Research on the Thermal Conductivity and Microstructure of Calcium Lignosulfonate-Magnesium Oxide Solidified Loess
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Experimental Tests
2.2.1. Sample Preparation Plan
2.2.2. Testing Methods
3. Results and Discussion
3.1. Basic Physical Properties Test
3.1.1. Atterberg Limits
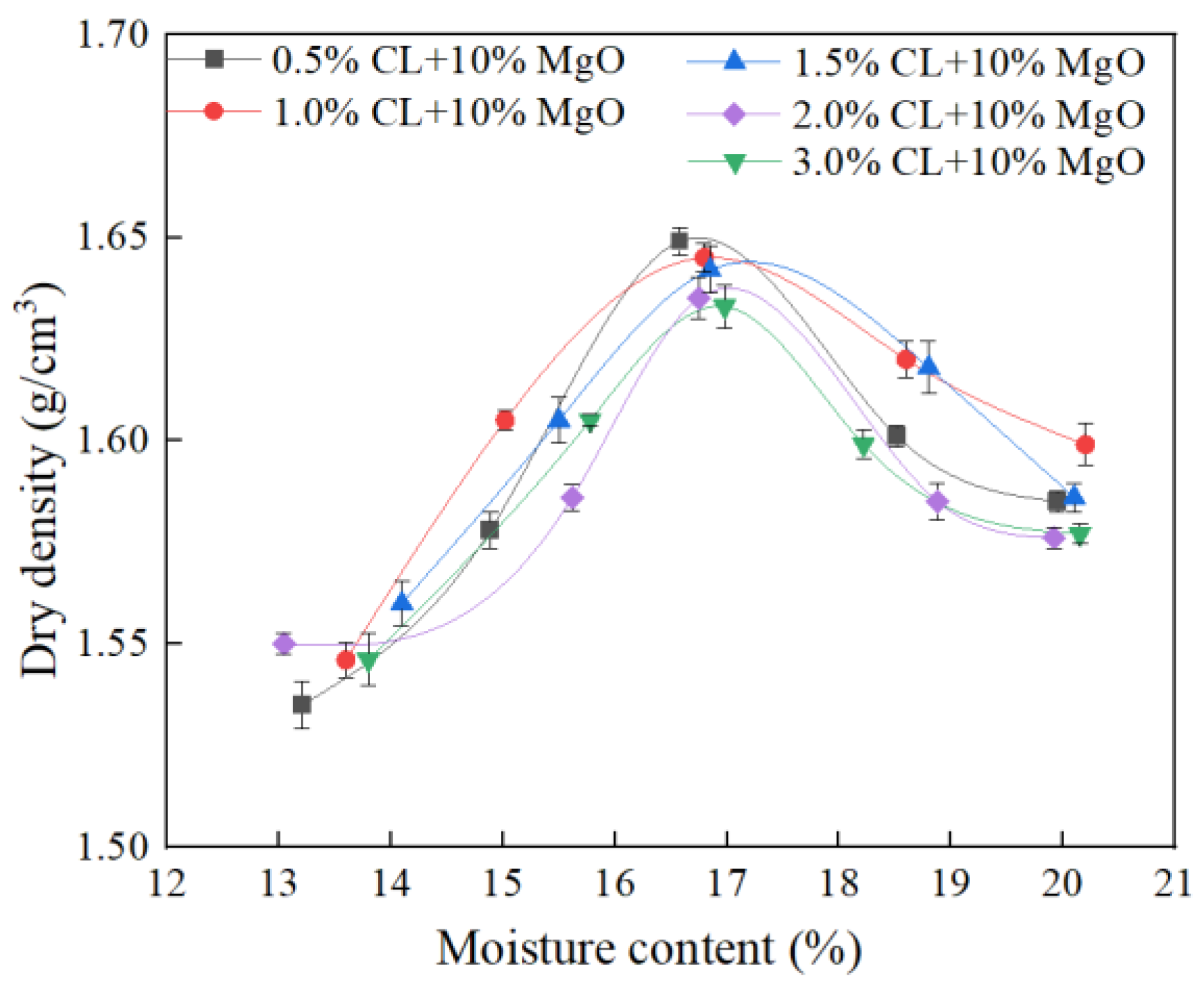
3.1.2. Compaction Curves
3.1.3. Collapsibility Test
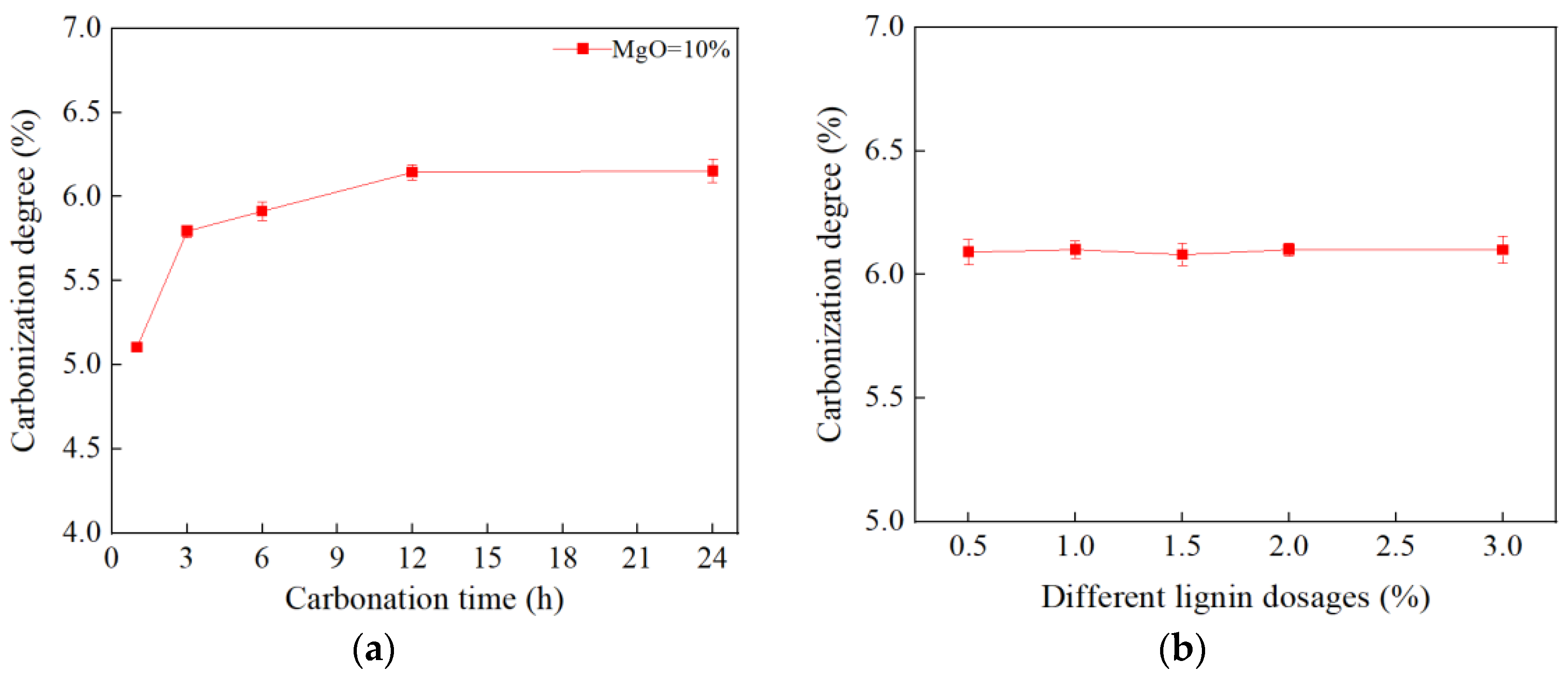
3.1.4. Carbonation Test
3.2. Thermal Conductivity Test
3.2.1. Admixture Ratios
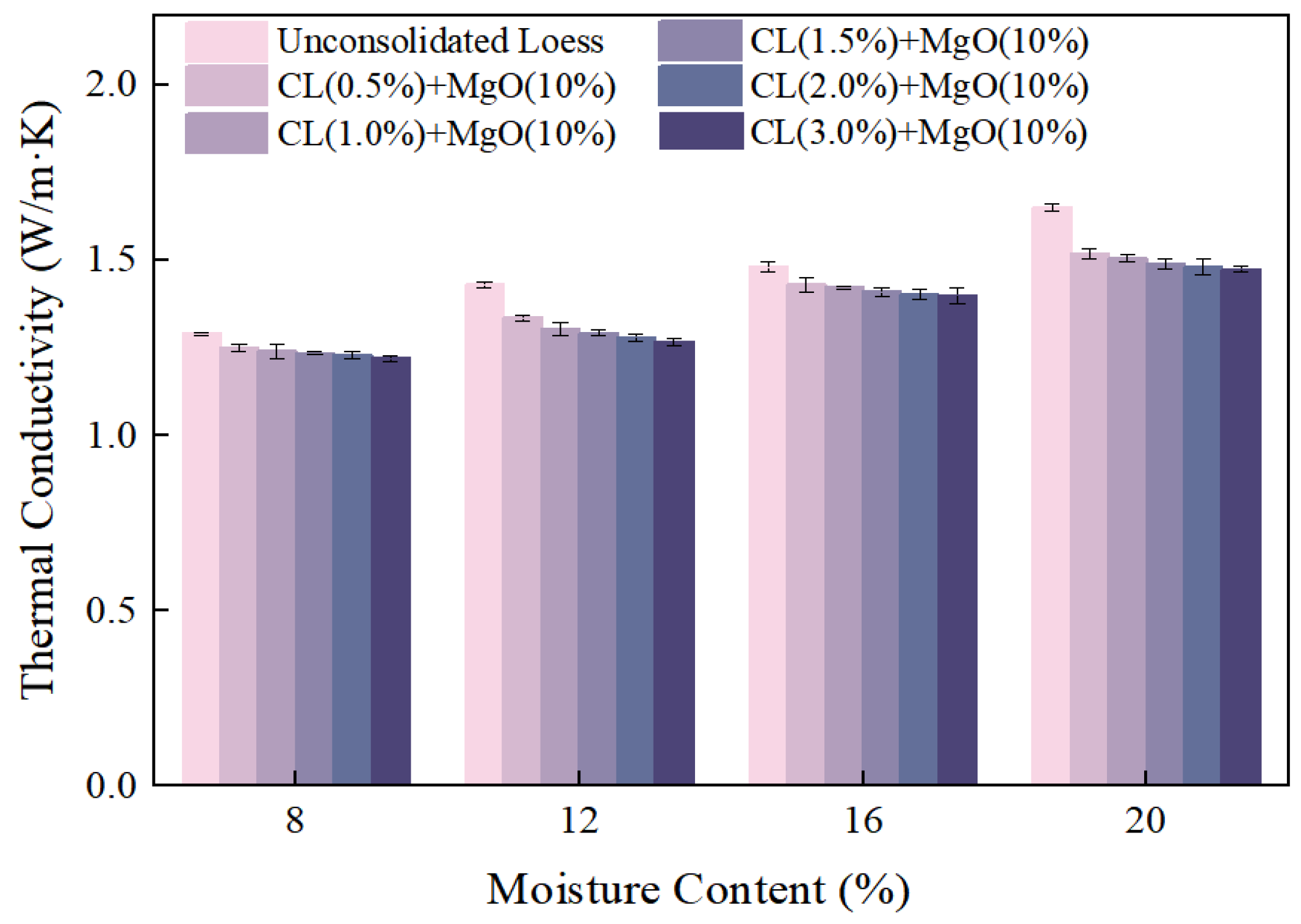
3.2.2. Moisture Content
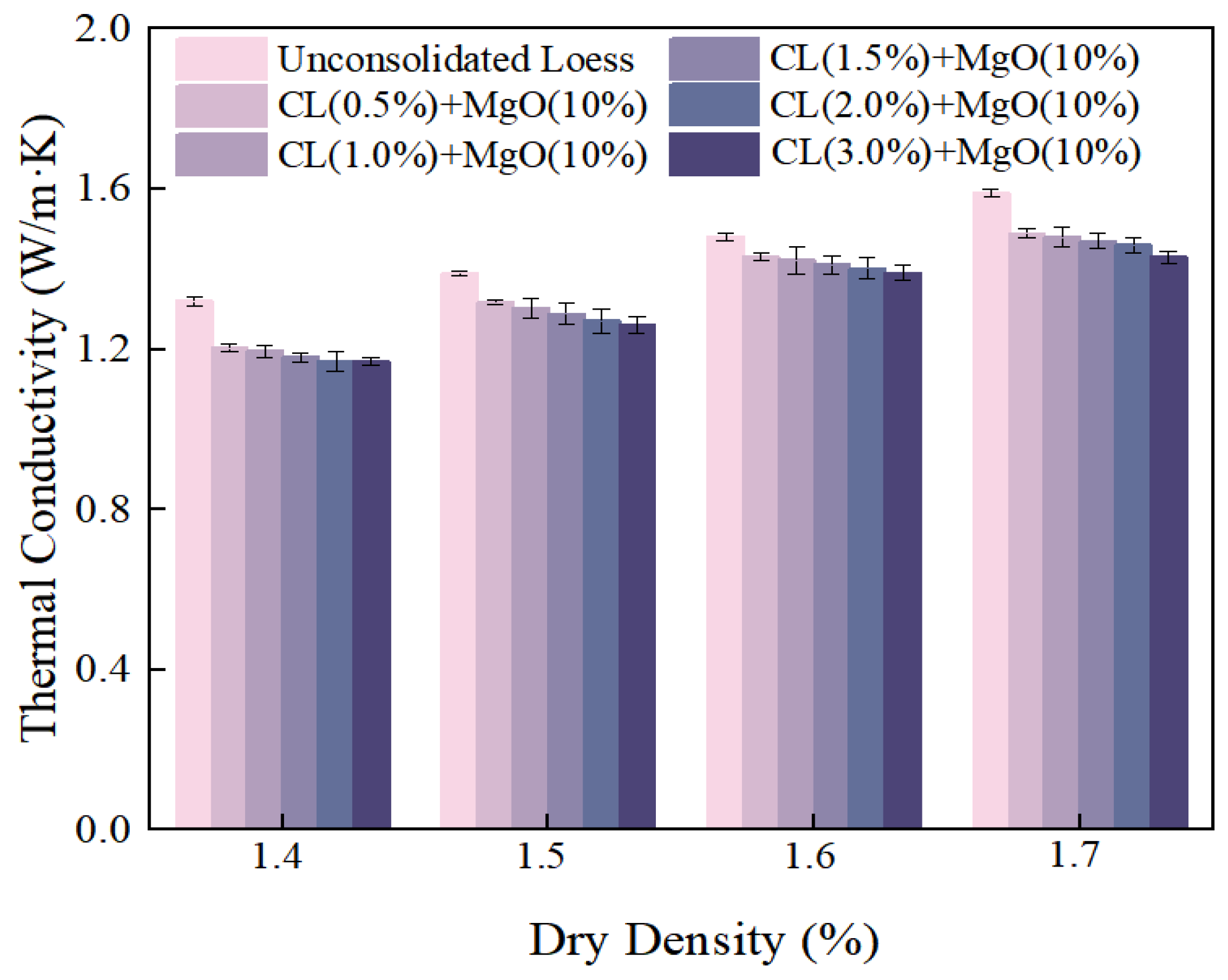
3.2.3. Dry Density
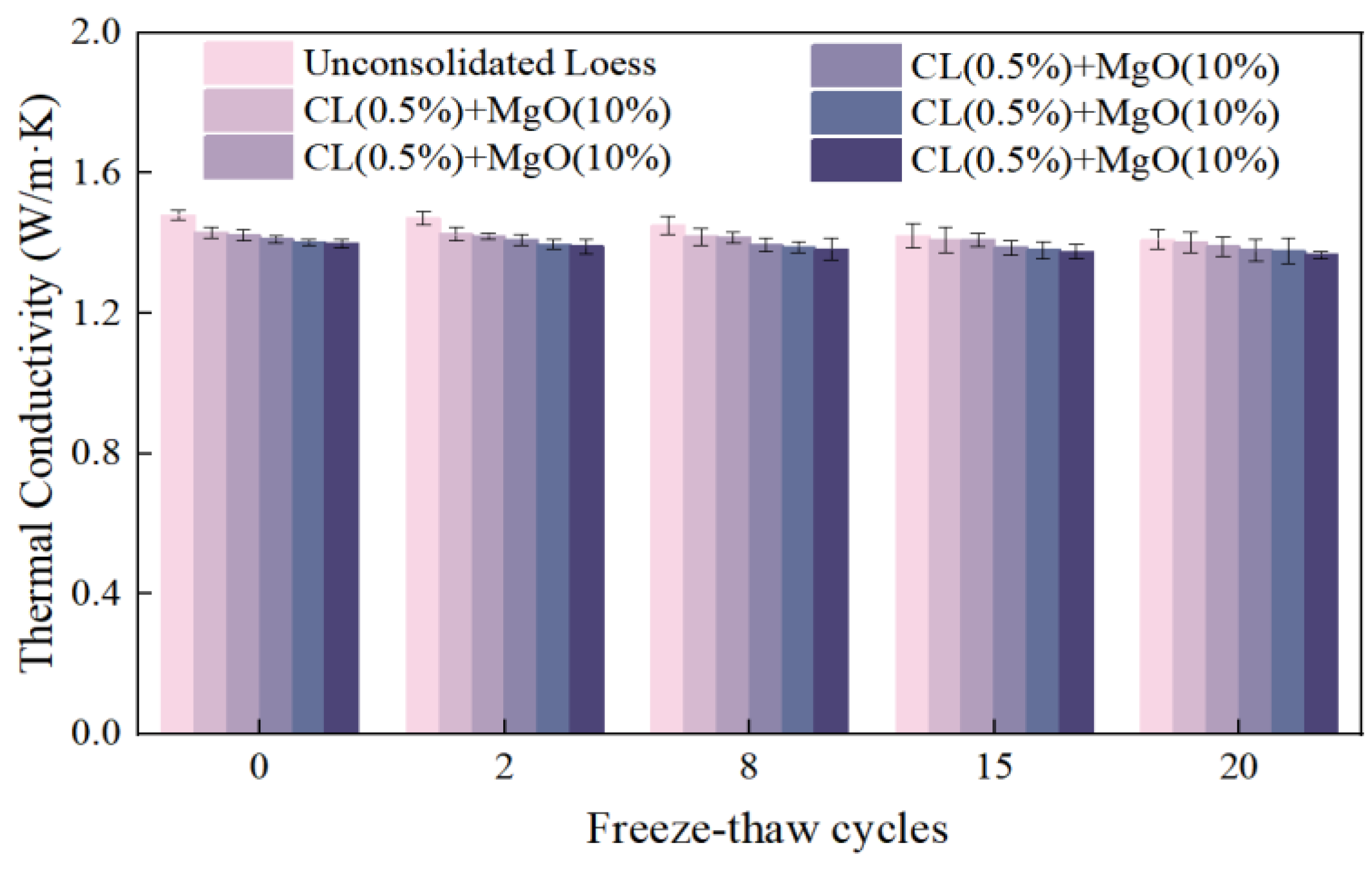
3.2.4. Freeze–Thaw Cycles
3.3. Microstructure and Mineralogical Analyses
3.3.1. Scanning Electron Microscope (SEM) with Particle and Crack Analysis System (PCAS)
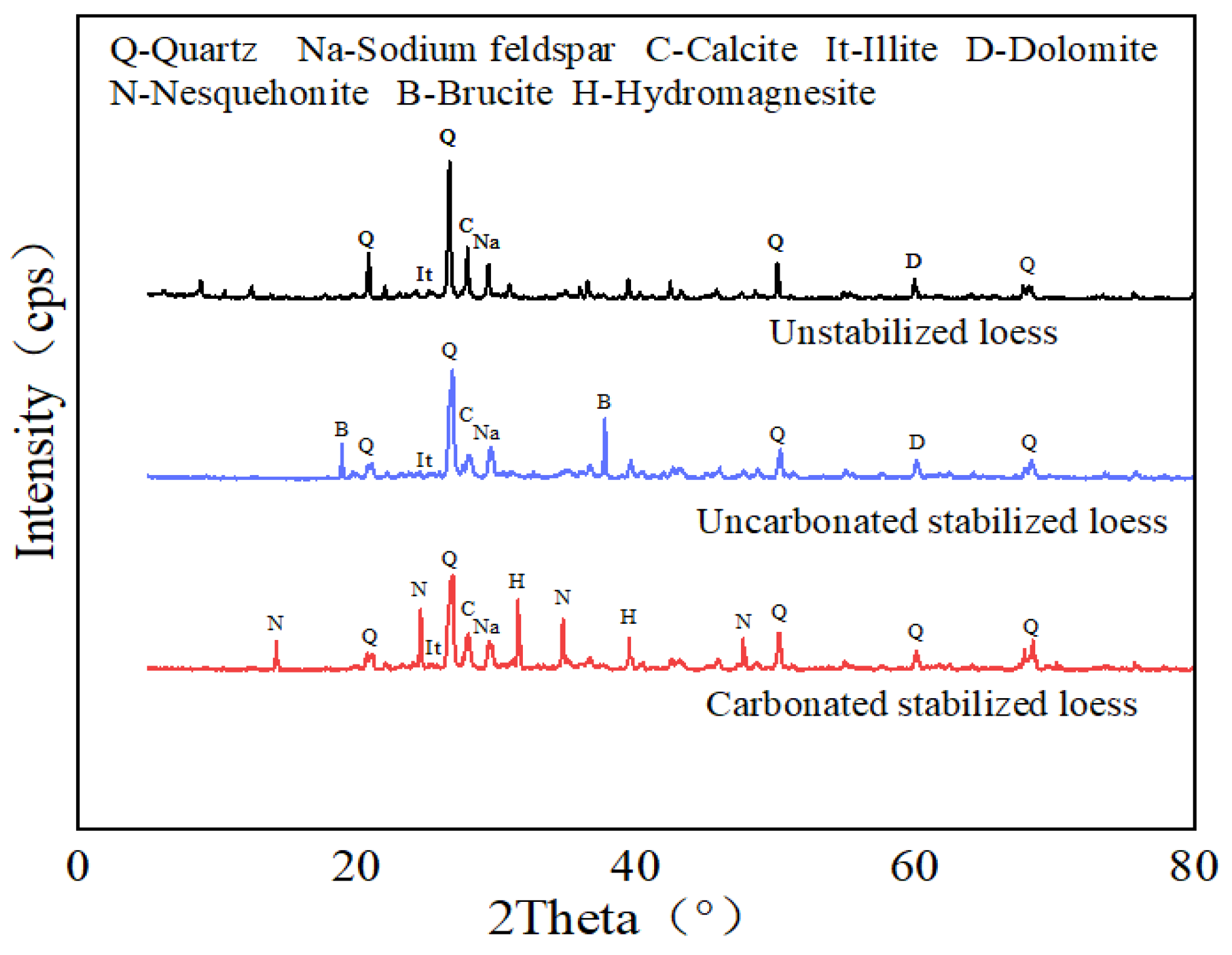
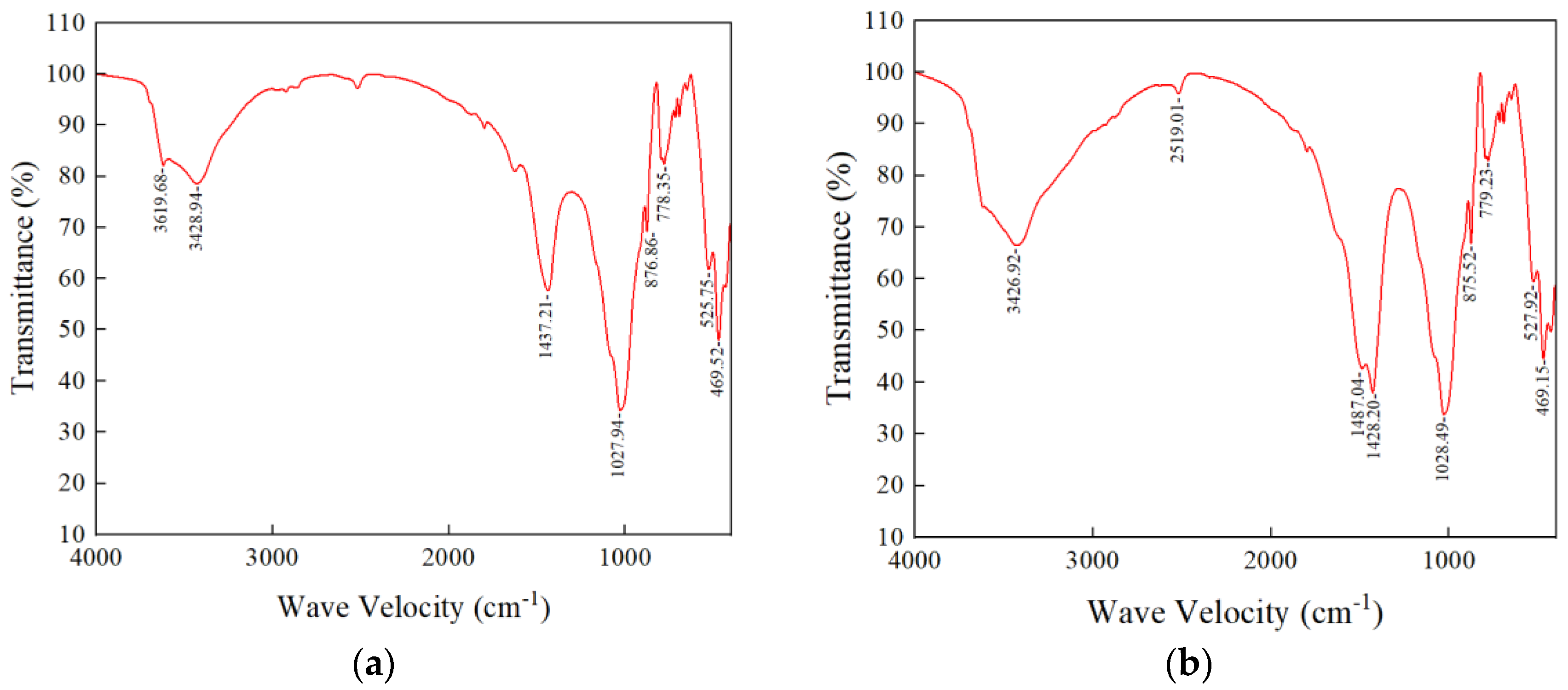
3.3.2. XRD and FT-IR
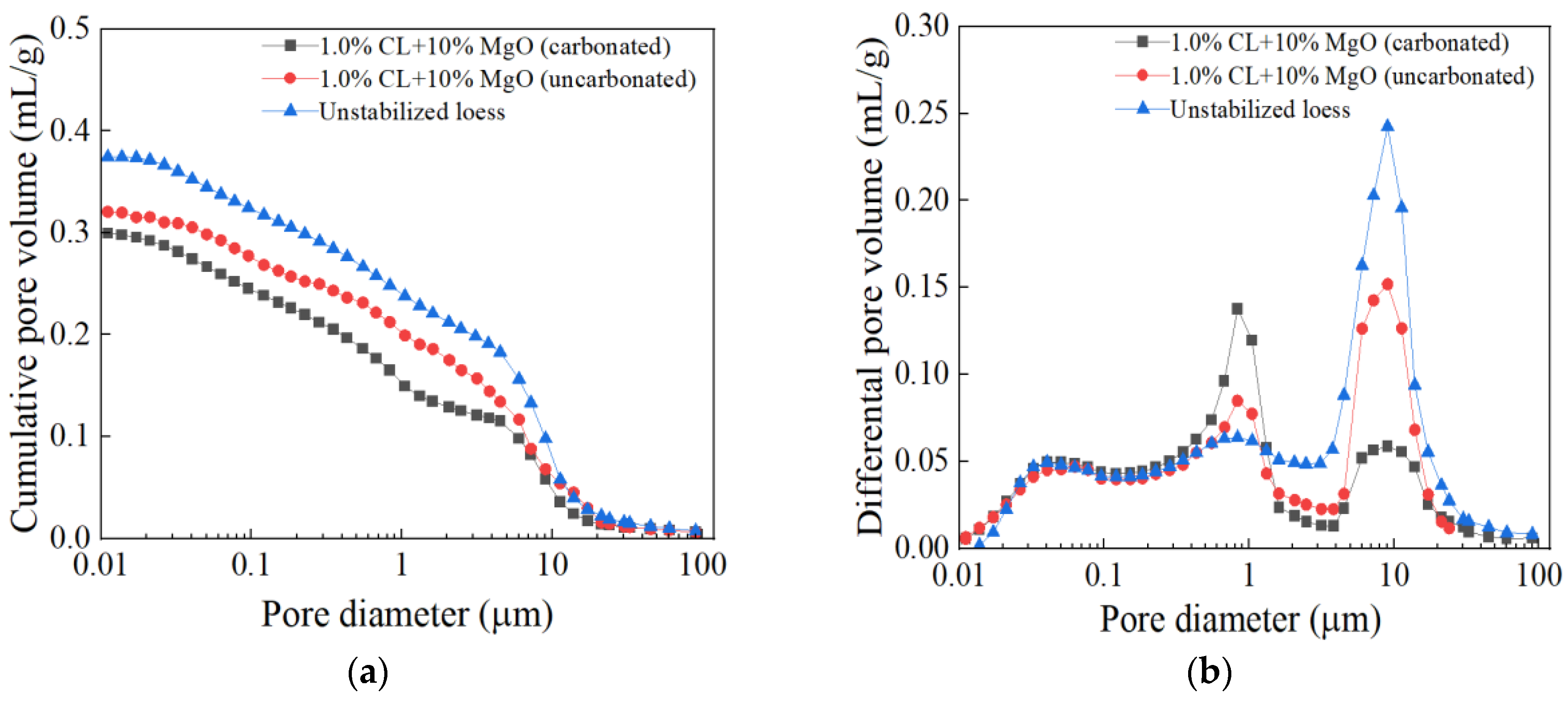
3.3.3. MIP
3.4. Proposed Mechanistic Model
4. Conclusions
- Carbonation Solidification Effect and Environmental Engineering Benefits: The CL-MgO composite material facilitated the solidification of loess, while simultaneously mineralizing and sequestering CO2. After 24 h of carbonation, the degree of carbonation reached 6%, with approximately 0.6 tons of CO2 fixed per ton of MgO, forming stable magnesium carbonate minerals (hydromagnesite and magnesite). The improved soil exhibited a collapsibility coefficient below 0.015, significantly enhancing stability. This dual-effect process effectively mitigated collapsible loess issues, while achieving CO2 emission reduction, demonstrating substantial environmental and engineering value.
- The Combination of CL and MgO Effectively Reduces Soil Thermal Conductivity Through Microstructural Modification: The combination of CL and MgO effectively reduced the soil thermal conductivity through microstructural modification. During carbonation, MgO’s carbonated products and CL molecules alter the soil’s pore structure, increasing porosity and the tortuosity of heat flow paths. Since both additives possess lower thermal conductivity than soil particles, this carbonation technique not only decreases the thermal conductivity of loess but also limits conductivity increases from environmental factors such as moisture. The improved thermal insulation properties benefit applications ranging from heating pipelines to cold-region infrastructure, while mitigating frost heave in permafrost areas. These findings can contribute to the development of sustainable building materials in loess regions.
- Microscopic Mechanism Elucidation: The primary products of MgO carbonated solidified loess are hydromagnesite and magnesite. The hydration of MgO, combined with the cementation effect of CL, optimizes the soil structure by enhancing inter-particle bonding and filling pores, resulting in a denser soil matrix. This structural densification suppresses thermal conduction pathways, leading to lower thermal conductivity than the loosely structured remolded loess, thereby exhibiting excellent thermal insulation properties. During carbonation, CO2 sequestration is achieved, contributing to carbon emission reduction. Even after undergoing freeze–thaw cycles, although cracks may develop between particles and carbonation products at the microscopic level, the soil maintains high stability and favorable thermal performance at the macroscopic level. This stability reflects the synergistic optimization of both microscopic and macroscopic properties.
- Future Outlook: Future research directions include validating the long-term performance of materials under cyclic freeze–thaw and wet–dry conditions to assess durability and CO2 mineralization stability in challenging environments; integrating molecular dynamics simulations with macro-scale performance data through machine learning for precise optimization of MgO–CL interactions; and exploring the co-utilization of industrial by-products to develop hybrid stabilization systems, while conducting lifecycle assessments to ensure scalable net-zero implementations. This would provide theoretical support for embankment engineering.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CL | Calcium Lignosulfonate |
| MgO | Magnesium Oxide |
References
- Smits, K.M.; Sakaki, T.; Limsuwat, A.; Illangasekare, T.H. Thermal conductivity of sands under varying moisture and porosity in drainage-wetting cycles. Vadose Zone J. 2010, 9, 172–180. [Google Scholar] [CrossRef]
- Derbyshire, E. Geological hazards in loess terrain, with particular reference to the loess regions of China. Earth-Sci. Rev. 2001, 54, 231–260. [Google Scholar] [CrossRef]
- Lu, S.; Ren, T.; Gong, Y.; Horton, R. An improved model for predicting soil thermal conductivity from water content at room temperature. Soil Sci. Soc. Am. J. 2007, 71, 8–14. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Zhang, T. Experimental study of thermal conductivity in soil stabilization for sustainable infrastructure. Sustainability 2024, 16, 946. [Google Scholar]
- Chen, X.; Liu, H.; Zhao, Q. Stabilization of expansive soils using chemical additives: A review. J. Rock Mech. Geotech. Eng. 2016, 15, 467–478. [Google Scholar] [CrossRef]
- Wang, Y. The effect of cement/lime on the performance of coastal saline soil for roadbed. J. Nanjing For. Univ. 2014, 38, 123–127. [Google Scholar]
- Zhang, H.; Wang, H. Experimental study on the performance of lime and cement- improved expansive soil. J. Geotech. Eng. 2019, 41, 1045–1052. [Google Scholar]
- Liu, M.; Li, X. The effect of polypropylene fibers and TG curing agents on the strength and stability of cement lime soil. J. Civ. Eng. 2016, 49, 89–95. [Google Scholar]
- Chen, X. Research on design methods for the strength ratio of alkali slag lime stabilized soil. Highw. Eng. 2021, 46, 112–117. [Google Scholar]
- Zhou, P.; Wu, J.; Chang, L. Subgrade improvement with mixed lime and cement as additives. Case Stud. Constr. Mater. 2023, 19, e01678. [Google Scholar]
- Miller, S.A.; Horvath, A.; Monteiro, P.J. Readily implementable techniques can cut annual CO2 emissions from the production of concrete by over 20%. Environ. Res. Lett. 2016, 11, 074029. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Xue, Z.; Zhang, B.; Sui, W.; Jia, H.; Si, C. Research and application progress of lignin in soil improvement and remediation. China Pulp Pap. 2024, 39, 140–153. [Google Scholar]
- Guo, F.; Hu, X.; Zhang, X.; Chen, Y.; Hou, J. Study on soil heavy metal contamination and its remediation using lignin-based adsorbents: A review. Environ. Technol. Innov. 2025, 37, 103958. [Google Scholar] [CrossRef]
- Xia, F. Application Research of MgO2-MgNCN/MgO System in Soil Pollution Remediation. Master’s Thesis, Huazhong University of Science and Technology, Wuhan, China, 2022. [Google Scholar]
- Ibrahim, M.M.; Guo, L.; Wu, F.; Liu, D.; Zhang, H.; Zou, S.; Xing, S.; Mao, Y. Field-applied biochar-based MgO and sepiolite composites possess CO2 capture potential and alter organic C mineralization and C-cycling bacterial structure in fertilized soils. J. Environ. Manag. 2022, 301, 113978. [Google Scholar] [CrossRef]
- Van den Heede, P.; De Belie, N. Environmental impact and life cycle assessment (LCA) of traditional and ‘green’ concretes: Literature review and theoretical calculations. Cem. Concr. Compos. 2012, 34, 431–442. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Accelerated carbonation–a potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic force microscopy reveals how relative humidity impacts the Young’s modulus of lignocellulosic polymers and their adhesion with cellulose nanocrystals at the nanoscale. Int. J. Biol. Macromol. 2020, 147, 1064–1075. [Google Scholar] [CrossRef]
- Michael, S.; Luis, Z.; Markus, K.; Markus, L.; Sebastián, S.-L.; Michael, H.; Olaf, L.; Valentin, S.; Josef, F. Characterization of mechanical properties of five hot-pressed lignins extracted from different feedstocks by microscopy-aided nanoindentation. Mater. Des. 2023, 227, 111765. [Google Scholar]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Chen, J.; Liu, C.; Chen, Y.; Zhang, G. Experimental investigation on thermal conductivity of MgO-improved loess under different densities and water contents. Constr. Build. Mater. 2022, 316, 125821. [Google Scholar]
- Zhang, T.; Liu, S.; Cai, G.; Puppala, A.J. Experimental investigation of thermal and mechanical properties of lignin treated silt under freeze-thaw cycling. Eng. Geol. 2021, 287, 106108. [Google Scholar]
- Pilkington, K.S.; Callaghan, C.A.; Murray, J.R. Initial investigation into the carbonation of MgO for soil stabilisation. Geomech. Geoengin. 2016, 11, 305–316. [Google Scholar]
- Guo, Z.Y.; Cai, G.H.; Liu, S.Y.; Zhong, Y.Q.; Liu, T.Y.; Poon, C.S. Influence of organic matter and carbonation time on engineering performance of reactive MgO carbonated soils. J. Build. Eng. 2025, 104, 112257. [Google Scholar] [CrossRef]
- Liu, Z.; He, H.; Li, M.; Zheng, S.; Liu, S.; Zhang, D. Factors and Mechanism of MgO Carbonation Enhanced Recycled Fine Aggregate. J. Southeast Univ. (Nat. Sci. Ed.) 2025, 1–11. Available online: http://kns.cnki.net/kcms/detail/32.1178.N.20250318.0955.002.html (accessed on 17 April 2025).
- Li Frazao, C.; Melo, T.C.; Zhang, J. Investigation of carbonation depth and its influence on the performance and microstructure of MgO cement and PC mixes. J. CO2 Util. 2020, 41, 101265. [Google Scholar]
- Wang, J.; Chen, L.; Li, Q. Effects of MgO particle size and density on microstructure development of MgO-based composite materials. Powder Technol. 2025, 412, 123456. [Google Scholar]
- Liu, Z.; Wang, Q.; Zhong, X.; Liu, F.; Liang, S.; Gao, Z. Water retention and water stability of loess modified by lignin. Chin. J. Rock Mech. Eng. 2020, 39, 2582–2592. [Google Scholar]
- Chen, L.; Wang, H.; Liu, J. Freeze-thaw performance of silt sand treated with lignin. Mater. Sci. Eng. A 2023, 806, 140568. [Google Scholar]
- Liu, S.; Zhang, T.; Cai, G. Research on the Technology and Application of Industrial Waste Lignin Stabilized Improved Fine Soil Subgrade. China Highw. Eng. J. 2018, 31, 1–11. [Google Scholar]
- Zhang, T.; Cai, G.; Liu, S.; Li, J.; Jie, D. Microscopic Mechanism of Lignin-Modified Subgrade Silt Using Industrial By-products. Rock Soil Mech. 2016, 37, 1665–1672. [Google Scholar]
- ASTM D4318-17; Standard Test Methods for Liquid Limit, Plastic Limit, and Plasticity Index of Soils. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D698-12e1; Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort. ASTM International: West Conshohocken, PA, USA, 2012.
- ASTM D5333-14; Standard Test Method for the Evaluation of the Collapsibility of Soils. ASTM International: West Conshohocken, PA, USA, 2014.
- ISO 1920-12:2015; Testing of Concrete-Part 12: Determination of the Carbonation Resistance of Concrete. International Organization for Standardization: London, UK, 2015.
- ASTM D560-16; Standard Test Methods for Frost Resistance of Concrete. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D5334-14; Standard Test Method for Thermal Conductivity of Soil and Soft Rock. ASTM International: West Conshohocken, PA, USA, 2014.
- Yi, Y.; Liška, M.; Albe-Tabar, A.; Liu, S.; Du, Y. A Green Low-Carbon Curing Agent for Soil Solidification. No. CN201010604325.2; Patent No. CN102071029A, 24 February 2025. [Google Scholar]
- Zheng, X.; Liu, S.; Cai, G.; Cao, Q. Experimental study on the wet-dry cycle characteristics of active MgO carbonized solidified soil. Chin. J. Geotech. Eng. 2016, 38, 297–304. [Google Scholar]
- Li, B.; Li, Y.; Hu, Z.; Yin, Z.; Zhang, S.; Min, F. Experimental Study on the Limit Water Content of MgO Modified Carbonized Shield Tunnel Centrifugal High Liquid Limit Silt. Open J. Transp. Technol. 2023, 12, 431–437. [Google Scholar]
- Jin, F.; Al-Tabbaa, A. Characterisation of different commercial reactive magnesia. Adv. Cem. Res. 2014, 26, 101–113. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Al-Tabbaa, A. Properties and microstructure of GGBS-MgO pastes. Adv. Cem. Res. 2014, 26, 114–122. [Google Scholar] [CrossRef]
- Liu, Z.; Cai, G.; Zhang, Y. Study on sulfate resistance of activated MgO carbonized solidified soil. Geotech. Mech. 2016, 37, 3003–3010. [Google Scholar]
- Zhang, T.; Cai, G.; Liu, S.; Zhang, Y. Study on the microscopic mechanism of improving subgrade silt based on industrial byproduct lignin. Geotech. Mech. 2016, 37, 3003–3010. [Google Scholar]
- Farouki, O.T. Thermal Properties of Soils; Trans Tech Publications: Clausthal-Zellerfeld, Germany, 1986. [Google Scholar]
- Sengul, O.; Azizi, S.; Karaosmanoglu, F.; Tasdemir, M.A. Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energy Build. 2011, 43, 671–676. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M.; Al-Tabbaa, A. MgO expansive cement and concrete in China: Past, present and future. Cem. Concr. Res. 2014, 57, 1–12. [Google Scholar] [CrossRef]
- Qi, J.; Vermeer, P.A.; Cheng, G. A review of the influence of freeze-thaw cycles on soil geotechnical properties. Permafr. Periglac. Process. 2006, 17, 245–252. [Google Scholar] [CrossRef]
- Vinod, J.; Indraratna, B.; Mahamud, M.A.A. Stabilization of an erodible soil using a chemical admixture. Proc. Inst. Civ. Eng.-Ground Improv. 2010, 163, 43–51. [Google Scholar] [CrossRef]
- Cai, G.; Liu, S.; Cao, J. Influence of initial water content on the strength and resistivity of MgO carbonized silt. China J. Highw. Transp. 2017, 30, 3–10. [Google Scholar]










| Natural Water Content (%) | Liquid Limit (%) | Plastic Limit (%) | Plasticity Index (%) | Dry Density (g/cm) | Optimum Water Content (%) | pH |
|---|---|---|---|---|---|---|
| 14.5 | 27.7 | 15.3 | 12.4 | 1.72 | 15.1 | 8.87 |
| Tests | Binder Content * | Freeze–Thaw (F-T) Cycles | Carbonation (h) | Moisture Content (%) | Dry Density (g·cm−3) | |
|---|---|---|---|---|---|---|
| Calcium Lignosulfonate (%) | MgO (%) | |||||
| Basic Physical Properties Test | 0, 0.5, 1, 1.5, 2, 3 | 10 | -- | U ** | 16.5 | 1.63 |
| Thermal Conductivity Test | 0, 0.5, 1, 1.5, 2, 3 | -- | 24 | 16.5 | 1.63 | |
| 1 | -- | 8, 12, 16, 20 | 1.63 | |||
| -- | 16.5 | 1.4, 1.5, 1.6, 1.7 | ||||
| 0, 2, 8, 15, 20 | 16.5 | 1.63 | ||||
| Microscopic tests | 1 | 0, 15 | 16.5 | 1.63 | ||
| Parameters | 1%CL + 10%MgO; FT = 0 | 1%CL + 10%MgO; FT = 15 |
|---|---|---|
| Number of Pores | 144 | 305 |
| Porosity | 3.08% | 6.66% |
| Probabilistic Entropy | 0.9559 | 0.9689 |
| Fractal Dimension | 1.1817 | 1.2099 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Zhang, W. Research on the Thermal Conductivity and Microstructure of Calcium Lignosulfonate-Magnesium Oxide Solidified Loess. Appl. Sci. 2025, 15, 4545. https://doi.org/10.3390/app15084545
Lu Y, Zhang W. Research on the Thermal Conductivity and Microstructure of Calcium Lignosulfonate-Magnesium Oxide Solidified Loess. Applied Sciences. 2025; 15(8):4545. https://doi.org/10.3390/app15084545
Chicago/Turabian StyleLu, Yuwen, and Wuyu Zhang. 2025. "Research on the Thermal Conductivity and Microstructure of Calcium Lignosulfonate-Magnesium Oxide Solidified Loess" Applied Sciences 15, no. 8: 4545. https://doi.org/10.3390/app15084545
APA StyleLu, Y., & Zhang, W. (2025). Research on the Thermal Conductivity and Microstructure of Calcium Lignosulfonate-Magnesium Oxide Solidified Loess. Applied Sciences, 15(8), 4545. https://doi.org/10.3390/app15084545





