Abstract
Background: Diabetic foot ulcers (DFUs) affect millions worldwide, significantly increasing the risk of amputation, mortality, and reduced quality of life. While conventional interventions such as specialized footwear and podiatric care can mitigate ulceration risks, they do not address the biomechanical factors contributing to ulcer recurrence. Emerging evidence suggests that lower limb exercises may play a role in secondary DFU prevention. This scoping review aims to synthesize available research on home-based lower limb exercise programs for individuals with diabetes mellitus, focusing on feasibility, adherence, and their impact on foot biomechanics and ulcer prevention. Methods: A search was conducted across six electronic databases (PubMed, Web of Science, Cochrane Library, EBSCO, Scopus, and ScienceDirect) for studies published between January 2014 and December 2024. Eligible studies included those assessing home-based lower limb exercises in diabetic individuals, with interventions lasting at least four weeks. Studies focusing on supervised exercises, pharmacological interventions, or non-diabetic populations were excluded. Results: Nine studies met the inclusion criteria, featuring a range of home-based exercise interventions, such as muscle strengthening, stretching routines, proprioceptive training, functional mobility exercises, and range-of-motion activities. These interventions demonstrated notable effectiveness, leading to improved foot biomechanics, more even plantar pressure distribution, enhanced balance, and reduced ulcer recurrence (in some cases). One study, for instance, reported a significant decrease in ulcer recurrence, with only 16% of participants in the intervention group experiencing relapse compared to 72% in the control group after 24 weeks. Adherence rates varied across studies but were generally higher when programs included structured guidance through educational booklets, mobile applications, or consistent phone follow-ups ranging from 41% to 92.5%. Nonetheless, the findings tend to be tempered by methodological differences between studies and a lack of robust long-term follow-up data. Conclusions: Home-based lower limb exercises show promise in improving foot function and preventing DFU recurrence. Further research is needed to standardize protocols, enhance adherence, and confirm long-term effectiveness.
1. Introduction
Diabetic foot ulcers (DFUs) impact around 18.6 million individuals globally each year [1], and approximately 20% of those affected will require a lower extremity amputation [2]. The development of these ulcers is influenced by a combination of neurological, vascular, and biomechanical factors [1]. Peripheral neuropathy and peripheral vascular disease are the main primary contributors to developing foot ulcers, which often lead to additional complications, such as restricted joint mobility, muscular changes, and foot deformities [3]. DFUs are characterized by a slow healing process; data from the US Wound Registry indicate that only 30% to 40% of these ulcers heal within 12 weeks, while 23% remain unhealed after a year [4]. Additionally, people with DFUs are characterized by a higher mortality risk compared to those with diabetes mellitus (DM) who do not have ulcers, with rates of 231 deaths per 1000 person-years versus 182 deaths per 1000 person-years, respectively [1]. Furthermore, the presence of DFU also increases the likelihood of depression among patients and, finally, negatively affects health-related quality of life [3]. The likelihood of DFUs recurring after healing is significant, with estimates suggesting a 42% recurrence rate within one year and 65% within five years [1]. In their guidelines, the International Working Group on the Diabetic Foot (IWGDF) has emphasized several essential strategies for DFU prevention. These include the regular inspection and assessment of at-risk feet, providing education not only to individuals with DM but also to their families and healthcare providers—incorporating psychological support as needed. Additionally, ensuring the consistent use of appropriate footwear and addressing risk factors that could lead to ulceration are vital components of a comprehensive prevention approach [5]. This review focuses specifically on secondary prevention, which refers to interventions designed to prevent the recurrence of diabetic foot ulcers (DFUs) in individuals who have already experienced ulceration. In contrast, primary prevention targets individuals at risk but without a prior history of DFUs. Clarifying this distinction is essential for appropriately contextualizing the scope and findings of the review.
As noticed in a systematic review and meta-analysis conducted by van Netten et al. [6] that analyzed 29 studies, detailed conventional interventions aimed at preventing diabetes-related foot ulcers include specialized footwear, insoles, podiatric care, and self-management strategies. However, although the above-mentioned interventions can reduce the risk of foot ulcers, they do not address the underlying causes contributing to ulcer formation, i.e., protective sensation, mechanical stress, range of motion, and foot strength and function. Consequently, the IWGDF [5] outlined the need for further research into foot–ankle exercise programs and emphasized that future studies must evaluate longer-term outcomes (typically programs lasting 8–12 weeks), with an assessment of the acceptability and feasibility of sustaining exercise routines after the initial supervised phase. Additionally, these studies should prioritize ulceration as a key outcome measure. Interestingly, a review by van Netten et al. [6] indicated that while foot-related exercises may not be effective in preventing the initial occurrence of foot ulcers, they could be helpful in the secondary prevention of DFUs. The need to introduce exercise to the prevention of diabetic foot ulcers has been discussed in previous studies conducted so far, highlighting the significant differences in kinetic and kinematic parameters among three groups: participants with type 2 diabetes mellitus (T2DM) having peripheral neuropathy, those with T2DM without neuropathy, and non-diabetic individuals, showed in a meta-analysis by Hazari et al. [7]. The study revealed that individuals in the diabetes group with neuropathy exhibited higher ground reaction force and plantar pressure values, which may increase the risk of ulceration and other foot complications.
Another meta-analysis, conducted by Wang et al. [8], including 499 patients with diabetic neuropathy and 467 diabetic controls without neuropathy, also noticed significant differences in gait parameters between the two groups. These parameters included gait velocity, stride length, stride time, stance time, and the maximum knee extension moment. Such variations may be linked to the onset of diabetic peripheral neuropathy (DPN), dysfunction in lower limb muscles, and cognitive decline, potentially explaining the increased incidence of falls among patients with DPN. In another narrative review by Francia et al. [3], it was concluded that Limited Joint Mobility is prevalent in individuals with DM, affecting over two-thirds of patients and serving as a significant risk factor for the development of foot ulcers.
The rationale for incorporating lower limb exercises as a secondary prevention strategy for DFU recurrence—extending beyond just the foot and ankle—stems from evidence indicating that patients with DM often experience notable alterations in lower limb biomechanics. Engaging in exercise therapy may serve as an effective means to enhance and sustain the biomechanics of the foot, thereby helping to lower peak plantar pressure during everyday activities [3].
This scoping review aims to explore and synthesize the available evidence on home-based lower limb exercise programs designed for individuals with DM, focusing on their role in the secondary prevention of DFUs. Specifically, the review seeks to (1) map the types of home-based exercises employed; (2) assess their feasibility, acceptability, and adherence; (3) evaluate their effectiveness in improving foot biomechanics and reducing ulcer recurrence; (4) fill the current gap in the literature regarding adherence to remote training and foot biomechanics; and (5) check the potential replicability or transferability of the exercises to implement exercise programs in real-world clinical and home settings.
2. Materials and Methods
This review was neither registered in the PROSPERO Register of Systematic Reviews nor the Open Science Framework. Nonetheless, its conduct and reporting adhered to the PRISMA 2020 guidelines [9,10,11]. This decision was based on the nature of the study as a scoping review without meta-analysis or formal outcome synthesis, which does not fall under the mandatory scope for PROSPERO registration [11].
2.1. Search Strategy
The search strategy for this review involved six electronic databases: PubMed, Web of Science (Core Collection), Cochrane Library, EBSCO (Rehabilitation and Sports Medicine Sources), Scopus, and ScienceDirect. Two independent authors (SB and PC) searched all databases on 27 December 2024. To identify relevant papers, the titles were screened using search terms outlined in Table 1. The review was restricted to studies published between January 2014 and December 2024.

Table 1.
The combination of applied search terms within each of the six electronic databases.
2.2. Eligibility
This review focused on studies that analyzed home-based lower limb exercises in individuals with DM. It included only full-text articles in English, with applied the eligibility criteria.
The inclusion criteria were as follows: (1) only human adults participated in studies; (2) participants with DM and/or with peripheral neuropathy induced by diabetes; (3) studies where the main intervention was exercised relevant to the lower limb; (4) home-based lower limb exercises performed by the study participant on their own after receiving instructions in various forms, e.g., using verbal instruction, booklets, cards, online applications, etc.; and (5) studies evaluated longer-term outcomes, i.e., exercises performed for at least 4 weeks or more.
The exclusion criteria applied in this review were as follows: (1) non-scientific publications; (2) articles in other language than English; (3) not full-text articles; (4) non-experimental articles, such as reviews or meta-analyses; (5) studies conducted for participants without DM or with DPN caused by other causes than diabetes; (6) pregnant participants of study; (7) studies that analyzed the effects of exercises not relevant to the lower limbs or combined with exercises for other parts of the body; (8) lower limb exercises combined with an additional type of intervention, i.e., pharmacological or electrical stimulation or physical therapy (i.e., combined therapies); (9) exercises performed in medical facilities or/and under supervision; (10) exercises conducted for improvement of healing the DFU; (11) exercises performed for less than 4 weeks; (12) home-based exercises performed only remotely or as follow-up to support supervised exercise protocol; (13) exercises conducted for/by amputees; and (14) papers analyzing the same data already included in this review, as well as proof-of-concept studies, feasibility studies, Delphi studies, or study protocols. Articles focusing on aspects outside the scope of this review, such as cost-effectiveness, were also removed (Figure 1).
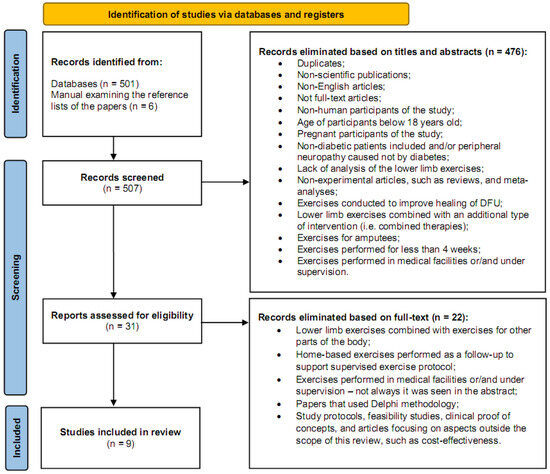
Figure 1.
A flowchart demonstrating the selection of articles through the review process [9,10].
2.3. Review Process
Two independent reviewers (SB and PC) screened articles in a two-step process. First, titles and abstracts were reviewed, with duplicates removed manually. Second, full-text publications were assessed to determine eligibility. A manual search of the reference lists of included articles was also performed to identify additional relevant studies. Any disagreements were resolved by JW, who reviewed the selected articles for relevance and made the final inclusion decisions. MB compiled all included articles using reference management software (EndNote X7.7, Clarivate Analytics, Philadelphia, PA, USA), ensuring duplicates from different sources were removed (Figure 1).
2.4. Quality Assessment
SB and JW evaluated the methodological quality of each eligible study using a standardized checklist for randomized and non-randomized studies [12].
The checklist included five subscales: (1) reporting (questions 1–10), ten items assessing the adequacy of information provided to ensure an unbiased evaluation of study outcomes; (2) external validity (questions 11–13), three items evaluating the generalizability of findings to the broader population; (3) internal validity—bias (questions 14–20), seven items addressing potential biases in intervention implementation and outcome measurement; (4) internal validity—confounding (selection bias: questions 21—26), six items identifying selection biases in participant recruitment; and (5) power (question 27), a single item assessing whether study outcomes could be influenced by insufficient statistical power.
Each item was scored 0 or 1, except for item 5 on the reporting subscale, which scored 0 to 2, and the power item, which scored 0 to 5. The maximum possible score for the checklist was 32.
3. Results
The search process initially identified a total of 507 papers. Of these, 501 articles were retrieved through searches conducted in six electronic databases: PubMed, Web of Science (Core Collection), Cochrane Library, EBSCO (Rehabilitation and Sports Medicine Sources), Scopus, and ScienceDirect, yielding 149, 129, 154, 51, 18, and 0 articles, respectively. An additional six papers were identified by analyzing the reference lists of articles included in this review. Following this, 31 full-text articles were selected and assessed for eligibility. Ultimately, nine articles met the inclusion criteria and were included in this review (Figure 1).
The results are presented in three tables (Table 2, Table 3 and Table 4). Table 2 summarizes the key features of the retrieved articles, offering detailed information about each study, including (1) the study type and country of origin, (2) participant characteristics, (3) inclusion criteria, (4) study objectives, (5) interventions applied, (6) descriptions of the exercises, (7) intervention duration and weekly dosage, and (8) the approach to exercise distribution.
Table 3 outlines adherence to exercise plans, dropout rates, and reported side effects or complications related to the intervention.
Table 4 presents additional information, such as (1) measurement devices used, (2) outcome measures and follow-up periods, and (3) key results from the studies.
3.1. Key Characteristics of Retrieved Articles
The studies presented in Table 2 focus on home exercise interventions aimed at improving foot and ankle health in patients with DM, particularly those affected by DPN and other diabetes-related foot complications. The studies encompass different interventions, including self-care programs, strengthening, stretching, and functional training, delivered through various methods, such as telephone support, web-based platforms, and in-person guidance. While Table 2 summarizes the basic features of each intervention, comprehensive details, including types of exercises, progression protocols, tools used (e.g., towel, balls, and resistance bands), and delivery formats (brochures, phone calls, and digital platforms) can be found in the Supplementary Materials.
The studies were conducted across countries such as Brazil, Turkey, Taiwan, and Indonesia, with the participant demographics largely consisting of individuals with T2DM and DPN, generally aged between 45 and 78 years. Many studies included a balanced gender distribution, with a higher proportion of female participants, and the body mass index (BMI) and DM duration varied among participants. The primary aim of these studies was to assess the effects of home exercise programs on improving foot and ankle function, enhancing mobility, reducing diabetic foot complications, and managing symptoms of neuropathy. Specific outcomes of interest included gait improvement, strength, flexibility, joint range of motion (RoM), and plantar pressure distribution.
Where terms such as “high adherence”, “feasible”, or “effective” are used, they tend to follow the quantifiable metrics reported in the studies. For example, adherence rates ranged from 72% to over 90% among the studies that used booklets and remote follow-up. Detailed adherence percentages and intervention durations are presented in Table 3 and elaborated in the Supplementary Materials. The complete protocols and implementation details—including frequency of sessions, progression, and use of digital or print support—are available in the Supplementary Materials.


Table 2.
Overview of study characteristics, country of origin, study type, quality assessment, participant demographics, objectives, and details of home exercise programs.
Table 2.
Overview of study characteristics, country of origin, study type, quality assessment, participant demographics, objectives, and details of home exercise programs.
| Study/ Country/ Type of Study/ Quality | Study Group Sex M/F; Age (Years); Body Mass (kg); Body Height (cm); BMI (kg/m2); Duration of Diabetes (DD) (Years) | Aim/ Inclusion Criteria | Description of Exercises/ Intervention Duration (Dose per Week)/ Approach to Exercise Distribution |
|---|---|---|---|
| Iunes et al. [13] Brazil Prospective, quasi-experimental clinical trial 20/32 | N: 97 (54F, 43M); Age: 62.12 ± 11.31; BMI: 17.64 ± 3.80; DD: 13.89 ± 7.57. | To verify self-care guidelines together with lower limb home exercises alter ankle and foot plantar pressure and alignment in patients with T2DM.
Inclusion criteria:
The performance of routine medical monitoring. | 11 exercises:
10 months (no data) Verbal guidance and an explanatory leaflet about self-care and lower limb exercises. |
| Sartor et al. [14] Brazil Randomized, controlled trial 23/32 | The control group: 29 (15 F, 14 M); Age: 60 ± 12; Body mass: 82.5 ± 16.4; Body height: 171 ± 30; BMI: 29 ± 4; DD: 18 ± 11. The intervention group: 26 (11 F, 15 M); Age: 59 ± 4; Body mass: 77.4 ± 14.1; Body height: 165 ± 9; BMI: 28 ± 4; DD: 17 ± 10. | To investigate the effects of strengthening, stretching, and functional training on the foot rollover process during gait. Inclusion criteria:
| Each session included exercises from four categories:
Exercises progressed gradually, starting with passive, then active movements, and ending with walking and functional skills, ensuring peripheral gains were integrated into functional movements. Pain and performance were monitored throughout. The intervention, lasting 40–60 min per session, twice a week for 12 weeks, began immediately after the patient was assigned to the intervention group. Receiving physical therapy and instructions to perform exercises at home. |
| Cerrahoglu et al. [15] Turkey Prospective, randomized, controlled trial 21/32 | With neuropathy: 38 (24 F, 14 M); The control group/ the intervention group: 19/19; Age: 56.87 ± 9.42; BMI: 31.92 ± 5.20; DD: 11.18 ± 6.86. Without neuropathy: 38 (24 F, 14 M); The control group/ The intervention group: 19/19; Age: 53.66 ± 9.36; BMI 31.84 ± 6.38; DD: 9.58 ± 7.07. | Investigation whether a home-exercise, self-care program that consists of RoM, stretching, and strengthening exercises could improve RoM for foot joints and plantar pressure distribution during walking in diabetic patients to prevent diabetic foot complications. Inclusion criteria:
| The home exercise program included RoM, stretching, and strengthening exercises for the ankle and metatarsophalangeal joints, with both weight-bearing and non-weight-bearing exercises.
The intervention lasted 4 weeks. Participants tracked their daily exercises in an activity log for self-motivation (not study data) and received weekly motivational calls to guide exercise progression. |
| Kuo et al. [16] Taiwan Secondary data analysis from a larger randomized controlled trial 22/32 | The intervention group: 88 (67 F, 21 M); Age: 78.6 ± 8.0; DD: 12.18 ± 8.8. | To examine adherence to home-based rehabilitation over 12 months post-discharge and its effects on postoperative recovery, including activities of daily living, hip and ankle RoM, quadriceps strengthening for weight-bearing and ambulation, and affected limb muscle strength. Inclusion criteria:
| The in-home rehabilitation program began with exercises such as ankle dorsiflexion with knee extension, isometric full knee extension, gentle vertical bouncing with knees semi-flexed and feet on the floor, and ball-rolling activities to improve proprioception. The specific exercises were adjusted based on the patient’s bone healing progress and physical condition. (Exercise details are not further described.) The intervention lasts 12 months. Home-based exercises were advised by a physical therapist. |
| Borges et al. [17] Brazil Single-blind, randomized, clinical trial 23/32 | The control group: 27 (17 F, 10 M); Age: 55.7 ± 5.4; Body mass: 80.4 ± 14.6; Body height 160 ± 0.1. The home training group: 27 (18 F, 9 M); Age: 55.8 ± 6.2; Body mass: 80.4 ± 14.4; Body height: 160 ± 0.1. The supervised training group: 26 (14 F, 12 M); Age: 56.8 ± 6.1; Body mass 81.1 ± 13.7; Body height: 160 ± 0.1. | To analyze postural control in the bipedal position as well as during gait and functional tests in patients with T2DM before and after supervised and unsupervised proprioceptive training. Inclusion criteria:
| Seven exercises:
The home training group received a card with exercise illustrations and a kit with the necessary materials but no supervision. |
| Silva et al. [18] Brazil Randomized controlled trial FOCA II (booklet intervention) 25/32 | The control group: 11 (9 F, 2 M); Age 55.3 ± 8.9; Body mass: 81.0 ± 15.7; Body height: 163.5 ± 0.1; BMI: 29.4 ± 3.1; DD: 20.3 ± 9.9; N with T2DM: 8. The intervention group: 9 (8 F, 1 M); Age: 58.1 ± 3.6; Body mass: 77.6 ± 14.7; Body height: 159.3 ± 0.1 BMI: 27.6 ± 12.4; DD: 19.1 ± 11.9; N with T2DM: 7. | Assessment of the feasibility of design, adherence, satisfaction, safety, and changes in outcomes followed by a home-based foot–ankle exercise guided by a booklet in individuals with DPN. Inclusion criteria:
| Six exercises with three difficulty levels. The first exercise session was supervised at the University of São Paulo to teach participants how to use the exercise booklet and deliver a kit with necessary materials (cotton balls, towel, pencil, elastic bands, balloons, massage ball, and finger separators). The program strengthened intrinsic and extrinsic foot–ankle muscles and increased RoM in interphalangeal, metatarsophalangeal, and ankle joints, including 3 warm-up exercises, 4 targeting intrinsic muscles, and 2 for extrinsic muscles. Difficulty was adjusted based on perceived effort, and participants recorded progress in the booklet. The control group received usual care, while the intervention group received usual care plus home-based foot–ankle exercises, performed three times a week for 8 weeks (24 sessions total). Weekly calls ensured adherence, addressed concerns and supported learning. |
| Suryani et al. [19] Indonesia Double-blind randomized clinical trial 23/32 | The control group: 25 (12 F, 13 M); Age: 56 ± 5.89; BMI: 24 ± 5.99; DD: 9.16 ± 5.13; N with T2DM: 25; HbA1c (%): 9.48 ± 1.99. The intervention group: 25 (12 F, 13 M); Age: 54 ± 5.96; BMI: 25 ± 5.37; DD: 9.6 ±8.59; N with T2DM: 25; HbA1c (%): 10.11 ± 2.04. | The study aimed to evaluate the effects of foot–ankle flexibility and resistance exercises on the recurrence rate of plantar foot diabetic ulcers, HbA1c levels, diabetic neuropathy examination (DNE) scores, ankle–brachial index (ABI), and walking speed within 12 and 24 weeks. Inclusion criteria:
| The control group:
The intervention group received the same education as the control group and exercises:
The intervention group performed foot–ankle exercises at home 3 times a week on non-consecutive days, including 30 repetitions of flexibility exercises and 5–50 repetitions of resistance exercises with an elastic band. Both intervention and the control group received foot care education. Foot–ankle exercises to perform at home with the help of modules and videos. |
| Silva et al. [20] Brazil Randomized controlled clinical trial FOCA II (booklet intervention) 26/32 | The control group: 25 (20 F, 5 M); Age: 56.5 ± 9.4; Body mass: 74.2 ± 14.8; Body height: 164.0 ± 0.1; BMI: 22.9 ± 3.6; DD: 18.2 ± 9.8; N with T2DM: 19. The intervention group: 25 (19 F, 6 M); Age: 59.1 ± 6.4; Body mass: 74.4 ± 15.6; Body height: 162.0 ± 0.1; BMI: 23.5 ± 4.8; DD: 13.8 ±10; N with T2DM: 22. | To investigate the effect of an 8-week home-based foot–ankle exercise program using an educational booklet on clinical outcomes (foot muscle strength and functionality; functional balance; diabetic neuropathy symptoms and severity; tactile and vibratory sensitivities; plantar pressure distribution; and foot–ankle, knee, and hip biomechanics during gait). Inclusion criteria:
| The control group received usual care, including:
The intervention group received usual care plus a home-based foot–ankle exercise program that included the following:
|
| Ferreira et al. [21] Brazil Randomized controlled trial FOCA I (SOPeD intervention) 25/32 | The control group: 31 (18 F, 13 M); Age: 57.0 ± 9.6; Body mass: 85.7 ± 16.3; Body height: 165.0 ± 0.1; BMI 31.7 ± 6.9; DD: 10.3 ± 6.7; N with T2DM: 30. The intervention group: 31 (20 F, 11 M); Age: 52.1 ± 9.3; Body mass: 78.8 ± 13.4; Body height: 167.0 ± 0.1 BMI: 28.2 ± 4.1; DD: 15.3 ± 9.4 N with T2DM: 26. | Assessment of the effectiveness of a web-based foot–ankle exercise program aiming to improve DPN-related outcomes, gait biomechanics and functional outcomes. Inclusion criteria:
| Both groups received self-care education, a personalized brochure, and consultations based on IWGDF guidelines, covering foot inspection, nail/skin care, proper footwear, glucose management, and injury prevention. The control group:
The intervention group:
Program Structure: Duration: 12 weeks (36 sessions, 3 per week). Session length: 20–30 min. Exercises included 39 types with 104 variations (progression via sublevels). Intensity progression was based on perceived effort and tailored to each user. Effort scores determined whether to progress, maintain, or revert levels. Weekly phone check-ins ensured adherence and addressed issues. Face-to-Face Support: The initial session included personalized guidance, SOPeD registration, and exercise kit distribution. Participants were advised to stop exercising if experiencing cramps, pain, or fatigue. Extended Program: After 12 weeks, participants were encouraged to continue the program remotely until week 24, with progress monitored via SOPeD. |
F—females; M—men; BMI—body mass index; DD—duration of diabetes; T2DM—type 2 diabetes mellitus; N with T2DM—number of individuals with type 2 diabetes mellitus; DPN—diabetic peripheral neuropathy; HbA1c—hemoglobin A1c; ADLs—activities of daily living; SOPeD—Sistema de Orientação ao Pé Diabético software (translation: Diabetic Foot Guidance System); FOCA I—FOotCAre Trial I (SOPeD intervention); FOCA II—FOotCAre Trial II (booklet intervention); RoM—range of motion; ABI—ankle–brachial index; DNE—diabetic neuropathy examination.
Several studies employed randomized controlled trials (RCTs) or prospective clinical trial designs. For instance, Iunes et al. [13] conducted a prospective trial involving 97 participants with T2DM, examining the impact of self-care guidelines and home exercises on lower limb plantar pressure and alignment over 10 months. Similarly, Sartor et al. [14] performed a 12-week RCT with 55 participants to evaluate the effects of strengthening, stretching, and functional exercises on foot rollover during walking, showing positive improvements in walking performance. Cerrahoglu et al. [15] also conducted an RCT on 76 participants to examine the effectiveness of a self-care home exercise program on joint RoM and plantar pressure distribution, incorporating motivational calls to encourage adherence over the 4-week intervention period.
Exercise programs in these studies typically included a combination of stretching, strengthening, range-of-motion exercises, and functional tasks. Common exercises incorporated dorsiflexion, plantarflexion, toe raises, balance training, and muscle strengthening activities, with varying durations and frequencies. Most interventions lasted between 4 and 12 weeks, with exercises performed 2 to 3 times per week. Many programs were home-based, with participants receiving detailed instructions through booklets, telephone support, or direct guidance from physical therapists. In some cases, participants were asked to track their progress in logs or receive motivational calls.
Other studies, such as Kuo et al. [16], focused on home-based rehabilitation for patients recovering from hip fractures, incorporating exercises on ankle dorsiflexion and proprioception over 12 months. Borges et al. [17] investigated the effects of proprioceptive training on postural control, gait, and functional tests in 80 participants, with exercises conducted at home and under supervision. Similarly, Silva et al. [18] explored the feasibility of a home-based foot–ankle exercise program for patients with DPN over 8 weeks, showing improvements in foot strength and functionality, with regular check-ins for support.
Suryani et al. [19] conducted a study to examine the effects of flexibility and resistance exercises in patients with recent plantar diabetic foot ulcers, with the primary aim of reducing ulcer recurrence and improving walking speed. The 12-week intervention involved patients performing exercises three times a week at home, with a focus on improving both the strength and flexibility of the foot and ankle. The study emphasized the importance of home-based exercise regimens, which have the potential to improve patient outcomes in managing diabetic foot ulcers. Highlighting clinical outcomes, such as ulcer recurrence, is crucial to understanding the effectiveness of these interventions in preventing further complications and improving long-term foot health in patients with diabetes. Additionally, Silva et al. [20] conducted the FOCA-II study, which focused on improving foot strength, functionality, and the management of DPN symptoms in 50 patients. This study utilized an 8-week home exercise protocol, with exercises tailored to strengthen the foot and improve overall functionality. Weekly check-ins were incorporated to monitor adherence and ensure proper exercise technique, further emphasizing the importance of ongoing support in achieving successful clinical outcomes. Both studies underscore the value of clinical outcomes such as ulcer recurrence and functional improvement in assessing the overall effectiveness of home exercise interventions for patients with diabetic foot complications.
Ferreira et al. [21] evaluated a 12-week web-based foot–ankle exercise program targeting DPN patients, showing effectiveness when combined with initial face-to-face support. The variety of approaches across these studies highlights the increasing interest in accessible, home-based methods for improving diabetic foot health and overall functionality.
In conclusion, these studies collectively demonstrate that home-based exercise programs, supported through different mediums, such as phone calls, web platforms, or in-person sessions, can significantly improve foot strength, balance, and overall function in individuals with DM, particularly those with DPN. The diversity in study methodologies, exercise types, and delivery methods underscores the potential benefits of such interventions for enhancing the health of diabetic patients, reducing complications, and improving quality of life.
3.2. Additional Details of Retrieved Articles
Table 3 provides an overview of nine studies that have examined adherence to exercise plans, dropout rates, and reported side effects or complications.


Table 3.
Overview of adherence to exercise plans, dropout rates, and reported side effects or complications.
Table 3.
Overview of adherence to exercise plans, dropout rates, and reported side effects or complications.
| Study/ Country/ Type of Study | Adherence to the Exercise Plan/ Dropout Rate | Side Effects/ Complications |
|---|---|---|
| Iunes et al. [13] Brazil Prospective, quasi-experimental clinical trial | On average, the individuals attended 5.12 ± 3.14 follow-up appointments during the monitoring period. However, 53.6% of the sample attended more than five follow-up sessions. | None of the individuals had any foot complications during the monitoring period. |
| Sartor et al. [14] Brazil Randomized, controlled trial | The dropout rate was 14.5%, with 4 participants leaving the intervention group and 4 from the control group. | There was no mention of side effects or complications. |
| Cerrahoglu et al. [15] Turkey Prospective, randomized controlled trial | Initially, 80 patients were included in the study. After 4 dropouts, 76 participants completed the first and second examinations after one month and were included in the statistical analysis. | There was no mention of side effects or complications. |
| Kuo et al. [16] Taiwan Secondary data analysis from a larger randomized controlled trial | Among all participants, adherence was highest before hospital discharge (31.7%) but gradually declined over 12 months post-discharge (17.7%). Patients adhered to more than 50% of the prescribed rehabilitation program. However, adherence to home-based rehabilitation dropped significantly after three months post-discharge. By the one-year follow-up, 62 participants remained in the study, 8 had passed away, and 18 were lost to follow-up (14 declined participation and 4 had relocated). | There was no mention of side effects or complications. |
| Borges et al. [17] Brazil Single-blind, randomized clinical trial | Adherence was high, with an average rate of 92.5%. | There was no mention of side effects or complications. |
| Silva et al. [18] Brazil Randomized controlled trial FOCA II (booklet intervention) | Adherence to the exercise program was 77.7%, with dropout rates of 11% in the intervention group and 9% in the control group. | There was no mention of side effects or complications. |
| Suryani et al. [19] Indonesia Double-blind, randomized clinical trial | Patients in the intervention group demonstrated excellent compliance. The dropout rate was 4 in the intervention group and 2 in the control group. | There was no mention of side effects or complications. In the intervention group, ulceration recurred in 2 patients (4%); in the control group, 17 patients (68%) experienced recurrence within 12 weeks. At 24 weeks, recurrence of plantar foot diabetic ulceration occurred in 4 patients (16%) in the intervention group and 18 patients (72%) in the control group. |
| Silva et al. [20] Brazil Randomized controlled clinical FOCA II (booklet intervention) | The intervention was simple to implement and demonstrated strong adherence, with a rate of 72%. |
|
| Ferreira et al. [21] Brazil Randomized controlled trial FOCA I (SOPeD intervention) | The compliance rate, indicating the percentage of the intervention group completing the exercise program 3 times a week for 12 weeks, was 41%. The completion rate, based on the SOPeD user bank, was 53%, and adherence at 12 weeks was 84%. Dropout rates were 19% in the intervention group (6 participants) and 16% in the control group (5 participants). | The 12-week web-based foot–ankle exercise program was feasible and well-accepted, demonstrating safety with minimal adverse events, including delayed-onset muscle soreness and foot muscle cramping. |
SOPeD—Sistema de Orientação ao Pé Diabético software (translation: Diabetic Foot Guidance System); FOCA II—FOotCAre Trial II (booklet intervention); FOCA I—FOotCAre Trial I (SOPeD intervention).
Iunes et al. [13] (Brazil) conducted a prospective, quasi-experimental clinical trial where participants attended an average of 5.12 follow-up sessions, with 53.6% of the sample attending more than 5 sessions. Notably, none of the individuals in the study experienced foot complications during the monitoring period. Sartor et al. [14] (Brazil) found a dropout rate of 14.5%, with four participants leaving each group (intervention and control), although there were no mentions of side effects or complications in their findings. Cerrahoglu et al. [15] (Turkey) conducted a prospective RCT with 80 patients. After 4 dropouts, 76 participants completed the study. The study did not report any side effects or complications.
Kuo et al. [16] (Taiwan) utilized secondary data analysis from a larger RCT. Their findings revealed that adherence was highest before hospital discharge (31.7%) but gradually declined to 17.7% after 12 months. By the one-year follow-up, 62 participants remained, while 8 had passed away and 18 were lost to follow-up. However, no side effects or complications were reported. Borges et al. [17] (Brazil) conducted a single-blind RCT with high adherence (92.5%), though dropout rates were not specified. There were also no reports of side effects or complications. Silva et al. [18] (Brazil) conducted an RCT as part of the FOCA II program, showing an adherence rate of 77.7%, with dropout rates of 9% to 11% in both the intervention and control groups. Again, no side effects or complications were mentioned.
Suryani et al. [19] (Indonesia) performed a double-blind RCT where patients in the intervention group demonstrated excellent compliance. The dropout rate was low, with only four participants dropping out from the intervention group and two from the control group. While no side effects were mentioned, the study noted recurrence rates of plantar foot diabetic ulceration. At 12 weeks, 4% of patients in the intervention group had recurrence, compared to 68% in the control group. At 24 weeks, the recurrence rate was 16% in the intervention group and 72% in the control group. Silva et al. [20] (Brazil) also conducted an RCT as part of the FOCA II program. The adherence rate was 72%, though dropout rates were not specified, and there were no mentions of side effects.
Lastly, Ferreira et al. [21] (Brazil) performed an RCT focusing on a 12-week web-based foot–ankle exercise program. The study showed a compliance rate of 41%, with 53% completion based on the user bank, and 84% adherence at 12 weeks. Dropout rates were 19% in the intervention group and 16% in the control group. The program was deemed feasible and well-accepted, with minimal adverse events, including delayed onset muscle soreness and foot muscle cramping.
In summary, the studies indicate varying adherence rates and dropout percentages, with some reporting excellent compliance, particularly in the Brazilian studies. While most studies did not report side effects or complications, a few, such as Suryani et al. [19] (Indonesia), noted the recurrence of foot ulceration as a complication; however, the recurrence rate of foot ulceration was reduced in the intervention group in comparison with the control group.
3.3. Results of Retrieved Articles
Table 4 includes previous studies analyzing various interventions for people with DPN using multiple measurement devices to assess outcomes related to foot health and function.


Table 4.
Overview of measurement devices, outcome measures, follow-up, and key results.
Table 4.
Overview of measurement devices, outcome measures, follow-up, and key results.
| Study/ Country/ Type of Study | Measure Devices | Outcome Measures and Follow-Up/ Results |
|---|---|---|
| Iunes et al. [13] Brazil Prospective, quasi-experimental clinical trial |
| All individuals had monthly follow-up examinations for 10 consecutive months. Health factors analyzed included sensitivity, circulation, risk rating, neuropathy score, ankle and foot alignment (photogrammetry), plantar pressures, and postural stability (baropodometry) before and after the guidelines and home exercises. The self-care guidelines and exercises led to improvements in the following:
|
| Sartor et al. [14] Brazil Randomized, controlled trial |
| Both groups were evaluated after 12 weeks. The intervention group was also evaluated after 24 weeks. Primary outcomes included changes in foot rollover during gait: peak pressure (PP). Secondary outcomes focused on time-to-peak pressure (TPP), pressure–time integral (PTI) across six-foot areas, center of pressure (CoP) velocity, ankle kinematics and kinetics, muscle function, and functional foot and ankle tests. The intervention group showed no significant change in PP, but improved heel strike (delayed heel TPP, p = 0.03), eccentric control of forefoot contact (decreased ankle extensor moment, p < 0.01; increased dorsiflexion, p < 0.05), earlier lateral forefoot contact (p < 0.01), and greater hallux (PP and PTI, p = 0.03) and toe participation (increased PTI, medium effect size). Slower CoP velocity (p = 0.05) and better foot and ankle function (p < 0.05) were also noted. Most values returned to baseline after follow-up (p < 0.05). The intervention subtly improved foot rollover toward a more physiological process, with better plantar pressure distribution and enhanced foot–ankle function. Continuous monitoring and patient education are vital to maintaining foot muscle and joint integrity in patients with polyneuropathy. |
| Cerrahoglu et al. [15] Turkey Prospective, randomized controlled trial | RoM Measurements:
| In the exercise group, significant improvements were observed in the RoM for the ankle and first metatarsophalangeal joints (p < 0.001). Static pedobarographic measurements revealed a significant reduction in right forefoot–medial pressure (p = 0.010). Dynamic pedobarographic values also showed significant decreases in peak plantar pressure in the left forefoot–medial (p = 0.007), right forefoot–lateral (p = 0.018), left midfoot (p < 0.001), and right hindfoot (p = 0.021) following the exercise intervention. No significant correlations were found between the neuropathy and non-neuropathy groups (p > 0.05). |
| Kuo et al. [16] Taiwan Secondary data analysis from a larger randomized controlled trial | RoM of Hip and Ankle Joints
Muscle Strength
| In the exercise group, significant improvements were observed in the RoM for the ankle and first metatarsophalangeal joints (p < 0.001). Static pedobarographic measurements showed a reduction in right forefoot–medial pressure (p = 0.010). Dynamic pedobarographic values revealed decreases in peak plantar pressure in the left forefoot–medial (p = 0.007), right forefoot–lateral (p = 0.018), left midfoot (p < 0.001), and right hindfoot (p = 0.021). No significant correlations were found between the neuropathy and non-neuropathy groups (p > 0.05). Patients received the intervention for one year, with outcome assessments at baseline, post-surgery, and at 1, 3, 6, 12, 18, and 24 months via home visits. Families kept weekly diaries. Adherence to home-based rehabilitation declined, but over 50% adhered. High-adherence patients showed better recovery, including improved ankle extension RoM, greater muscle strength, and better self-care abilities than the low-adherence group. |
| Borges et al. [17] Brazil Single-blind, randomized clinical trial | Tactile Sensitivity Test:
Test Procedures (each test was repeated three times, and the mean was calculated):
Vibratory Sensitivity Test:
Neuropathy Symptoms Score:
Physical Activity Assessment:
Postural Control Evaluation:
| Bipedal balance, gait, and functional performance were assessed before and after 12 weeks using the Balance Evaluation Systems Test (BESTest) and a force plate. The proposed proprioceptive training did not improve postural control in patients with type 2 diabetes who showed no clinical signs of diabetic distal polyneuropathy. This conclusion was based on analyses of bipedal stance, gait, and functional tests using the BESTest and force plate measurements. |
| Silva et al. [18] Brazil Randomized controlled trial FOCA II (booklet intervention) | Clinical and Biomechanical Assessments:
Kinematic Analysis:
DPN Symptoms:
Foot–Ankle RoM:
| Feasibility Outcomes: Recruitment spanned 20 weeks from 1310 individuals with diabetes. Of these, 1168 were contacted (89%), 323 (28%) met initial criteria, and 20 (6.2%) with moderate DPN (fuzzy score ≥ 2) were enrolled (1 participant/week). Adherence was 77.7%, with 7 of 9 participants completing at least 75% of sessions and 5 completing all. The average was 21.6 (±3.5) sessions. Two participants dropped out due to personal reasons and travel distance (CG: 9.1%, IG: 11.1%). Satisfaction was high (median: 4, IQR: 4–5); 44.1% rated it excellent, 51.9% great, and all would recommend the booklet. Safety was rated 3 (IQR: 3–5), with no adverse events. Exercise progression peaked at session 11, with 90% standing on both legs and 10% on one. By the final sessions, most exercised on one leg, averaging 25 ± 4.5 repetitions. Clinical and Biomechanical Outcomes: At baseline, both groups were similar in characteristics and outcomes. After 8 weeks, the intervention group showed a significant decrease in DPN severity, an increase in hallux relative to forefoot (first metatarsal joint) RoM, and a reduction in maximum forefoot-to-hindfoot dorsiflexion during gait. There was also a trend toward increased maximum hallux dorsiflexion relative to the forefoot, though this was not statistically significant. |
| Suryani et al. [19] Indonesia Double-blind, randomized clinical trial |
| There were significant differences in ulcer recurrence between groups:
|
| Silva et al. [20] Brazil Randomized controlled clinical trial FOCA II (booklet intervention) | Primary Outcomes:
Secondary Outcomes Clinical Variables:
|
|
| Ferreira et al. [21] Brazil Randomized controlled trial FOCA I (SOPeD intervention) | Primary Outcomes:
Secondary Outcomes:
Measurement Procedure:
| DPN Symptoms and Severity:
Plantar Pressure and Kinetics:
Ankle RoM:
|
FPP—FootWork Pressure Plate; SOPeD—Sistema de Orientação ao Pé Diabético software (translation: Diabetic Foot Guidance System); FOCA I—FOotCAre Trial I (SOPeD intervention); FOCA II—FOotCAre Trial II (booklet intervention); PP—peak pressure; TPP—time-to-peak pressure; PTI—pressure–time integral; CoP—center of pressure; p—probability value; RoM—range of motion; DPN—diabetic peripheral neuropathy; NDS—Neuropathy Disability Score; DNE—diabetic neuropathy examination; mCTSIB—Modified Clinical Test of Sensory Interaction on Balance; BESTest—Balance Evaluation Systems Test; IPAQ—International Physical Activity Questionnaire; CG—control group; IG—intervention group; IQR—interquartile range; RR—relative risk; CI—confidence interval; MNSI—Michigan Neuropathy Screening Instrument; FHSQ—Foot Health Status Questionnaire.
One study utilized podoscope and baropodometric pressure plates to assess plantar pressure, foot movement, and alignment. These devices helped measure the changes in foot alignment, body balance, and center of pressure (CoP) displacement, which significantly improved with the intervention. Results highlighted improvements in foot alignment and stability, particularly in the mediolateral direction, suggesting that home exercises contributed positively to preventing complications in diabetic patients [13]. Another study focused on kinematic analysis, using cameras and pressure plates to monitor foot rollover, ankle kinetics, and plantar pressure distribution during gait. The intervention group showed improved control during forefoot contact and better foot–ankle function, though some measures reverted after follow-up [14].
In other research, RoM was assessed by using manual goniometers, and dynamic walking assessments were performed on pressure platforms. These studies found significant improvements in the ankle and first metatarsophalangeal joint RoM following exercise interventions. Additionally, reductions in peak plantar pressures were observed across various foot areas, indicating that exercises effectively reduced pressure in the forefoot and hindfoot [15].
A different trial assessed tactile sensitivity and vibration perception using monofilaments and tuning forks, revealing improvements in plantar sensitivity among participants following the intervention [17]. Meanwhile, the analysis of gait speed and balance, using tools like the BESTest and a force plate, showed no significant postural control improvements despite interventions, suggesting the complexity of managing neuropathy symptoms in the absence of overt signs of DPN [19].
The most notable outcome across these studies was the improvement in foot function, muscle strength, and plantar pressure distribution, as well as the enhancement of ankle RoM, which was often observed in the intervention groups. However, some measures returned to baseline after follow-up, emphasizing the need for continuous management and monitoring [14,15,17].
Figure 2 provides a visual summary of the relative effectiveness of each intervention across four key clinical outcome domains. The chart highlights that studies such as those by Cerrahoglu et al. [15], Silva et al. [18], and Ferreira et al. [21] demonstrated higher overall effectiveness, particularly in foot biomechanics and plantar pressure reduction. Suryani et al. [19] showed strong performance in ulcer recurrence prevention. Conversely, interventions like those by Kuo et al. [16] and Borges et al. [17] exhibited comparatively lower scores, especially in balance and ulcer prevention. This chart complements the narrative synthesis by enabling a clear, at-a-glance comparison of the interventions’ multidimensional impacts.
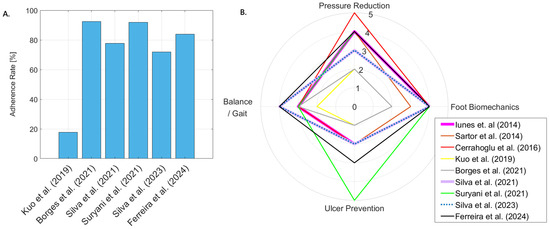
Figure 2.
(A). Adherence rates (%) for home-based lower limb exercise interventions reported in the reviewed studies [16,17,18,19,20,21] (B). Comparative effectiveness of interventions based on outcomes, including foot biomechanics, plantar pressure, gait/balance, and ulcer recurrence. Each intervention is scored on a 1–5 scale based on a qualitative synthesis of the results reported in the reviewed studies [13,14,15,16,17,18,19,20,21].
4. Discussion
This scoping review aimed to comprehensively explore and synthesize the available evidence on home-based lower limb exercise programs designed for individuals with diabetes, focusing on their role in the secondary prevention of DFUs. This review sought to systematically map the types of home-based exercises that have been implemented; assess their feasibility, acceptability, and adherence among individuals with diabetes; and evaluate their effectiveness in enhancing foot biomechanics, improving balance, and reducing plantar pressure distribution. Additionally, this review aimed to determine whether such interventions contributed to a reduction in ulcer recurrence. By identifying existing research gaps, methodological challenges, and limitations in the current body of literature, this scoping review provided valuable insights to inform the design of future studies and guide the development of more effective, evidence-based home exercise programs for diabetic foot ulcer prevention.
4.1. Validation and Implementation of Remote Exercise Programs for Individuals with Diabetes
The relatively narrow scope of included studies—9 out of more than 500 initially identified—may raise concerns regarding the perceived significance of home-based lower limb exercise programs within the broader context of diabetes research. This limitation is primarily a reflection of the current scarcity of high-quality, focused studies in this specific area, rather than an indication of the topic’s lack of relevance. Although global diabetes management often emphasizes glycemic control and pharmacological interventions, diabetic foot ulcers remain a leading cause of morbidity, amputation, and healthcare burden. Consequently, the potential of low-cost, accessible strategies such as home-based exercise programs for secondary prevention should not be underestimated. Before introducing remote exercise programs for individuals with DM or DPN, researchers used the Delphi technique to validate these interventions among experts and patients. This method has supported the development and usability assessment of educational booklets and web-based software applications for desktop and mobile platforms [21]. Such studies offer valuable insights into ensuring the safety and acceptability of home-based exercise programs for both healthcare professionals and end-users.
Some studies specifically focused on using the Delphi technique to establish a structured approach before clinical implementation. For instance, Ferreira et al. [21] detailed the validation and usability evaluation of a web-based software [22] and an educational booklet [23]. These efforts highlight the importance of expert and patient involvement in refining home-based exercise programs.
Several studies were excluded from this review, as they primarily described study protocols rather than the direct application of exercise interventions. These included research outlining the development of RCTs assessing the effects of an educational booklet [24] and the Diabetic Foot Guidance System (SOPeD) software [25]. One feasibility study, though not included, evaluated an internet-based foot–ankle exercise program using SOPeD [26], involving 14 participants to determine its scalability. Additionally, an economic evaluation of SOPeD [27] examined its cost-effectiveness in an RCT with 62 participants [21]. The results suggested that SOPeD could be a cost-effective tool for improving foot pain, foot function, and DPN symptoms, with cost-effectiveness probabilities of 98.7%, 82.5%, and 77.6%, respectively. However, no significant cost–utility benefit was observed for quality-adjusted life years (QALYs). These studies reinforce the necessity of validating remote exercise interventions while considering their economic viability.
Other research, also not included in this review, explored hybrid exercise interventions that combined remote, unsupervised home-based exercises with in-person supervision. In some studies, participants engaged in twice-weekly supervised physiotherapy sessions alongside twice-weekly remote exercises using SOPeD [28,29]. The findings indicated improvements in hip extension at push-off, plantar arch height, and plantar pressure distribution during gait, demonstrating the potential benefits of integrating supervised and remote exercise strategies for individuals with diabetes.
Although these studies were not included in the final synthesis due to methodological scope, they provide valuable context regarding the development, validation, feasibility, and cost-effectiveness of home-based lower limb exercise programs. The inclusion of such supporting evidence reinforces the relevance of the topic and highlights the need for continued investigation in this underexplored yet clinically significant domain.
4.2. Types of Home-Based Exercises Included in the Review
The studies included in this review primarily focused on structured lower limb exercise programs designed to prevent DFUs. While most research on exercise therapy for individuals with diabetes emphasizes general physical activity, such as aerobic and resistance training to enhance insulin sensitivity and cardiovascular health, this review specifically examined interventions targeting foot biomechanics, muscle strength, and proprioception.
Exercise programs varied in duration, ranging from a minimum of 4 weeks [15] to a maximum of 12 months [16], with follow-up assessments conducted between 4 weeks and 24 months to evaluate both short- and long-term effects. Exercise sessions typically lasted between 20 and 60 min and were performed two or three times per week at a time and place convenient for participants. In addition to exercise prescriptions, most studies incorporated self-care education through written or verbal instructions, aiming to increase awareness of potential complications and encourage early reporting of symptoms. Some participants also received exercise kits containing materials such as cotton balls, pencils, and massage balls to help them perform the exercises correctly [17,20,21]. Among the nine studies analyzed, six provided detailed exercise protocols that included a warm-up and a structured exercise regimen, and two studies introduced a cool-down phase.
The warm-up phase, lasting between five and ten minutes, consisted of one to three exercises, such as walking, seated foot tapping, leg stretching, massaging the sole in circular motions, rolling a ball underfoot, or gently twisting toes [15,17,18,19,20,21].
The main exercise component incorporated various movements to improve mobility, strength, proprioception, and balance. Ankle mobility exercises included passive and active dorsiflexion and plantarflexion; eversion and inversion; subtalar joint pronation and supination; and walking on different foot surfaces, such as the heel, forefoot, and lateral or medial borders of the foot [14,15,18,20,21].
Toe and intrinsic foot muscle-strengthening activities included grasping objects with the toes, moving a cloth underfoot, toe extensions and raises, spreading, and walking with toe-gripping motions [13,14,18,20,21].
Some exercises focused on proprioception training, such as double-leg support on a rubber disc, and rolling a curly ball under the foot to enhance sensory feedback and stability [13,14].
Calf muscle-strengthening exercises included standing on tiptoes and lowering slowly, as well as self-stretching techniques for the triceps surae muscle, such as standing with one foot forward, the knee bent, and the opposite leg extended back with the heel in contact with the ground [13,15,18,20,21].
Balance and coordination exercises involved isometric knee extension, one-leg stance, forefoot stamping, tandem walking, and controlled heel-to-toe foot rollover during gait to promote functional stability [14,16].
Some studies (three out of nine) also included additional movements, such as foot circumduction, drawing the alphabet with the feet, and sliding feet on the floor [13,15,17]. Others (two out of nine) incorporated hip exercises, including flexion, extension, abduction, adduction, and circular hip motions in a standing position to support overall lower limb function [13,17]. The cool-down phase, lasting around five minutes, consisted of relaxation techniques such as deep-breathing exercises and slow foot movements, aimed at reducing muscle tension and promoting circulation [17,19].
Although specific exercise variations and progressions differed across studies, the reviewed interventions consistently emphasized simplicity, accessibility, and ease of adherence. Some studies allowed exercise difficulty to be tailored to individual capabilities, ensuring that participants could gradually increase intensity based on their functional status [18,20,21]. These findings highlight the structured nature of home-based lower limb exercise programs for individuals with diabetes and their potential role in improving foot biomechanics, muscle function, and overall lower limb health. Future research should further explore the long-term effectiveness of these interventions and optimize strategies to enhance adherence and patient outcomes.
4.3. Feasibility, Acceptability, and Adherence to Home-Based Exercise Programs
The effectiveness of home-based and remotely supervised exercise programs for individuals with diabetes has been demonstrated in various studies. Akinci et al. [30] conducted an RCT comparing supervised group exercises with internet-based home programs. Both interventions significantly improved glycemic control, functional capacity, and quality of life, with no major differences in effectiveness between the two methods. Similarly, Dadgostar et al. [31] assessed supervised versus home-based exercise interventions for women with T2DM. Their findings indicated that while supervised training led to better health-related quality of life outcomes, home-based exercises were still effective in improving glycemic control, body composition, and lipid profiles. These studies confirm the potential of structured home exercise programs to enhance health outcomes in individuals with T2DM.
Despite these benefits, adherence to remote exercise interventions remains a challenge. Some studies incorporated adherence-enhancing strategies, such as checking exercise logbooks every three weeks or conducting weekly follow-up phone calls [18,22]. In contrast, others provided no interim supervision, even in long-term interventions spanning up to 10 months [28]. Lack of ongoing support can hinder engagement and reduce the effectiveness of these interventions. Research suggests that structured guidance, including exercise booklets, mobile applications, and telehealth support, can improve adherence and ensure program continuity [17,18,19].
Technology use in diabetes care is emerging as a promising tool to support remote exercise programs. Mobile health (mHealth) applications have been shown to enhance motivation and patient engagement by providing interactive guidance and real-time monitoring [32]. Web-based recruitment methods for digital exercise programs also demonstrate the potential to increase access to high-risk populations, enabling better participation in clinical trials and structured interventions. Moreover, the inclusion of self-determined motivation strategies can help patients overcome perceived barriers to exercise, further supporting long-term adherence [33].
In addition to digital solutions, qualitative research highlights the importance of addressing practical barriers to exercise participation. Sharifi et al. [34] identified key factors influencing non-participation in diabetes self-care programs, including accessibility, quality of education, and the need for flexible, distance-learning options. By offering modern training methods that combine virtual education with individualized support, adherence to exercise programs can be improved.
Several studies have employed expert validation methods, such as the Delphi technique, to refine remote exercise therapy programs before implementation. This approach has been used to develop educational booklets and web-based software applications that ensure the safety, feasibility, and acceptability of home-based interventions [18,22]. Although certain feasibility studies on internet-based foot and ankle exercise programs, such as SOPeD, were excluded from this review, they provide valuable insights into scalable intervention models for future research [28,29].
Overall, home-based exercise programs for individuals with diabetes show promise in improving glycemic control and functional outcomes. However, adherence remains a critical factor in their long-term success. Future interventions should incorporate structured supervision, technological support, and tailored motivational strategies to enhance patient engagement and ensure sustainable benefits.
4.4. Evaluation of the Effectiveness in Improving Foot Biomechanics and Reducing Ulcer Recurrence
Hyperglycemia-induced oxidative stress, impaired nerve regeneration, and vascular complications significantly impact neuromuscular integrity, leading to muscle mass loss and increased susceptibility to sarcopenia. Studies have highlighted that individuals with T2DM, regardless of age, experience muscle weakness and physical function decline, particularly in men [35,36]. This muscle deterioration contributes to biomechanical alterations that increase the risk of DFUs. Diabetes and diabetic peripheral neuropathy result in reduced lower limb muscle strength. Research utilizing handheld dynamometry has demonstrated diminished strength in various foot and ankle movements in diabetic patients [37]. Additionally, LJM exacerbates DFU risk by increasing plantar pressure, prolonging the stance phase during gait, and altering joint kinematics [6,38].
Exercise interventions targeting foot–ankle flexibility and resistance training have shown promising results in reducing DFU recurrence. A study assessing lower limb exercises for DFU secondary prevention demonstrated a 72% reduction in ulcer recurrence within 12 weeks and a 78% reduction within 24 weeks, with no reported adverse effects [17]. Similar findings were reported in an interventional exercise program, which improved muscle strength and body flexibility in diabetic patients in remission [39]. Furthermore, weight-bearing activities have been deemed safe for diabetic individuals with DPN with proper assessment and counseling [40]. High-intensity interval training (HIIT) has emerged as an effective intervention. Research analyzing one-legged HIIT found that it significantly improved skeletal muscle insulin sensitivity, with a ~30% increase in insulin-stimulated glucose clearance in the trained leg compared to the untrained leg [41]. This suggests that localized lower limb exercises can substantially enhance metabolic health in diabetes patients. Exercise training has also been recognized as a key strategy in counteracting the neuromuscular decline in DPN, effectively alleviating sensory–motor deficits [19,38].
Despite these benefits, adherence to exercise programs remains a challenge. Structured interventions incorporating remote monitoring tools, such as exercise booklets, mobile applications, and phone follow-ups, have reported higher adherence rates [24,25]. Conversely, interventions lacking reinforcement mechanisms showed lower adherence, underscoring the importance of continuous engagement strategies. Additionally, research indicates that self-reported activity logs and supervised initial training sessions lead to better adherence compared to written instructions alone [17,18].
Only a few studies monitored ulcer recurrence as a primary clinical outcome, despite its critical relevance in evaluating long-term effectiveness. Future research should prioritize ulcer recurrence alongside functional metrics to improve clinical decision-making and risk stratification.
Overall, structured exercise programs focusing on foot biomechanics and lower limb strength play a critical role in reducing DFU recurrence and improving functional outcomes. The integration of targeted resistance training, flexibility exercises, and HIIT into diabetes management strategies offers a promising approach to mitigating neuromuscular decline and enhancing quality of life for individuals with diabetes and DPN. In addition to standardizing exercise protocols and improving long-term adherence, future studies are needed to determine which specific types and intensities of exercise are most effective for the secondary prevention of diabetic foot ulcers. These investigations should aim to establish evidence-based guidelines for exercise timing, dosage, and intensity, enabling the development of personalized strategies tailored to individual patient needs. Factors such as diabetes severity, age, baseline physical activity level, and comorbid conditions should be considered to optimize safety, engagement, and clinical outcomes.
4.5. Gaps in the Literature and Future Research Directions
The field of home-based lower limb exercises for individuals with diabetes, particularly in secondary prevention of DFUs, remains underexplored. While there is a growing interest in exercise interventions, few studies specifically focus on their effectiveness in preventing DFU recurrence, a critical concern given the chronic and progressive nature of diabetes-related foot complications. Home-based exercise programs, particularly those targeting RoM improvement and reducing peak plantar pressures, are essential components for secondary DFU prevention. However, the literature reveals a distinct gap in examining the long-term benefits and effectiveness of these exercises.
One notable gap is the absence of standardized protocols for home-based lower limb exercises in diabetic populations. Existing studies employ varied exercise regimens, from basic mobility exercises to complex resistance training. This diversity complicates comparisons and makes it difficult to draw firm conclusions regarding the optimal exercise intervention. Future research must focus on developing and validating standardized exercise protocols tailored to individuals with diabetes, accounting for varying levels of DPN severity. Establishing such protocols would allow for more robust comparisons and a clearer understanding of the most effective exercise interventions for DFU prevention.
Another significant gap is the short duration of most studies in this area. While some studies have demonstrated benefits from home-based exercises over 4–12-week periods, very few extend follow-up beyond six months. Given the chronic nature of diabetes and its associated complications, longer-term follow-up is essential to assess whether exercise interventions yield sustainable benefits in terms of preventing DFU recurrence. Research that examines long-term outcomes, ideally over 12 months or more, would provide more valuable insights into the lasting impact of home-based exercises on foot health and overall diabetes management.
Furthermore, while many studies focus on functional and biomechanical outcomes, few specifically investigate the impact of home-based exercise interventions on ulcer recurrence as a primary outcome. Only a limited number of studies, such as Suryani et al. [19], have directly assessed ulcer recurrence rates in response to exercise interventions, and they reported significantly lower recurrence in the intervention group. Expanding this line of inquiry is essential for understanding how exercise affects not only functional and biomechanical measures but also clinically relevant outcomes like DFU recurrence. Future research should prioritize ulcer recurrence as a primary outcome to better assess the direct impact of exercise interventions on preventing foot complications in individuals with diabetes.
Technological advancements also present an important opportunity to enhance the effectiveness and engagement of home-based exercise programs. Mobile health (mHealth) applications, wearable sensors, and real-time feedback mechanisms are underutilized in the current literature but have great potential to support patients’ self-management behaviors. Integrating these technologies into home-based exercise interventions could significantly improve adherence, provide personalized recommendations, and facilitate remote monitoring by healthcare providers. The incorporation of AI-driven exercise monitoring could also offer tailored exercise adjustments, ensuring safety and efficacy for individuals with varying levels of physical ability and disease severity. As such, future research should explore the integration of these technologies to enhance patient engagement and optimize clinical outcomes.
Additionally, there is a growing trend toward remotely delivered exercise programs, which gained momentum during the COVID-19 pandemic. These programs have the potential to reach a large number of individuals with diabetes, offering scalable, accessible interventions that may be more cost-effective and convenient than traditional in-person therapies. With the increasing prevalence of diabetes, there is a pressing need for preventive care strategies that can be widely implemented. Remote exercise programs, supported by digital tools, could be an effective solution to meet this demand. Future research should investigate how remote delivery of lower limb exercise programs can be optimized to improve patient outcomes and expand access to preventive care.
Lastly, early screening and biomechanical assessment of diabetic patients are crucial in preventing foot-related complications, including DFUs. Targeted exercise interventions aimed at improving muscle strength, gait parameters, and overall lower limb function have the potential to reduce the risk of foot ulcers. However, more studies are needed to explore the role of early detection of biomechanical abnormalities and how targeted exercises can improve these parameters over time. Implementing a holistic approach that includes early screening, exercise, and technological support could enhance managing diabetes-related foot complications.
5. Conclusions
In conclusion, the current literature on home-based lower limb exercises for the secondary prevention of DFUs is limited by a lack of standardized protocols, short study durations, and a focus on functional outcomes rather than ulcer recurrence. Future research should prioritize the development of standardized exercise regimens, referencing the availability of tools such as SOPeD and educational booklets used in some studies; investigate long-term effects; and incorporate ulcer recurrence as a primary outcome. Additionally, the role of longitudinal follow-up in assessing the clinical sustainability of home-based exercises should be more clearly explained; integrating digital health tools and remote exercise delivery presents exciting opportunities to improve patient engagement and self-management, ultimately reducing the risk of DFUs and improving overall diabetes care.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/app15084552/s1, Description of the exercises/interventions used in each of the papers included in this review. Table S1: Outline of steps and time required for at-home and guided training sessions. Table S2: Outline of steps and time required for at-home and guided training sessions. Table S3: Outline of steps and time required for at-home and guided training sessions.
Author Contributions
Conceptualization, S.B. and M.B.; methodology, S.B., P.C., J.W. and M.B.; software, S.B. and M.B.; validation, S.B., P.C. and M.B.; formal analysis, S.B. and T.R.; investigation, S.B., P.C. and M.B.; resources, S.B., P.C. and M.B.; data curation, S.B. and M.B.; writing—original draft preparation, S.B.; writing—review and editing, S.B. and T.R.; visualization, S.B. and M.B.; supervision, P.C., T.R. and J.W.; project administration, S.B., P.C. and M.B.; funding acquisition, J.W. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed toward the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.W. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef]
- Francia, P.; Gulisano, M.; Anichini, R.; Seghieri, G. Diabetic foot and exercise therapy: Step by step the role of rigid posture and biomechanics treatment. Curr. Diabetes Rev. 2014, 10, 86–99. [Google Scholar] [CrossRef]
- Fife, C.E.; Eckert, K.A.; Carter, M.J. Publicly Reported Wound Healing Rates: The Fantasy and the Reality. Adv. Wound Care 2018, 7, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Sacco, I.C.N.; Monteiro-Soares, M.; Raspovic, A.; Paton, J.; Rasmussen, A.; Lavery, L.A.; van Netten, J.J. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2023 update). Diabetes/Metab. Res. Rev. 2024, 40, e3651. [Google Scholar] [CrossRef]
- van Netten, J.J.; Sacco, I.C.N.; Lavery, L.; Monteiro-Soares, M.; Paton, J.; Rasmussen, A.; Raspovic, A.; Bus, S.A. Clinical and biomechanical effectiveness of foot-ankle exercise programs and weight-bearing activity in people with diabetes and neuropathy: A systematic review and meta-analysis. Diabetes/Metab. Res. Rev. 2024, 40, e3649. [Google Scholar] [CrossRef] [PubMed]
- Hazari, A.; Maiya, A.G.; Shivashankara, K.N.; Agouris, I.; Monteiro, A.; Jadhav, R.; Kumar, S.; Shashi Kumar, C.G.; Mayya, S.S. Kinetics and kinematics of diabetic foot in type 2 diabetes mellitus with and without peripheral neuropathy: A systematic review and meta-analysis. SpringerPlus 2016, 5, 1819. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, S.; Zhang, H.; Sun, H.; Hu, J. Gait Parameters and Peripheral Neuropathy in Patients With Diabetes: A Meta-Analysis. Front. Endocrinol. 2022, 13, 891356. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Iunes, D.H.; Rocha, C.B.; Borges, N.C.; Marcon, C.O.; Pereira, V.M.; Carvalho, L.C. Self-care associated with home exercises in patients with type 2 diabetes mellitus. PLoS ONE 2014, 9, e114151. [Google Scholar] [CrossRef]
- Sartor, C.D.; Hasue, R.H.; Cacciari, L.P.; Butugan, M.K.; Watari, R.; Pássaro, A.C.; Giacomozzi, C.; Sacco, I.C. Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: Results of a randomized controlled trial. BMC Musculoskelet. Disord. 2014, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Cerrahoglu, L.; Koşan, U.; Sirin, T.C.; Ulusoy, A. Range of Motion and Plantar Pressure Evaluation for the Effects of Self-Care Foot Exercises on Diabetic Patients with and Without Neuropathy. J. Am. Podiatr. Med. Assoc. 2016, 106, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.Y.; Shyu, Y.L.; Wang, J.S.; Chen, M.C.; Wu, C.C.; Chen, M.L. Adherence to Home-Based Rehabilitation in Older Adults with Diabetes After Hip Fracture. Nurs. Res. 2019, 68, 383–389. [Google Scholar] [CrossRef]
- Borges, N.C.S.; Pletsch, A.H.M.; Buzato, M.B.; Terada, N.A.Y.; Cruz, F.; Guirro, R.R.J. The effect of proprioceptive training on postural control in people with diabetes: A randomized clinical trial comparing delivery at home, under supervision, or no training. Clin. Rehabil. 2021, 35, 988–998. [Google Scholar] [CrossRef]
- Silva, É.Q.; Santos, D.P.; Beteli, R.I.; Monteiro, R.L.; Ferreira, J.S.S.P.; Cruvinel-Junior, R.H.; Donini, A.; Verissímo, J.L.; Suda, E.Y.; Sacco, I.C.N. Feasibility of a home-based foot–ankle exercise programme for musculoskeletal dysfunctions in people with diabetes: Randomised controlled FOotCAre (FOCA) Trial II. Sci. Rep. 2021, 11, 12404. [Google Scholar] [CrossRef]
- Suryani, M.; Samekto, W.; Heri, N.; Susanto, H.; Dwiantoro, L. Effect of foot-ankle flexibility and resistance exercise in the secondary prevention of plantar foot diabetic ulcer. J. Diabetes Complicat. 2021, 35, 107968. [Google Scholar] [CrossRef]
- Silva, É.Q.; Veríssimo, J.L.; Ferreira, J.S.S.P.; Cruvinel-Júnior, R.H.; Monteiro, R.L.; Suda, E.Y.; Sacco, I.C.N. Effects of a Home-Based Foot–Ankle Exercise Program with Educational Booklet for Foot Dysfunctions in People with Diabetic Neuropathy: Results of the FOCA-II Randomized Controlled Clinical Trial. Appl. Sci. 2023, 13, 1423. [Google Scholar] [CrossRef]
- Ferreira, J.; Cruvinel-Júnior, R.H.; da Silva, E.Q.; Veríssimo, J.L.; Monteiro, R.L.; Duarte, M.; Giacomozzi, C.; Sacco, I.C.N. Effectiveness of a web-based foot-ankle exercise program for treating ulcer risk factors in diabetic neuropathy in a randomized controlled trial. Sci. Rep. 2024, 14, 27291. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Sacco, I.C.N.; Siqueira, A.A.; Almeida, M.H.M.; Sartor, C.D. Rehabilitation technology for self-care: Customised foot and ankle exercise software for people with diabetes. PLoS ONE 2019, 14, e0218560. [Google Scholar] [CrossRef] [PubMed]
- Veríssimo, J.L.; Sacco, I.C.N.; Almeida, M.H.M.d.; Sartor, C.D.; Suda, E.Y. Development of a customized booklet of foot-ankle exercises for people with diabetes mellitus as a management and prevention tool for musculoskeletal complications. Braz. J. Phys. Ther. 2022, 26, 100402. [Google Scholar] [CrossRef]
- Silva, E.Q.; Suda, E.Y.; Santos, D.P.; Veríssimo, J.L.; Ferreira, J.S.S.P.; Cruvinel Júnior, R.H.; Monteiro, R.L.; Sartor, C.D.; Sacco, I.C.N. Effect of an educational booklet for prevention and treatment of foot musculoskeletal dysfunctions in people with diabetic neuropathy: The FOotCAre (FOCA) trial II, a study protocol of a randomized controlled trial. Trials 2020, 21, 180. [Google Scholar] [CrossRef]
- Ferreira, J.; Cruvinel Junior, R.H.; Silva, E.Q.; Veríssimo, J.L.; Monteiro, R.L.; Pereira, D.S.; Suda, E.Y.; Sartor, C.D.; Sacco, I.C.N. Study protocol for a randomized controlled trial on the effect of the Diabetic Foot Guidance System (SOPeD) for the prevention and treatment of foot musculoskeletal dysfunctions in people with diabetic neuropathy: The FOotCAre (FOCA) trial I. Trials 2020, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Cruvinel Júnior, R.H.; Ferreira, J.; Beteli, R.I.; Silva, É.Q.; Veríssimo, J.L.; Monteiro, R.L.; Suda, E.Y.; Sacco, I.C.N. Foot-ankle functional outcomes of using the Diabetic Foot Guidance System (SOPeD) for people with diabetic neuropathy: A feasibility study for the single-blind randomized controlled FOotCAre (FOCA) trial I. Pilot Feasibility Stud. 2021, 7, 87. [Google Scholar] [CrossRef]
- Cruvinel-Júnior, R.H.; Ferreira, J.; Veríssimo, J.L.; Monteiro, R.L.; Silva, É.Q.; Suda, E.Y.; Sacco, I.C.N. Affordable web-based foot-ankle exercise program proves effective for diabetic foot care in a randomized controlled trial with economic evaluation. Sci. Rep. 2024, 14, 16094. [Google Scholar] [CrossRef]
- Monteiro, R.L.; Ferreira, J.; Silva, É.Q.; Cruvinel-Júnior, R.H.; Veríssimo, J.L.; Bus, S.A.; Sacco, I.C.N. Foot-ankle therapeutic exercise program can improve gait speed in people with diabetic neuropathy: A randomized controlled trial. Sci. Rep. 2022, 12, 7561. [Google Scholar] [CrossRef]
- Monteiro, R.L.; Ferreira, J.S.S.P.; Silva, É.Q.; Cruvinel-Júnior, R.H.; Veríssimo, J.L.; Bus, S.A.; Sacco, I.C.N. Effects of foot-ankle exercises on foot-ankle kinematics, plantar pressure, and gait kinetics in people with diabetic neuropathy: Secondary outcomes from a randomized controlled trial. Braz. J. Phys. Ther. 2023, 27, 100517. [Google Scholar] [CrossRef]
- Akinci, B.; Yeldan, I.; Satman, I.; Dirican, A.; Ozdincler, A.R. The effects of Internet-based exercise compared with supervised group exercise in people with type 2 diabetes: A randomized controlled study. Clin. Rehabil. 2018, 32, 799–810. [Google Scholar] [CrossRef]
- Dadgostar, H.; Firouzinezhad, S.; Ansari, M.; Younespour, S.; Mahmoudpour, A.; Khamseh, M.E. Supervised group-exercise therapy versus home-based exercise therapy: Their effects on Quality of Life and cardiovascular risk factors in women with type 2 diabetes. Diabetes Metab. Syndr. 2016, 10, S30–S36. [Google Scholar] [CrossRef]
- Ash, G.I.; Griggs, S.; Nally, L.M.; Stults-Kolehmainen, M.; Jeon, S.; Brandt, C.; Gulanski, B.I.; Spanakis, E.K.; Baker, J.S.; Whittemore, R.; et al. Evaluation of Web-Based and In-Person Methods to Recruit Adults with Type 1 Diabetes for a Mobile Exercise Intervention: Prospective Observational Study. JMIR Diabetes 2021, 6, e28309. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Wang, J.C.K.; Burns, S.F.; Leow, M.K. Is Self-Determined Motivation a Useful Agent to Overcome Perceived Exercise Barriers in Patients with Type 2 Diabetes Mellitus? Front. Psychol. 2021, 12, 627815. [Google Scholar] [CrossRef]
- Sharifi, T.; Javan-Noughabi, J.; Asadi, Z.; Zarqi, M. Reasons for non-participation in a self-care training program for diabetic patients: A qualitative study. BMC Health Serv. Res. 2022, 22, 127. [Google Scholar] [CrossRef] [PubMed]
- Naruse, A.; Yamada, Y.; Miyamoto, T. Skeletal Muscle Mass Loss and Physical Function in Young to Middle-Aged Adult Patients With Diabetes: Cross-Sectional Observational Study. Interact. J. Med. Res. 2024, 13, e58038. [Google Scholar] [CrossRef] [PubMed]
- Pechmann, L.M.; Jonasson, T.H.; Canossa, V.S.; Trierweiler, H.; Kisielewicz, G.; Petterle, R.R.; Moreira, C.A.; Borba, V.Z.C. Sarcopenia in Type 2 Diabetes Mellitus: A Cross-Sectional Observational Study. Int. J. Endocrinol. 2020, 2020, 7841390. [Google Scholar] [CrossRef]
- Błażkiewicz, M.; Sundar, L.; Healy, A.; Ramachandran, A.; Chockalingam, N.; Naemi, R. Assessment of lower leg muscle force distribution during isometric ankle dorsi and plantar flexion in patients with diabetes: A preliminary study. J. Diabetes Complicat. 2015, 29, 282–287. [Google Scholar] [CrossRef]
- Lecce, E.; Bellini, A.; Greco, G.; Martire, F.; Palumbo, A.; Sacchetti, M.; Bazzucchi, I. Physiological mechanisms of neuromuscular impairment in diabetes-related complications: Can physical exercise help prevent it? J. Physiol. 2025. [Google Scholar] [CrossRef]
- Vrátná, E.; Husáková, J.; Jarošíková, R.; Dubský, M.; Wosková, V.; Bém, R.; Jirkovská, A.; Králová, K.; Pyšková, B.; Lánská, V.; et al. Effects of a 12-Week Interventional Exercise Programme on Muscle Strength, Mobility and Fitness in Patients with Diabetic Foot in Remission: Results from BIONEDIAN Randomised Controlled Trial. Front. Endocrinol. 2022, 13, 869128. [Google Scholar] [CrossRef]
- Lemaster, J.W.; Mueller, M.J.; Reiber, G.E.; Mehr, D.R.; Madsen, R.W.; Conn, V.S. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet first randomized controlled trial. Phys. Ther. 2008, 88, 1385–1398. [Google Scholar] [CrossRef]
- Dela, F.; Ingersen, A.; Andersen, N.B.; Nielsen, M.B.; Petersen, H.H.H.; Hansen, C.N.; Larsen, S.; Wojtaszewski, J.; Helge, J.W. Effects of one-legged high-intensity interval training on insulin-mediated skeletal muscle glucose homeostasis in patients with type 2 diabetes. Acta Physiol. 2019, 226, e13245. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).