bilateral Anterior Capsulotomy in Treatment-Resistant Depression. Psychol. Med. 2017, 47, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Bossa, M.; Manocchio, N.; Argento, O. Non-Pharmacological Treatments of Cognitive Impairment in Multiple Sclerosis: A Review. NeuroSci 2022, 3, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Tramontano, M.; Argento, O.; Manocchio, N.; Piacentini, C.; Orejel Bustos, A.S.; De Angelis, S.; Bossa, M.; Nocentini, U. Dynamic Cognitive–Motor Training versus Cognitive Computer-Based Training in People with Multiple Sclerosis: A Preliminary Randomized Controlled Trial with 2-Month Follow-Up. J. Clin. Med. 2024, 13, 2664. [Google Scholar] [CrossRef] [PubMed]
- McDowell, F.H. Neurorehabilitation. West. J. Med. 1994, 161, 323–327. [Google Scholar]
- King, R.L.; Brown, J.R.; Newsome, W.T.; Pauly, K.B. Effective Parameters for Ultrasound-Induced In Vivo Neurostimulation. Ultrasound Med. Biol. 2013, 39, 312–331. [Google Scholar] [CrossRef]
- Younan, Y.; Deffieux, T.; Larrat, B.; Fink, M.; Tanter, M.; Aubry, J.-F. Influence of the Pressure Field Distribution in Transcranial Ultrasonic Neurostimulation: Influence of Pressure Distribution in Transcranial Ultrasonic Neurostimulation. Med. Phys. 2013, 40, 082902. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.D.; Park, M.Y.; Foley, L.; Purcell-Estabrook, E.; Kim, H.; Fischer, K.; Maeng, L.-S.; Yoo, S.-S. Image-Guided Focused Ultrasound-Mediated Regional Brain Stimulation in Sheep. Ultrasound Med. Biol. 2016, 42, 459–470. [Google Scholar] [CrossRef]
- Manocchio, N.; Ljoka, C.; Piacentini, N.; Sorge, R.; Vita, G.; Foti, C. Intra-Articular Injections with Carboxymethyl-Chitosan in Patients Affected by Knee Osteoarthritis Non-Responders to Hyaluronic Acid: A Pilot Study. Eur. J. Transl. Myol. 2024, 34, 12413. [Google Scholar] [CrossRef]
- Pirri, C.; Sorbino, A.; Manocchio, N.; Pirri, N.; Devito, A.; Foti, C.; Migliore, A. Chondrotoxicity of Intra-Articular Injection Treatment: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 7010. [Google Scholar] [CrossRef] [PubMed]
- Manocchio, N.; Pirri, C.; Ljoka, C.; Sorbino, A.; Piacentini, N.; Monello, C.; Vita, G.; Foti, C. Long-Term Efficacy of Carboxymethyl-Chitosan in Advanced Knee Osteoarthritis: A Twelve-Month Follow-Up Study on Non-Responders to Hyaluronic Acid. Biomedicines 2025, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Xu, G.; Bai, X.; Li, Z. Facet Joint Syndrome: Pathophysiology, Diagnosis, and Treatment. J. Pain Res. 2022, 15, 3689–3710. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.P.; Bhaskar, A.; Bhatia, A.; Buvanendran, A.; Deer, T.; Garg, S.; Hooten, W.M.; Hurley, R.W.; Kennedy, D.J.; McLean, B.C.; et al. Consensus Practice Guidelines on Interventions for Lumbar Facet Joint Pain from a Multispecialty, International Working Group. Reg. Anesth. Pain Med. 2020, 45, 424–467. [Google Scholar] [CrossRef]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What Proportion of Patients Report Long-Term Pain after Total Hip or Knee Replacement for Osteoarthritis? A Systematic Review of Prospective Studies in Unselected Patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Thermal Dose Determination in Cancer Therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Bobola, M.; Ezeokeke, C.; Kuznetslova, K.; Lahti, A.; Loeser, J.; Olmstead, T.; Friedly, J.; Mourad, P. A Pre-Clinical Study of the Response Threshold of Intact and Transected Nerves to Stimulation by Transcutaneous Intense Focused Ultrasound. Ultrasound Med. Biol. 2019, 45, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Cotero, V.; Fan, Y.; Tsaava, T.; Kressel, A.M.; Hancu, I.; Fitzgerald, P.; Wallace, K.; Kaanumalle, S.; Graf, J.; Rigby, W.; et al. Noninvasive Sub-Organ Ultrasound Stimulation for Targeted Neuromodulation. Nat. Commun. 2019, 10, 952. [Google Scholar] [CrossRef]
- Mesik, L.; Parkins, S.; Severin, D.; Grier, B.D.; Ewall, G.; Kotha, S.; Wesselborg, C.; Moreno, C.; Jaoui, Y.; Felder, A.; et al. Transcranial Low-Intensity Focused Ultrasound Stimulation of the Visual Thalamus Produces Long-Term Depression of Thalamocortical Synapses in the Adult Visual Cortex. J. Neurosci. 2024, 44, e0784232024. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Oh, J. Neuropathic Cancer Pain: Prevalence, Pathophysiology, and Management. Korean J. Intern. Med. 2018, 33, 1058–1069. [Google Scholar] [CrossRef]
- Edwards, H.L.; Mulvey, M.R.; Bennett, M.I. Cancer-Related Neuropathic Pain. Cancers 2019, 11, 373. [Google Scholar] [CrossRef]
| Application Area | Condition/Target | FUS Technique | Mechanism | Key Outcomes | Adverse Effects |
|---|---|---|---|---|---|
| Movement Disorders | Essential tremor, Parkinson’s | MRgFUS thalamotomy | Ablation of ventral intermediate thalamic nucleus | 56.4–59.4% tremor reduction; sustained QoL improvements | Transient gait disturbances (23.7%), numbness (28.9%) |
| Psychiatric Disorders | OCD, MDD | MRgFUS anterior capsulotomy | Modulation of cortico-striato-thalamo-cortical circuits | Symptom improvement without cognitive deficits | None significant reported |
| Epilepsy | Refractory seizures | Anterior thalamic ablation | Disruption of seizure propagation pathways | Reduced seizure frequency | Transient verbal fluency deficits |
| Stroke Rehabilitation | Chronic motor deficits | LIFUS to ipsilesional motor cortex | Enhanced neuroplasticity via corticospinal excitability modulation | ≥20% motor learning improvement (high-intensity group) | None significant reported |
| Neuropathic Pain | Cancer-related pain | Low-intensity FUS | Non-thermal neuromodulation | 85.7% response rate | Not specified |
| Phantom Limb Pain | Amputation-related sensations | High-intensity FUS (71.5 W/cm2) | Peripheral nerve reactivation | Restored sensation; pain modulation | Not specified |
| Musculoskeletal Pain | Knee osteoarthritis, facet joint | MRgFUS thermal ablation | Targeted denervation of nociceptive fibers | 73.7% with ≥50% pain reduction; functional improvement | Mild post-procedural discomfort |
| Cognitive Modulation | Executive function | Transcranial FUS to prefrontal cortex | Cortical network modulation | Improved anti-saccade task performance | Transient headaches (64 participants) |
| Neurodegenerative Research | Alzheimer’s disease | MRgFUS-mediated BBB opening | Enhanced drug delivery via blood-brain barrier disruption | Increased CSF neurofilament light chain | No cognitive improvements observed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirri, C.; Manocchio, N.; Polisano, D.; Sorbino, A.; Foti, C. Focused Ultrasounds in the Rehabilitation Setting: A Narrative Review. Appl. Sci. 2025, 15, 4743. https://doi.org/10.3390/app15094743
Pirri C, Manocchio N, Polisano D, Sorbino A, Foti C. Focused Ultrasounds in the Rehabilitation Setting: A Narrative Review. Applied Sciences. 2025; 15(9):4743. https://doi.org/10.3390/app15094743
Chicago/Turabian StylePirri, Carmelo, Nicola Manocchio, Daniele Polisano, Andrea Sorbino, and Calogero Foti. 2025. "Focused Ultrasounds in the Rehabilitation Setting: A Narrative Review" Applied Sciences 15, no. 9: 4743. https://doi.org/10.3390/app15094743
APA StylePirri, C., Manocchio, N., Polisano, D., Sorbino, A., & Foti, C. (2025). Focused Ultrasounds in the Rehabilitation Setting: A Narrative Review. Applied Sciences, 15(9), 4743. https://doi.org/10.3390/app15094743







