Abstract
Minerals have played a fundamental part in prebiotic chemistry on Earth, catalyzing the synthesis of inorganic and even organic molecules, including macromolecules such as RNA or DNA. Minerals based on silica are some of the first inorganics to be found in very ancient mineral fossils. These minerals or even volcanic glasses rich in silica, such as obsidians (a naturally volcanic glass, which is in fact an igneous rock), play an important role as supporting materials for obtaining the silico-carbonates of alkaline earth metals (usually called biomorphs). This is because, in most radiolarians, diatoms, and foraminifera, their external shells are made up of silica (SiO2). However, it has yet to be evaluated whether the silica contained in the minerals present in the prebiotic era of the Earth interacted with the chemical elements that were also present during that era. To evaluate whether obsidian participated in the formation of the first inorganic structures of pioneering organisms, this study aimed to synthesize calcium and barium biomorphs on igneous rock and to show that dissolved organic and inorganic molecules might have interacted with the molecules of obsidian, producing a plethora of shapes that mimicked the cherts of the Precambrian.
1. Introduction
At present, some 6000 known types of minerals have been recorded; however, it has been documented that the abundance of minerals on the surface of the Earth has changed drastically throughout the millennia due to various physical, chemical, and biological processes [1]. Some scientists have postulated the possibility that the formation of minerals (made up mainly of iron and silica compounds) began when the planets of our solar system were formed from clouds of dust and cosmic gas that were at first cold and chemically homogeneous. The oxide reduction processes that took place during the formation of the solar system played a very important role in the planet’s development since they allowed the beginning of the formal synthesis of minerals on the Earth’s crust [2]. It was the fusion of these substances that favored the reduction in metal oxides, thereby producing carbonaceous compounds, which were trapped during the formation of the Earth. Additionally, the most abundant metals were thereby segregated, forming the nucleus of the planet [3]. It is due to this process that there was the evolution of the minerals, as well as the volcanic glasses, formed when lava was extruded from volcanos and formed igneous rock; the interaction between the chemical elements that make up each mineral contributed to the origin of life [1,2,3,4,5]. It is important to mention that each type of mineral has a characteristic crystal structure and a specific chemical composition that results from the combination of the elements present in the Earth’s crust. For example, oxygen has been described as the most abundant element in characterized minerals and igneous rocks, followed by silicon [5].
A high percentage of oxides and minerals rich in silica (the majority of which are called silicates) have been found in the Earth’s crust, in which elements such as aluminum, iron, calcium, magnesium, sodium, and potassium are combined. Silica minerals were among the first minerals to be formed and can be found in very ancient rock fossils (pseudomorphs of silica have also been found in Precambrian cyanobacteria) [6]. As such, silicates make up approximately 90% of the Earth’s crust. Some of the more common silica minerals include feldspar, micas, pyroxenes, amphiboles, and olivines, among others. Silica is also found in nature in seven different polymorphs, namely, quartz, cristobalite, tridymite, coesite, stishovite, and amorphous polymorphs like lechatelierite and opal [7,8]. Obsidian is a volcanic glass rich in silica, oxygen, aluminum, sodium, and potassium. Therefore, it is a good candidate for emulating the minerals rich in silica [9,10]. Obsidian has been described by some authors as similar to glass, due to the fact that it is the result of the rapid cooling of viscous volcanic lava rich in silicon, oxygen, aluminum, and potassium and, therefore, does not crystallize adequately. There are various types of obsidian, the most well known being rhyolitic obsidian (containing more than 66% silicon by weight), dacitic obsidian (containing between 52 and 66% silicon by weight), and tachylite (basaltic glass containing less than 52% silicon by weight) [9,10]. Obsidian is generally black or gray in color, although other colors are also possible, depending on the composition and the formation circumstances [11]. The importance of analyzing the trace elements in obsidian is due to their unique chemical properties and to the fact that they have a greater variation in concentration with respect to the other elements [12,13,14].
Previous studies on the role of mineral surfaces for the origin of life [15] have been conducted on the surfaces of oxides, silicates, and carbonates, as these are the major components of rocks, allowing the selection and concentration of macromolecules such as amino acids, sugars, and other types of specific molecules; moreover, clays also allowed for the self-organization of lipids. Therefore, the surfaces of these minerals have been helpful in solving the unknowns of the main prebiotic problems, since they were able to synthesize, contain, and organize biomolecules in a prebiotic soup in which the origin of life might have been favored during the Precambrian era. It is important to mention that obsidian, being rich in silica, in recent times might have played a fundamental role as a supportive substance to preserve the collection of silica carbonate compound inorganic structures in alkaline earth metals. This interaction was first studied many years ago. The results obtained by Alfonso Herrera in the last decade of the XIX century demonstrated that these structures are frequently obtained in the presence of the alkaline earth metals [16,17]. However, whether the silica contained in the minerals or amorphous rocks present in the primeval era of the Earth interacted with the chemical elements that were also present in that era has not yet been evaluated. It has been shown that, regardless of the natural environment in which the mineral–water interfaces interact, a release of ions from the mineral takes place when it dissolves [18]. This possible interaction, together with atmospheric factors [19], might have formed the structure of the protocell, therefore giving rise to the first organism on our planet. With the passing of time, silica was probably replaced by other chemicals like the carbon-based structures in some organisms while still being preserved nowadays in others such as radiolarians, diatoms, and foraminifera. To evaluate whether the silica in igneous rocks participated in the formation of these first inorganic structures protecting the pioneering organisms like cyanobacteria covered with silica–iron (III) layers [20], we needed to synthesize calcium and barium silico-carbonates (usually called biomorphs) on obsidian, which, as we have already mentioned, is rich in silica. Biomorphs are the ideal models to carry out this type of research because, in addition to being one of the first inorganic structures that protected and isolated the first Precambrian biomolecules on Earth, they are possibly reminiscent of the Precambrian cherts [21]. These biomorphs were obtained for the first time by the Mexican biologist Alfonso L. Herrera in the last part of the nineteenth century and at the beginning of the twentieth century [16,17]. Calcium, barium, or strontium silica–carbonate biomorphs are self-organized crystalline materials that display a variety of biomimetic morphologies and a variety of lifelike shapes. These biomorphs show unique curved forms resembling worms, corals, stems, flowers, and leaves, among others, which greatly deviate from the restrictions of classic crystallographic symmetry. Biomorphs are all formed on a silica network (chemically defined as polysiloxane) with calcium, barium, or strontium carbonates deposited on the surface of the silica films [22,23,24,25,26,27].
In this contribution, we carried out the synthesis of calcium and barium silica–carbonates on obsidian under two separate atmospheric conditions in order to see how this igneous rock influences the shape of the biomorphs.
2. Materials and Methods
2.1. Composition of Obsidian
The obsidian used in this work was obtained from the county called Teotihuacan located in the State of Mexico (Mexico). Its chemical composition was determined by inductively coupled plasma mass spectrometry. The analysis procedure was carried out from the acid dissolution of the samples according to the modified protocol of Eggins et al. (1997) [28]. The elements analyzed are reported in terms of the elements with the highest concentrations indicated in percentage (%): SiO2 (74.47), Na (4.4), Al (5.2), K (3.9), and Fe (1.60). The trace elements, identified in parts per million (ppm), were Sc (3.39), Mn (1105), V (7.4), Cr (31.4), Ni (1.24), Cu (1.39), Zn (180.2), Co (1.7), Rb (258), Sb (0.24), Cs (4.7), Ba (15), La (39.5), Ce (98.9), Y (95.1), Zr (94.0), and Th (20.6).
2.2. Synthesis of Biomorphs
The synthesis of biomorphs was carried out using the gas diffusion method adapted by our work group [22]. Briefly, the synthesis mixtures were carried out as follows:
2.2.1. Synthesis of Control Biomorphs
Obsidian plates 5 mm in length, 5 mm in width, and 1 mm thick were used. A glass plate was placed in a crystallization cell with a final volume of 200 µL, which contained sodium metasilicate (1000 ppm) and calcium or barium chloride (20 mM), and the pH of the mixture was adjusted to 11.0 with NaOH (at this pH, the silicate layers to form the biomorphs are available in the solution) [22]. All the reagents used were purchased from Sigma-Aldrich (Saint Louis, MO, USA). The synthesis was carried out in two separate atmospheric conditions: one that emulated some of the conditions of the Precambrian era (5% CO2 and 50 °C) and the other in an ambient temperature (20 °C) and 0.03% CO2.
2.2.2. Synthesis of Biomorphs on Obsidian
For the synthesis of the biomorphs, the specimens were initially grown on borosilicate glass (for the control) and then grown on obsidian. In particular, for this second experiment, the glass plates were replaced with slabs of obsidian (previously cut to the same dimensions as the glass plates). The pieces of obsidian were placed in the crystallization cell in the same conditions as described in the control experiment for the synthesis of biomorphs. Both biomorphs, i.e., the controls as well as those synthesized on the obsidian, were obtained under two different atmospheric conditions: The first was 5% CO2, 50 °C (conditions that emulated the Precambrian era). It has been reported that in the Precambrian the temperatures on our planet ranged from 20 to 80 °C, and, because of this, it was decided to work at 50 °C [29,30]. Furthermore, it has also been documented that the concentration of CO2 in that era of the Earth was high compared to nowadays [30]. On the other hand, the biomorphs obtained at room temperature and an ambient concentration of CO2 were allowed to form for 24 h. All the experiments were carried out in triplicate.
2.3. Characterization of the Biomorphs
The morphology of the biomorphs was described via scanning electron microscopy (SEM). The chemical composition and crystalline structure of the biomorphs were both determined by Raman spectroscopy, Fourier transform infrared spectroscopy (FTIR), and X-ray diffraction.
- (a)
- Scanning electron microscopy (SEM)
The description of the morphology of the biomorphs synthesized in the different conditions was carried out with a TESCAN VEGA3 SB model microscope (Brno, Czech Republic), with a secondary electron detector (SE) from 10 to 20 kV in high-vacuum conditions (at a working distance of 10 mm). The samples were coated with gold by using a sputtering deposition system (physical vapor deposition, PVD). The SPI-Module Sputter coater (West Chester, PA, USA) was used (110 v/60 Hz). The time for the gold coating was 30 s to avoid any burning when scanning with the secondary electrons (SEs) of the scanning electron microscope (SEM) [22,25,26,31].
- (b)
- Raman spectroscopy
The chemical analysis of the biomorphs was carried out according to the methodology described previously by the research group. The Raman spectroscopy was performed using an alpha300 R spectrometer (WITec GmbH, Ulm, Germany) using a Nd: YVO4 green laser with a wavelength of 532 nm, equipped with a 300 lines/mm grating and a thermo-cooled CCD. The incident laser beam was focused using 20×, 50×, and 100× (Zeiss, Oberkochen, Ostalb, Baden-Würtemberg, Germany) objectives at 0.4, 0.75, and 0.9 NA, respectively. The Raman map was obtained using 0.03 s of integration time and a power of 31.7 mW. The processing and analysis of the data were carried out using the WITec Project Version 5.1 software [22,23].
- (c)
- Fourier transform infrared spectroscopy (FTIR)
The characterization of the biomorphs was additionally carried out by means of Fourier transform infrared spectroscopy (FTIR). This technique was carried out in a Nicolet iS50R Thermo Scientific (Waltham, MA, USA) spectrometer equipped with an attenuated total reflectance (ATR) accessory (Smart-iTX) with a diamond crystal. The spectra acquisitions were collected with 32 scans at 4 cm−1 of spectral resolution, in the range of 525 to 4000 cm−1. Additionally, iron salts were used to form KBr pellets for the FT-IR spectrum measurements on the same spectrophotometer in the transmittance mode. The data processing and analysis were performed with the OriginPro version 2021 software [31].
- (d)
- Powder X-Ray diffraction (PXRD)
The identification of the partially crystalline phases of the obsidian that contained the biomorphs was carried out by means of powder X-ray diffraction (PXRD) INEL Equinox 2000 (Thermo Scientific, Waltham, MA, USA). The diffractometer was used with an X-ray cobalt anode tube operating at 30mA and 25 kV. The samples obtained via the synthesis of the biomorphs on the obsidian were carefully transferred to a circular aluminum specimen holder (ϕ = 10 mm and 2 mm in depth) that was placed horizontally in the center of the goniometer of the diffractometer (ϕ = 185 mm). The Debye–Scherrer configuration of geometry and reflection was used; the detector used was sensitive to the position of a curve that covered a range angle of 110°. The simultaneous detection of the totality of the Bragg 20 range of this detector was divided into 4096 channels, which resulted in an average pass size of 0.031° (This value represents the step of the diffractometer. Thus, every 0.031 degrees the X-rays strike the sample). Each experiment was scanned from ~5° to ~120° 2θ using CoKα1 radiation obtained through a Ge monochromator situated in the primary beam, which allowed for the experimental extraction of the Kα2 line. These experimental conditions and the geometric characteristics of the diffractometer assured a sufficient representation of the diffracted planes to optimize the a priori identification of the crystalline or semicrystalline phases in our complex geological samples. The identification of these mineral phases was carried out through a combination of searches for automatic and manual peaks based on the database in the powder diffraction file (version 4+, published by the International Center of Data Diffraction) and were implemented in the Jade 6.5 program [32].
3. Results and Discussion
3.1. The Interaction of Obsidian with the Reaction Mixture Favored the Synthesis of Biomorphs with a Crystalline Phase of the Röntgenite Polymorph
When the SEM samples were analyzed, we observed that the morphology that the biomorphs adopted was dependent on the conditions of the method of synthesis.
In the case of the control biomorphs using calcium synthesized on glass under standard pressure and temperature conditions (that is, the current conditions), they adopted a conventional polycrystalline shape (Figure 1a). In contrast, the control biomorphs obtained at 5% CO2 and 50 °C (that is, Precambrian conditions) presented a rhombohedral morphology (Figure 1b). In the case of the silico-carbonate of the calcium biomorphs obtained on the obsidian at an ambient temperature (Figure 1c), the shape that appeared was polycrystalline, just like the biomorphs produced on glass (Figure 1a). Meanwhile, the silico-carbonate of the calcium biomorphs synthesized on the obsidian under conditions that emulated the Precambrian atmosphere (50 °C in the presence of 5% CO2) adopted a rhombohedral morphology (Figure 1d), as did those synthesized on glass (Figure 1b).
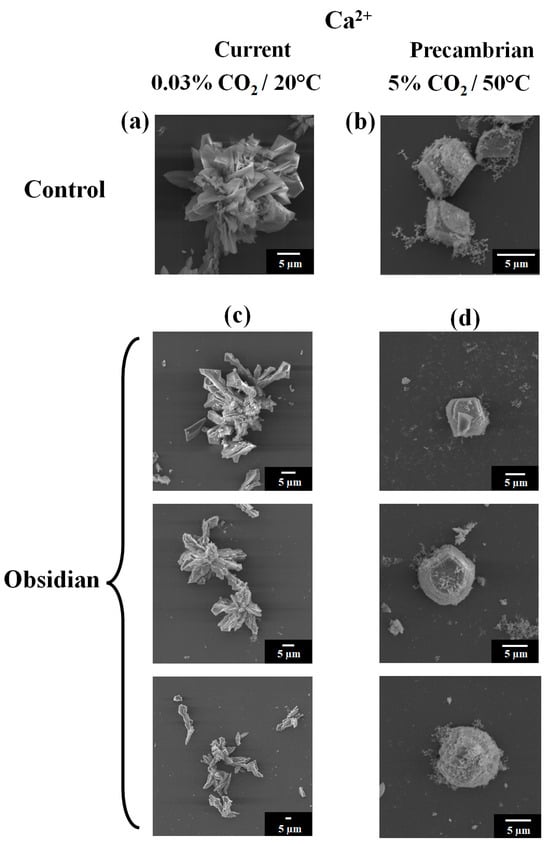
Figure 1.
Micrographs of calcium silica–carbonate biomorphs obtained via SEM on glass (a,b), on obsidian (c,d), under (a,c) ambient temperature and (b,d) conditions emulating the Precambrian atmosphere.
The difference in the shapes adopted by the biomorphs under the two synthesis conditions is due to the fact that the calcium biomorphs obtained under the Precambrian conditions were synthesized at a high concentration of CO2 and a higher temperature, compared with the calcium biomorphs produced under the standard atmospheric conditions of ambient CO2 and temperature. It has been shown in work by this research group and other groups that atmospheric conditions play an important role in the form that biomorphs adopt [27,31,33]. The morphology or crystalline aggregate of the silico-carbonate of the calcium biomorphs observed in these two tested conditions was found to be in agreement with that previously reported [27,31,33]. It has been reported that the morphology adopted by the biomorphs depends on various factors, such as the nucleation conditions of the crystalline cell and the interactions of the elements between the support and the medium where this interaction takes place, thus forming the crystalline structures of the different morphologies seen in the resulting biomorphs [15,16,17,19,20,21,22,23,24,25,26,27,33,34,35]. Additionally, our results are in agreement with a surface engineering study in which spontaneous growth produces surface microstructure engineering through a natural surface phenomenon similar to ion secretion precipitation, that is, coupled dissolution precipitation [36,37,38,39].
To identify and characterize the biomorphs (the control), as well as those synthesized on obsidian, in both atmospheric conditions, analyses were carried out using Raman and FTIR spectroscopies and PXRD. The biomorphs synthesized under ambient conditions showed Raman bands at 160, 285, 714, and 1087 cm−1 (Table 1). Whereas in the biomorphs obtained under Precambrian-like conditions, bands at 162, 287, 717, and 1089 cm−1 were identified (Table 1). The vibrations identified correspond to the calcite polymorph [40]. These results are consistent with those reported in other studies under these synthesis conditions [31]. The polymorphs were corroborated by IR (Table 1). When performing the Raman analysis of the biomorphs synthesized on obsidian at room temperature, bands were observed at 157, 282, 713, and 1086 cm−1 (Figure 2b, Table 1).

Table 1.
Identification through Raman and FTIR spectroscopies of the polymorphs of calcium or barium silica–carbonate biomorphs.
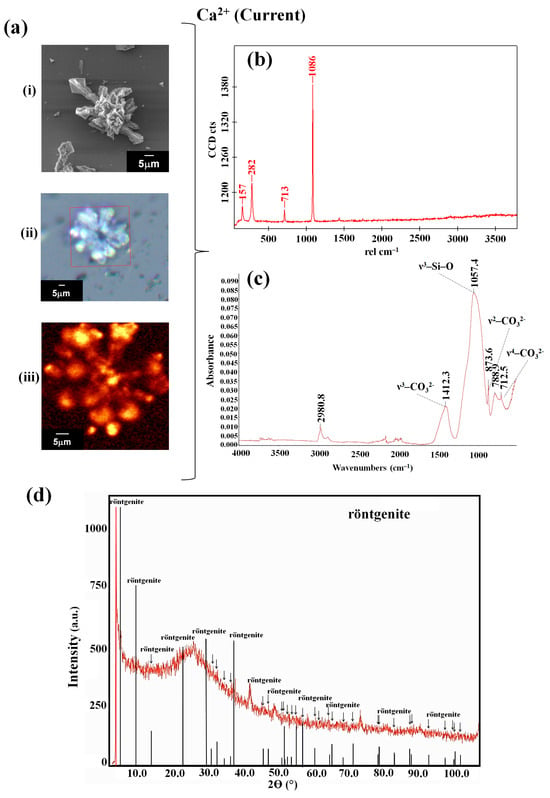
Figure 2.
Characterization and identification of the calcium silica–carbonate biomorphs synthesized on obsidian under standard atmospheric conditions. (a) Micrographs: (i) SEM, (ii) optical image, (iii) Raman map (1086 cm−1); (b) Raman spectra; (c) FTIR spectra; and (d) PXRD.
Bands on the biomorphs obtained under the conditions emulating the Precambrian atmosphere were identified via Raman spectroscopy at 158, 282, 713, and 1087 cm−1 (Figure 3b, Table 1).
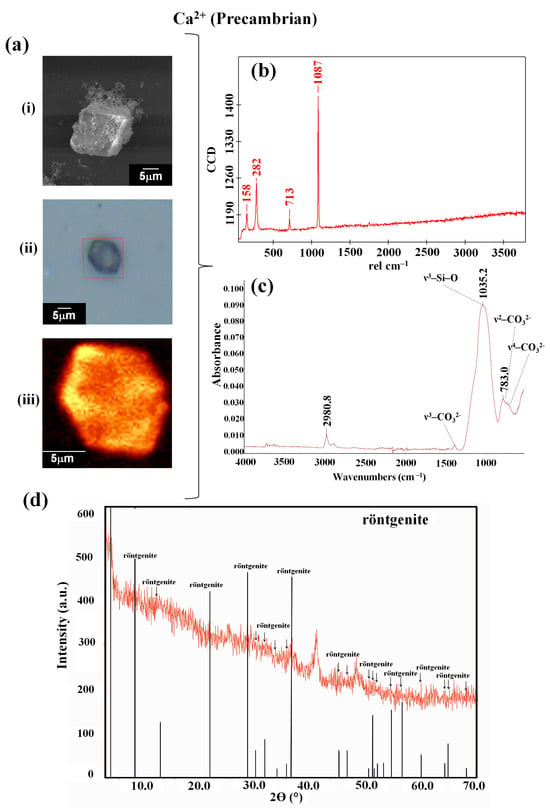
Figure 3.
Identification and characterization of the calcium silica–carbonate biomorphs synthesized on obsidian under conditions that emulated the Precambrian atmosphere. (a) Micrographs: (i) SEM, (ii) optical image, (iii) Raman map (1086 cm−1); (b) Raman spectra; (c) FTIR spectra; and (d) PXRD. The peak at 40° and 2θ corresponds to the aluminum sample’s holder.
The Raman signals identified in the biomorphs might correspond to the calcite (CaCO3) polymorph (Figure 2b,c and Figure 3b,c). However, these calcium silico-carbonate biomorphs, having been synthesized on an igneous rock such as obsidian, made us wonder whether their synthesis under these conditions favored the interaction of the molecules dissolved in the aqueous solution with the obsidian molecules. If this were so, then the chemical composition of the biomorphs obtained might correspond mainly to CaCO3 crystallites, in addition to the chemical elements that were taken from the obsidian. Should that be the case, there was a high possibility that we might be dealing with some polymorphs, such as parisite or röntgenite, considering the chemical composition of obsidian. The composition of the obsidian is important, as it has been shown in other contributions that when there is an interaction between a mineral and a solution, the chemical elements that make up both parts favor the synthesis of the new chemical molecule, as happens in a reaction mixture between solutions [18,43,44].
In order to answer this question, we must corroborate whether there was an interaction between the molecules in the solution with those of obsidian. The characterization of the FTIR spectra of the samples allowed us to observe the vibration bands that corresponded to the elements that made up the biomorphs.
The biomorphs synthesized under the standard atmospheric conditions showed vibration bands at 712.5, 788.9, 873.6, 1057.4, 1412.3, and 2980.8 cm−1 (Figure 2c, Table 1), while, in the case of the calcium silico-carbonates obtained under the conditions that emulated the Precambrian atmosphere, bands were observed at 783.0, 1035.2, 1450.0, and 2980.8 cm−1 (Figure 3c, Table 1). Therefore, when observing the bands in the FTIR spectra, characteristic CO32− bands can be identified, as the FTIR technique is especially sensitive to the detection and quantification of carbonates.
CO32− is a type of triangular symmetry, D3h, presenting a strong absorption at 1426 cm−1, a typical band assigned to ν3–CO32−, which is sometimes unfolded; a fine band at 875 cm−1, assigned to ν2–CO32−; and a very fine one at 712 cm−1, assigned to ν4–CO32−. It is important to mention that the weak band observed between 1057 and 1035 cm−1 overlaps with the band of the characteristic ν3–Si–O vibration, which can be observed in the interval of 966–970 cm−1. Additionally, intense bands at 677 cm−1 in the region of low-frequency bands can be observed, corresponding to Si–O–Si deformation. However, using FTIR, we were unable to identify whether there was any interaction between the molecules of the reaction mixture with the obsidian in the solution; this would rather be identified in the chemical and crystalline structure of the synthesized biomorphs. Thus, if the interaction with obsidian did not take place, then the calcite would be confirmed. Meanwhile, if there was an interaction with the minerals, it would be highly probable that calcium silico-carbonate would be identified, as this is an element characteristic of obsidian forming a part of the biomorph. Therefore, for the purpose of determining which of the calcium carbonate polymorphs (calcite obtained at ambient conditions (trigonal) and at high pressure (aragonite orthorhombic) or vaterite at low pressure (hexagonal)) was present in the samples analyzed, an analysis with powder X-ray diffraction (PXRD) was carried out.
Each diffractogram shows relevant information about the mineralogic phases associated with the formation of biomorphs on obsidian. In general, the resolution and the optics of the technique showed the 2θ-I data that allow us to identify a great number of phases in each of the diffractograms. In addition, although the differences between the two types of diffractograms are not easy to discern, the superimposed peaks, together with previous knowledge of obsidian, permitted us to make some inferences. In the diffractograms corresponding to the calcium silica–carbonate biomorphs, the main peaks were found in the phase with a structure in which the röntgenite polymorph was identified (from the homologous bastnäsite–vaterite) (Table 2). A low percentage of calcium zeolite–wairakite (CaAl2SiO4·2H2O, the topological structure of analcime, ANA) and the phyllosilicate nontronite, a Fe3+ rich smectite (NaO3Fe3+2(Si,Al)4O10(OH)2·n(H2O)−), was found.

Table 2.
Results of a unit cell of röntgenite for the R3 spatial group; rhombohedral cell expressed with hexagonal axes (a = b ≠ c; α = β = 90°, γ = 120°).
This finding shows that the synthesis of the biomorphs took place through the interaction between the reaction mixture and the minerals or rocks in the solution, since the chemical formula of röntgenite is 3CeFCO3•2CaCO3. Therefore, the presence of calcium silico-carbonates with fluorocarbonates and cerium can be proven in the obtained biomorphs. The result shows that there is an interaction between the obsidian and the reaction mixture. Our data provide evidence supporting the hypothesis that minerals or amorphous silicates in the form of igneous rocks present in the Precambrian were what favored the catalysis of the synthesis of the first biomolecules (from small to larger organic molecules) [15,45]. To corroborate this hypothesis, various experiments have been carried out; for example, it has been shown that biomolecules such as peptides, proteins, nucleic acids, ribose, lipids, or phosphates are absorbed efficiently by minerals such as pyrite, hematite, silica gel, mesoporous, zeolite, hydroxyapatite, titanium dioxide, and clays [46,47,48,49,50,51,52,53,54]. It has also been reported that the polymerization of biomolecules can be carried out efficiently on minerals or even amorphous materials, due to the fact that they catalyze the union of monomers to form various biomolecules [54,55]. In other published contributions, it has been observed that the abiotic synthesis of histidine in conditions that emulate the prebiotic environment is possible. This was performed by using DL-histidine on minerals such as pyrite, aragonite, and antigorite [56]. In this work, the formation of amide I and amide II vibration bands were observed, which was possible because of the role of the surface of the igneous rock in increasing organic stability during prebiotic evolution [56]. Additionally, our results reinforce what other work groups have postulated: the first biomolecules, and therefore pioneer organisms, originated in the prebiotic soup with the participation of minerals or amorphous silicates [56,57,58,59,60,61,62,63,64,65,66,67]. In another work, the interactions between amino acids and the surface of layered double hydroxides (LDHs) were evaluated, where the results showed that all the amino acids analyzed were absorbed on the surface of the LDHs with positive charges from C-terminal oxygen through the formation of hydrogen bridges [54]. The interaction of the obsidian with other biomolecules such as nucleic acids was also evaluated, and the results obtained suggest that, in the origin of life, minerals or even some igneous rocks permitted DNA to reside outside the molecule that stored genetic information, while RNA (ribose nucleic acid) and PNA (peptide nucleic acid) served other functions [68]. In this way, the presence of organic molecules, water, and obsidian pieces function as catalysts for the synthesis of complex molecules and as protectors of the biomolecules from the external environment [55,69]. However, in these works, whether organic and inorganic molecules in dissolution can interact with the molecules of obsidian was not evaluated, unlike our results presented here (Figure 2 and Figure 3, Table 1). Additionally, our results favor our hypothesis as follows: biomorphs might have been one of the first inorganic structures that were formed in the Precambrian where protomolecules could be concentrated, aligned, polymerized, and protected from the atmospheric conditions of that time, which have been reported to not have been the best conditions for maintaining and perpetuating life [19,21]. Having identified that the calcium silica–carbonate biomorphs synthesized on obsidian are formed by röntgenite, we propose that volcanic glasses that were rich in silica (such as obsidian) possibly played a leading role in concentrating and aligning biomolecules in the Precambrian era, acting as a template in their synthesis, as well as protecting the first biomolecules that gave rise to the pioneer organisms.
3.2. Barium Silico-Carbonate Biomorphs Are Possibly the First Inorganic Structure That Formed Parts of Various Forms of Life in the Precambrian
In the case of the barium control biomorphs synthesized under the current conditions, a leaf morphology was observed (Figure 4a). The biomorphs obtained under the conditions that emulated the Precambrian showed a spherical shape (Figure 4b). To evaluate the shape, chemical composition, and crystalline structure of the barium silica–carbonate biomorphs obtained on the obsidian, the biomorphs were synthesized under the current atmospheric conditions and under the conditions that emulated the Precambrian era (50 °C in the presence of 5% CO2). The barium carbonate biomorphs synthesized under the current atmospheric conditions presented crystalline aggregates that resembled leaves, spirals, flowers, and stems (Figure 4c). Meanwhile, the barium silica–carbonate biomorphs produced under an atmosphere that emulated the Precambrian conditions presented a polycrystalline sphere-type morphology (Figure 4d). Shapes have been previously observed in other works under both atmospheric conditions that emulated the Precambrian era [19,25]. In addition, these data show that, regardless of the material or mineral on which the biomorphs are synthesized, atmospheric conditions seem to be directly involved in the morphology they adopt (Figure 4). Thus, the results obtained in this work show that, under the conditions that emulated the Precambrian, the biomorphs obtained tend to be sphere-like structures (Figure 4). This spherical shape has also been shown in biomorphs synthesized in the presence of biomolecules [19,22,34,70] and of different clays [31], as well as in atmospheric conditions that emulated the Precambrian [70], which is possibly due to the fact that it is the most thermodynamically stable morphology, considered to be characteristic of life [70].
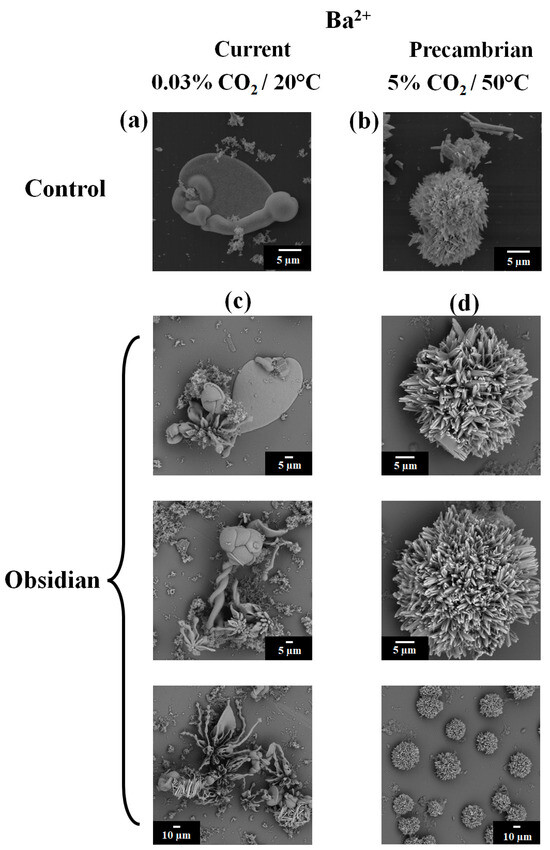
Figure 4.
SEM micrographs of the barium silica–carbonate biomorphs synthesized on glass (a,b), on obsidian (c,d), under (a,c) ambient temperature, and (b,d) conditions emulating the Precambrian atmosphere.
The fact that previously observed crystalline aggregates under these same atmospheric conditions have been identified indicates that obsidian is a good support for the synthesis of biomorphs. This fact is important because it has been described that biomorphs, when obtained on other support surfaces under the current atmospheric conditions, present the shapes of leaves, flowers, and helixes (twisted structures) [27], similar to the barium carbonate biomorphs synthesized on obsidian (Figure 4c). In the case of the biomorphs produced under the conditions that emulated the Precambrian era, which present a sphere morphology (Figure 4d), it is noted that this is the morphology that also appears when biomorphs are synthesized at low temperatures of 4 °C and −20 °C [33], as well as in the presence of biomolecules such as proteins and nucleic acids [22,23,34]. The evidence indicates that crystalline aggregates of barium, which took part in supporting the first forms of life, were possibly spherical, which, by being the most stable shape, allowed for improved survival, together with other environmental factors and the primitive metabolism of these lifeforms (which, in some cases, have continued to exist up to the present day). It has been reported that Ba2+ has been identified in algae, in some invertebrate species, and in fish in the Irish Sea [71]. Once the morphology of the barium silica–carbonate biomorphs obtained under both atmospheric conditions was observed, the identification of their chemical composition and crystalline structure was carried out via Raman and FTIR spectroscopies and PXRD analysis. The Raman spectra corresponding to the biomorphs synthesized under the current atmospheric conditions showed bands at 95, 138, 53, 691, 1059, and 2907 cm−1 (Figure 5b, Table 1), which were similar to the bands observed in the biomorphs synthesized under the atmospheric conditions that emulated the Precambrian period, for which bands were recorded at 95, 138, 155, 224, 691, and 1509 cm−1 (Figure 6b, Table 1).
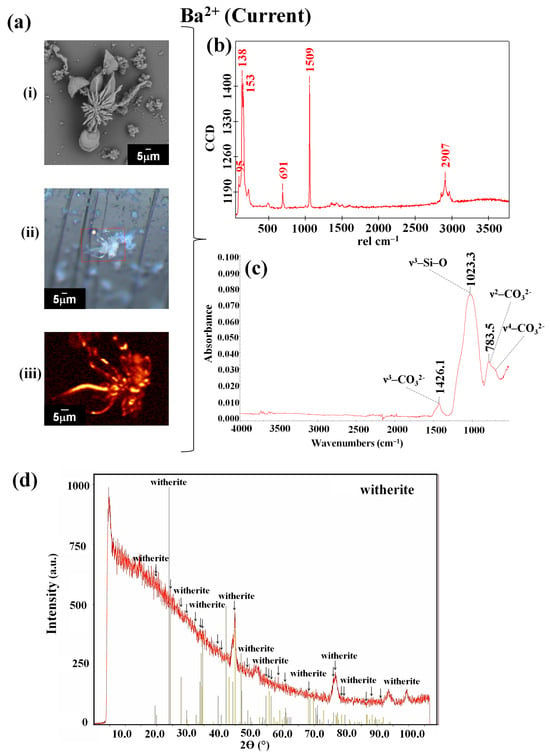
Figure 5.
Identification via Raman spectroscopy (b), IR (c), and PXRD (d) of the barium silica–carbonate biomorphs synthesized under current atmospheric conditions. (a): (i) SEM micrograph; (ii) optical image obtained with the Raman microscope; (iii) Raman map (1509 cm−1).
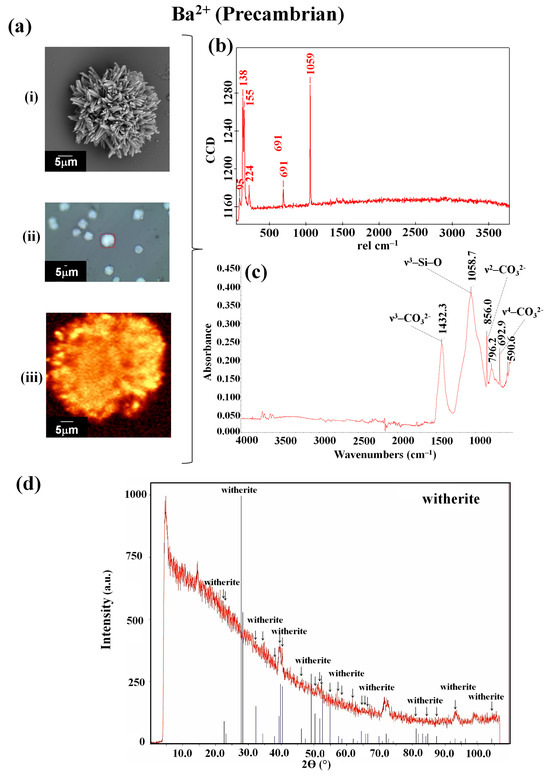
Figure 6.
Identification via Raman spectroscopy (b), IR (c), and DRX (d) of the barium silica–carbonate biomorphs synthesized on obsidian under conditions that emulated the Precambrian. (a): (i) SEM micrograph; (ii) optical image obtained with a coupled RAMAN; (iii) Raman map (1509 cm−1).
These vibrations were attributed to the presence of BaCO3 in witherite form in both samples. This crystalline polymorph coincides with the bands characteristic of those reported in previous works, where reticular vibration bands were found at 136, 155, 180, and 225 cm−1 and internal vibration ones were shown at 691 and 1059 cm−1. To corroborate whether it is the witherite, the FTIR spectra of the aforementioned samples were characterized and the corresponding vibration bands of the elements that made up the biomorphs were observed. Therefore, in the FTIR spectra, the peaks that are characteristic of the CO32− groups can be identified. It is important to mention that, for the case of the FTIR spectrum corresponding to the biomorph samples obtained under the current atmospheric conditions, the band n3-CO32− at 1426.1 cm−1 and the band n1-CO32− at 1023.3 cm−1 were both observed.
In the case of the FTIR spectrum obtained for the biomorphs synthesized under the conditions that emulated the Precambrian period, analog vibration bands were observed for the biomorphs obtained at the environmental temperature. Therefore, in the spectra, the n3-CO32− band at 1432.3 cm−1, the n1-CO32− band at 1058.7 cm−1, and the n2-CO32− band at 856.0 cm−1 can be observed. The signals present on the FTIR spectra of the reported witherite were compared, where the bands were attributed to the BaCO3 polymorph [42]. Additionally, in the diffractograms of the barium biomorphs, the main peaks of a phase comprising a witherite structure with a BaCO3 composition were observed. These results confirm the chemical composition and crystalline structure of the obtained biomorphs.
The identification of BaCO3 biomorphs presenting the witherite polymorph, as was identified under other synthesis conditions [22,23,33], is relevant because, as has been mentioned, Ba2+ has been identified in some organisms of our present time, such as algae, invertebrates, and fish [71]. This allows us to infer that in the primeval era of our planet, barium was present in the form of barite in the Earth’s crust [72] and formed part of various forms of primitive life since it seems that, independent of the mineral or substrate within which it is found, it is capable of carrying out chemical reactions with the elements present in diverse atmospheric and environmental conditions. In addition, the barium silica–carbonate biomorphs, as well as the calcium silica–carbonate biomorphs, obtained on obsidian show (as we have already mentioned) that minerals and perhaps some igneous rocks had a prominent role in the rise of the first forms of life on Earth, currently forming part of the hard and rigid structures of various organisms, through a process called biomineralization [73,74,75,76,77,78,79,80,81,82]. This mechanism of biomineralization is carried out in the organisms of practically all five kingdoms (animalia, plantae, fungi, protista, and monera).
4. Conclusions
Our results show that organic and inorganic molecules in dissolution can interact with the molecules of obsidian, as was shown in the biomorphs formed. Most of the borosilicate glasses and even igneous rocks like obsidian, when interacting with alkaline solutions, will be dissolved in the form of polysiloxanes. The groups silanol will interact with are alkaline metals (Ca and Ba), producing a massive crystallization of carbonates (obtained from the CO2) and forming the self-organized structures called biomorphs. When organic molecules are present in the synthesis of biomorphs, these biomolecules used to be incorporated into the structure via the silanol groups.
Independent of the atmospheric conditions used in their synthesis, the biomorphs took the form of a röntgenite polymorph. Firstly, these findings contribute to the hypothesis that minerals or even igneous rocks participated in the concentration, alignment, polymerization, and protection of proto-biomolecules at the early stages when cyanobacteria were formed. Secondly, these findings also favor the hypothesis that biomorphs were one of the first inorganic structures that were formed in the first era of our planet and mimicked the shapes of living organisms. Finally, we observed that silica–carbonate biomorphs of barium synthesized under conditions emulating the Precambrian era adopted a spherical morphology, which may indicate that the first morphologies that protocells adopted, in which barium was present, were indeed spherical to properly protect them. This morphology is the most stable and, thus, allowed for increased survival, together with other environmental factors and the primitive metabolism of that era (which, in some cases, persists even up to our own time).
Author Contributions
M.C.-C. and A.M. Methodology, M.C.-C., S.R.I., E.A.Z.-E. and M.A.Z.-E.; validation, M.C.-C., S.R.I. and A.M.; formal analysis, M.C.-C. and A.M.; investigation, E.A.Z.-E., M.A.Z.-E., M.C.-C., S.R.I. and A.M.; writing—original draft preparation, M.C.-C.; writing—review and editing, E.A.Z.-E., M.A.Z.-E., S.R.I., M.C.-C. and A.M.; supervision, M.C.-C. and A.M.; project administration, M.C.-C.; and funding acquisition, M.C.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out with the financial support granted to M. Cuéllar-Cruz by project no. CF2019-39216 from the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECIHTI) and Proyecto-Institucional-UGTO-005/2024 from the Universidad de Guanajuato, México.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Laboratorio Universitario de Caracterizacion Espectroscópica (LUCE) of the Instituto de Ciencias Aplicadas y Tecnología, UNAM, for the Raman and FTIR measurements, and José Guadalupe Bañuelos for their technical support. The authors thank the Universidad Autónoma del Estado de Hidalgo for providing the facilities in the Earth and Materials Sciences Department laboratories for the analysis of the PXRD data. Marcelino Antonio Zúñiga-Estrada (CVU: 784081) and Erick Alfredo Zúñiga-Estrada (CVU: 350391) thank CONAHCYT for the postdoctoral support nos. 2365907 and 2891301, respectively. The authors acknowledge Antonia Sanchez for the English style corrections of this manuscript.
Conflicts of Interest
The authors declare that they have no competing financial interests.
References
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral evolution. Am. Miner. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Ringwood, A.E. Chemical evolution of the terrestrial planets. Geochim. Cosmochim. Acta 1966, 30, 41–104. [Google Scholar] [CrossRef]
- Ringwood, A.E. On the chemical evolution and densities of the planets. Geochim. Cosmochim. Acta 1959, 15, 257–283. [Google Scholar] [CrossRef]
- Scott, E.R.D.; Krot, A.N. Chondrites and Their Components. In Treatise on Geochemistry; Elsevier Ebooks: Amsterdam, The Netherlands, 2003; pp. 1–72. [Google Scholar]
- Skinner, B.J. Earth resources. Proc. Natl. Acad. Sci. USA 1979, 76, 4212–4217. [Google Scholar] [CrossRef]
- Monger, H.C.; Kelly, E.F. Silica Minerals; Soil Science Society of America Book Series; Wiley: Hoboken, NJ, USA, 2018; pp. 611–636. [Google Scholar]
- Huang, P.M. Feldspars, Olivines, Pyroxenes, and Amphiboles; Soil Science Society of America Book Series; Wiley: Hoboken, NJ, USA, 2018; pp. 975–1050. [Google Scholar]
- Drees, L.R.; Wilding, L.P.; Smeck, N.E.; Senkayi, A.L. Silica in Soils: Quartz and Disordered Silica Polymorphs; Soil Science Society of America Book Series; Wiley: Hoboken, NJ, USA, 2018; pp. 913–974. [Google Scholar]
- Ericson, J.E.; Makishima, A.; Mackenzie, J.D.; Berger, R. Chemical and physical properties of obsidian: A naturally occuring glass. J. Non-Cryst. Solids 2018, 17, 129–142. [Google Scholar] [CrossRef]
- Glascock, M.D. Obsidian Provenance Research in the Americas. Acc. Chem. Res. 2002, 35, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R.E. The mode of origin of the color of certain varicolored obsidians. J. Geol. 1927, 35, 570–573. [Google Scholar] [CrossRef]
- Cann, J.; Renfrew, C. The Characterization of Obsidian and its Application to the Mediterranean Region. Proc. Phys. Soc. 1964, 30, 111–133. [Google Scholar] [CrossRef]
- Carballo, D.M.; Carballo, J.; Neff, H. Formative and Classic Period Obsidian Procurement in Central Mexico: A Compositional Study Using Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry. Lat. Am. Antiq. 2007, 18, 27–43. [Google Scholar] [CrossRef]
- Charlton, T.H.; Grove, D.C.; Hopke, P.K. El Paredón Mexico, Obsidian Source and Early Formative Exchange. Science 1978, 201, 807–809. [Google Scholar] [CrossRef]
- Hazen, R.M.; Sverjensky, D.A. Mineral Surfaces, Geochemical Complexities, and the Origins of Life. Cold Spring Harb. Perspect. Biol. 2010, 2, a002162. [Google Scholar] [CrossRef]
- Mary, A.; Mary, A. Observations sur la morphogénèse en Plasmologie. Memoires Soc. Sci. “Antonio Alzate” 1909, 29, 242–250. [Google Scholar]
- Lillie, R.S.; Johnston, E.N. Precipitating-structures simulating organic growth. II. A Contribution to the Physico-Chemical Analysis of Growth and Heredity. Biol. Bull. 1919, 36, 225–272. [Google Scholar] [CrossRef]
- Ober, P.; Kolbinger, S.H.; Backus, E.H.G.; Bonn, M. Ion-Specific Interactions at a Mineral-Water Interface Revealed by Surface-Sensitive Spectroscopy under Flow Conditions. J. Phys. Chem. C 2023, 127, 13005–13010. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M. The equation of life in the Universe: Biomorphs as reminiscence of the first forms of life. Prog. Cryst. Growth Charact. Mater. 2024, 70, 100624. [Google Scholar] [CrossRef]
- González-Ramírez, L.A.; Moreno, A.; Ng, J.D.; García-Ruiz, J.M.; González-Ramírez, L.A.; Moreno, A.; Ng, J.D.; García-Ruiz, J.M. Investigations on the role of iron (III) and silica-iron (III) for the DNA protection against highly intense UV radiation: Tracking the connection of prebiotic chemistry to biology. Astrobiology 2023, 23, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Cuéllar-Cruz, M. New insights on the origin of life: The role of silico-carbonates of Ba (II) to preserve DNA against highly intense UV radiation. ACS Omega 2023, 8, 29585–29594. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Islas, S.R.; González, G.; Moreno, A. Influence of nucleic acids on the synthesis of crystalline Ca (II), Ba (II), and Sr (II) silica-carbonate biomorphs: Implications for the chemical origin of life on primitive Earth. Cryst. Growth Des. 2019, 19, 4667–4682. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Moreno, A. The role of calcium and strontium as the most dominant elements during combinations of different alkaline Earth metals in the synthesis of crystalline silica-carbonate biomorphs. Crystals 2019, 9, 381. [Google Scholar] [CrossRef]
- Bittarello, E.; Aquilano, D. Self-assembled nanocrystals of barium carbonate in biomineral-like structures. Eur. J. Mineral. 2017, 19, 345–351. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Moreno, A. Synthesis of crystalline silica-carbonate biomorphs of Ba (II) under the presence of RNA and positively- and negatively charged ITO electrodes: Obtainment of graphite via bio-reduction of CO2 and its implications to the chemical origin of life on primitive Earth. ACS Omega 2020, 5, 5460–5469. [Google Scholar] [CrossRef]
- Islas, S.R.; Cuéllar-Cruz, M. Silica-carbonate of Ba(II) and Fe2+/Fe3+ Complex as Study Models to Understand Prebiotic Chemistry. ACS Omega 2021, 6, 35629–35640. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.M.; Melero-Garcia, E.; Hyde, S.T. Morphogenesis of Self-Assembled Nanocrystalline Materials of Barium Carbonate and Silica. Science 2009, 323, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Eggins, S.M.; Woodhead, J.D.; Kinsley, L.P.J.; Mortimer, G.E.; Sylvester, P.; McCulloch, M.T.; Hergt, J.M.; Handler, M.R. A Simple Method for the Precise Determination of >=40 Trace Elements in Geological Samples by ICP-MS Using Enriched Isotope Internal Standardization. Chem. Geol. 1997, 134, 311–326. [Google Scholar] [CrossRef]
- Chaussidon, R.F.; M. A palaeotemperature curve for the Precambrian oceans based on silicon isotopes in cherts. Nature 2006, 443, 969–972. [Google Scholar] [CrossRef]
- Kasting, J.F.; Howard, M.T. Atmospheric composition and climate on the early Earth. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1733–1741; discussion 1741–1742. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Ramírez-Cardona, M.; Moreno, A. Influence of different types of clay minerals on the shape and form of silica-carbonates (Biomorphs) of Ca(II), Ba(II), and Sr(II). ACS Earth Space Chem. 2022, 6, 3054–3065. [Google Scholar] [CrossRef]
- Lilli, M.A.; Nikolaidis, Ν.P.; Karatzas, G.P.; Kalogerakis, N. Identifying the controlling mechanism of geogenic origin chromium release in soils. J. Hazard. Mater. 2019, 366, 169–176. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Schneider, D.K.; Stojanoff, V.; Islas, S.R.; Sánchez-Puig, N.; Arreguín-Espinosa, R.; Delgado, J.M.S.; Moreno, A. Formation of Crystalline Silica–Carbonate Biomorphs of Alkaline Earth Metals (Ca, Ba, Sr) from Ambient to Low Temperatures: Chemical Implications during the Primitive Earth’s Life. Cryst. Growth Des. 2019, 20, 1186–1195. [Google Scholar] [CrossRef]
- Sánchez-Puig, N.; Cuéllar-Cruz, M.; Islas, S.R.; Tapia-Vieyra, J.V.; Arreguín-Espinosa, R.A.; Moreno, A. The influence of silicateins on the shape and crystalline habit of silica carbonate biomorphs of alkaline Earth metals (Ca, Ba, Sr). Crystals 2021, 11, 438. [Google Scholar] [CrossRef]
- Opel, J.; Wimmer, F.P.; Kellermeier, M.; Colfen, H. Functionalisation of silica-carbonate biomorphs. Nanoscale Horiz. 2016, 1, 144–149. [Google Scholar] [CrossRef]
- Voinescu, A.E.; Touraud, D.; Lecker, A.; Pfitzner, A.; Kunz, W.; Ninham, B.W. Mineralization of CaCO3 in the presence of egg White lysozyme. Langmuir 2007, 23, 12269–12274. [Google Scholar] [CrossRef]
- Frampton, M.B.; Zelisko, P.M. Organosilicon Biotechnology. Silicon 2009, 1, 147–163. [Google Scholar] [CrossRef]
- Noorduin, W.L.; Grinthal, A.; Mahadevan, L.; Aizenberg, J. Rationally Designed Complex, Hierarchical Microarchitectures. Science 2013, 340, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Zhang, J.; Wang, L.; Lu, Z.; Wang, F.; Liu, Z.; Zeng, H. Spontaneous hierarchical surface engineering of minerals through coupled dissolution-precipitation chemistry. Aggregate 2023, 5, e452. [Google Scholar] [CrossRef]
- Buzgar, N.; Apopei, A.I. The Raman study of certain carbonates. Geol. Tomul L 2009, 55, 97–112. [Google Scholar]
- Kasatkin, A.V.; Škoda, R.; Nestola, F.; Kuznetsov, A.M.; Belogub, E.V.; Agakhanov, A.A. Röentgenite-(Ce) and other REE fluorcarbonates from vein no. 35, Vishnevye Mountains, southern Urals. Mineralogija 2019, 5, 10–22. [Google Scholar] [CrossRef]
- Lin, C.C.; Liu, L.G. High-pressure Raman spectroscopic study of post-aragonite phase transition in witherite (BaCO3). Eur. J. Miner. 1997, 9, 785–792. [Google Scholar] [CrossRef]
- Morse, J.W.; Mackenzie, F.T. Chapter 2 Interactions Between Carbonate Minerals and Solutions. Dev. Sedimentol. 1990, 48, 39–86. [Google Scholar] [CrossRef]
- Hunger, J.; Schaefer, J.; Ober, P.; Seki, T.; Wang, Y.; Prädel, L.; Nagata, Y.; Bonn, M.; Jan Bonthuis, D.J.; Backus, E.H.G. Nature of cations critically affects water at the negatively charged silica interface. J. Am. Chem. Soc. 2022, 144, 19726–19738. [Google Scholar] [CrossRef]
- Hazen, R.M. Genesis: Rocks, Minerals, and the Geochemical Origin of Life. Elements 2005, 1, 135–137. [Google Scholar] [CrossRef]
- Benetoli, L.O.B.; De Souza, C.M.D.; Da Silva, K.L.; De Souza, I.G.; De Santana, H.; Paesano, A.; Da Costa, A.C.S.; Zaia, C.T.B.V.; Zaia, D.A.M. Amino Acid Interaction with and Adsorption on Clays: FT-IR and Mössbauer Spectroscopy and X-ray Diffractometry Investigations. Orig. Life Evol. Biosph. 2007, 37, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Beltrami, G.; Martucci, A.; Pasti, L.; Chenet, T.; Ardit, M.; Gigli, L.; Cescon, M.; Suard, E. L-Lysine Amino Acid Adsorption on Zeolite L: A Combined Synchrotron, X-Ray and Neutron Diffraction Study. ChemistryOpen 2020, 9, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Villafanñe-Barajas, S.A.; Baú, J.P.T.; Colin-Garcia, M.; Negron-Mendoza, A.; Heredia-Barbero, A.; Pi-Puig, T.; Zaia, D.A.M. Salinity Effects on the Adsorption of Nucleic Acid Compounds on Na-Montmorillonite: A Prebiotic Chemistry Experiment. Orig. Life Evol. Biosph. 2018, 48, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Pitsch, S.; Eschenmoser, A.; Gedulin, B.; Hui, S.; Arrhenius, G. Mineral Induced Formation of Sugar Phosphates. Orig. Life Evol. Biosph. 1995, 25, 297–334. [Google Scholar] [CrossRef]
- Hartmann, M. Ordered Mesoporous Materials for Bioadsorption and Biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Hashizume, H. Adsorption of Nucleic Acid Bases, Ribose, and Phosphate by some Clay minerals. Life 2015, 5, 637–650. [Google Scholar] [CrossRef]
- Lin, R.; Wang, Y.; Li, X.; Liu, Y.; Zhao, Y. pH-Dependent Adsorption of Peptides on Montmorillonite for Resisting UV Irradiation. Life 2020, 10, 45. [Google Scholar] [CrossRef]
- Gillams, R.J.; Jia, T.Z. Mineral Surface-Templated Self-Assembling Systems: Case Studies from Nanoscience and Surface Science towards Origins of Life Research. Life 2018, 8, 10. [Google Scholar] [CrossRef]
- Erastova, V.; Degiacomi, M.T.; Fraser, D.G.; Greenwell, H.C. Mineral Surface Chemistry Control for Origin of Prebiotic Peptides. Nat. Commun. 2017, 8, 2033. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Hartman, H. Clays and the Origin of Life: The Experiments. Life 2022, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Madrigal-Trejo, D.; Villanueva-Barragan, P.S.; Zamudio-Ramirez, R.; Cervantes-de la Cruz, K.E.; Mejia-Luna, I.; Chacon-Baca, E.; Negron-Mendoza, A.; Ramos-Bernal, S.; Heredia-Barbero, A. Histidine Self-assembly and Stability on Mineral Surfaces as a Model of Prebiotic Chemical Evolution: An Experimental and Computational Approach. Orig. Life Evol. Biosph. 2021, 51, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Aguilar, C.D.; Cuéllar-Cruz, M. The formation of crystalline minerals and their role in the origin of life on Earth. Prog. Cryst. Growth Charact. Mater. 2022, 68, 100558. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W. Chemistry of Iron Sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Poulton, S.W.; Canfield, D.E. Ferruginous Conditions: A Dominant Feature of the Ocean through Earth’s History. Elements 2011, 7, 107–112. [Google Scholar] [CrossRef]
- Rasmussen, B. Filamentous microfossils in a 3,235-million-year-old volcanogenic massive sulphide deposit. Nature 2000, 405, 676–679. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.A.; Kelley, D.S.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef]
- Hazen, R.M.; Filley, T.R.; Goodfriend, G.A. Selective adsorption of L- and D-amino acids on calcite: Implications for biochemical homochirality. Proc. Natl. Acad. Sci. USA 2001, 98, 5487–5490. [Google Scholar] [CrossRef]
- Mendoza-Torres, E.; Cruz-Cataneda, J.; Negron-Mendoza, A.; Heredia, A. Computer and Experimental Simulation of Alloxazine Synthesis from Gamma Irradiation of Amino Acids on Iceland Spar: A Prebiotic Chemistry Perspective. J. Mol. Evol. 2020, 88, 284–291. [Google Scholar] [CrossRef]
- Schrenk, M.O.; Brazelton, W.J.; Lang, S.Q. Serpentinization, carbon, and deep life. Rev. Mineral. Geochem. 2013, 75, 575–606. [Google Scholar] [CrossRef]
- Sojo, V.; Herschy, B.; Whicher, A.; Camprubi, E.; Lane, N. The origin of life in alkaline hydrothermal vents. Astrobiology 2016, 16, 181–197. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Martin, W. Serpentinization as a source of energy at the origin of life. Geobiology 2010, 8, 355–371. [Google Scholar] [CrossRef]
- Russell, M.J.; Ponce, A. Six ‘Must-Have’ Minerals for Life´s Emergence: Olivine, Pyrrhotite, Bridgmanite, Serpentine, Fougerite and Mackinawite. Life 2020, 10, 291. [Google Scholar] [CrossRef] [PubMed]
- Swadling, J.B.; Coveney, P.V.; Greenwell, H.C. Stability of free and mineral-protected nucleic acids: Implications for the RNA world. Geochim. Cosmochim. Acta 2012, 83, 360–378. [Google Scholar] [CrossRef]
- Hartman, H. Photosynthesis and the Origin of Life. Orig. Life Evol. Biosph. 1998, 28, 515–521. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M. The equation of the origin of life in the Universe (Part II): The combination of chemical elements does not determine the emergence of life on Earth. Prog. Cryst. Growth Charact. Mater. 2024, 70, 100625. [Google Scholar] [CrossRef]
- Templeton, W.L.; Brown, V.M. The Relationship between the concentrations of calcium, strontium and strontium-90 in wild brown trout, Salmo-Trutta L. and the concentrations of the stable elements in some waters of the United-Kingdom, and the implications in radiological health studies. Air Water Pollut. 1964, 8, 49–75. [Google Scholar]
- Madejón, P. Barium. In Heavy Metals in Soils; Alloway, B., Ed.; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2013; pp. 507–514. [Google Scholar]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Margulis, L. Evolutionary prerequisites for early phanerozoic calcareous skeletons. Biosystems 1980, 12, 27–41. [Google Scholar] [CrossRef]
- Ruiz-Arellano, R.; Moreno, A. Obtainment of Spherical-Shaped Calcite Crystals Induced by Intramineral Proteins Isolated from Eggshells of Ostrich and Emu. Cryst. Growth Des. 2014, 14, 5137–5143. [Google Scholar] [CrossRef]
- Ruiz-Arellano, R.; Medrano, F.; Moreno, A.; Romero, A. Structure of struthiocalcin-1, an intramineral protein from Struthio camelus eggshell, in two crystal forms. Acta Crystallogr. Sect. D-Biol. Crystallogr. 2015, 71, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Grajeda, J.P.; Moreno, A.; Romero, A. Crystal Structure of Ovocleidin-17, a Major Protein of the Calcified Gallus gallus Eggshell: Implications in the calcite mineral growth pattern. J. Biol. Chem. 2004, 279, 40876–40881. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H. A crystal-clear view. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef]
- Khan, S.R. Histological aspects of the “fixed particle” model of stone formation: Animal studies. Urolithiasis 2016, 45, 75–87. [Google Scholar] [CrossRef]
- Mann, K. The calcified eggshell matrix proteome of a songbird, the zebra finch (Taeniopygia guttata). Proteome Sci. 2015, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Mann, M. The proteome of the calcified layer organic matrix of turkey (Meleagris gallopavo) eggshell. Proteome Sci. 2013, 11, 40. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M. Synthesis of inorganic and organic crystals mediated by proteins in different biological organisms. A mechanism of biomineralization conserved throughout evolution in all living species. Prog. Cryst. Growth Charact. Mater. 2017, 63, 94–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).