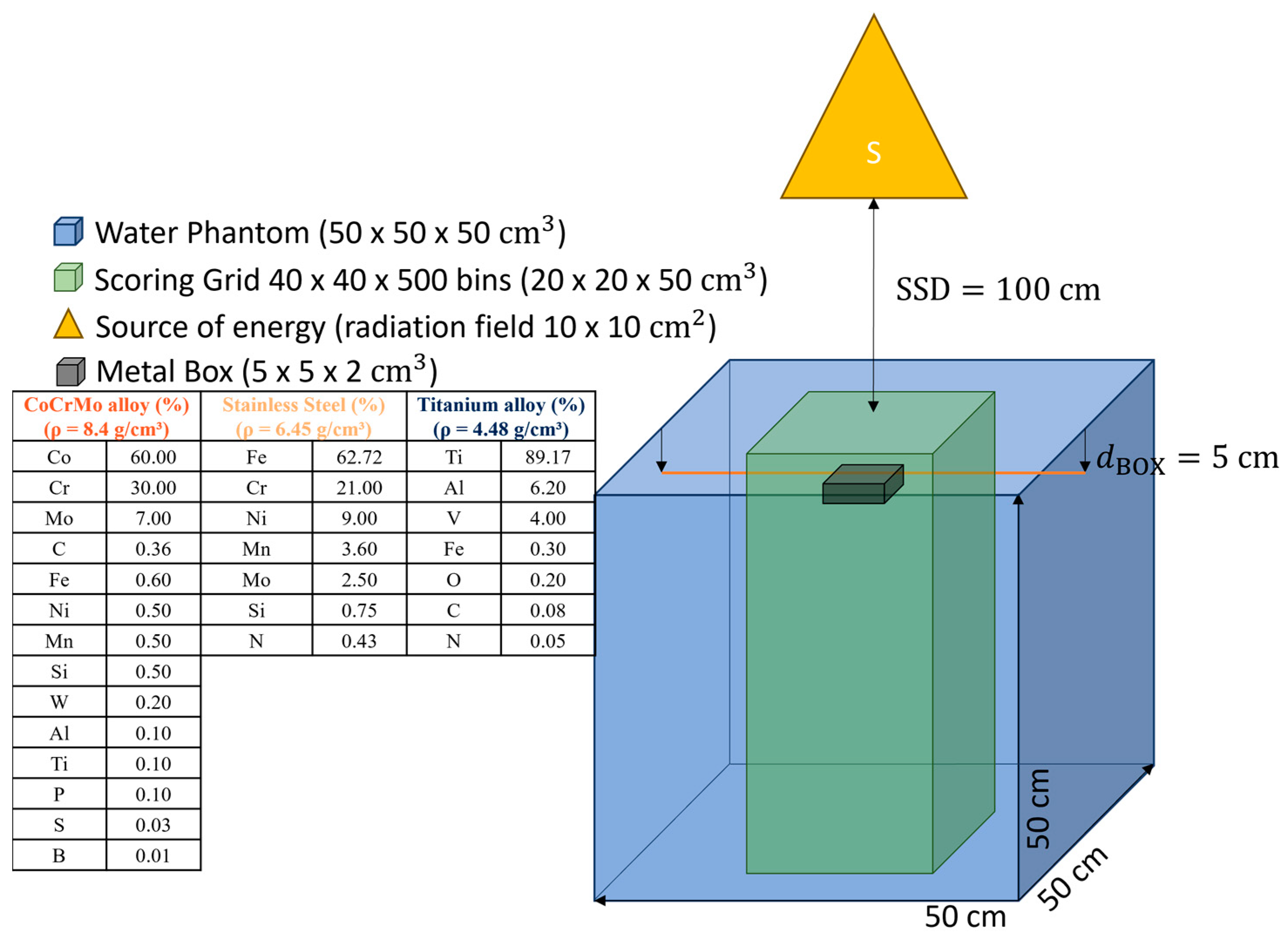
1. Introduction
Metallic implants are becoming increasingly popular in medical operations. Many patients receiving joint replacements or cardiac stents also receive radiotherapy (RT). This raises questions about possible patient risks and the interactions between these implants and the ionizing radiation used in radiotherapy. However, metallic implants have the potential to drastically change the intended radiation dose distribution, possibly resulting in over-irradiation of healthy tissues or under-treatment of the tumor [
1]. This may lessen the effectiveness of treatment and raise the possibility of side effects. It is essential to comprehend these relationships. Various factors influence radiation beam dispersion, absorption, and attenuation, including the implant’s location, size, and composition. Localized dose changes that arise from this may impact treatment outcomes [
2].
Researchers are examining how metallic implants affect radiotherapy to optimize treatment and reduce risk [
3]. Effects are significant for recently developed radiation methods like FLASH radiotherapy with very high electron energy (VHEE), which may interact differently with metallic implants than more conventional methods. VHEE is a new modality with unique physical diametric properties that is promising for dose delivery. If we compare VHEE with other modalities, they show lower lateral scattering than photon beams [
4]. They are more insensitive to inhomogeneities than other charged particle therapies [
5]. VHEE may also have the advantage of ultra-high dose rate (UHDR) capability [
6]. The combination of VHEE and UHDR contributes to faster and, therefore, more precise radiation treatment of the patient and limits the occurrence of inhomogeneities associated with patient movement or patient organ movement.
Metal prostheses are widely employed in orthopedics to substitute damaged joints and bones. There is a growing trend among patients to choose surgical procedures, such as metal prostheses, due to the increasing prevalence of arthritis, osteoporosis, and other degenerative joint disorders. In the case of metastatic bone disease in cancer patients, metal plates and screws are used for stabilization. Orthopedics practitioners commonly employ knee and hip joint replacements because of the high incidence of degenerative alterations that affect these weight-bearing articulations [
7]. The occurrence of degenerative alterations frequently necessitates joint replacement procedures to restore functionality and reduce the accompanying pain. Patients’ prosthesis replacement procedures do not consider radiotherapy treatment; thus, problematic situations may arise in the future during the treatment of oncological diseases. In contemporary orthopedics, various materials for fabricating metallic prostheses are being used. Each of these materials has significant implications for their application in the human body. Stainless steel has become known for its excellent robustness and corrosion resistance [
8]. This characteristic renders it a preferred option for a wide range of joint replacements. In recent times, titanium and its alloys have garnered significant attention as formidable candidates due to their remarkable biocompatibility and low mass [
9]. The attributes above contribute to mitigating stress shielding and enhancing implant durability, particularly in hip and knee replacement procedures. The utilization of cobalt-chromium alloys in joint replacements is prevalent due to their exceptional mechanical strength and resistance to wear [
10].
Metal implants in radiation oncology pose ongoing issues for doctors and medical physicists. They impact the precision and efficacy of treatment. Prostheses with high proton number Z mainly cause the complexity of image processing and dose calculations for the specified volume. These prostheses have a dosimetric impact on the treatment results, particularly for patients with pelvic area malignancies. Comprehending and resolving the difficulties that arise from this problem is crucial in guaranteeing secure and efficient radiation treatment. Recent research has highlighted the significance of reducing dosimetric inaccuracies, as demonstrated in the study by James D. Rijken [
11], which emphasizes the importance of optimizing the density of the metal implant in treatment planning systems (TPSs). Researchers have observed a dosimetric inaccuracy of up to 10% in the planning tumor volume (PTV) areas for the rays passing through the implant [
12]. A thermoluminescent detector (TLD), an anthropomorphic human pelvic phantom, was used to investigate spot doses in the PTV areas. The findings revealed notable fluctuations in administered doses, which were influenced by the presence of a metal prosthesis.
Why is VHEE interesting to us? Precisely because FLASH radiotherapy has the potential of highly advantageous oncological treatment. Developments in this field have been advancing significantly over the last decade. Experts in radiotherapy have focused on the possibility that damage to surrounding healthy tumor tissue is minimized when using an ultra-high dose rate (UHDR ≥ 40 Gy/s) and have called this the FLASH effect [
13]. Hart et al. [
14] irradiated Drosophila melanogaster larvae with X-ray UHDR compared to conventional radiotherapy (CONV RT), thus setting a possible limit to observe the FLASH effect. Based on their results, it is possible to increase the therapeutic window by using UHDR RT for some intermediate doses, focusing on protecting healthy tissue. Bazalova-Carter et al. [
15] performed treatment planning for RT with VHEE and compared this with VMAT plans. The research group showed in three cases that treatment with VHEE may have a dosimetric advantage over photon VMAT plans, so it should be investigated further as a treatment method. This was followed up by Fisher et al. [
16], who point out the possibility of using Spatially Fractionated Radiotherapy (SFRT) in combination with VHEE. One of the main factors in FLASH RT with VHEE is dosimetry, which, according to Vanreusel et al. [
17], can be performed using point scintillation detectors. Hart et al. [
18] performed dosimetric measurements using a plastic scintillator. The research groups emphasize the importance of developing suitable dosimeters, exhibiting dose rate independence and sufficient radiation hardness. Studies have shown the possibility of using VHEE for FLASH RT and deeply oriented tumors. This is why it is also necessary to focus on how VHEE reacts with metal implants in the human body.
This work aims to investigate the effect of metal implants on dose distribution in radiotherapy by using Monte Carlo simulations that demonstrate the ideal case of a metal box interacting with ionizing radiation. We used different energies in radiotherapy as ionizing radiation: photons with energy 6 MV, electrons with energy 15 MV, protons with energy 170 MeV, and experimental VHEE with 100 MeV and 150 MeV. Finally, we simulated the case of a patient with two stainless steel femoral metal replacements. The simulation was a single direct field using VHEE, showing the appearance of attenuation behind the metal replacement in the computed tomography (CT) image.
4. Discussion
We focused on a comprehensive investigation of the impact of metal prostheses (metal boxes), primarily on dose profiles and PDDs. We used TOPAS MC simulations. We dedicated ourselves to the study by focusing on professional practice in Slovakia’s hospital setting. The AAPM RT TG 63 [
3] report discusses the problem of a high atomic number of hip prostheses in patients receiving radiotherapy treatment in the pelvic region. It is noted that prostheses can lead to differences in the dose, consequently impacting the treatment’s results. The research also highlights the need for more scientific comprehension and methodology in clinically measuring radiation doses for these individuals. Nevertheless, numerous other studies have been released that specifically examine this matter.
Considering theoretical assumptions and investigations in metal implants, it is imperative to prioritize locations where metal replacements are present. Designing the radiation therapy treatment for patients with metal implants is a complex task, as the presence of the implant affects how the radiation dose is distributed. The issue’s historical origins can be traced back to 1984, when Hudson et al. [
22] documented notable alterations in the dose distribution with exposure to metal restorations. Specific implants induced the alterations using 8 MV gamma beams (Cobalt) in the treatment. Biggs and Russell [
23] found that 25 MV X-ray beams had an average dose decrease of 2%, whereas Cobalt had a 5% reduction. The magnitude of the disturbance is contingent upon the specific model and composition of the implant, as well as the energy level of the radiation beam being used. Hence, it is imperative to possess knowledge of a particular implant, precisely the technique employed for dose calculation.
Accuracy is crucial in radiation therapy when calculating doses using algorithms. The uniformity of the surroundings significantly influences the estimation of the dose. When there is a disruption, the significance of algorithms in the computation becomes even more crucial, particularly when it comes to metal implants. The accuracy of the algorithms used to calculate the dose in the field of hip prostheses is directly related to the complexity of the physical modeling of the processes. One notable limitation of the superposition method occurs at interfaces where there are distinct atomic numbers. This limitation is supported by our experimental findings, which align with the results reported in the study conducted by Keall et al. [
24]. One possible option to address these restrictions is to introduce nuclei produced in other media [
23] or to apply the modifications proposed by [
25,
26]. Using a Monte Carlo approach, Bazalova et al. [
27] examined the impact of artefacts produced by metallic materials from three commonly used prosthetic materials (Ti alloys, stainless steel, and Co-Cr-Mo) on dose estimation. During the calculation, they utilized the sinogram interpolation approach to rectify metal artefacts and subsequently assessed their influence. They acquired data demonstrating 11% differences in the prostate region when using bilateral hip endoprosthesis made from Co-Cr-Mo material. For patients with one-sided endoprosthesis, it is advisable to refrain from threading the bundle across the specified region. For bilateral endoprosthesis cases, employing the sinogram interpolation correction algorithm is advisable to compensate for artefacts in the beam going through the affected area. If any bundles traverse the region impacted by the artefact, the correction method should be applied, even for individuals with unilateral endoprosthesis.
Four fundamental strategies are employed in the realm of radiation therapy for patient exposure using photon beams: 3D Conformal Radiation Therapy (3D CRT), forward Intensity Modulated Radiation Therapy (fIMRT), Intensity Modulated Radiation Therapy (IMRT), and Volumetric Modulated Arc Therapy (VMAT). Techniques are selected according to the extent of the exposed area. The writers in the field of research address the problem of mitigating the adverse impacts of metal implants on dose distribution by utilizing an appropriately selected calculating technique. VMAT technology offers exceptional precision and flexibility in preventing irradiation of metal implants, hence assuring a more uniform distribution of doses. Most studies concentrate on the pelvic region, specifically the site of hip replacements. To et al. [
28] examined the planning approach of VMAT at LU for prostate patients with bilateral hip arthroplasties. They focused on ensuring the plan’s quality and feasibility while minimizing the radiation exposure to the prosthesis. Significant disparities were observed in fulfilling these goals across the three retrospectively constructed VMAT plans for 20 patients. The PA designs comprised six half-arcs that circumvented the need to enter each prosthesis.
Nevertheless, it was demonstrated that these plans were suboptimal compared to plans that employed two complete arcs with optimized maximum dose (MD) and two complete arcs with maximized DVH constraint (MDVH). The MD and MDVH plan achieved comparable dosimetric quality assurance and demonstrated considerable enhancements in rectal and bladder DVH measures compared to the PA plan. The findings of this study unequivocally demonstrate that MD and MDVH plans surpass PA plans in terms of dosimetric QA and feasibility. Simultaneously, these two strategies efficiently minimized the input doses to the prosthesis, reaching a mere 1% of the authorized dose. The study emphasized the significance of utilizing the VMAT technique in bilateral hip joint endoprosthesis, which allowed for the testing of dosimetric values using a human pelvic phantom. There is much focus on selecting the appropriate exposure approach in ongoing research. The VMAT approach has great potential in minimizing irradiation of the metal implant region and achieving improved dose distribution. A study by To et al. [
28] showcased the efficacy of this strategy and yielded significantly enhanced outcomes compared to conventional methods. The findings of the studies highlight the significance of selecting an appropriate radiation therapy planning approach and the necessity for ongoing research to enhance the treatment of patients with metal implants.
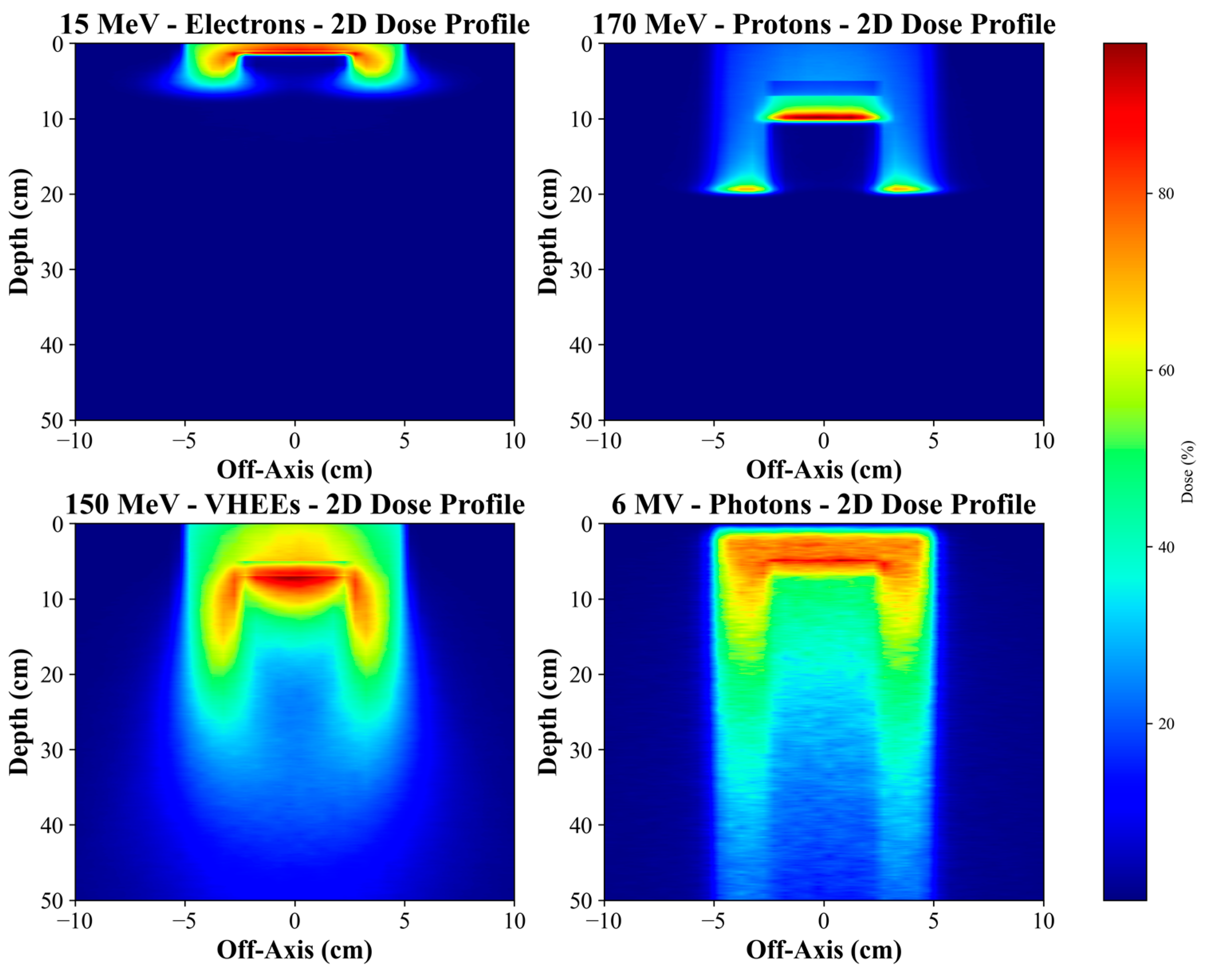
The results of our study obtained for different energy modalities (electrons, VHEE and protons) show the consistent and significant effects of metallic materials on the dose distribution and the extent of energy deposition in RT scenarios. Metallic implants, such as Co-Cr-Mo alloys, titanium, and stainless steel, greatly influence the physical processes of particle–tissue interaction, manifested in dose reduction and a shift in the depth of maximum deposition (Bragg peak for protons).
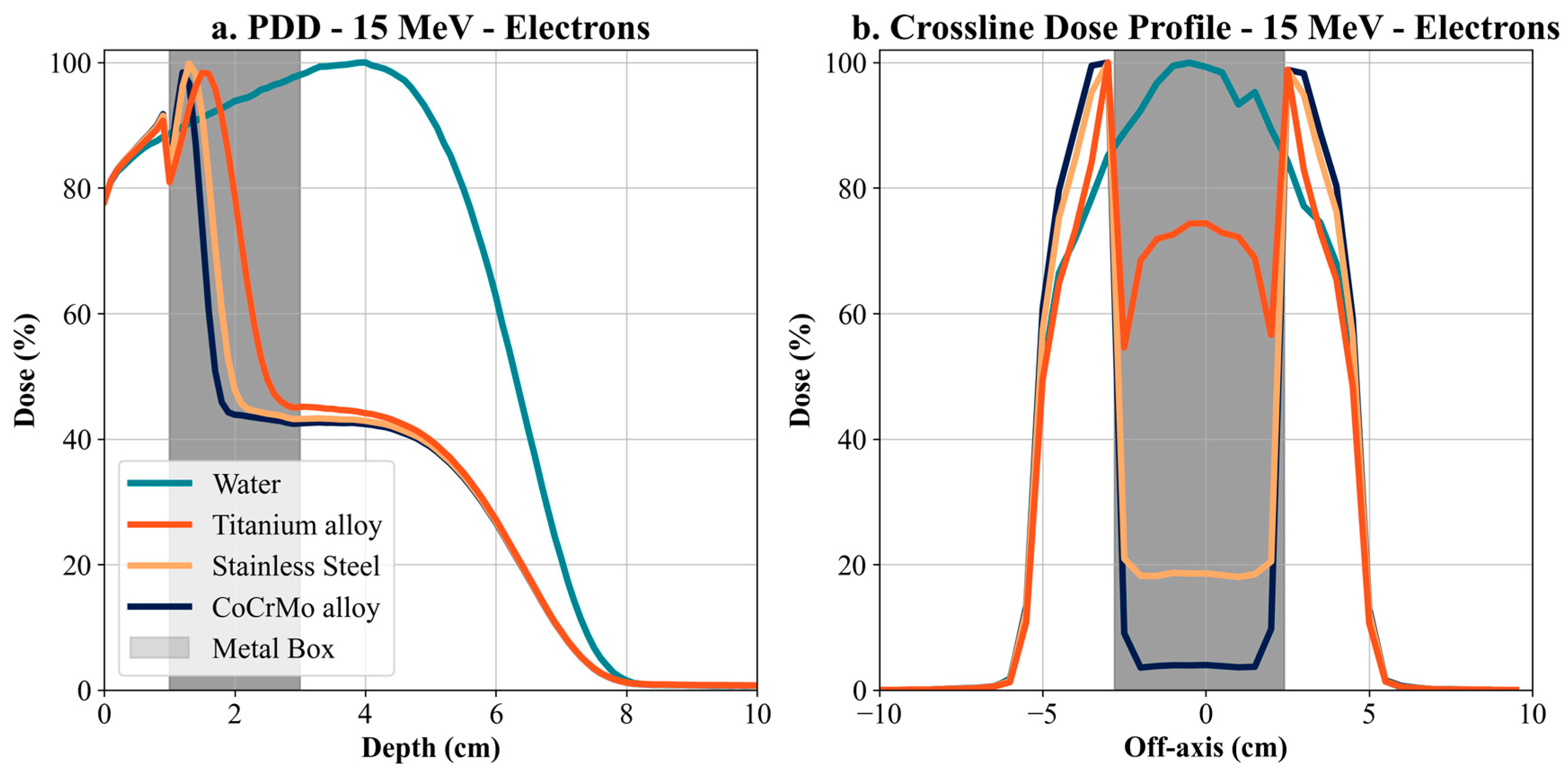
Simulations for 15 MeV electrons show that metallic implants, especially Co-Cr-Mo alloy, cause a dramatic dose attenuation behind the implant. At a depth of 1 cm, the dose for Co-Cr-Mo alloy was more than 6% lower than for water. This difference increased with increasing depth, with a significant dose decrease behind the implant, resulting in a shadowing zone with low energy deposition. Lower-density alloys, such as titanium and steel, showed less but still significant attenuation. This phenomenon is a direct result of the high density of the metals causing intense scattering and absorption of electrons, leading to a dose reduction in the target region behind the implant. Electrons have a significantly limited range compared to VHEE or protons, meaning their interaction with metallic materials can be limited to the surface irradiation areas. Clinical implications include potential under-dosing in target areas, particularly behind metal implants, critical for near-surface irradiation. Thus, for patients with metal implants, it is essential to consider modification of the radiation schedule or a change in modality. While adapting the radiation modality or modifying the treatment schedule may be clinically desirable in patients with metallic implants, the feasibility of such changes can be limited by institutional resources and infrastructure. Not all treatment centers have access to advanced modalities such as VHEE or proton therapy, and even within a center, re-planning may depend on the availability of trained personnel and appropriate imaging and verification systems. Therefore, any proposed change must be evaluated within the context of the specific clinical environment, and decisions should be based on a multidisciplinary assessment involving medical physicists, radiation oncologists, and treatment planning staff.
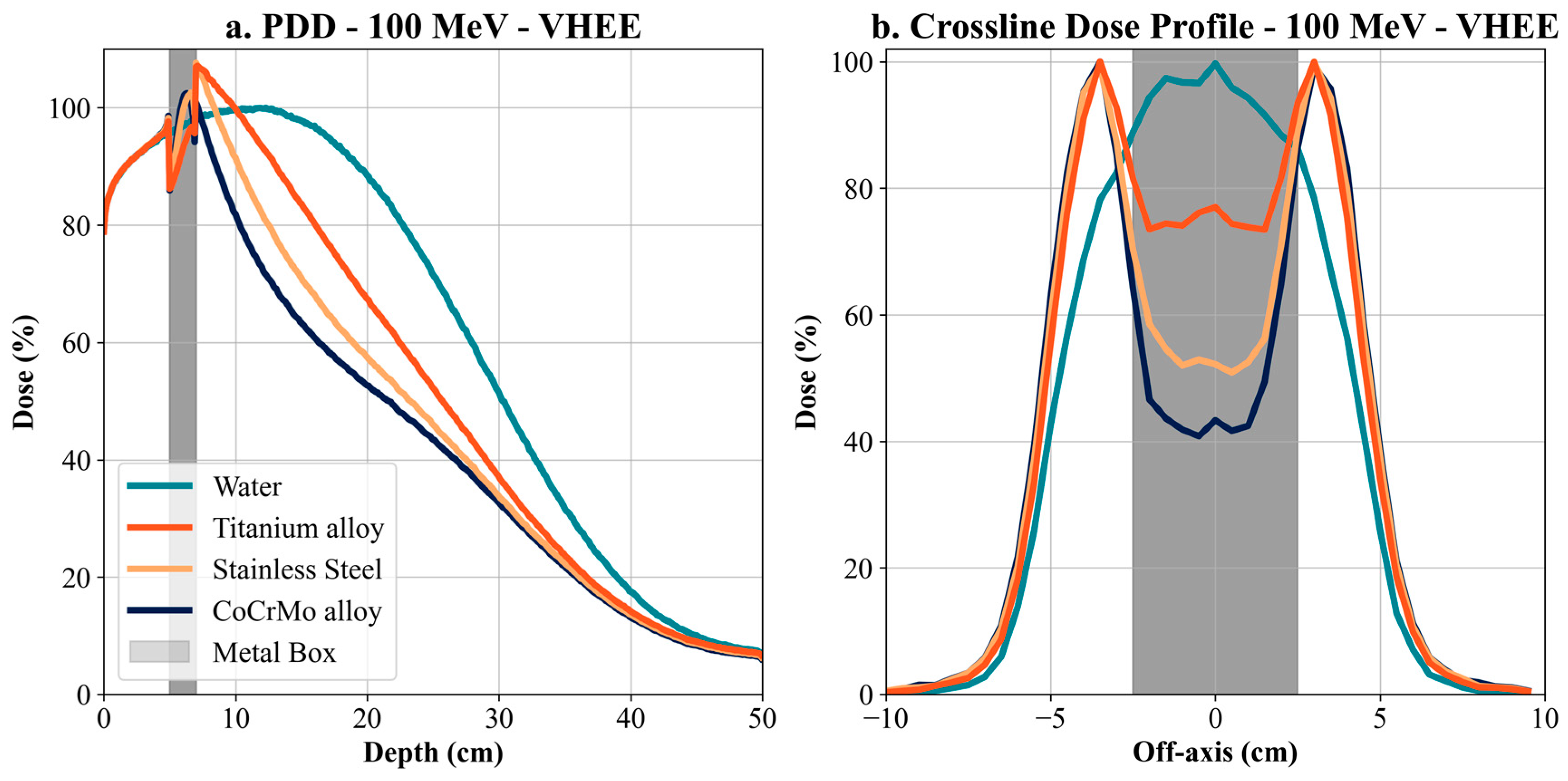
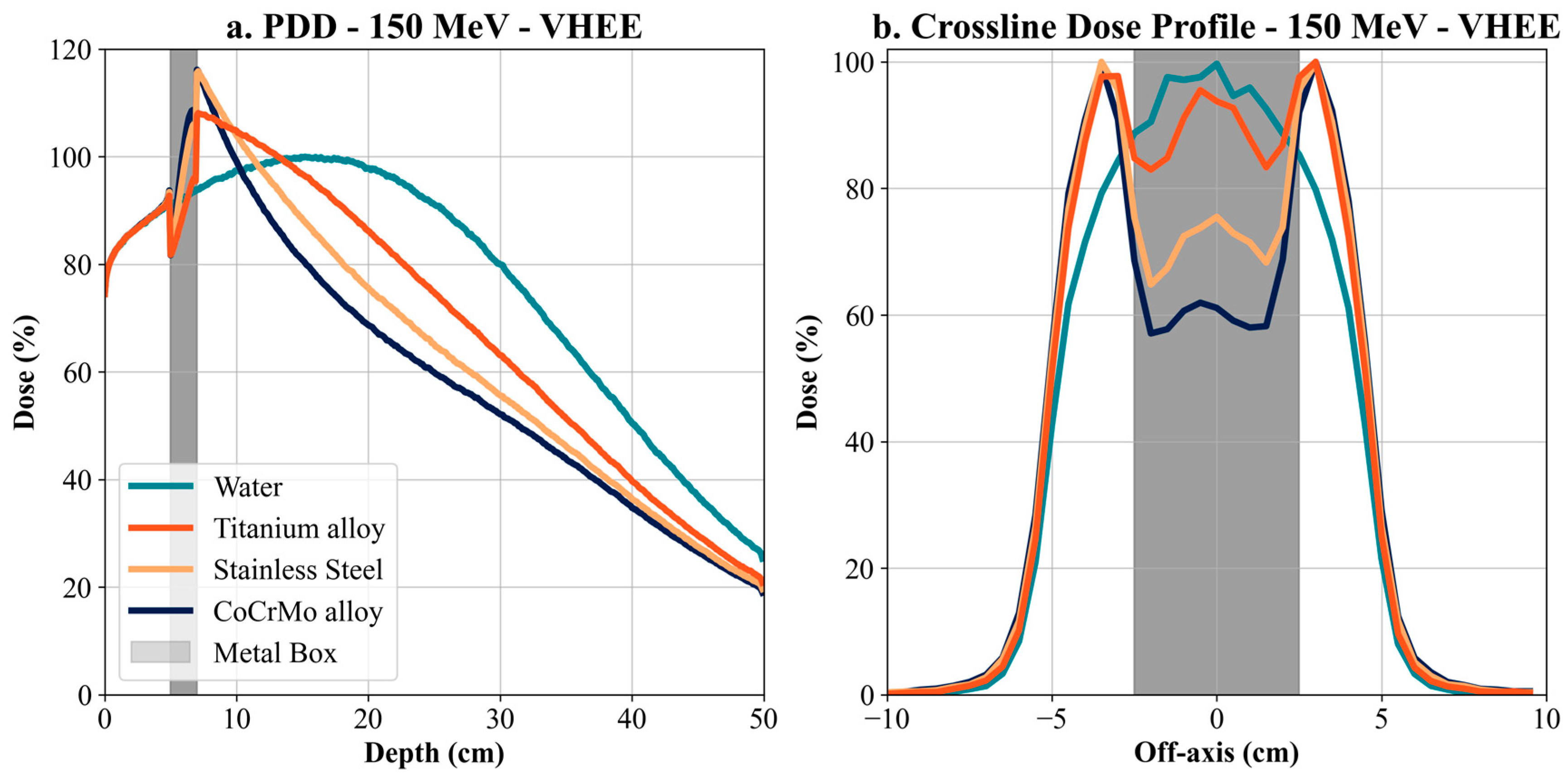
To improve the consistency and safety of patient care across different institutions, it is essential to establish and implement clear, accessible, and standardized clinical guidelines for managing radiotherapy in patients with metallic implants. These guidelines should be readily available and applicable in daily clinical practice, supporting harmonized treatment planning workflows across centers worldwide. Simulations for VHEE (100 MeV and 150 MeV) showed a more modest dose attenuation than electrons but still significant differences between materials. For 100 MeV VHEE, the Co-Cr-Mo alloy proved to be the most influential on the dose profile, with a decrease in maximum dose of up to 30% compared to water, with titanium and stainless steel causing a more minor but still significant decrease. At 150 MeV VHEE, this effect was even more pronounced, with Co-Cr-Mo causing a shift in the depth of the maximum dose and a decrease in its value, indicating the high ability of metallic materials to affect energy deposition even at high energies.
VHEEs are an exciting alternative for deep irradiation because they have significant penetration compared to lower electron energies. However, interaction with metal implants remains a significant challenge. Although lower-density alloys such as titanium show less dose reduction, there is still a shift of maximum energy deposition towards the surface, which could lead to underdosing in deeper target tissues. This effect could be addressed by adjusting the beam energy or optimizing the irradiation angle.
Protons pose a specific challenge in the presence of metal implants due to the shift of the Bragg peak. The results for 170 MeV protons showed a dramatic shift of the Bragg peak due to the presence of metal, particularly Co-Cr-Mo alloy. In water, the Bragg peak was at a depth of 19.4 cm, while for Co-Cr-Mo, this peak shifted to 9.9 cm, indicating a nearly 50% reduction in rank. Titanium and steel also affected the shift of the Bragg peak, but to a lesser extent, with values of 15 cm and 12.4 cm, respectively. In addition to the shift of the Bragg peak, a significant reduction of the maximum dose in metals was also observed. The maximum dose reached 100% in water, while Co-Cr-Mo reduced this value to 70.87%, a more than 29.00% reduction. Titanium and steel also reduced the maximum dose, with titanium reaching 73.07% and steel reaching 71.36%. These results are significant for proton treatment, where precise planning depends on the exact location of the Bragg peak. Metal implants can significantly shorten the proton range and reduce treatment efficacy if these factors are not considered.
The results obtained for all modalities demonstrate that metal implants significantly impact distribution and depth of maximum energy deposition. High-density metallic materials, such as Co-Cr-Mo, have the greatest impact, leading to a significant shift of the Bragg peak for protons and a sharp drop in dose behind the implant for electrons and VHEE. On the other hand, lower-density materials, such as titanium, have a more moderate effect but still cause measurable changes in the dose profiles.
Clinically, these results are significant in radiation planning for patients with metal implants. It is essential to accurately model the effect of metal on dose distribution to avoid underdosing tumor tissue or over-irradiating surrounding healthy tissue. For proton therapy, the Bragg peak shift should be considered, and the treatment plan should be adjusted based on the type of implant. In the case of electron therapy, consideration could be given to using a different modality in areas with significant dose attenuation behind the metal. The ideal case is to avoid irradiation through the metal implant.
The advantage of the new planning systems is that they can account for heterogeneities in the patient’s body. They often also consider the characteristics of the implants, which contributes to accurate and efficient dose delivery.
The findings directly relate to radiation treatment planning in oncology patients with metallic implants. High-density metals such as Co-Cr-Mo cause significant dose perturbations—both dose build-up in the proximal region and attenuation beyond the implant—potentially resulting in underdose of the target volume and overdose (hot spots) or underdose (cold spots) in surrounding tissues due to dose scattering and backscatter effects.
Additionally, in the high-gradient dose regions around implants, the interaction of radiation with metallic structures may lead not only to complex spatial redistribution of the dose but also to structural changes in the implant material itself, especially under repeated exposure or with certain beam types (e.g., high-energy electrons or protons). While this effect may be less pronounced with clinical photon beams, it remains an important consideration for future studies and quality assurance protocols.
To mitigate these effects, we strongly recommend using avoidance sectors during VMAT plan optimization, preventing beam paths from crossing the implant. Similarly, for 3DCRT, IMRT, or FIMRT techniques, it is crucial to carefully select beam angles to avoid traversing metallic implants, regardless of their composition.
These findings are summarized in
Table 1, which serves as a practical reference for clinicians involved in treatment planning. Integrating such strategies alongside advanced calculation algorithms (e.g., Monte Carlo) and photon counting CT can improve dose accuracy and patient safety. This table summarizes the dose attenuation at a depth of 15 cm for VHEE and photon beams and the Bragg peak shift for proton beams across different metallic implant materials. Dose values were extracted using the following methods:
For VHEE, electrons and photons: at 15 cm depth.
For protons, Dmax range shift, defined as the difference in Bragg peak position compared to water Dmax.
These metrics quantify the relative impact of each material on the dose distribution. The table also includes clinical recommendations for treatment planning, such as using avoidance sectors in VMAT or beam angle restrictions in 3DCRT/IMRT techniques.
This study was performed using simplified geometries and homogeneous water phantoms, which do not fully represent the complexity of human anatomy. Although Monte Carlo simulations inherently account for both primary and scattered radiation, including contributions from surrounding materials and structures, the use of simplified phantoms may limit the realism of anatomical scatter conditions. Future work will address these limitations by validating the results in anthropomorphic phantoms and incorporating anatomical heterogeneities such as bone and soft tissue into the simulations. Additionally, further research will explore potential structural changes in metallic implants due to irradiation, including microcrack formation, material density changes, and early signs of corrosion. A 3D pelvic phantom representing the male anatomy will also be developed, allowing the replacement of metallic implants with bone to assess their impact on treatment planning. This phantom will be used to compare VMAT (with a single arc) and conventional 3D-CRT box techniques in detail. The study will include experimental verification through physical measurements conducted at the Department of Clinical and Radiation Oncology of the Faculty Hospital Žilina, providing real-world insight into dose distribution effects. The ultimate aim is to offer precise and clinically applicable recommendations for medical physicists involved in radiotherapy planning for patients with metallic implants.
5. Conclusions
Monte Carlo simulations demonstrated how metals influence distribution as they traverse the metal implant. Our findings indicate that radiation therapy beams should avoid metal implants whenever possible due to the production of low- and high-dose regions before and after the implant, respectively. Owing to the potential for hot spots and cold spots in dose distribution. When treating these patients, it is essential to consider the position of individual metal components throughout their bodies to prevent them from reaching the irradiation area. This entails adhering to the principles of IGRT while ensuring adequate avoidance zones around metallic implants. It is essential to prevent superfluous bremsstrahlung and backscatter effects in the irradiated region. In the context of VHEE, evaluating the consequences induced by metallic implants within the human body is essential.
Our results offer a critical understanding of the significant influence of metallic implants on dose distributions, particularly for high-density materials like Co-Cr-Mo. High-density materials like Co-Cr-Mo exhibit significant dose attenuation. For example, at 15 cm depth, dose attenuation for 6 MV photons was −15.16% with Co-Cr-Mo, −8.11% with titanium, and −13.23% with steel. For 150 MeV VHEE, the impact was even more pronounced, with dose attenuation of −19.26% for Co-Cr-Mo, −3.46% for titanium, and −11.8% for steel. This trend continues with 100 MeV VHEE, demonstrating significant sensitivity to high-density materials. Furthermore, for electron therapy at 15 MeV, dose attenuation at 3 cm depth was −55.52% for Co-Cr-Mo, −54.79% for titanium, and −52.87% for steel, highlighting substantial attenuation even at lower energies and shallower depths. The Bragg peak shift in proton therapy, with a displacement of −9.5 cm for Co-Cr-Mo, −4.4 cm for titanium, and −7 cm for steel at 170 MeV, illustrates the challenges of dose accuracy around metallic implants for proton therapy.
The findings of this work demonstrate that all treatment beams, including novel VHEE beams, must carefully consider patient implants to produce optimal dose distributions. The pronounced impact of titanium, stainless steel, and Co-Cr-Mo alloy on dose attenuation indicates that meticulous material selection and beam angle modification are essential when treating patients with metallic implants. Our research establishes a basis for enhancing radiotherapy planning systems, promoting comprehensive simulations between metal and ionizing radiation. The research substantiates the imperative to customize radiation treatments according to each patient’s anatomical and material characteristics, providing a pathway to safer and more successful treatment for individuals with metal implants.