Abstract
Hydrogen (H2) production from magnesium and its alloys offers an efficient alternative to traditional methods. In magnesium-based hydrolysis, the formation of a Mg(OH)2 passivation layer significantly hinders the reaction, thereby reducing hydrogen production efficiency. To address this issue, increasing the reaction temperature and selecting appropriate materials are essential. This study adjusted the compositions of magnesium alloys (pure Mg, Mg-Al, Mg-Zn) to form low-melting phases (Mg17Al12 and Mg7Zn3). The results demonstrate that as-cast Mg-Al and Mg-Zn alloys exhibit faster reaction rates and lower critical temperatures compared to pure Mg. Specifically, Mg-30wt.% Al and Mg-30wt.% Zn show the fastest reaction rates, with hydrogen purity reaching 99%. Mg-Zn alloys have an induction time of merely 98 s and can achieve complete reaction at 300 °C, while Mg-Al alloys require 174 s and 420 °C. Pure Mg, on the other hand, requires 270 s and 520 °C. Therefore, regarding the critical reaction temperature, the Mg-Zn alloy requires the lowest critical reaction temperature, the Mg-Al alloy requires a moderate reaction temperature, and pure Mg requires the highest critical reaction temperature. In addition, in terms of the performance of hydrogen production through hydrolysis, compared with pure Mg, Mg-30wt.%Al, Mg-20wt.%Zn, and Mg-30wt.%Zn all exhibit good hydrogen production performance through hydrolysis. Their final conversion rates are all higher than that of pure Mg. Among them, Mg-30wt.%Zn has the most excellent hydrogen production performance, followed by Mg-30wt.%Al. The specific conclusions will be analyzed and discussed in detail in the subsequent text.
1. Introduction
In the case of environmental pollution problems and the shortage of fossil fuels, hydrogen is considered an ideal renewable energy carrier due to its abundant sources, high energy density, and water being the sole emission [1,2]. Yet, in the process of practical application, hydrogen production, storage, and transportation are three key points that restrict its development [3,4]. To this day, with regard to hydrogen production, there have been various methods including the electrolysis of water, photolysis of water, gasification of heavy oil or biomass, and steam reforming of hydrocarbons [5,6,7]. The disadvantages of these methods, such as low efficiency and high pollution, seriously restrict the efficiency of hydrogen production and commercial application [8,9]. In recent years, lightweight metal production of hydrogen, i.e., with magnesium (Mg) and its alloys, via a hydrolysis reaction has attracted great attention due to its safety, low cost, and high efficiency [10,11]. Currently, numerous studies have focused on the use of titanium-based materials, aluminum-based materials, and magnesium-based materials for hydrogen production through the hydrolysis process. Titanium-based materials, due to their excellent corrosion resistance, chemical stability, and good electrical conductivity, are widely used as electrode materials and catalysts in the process of hydrogen production via hydrolysis [2,12,13,14,15]. However, the preparation cost of titanium-based materials is relatively high, and they do not possess the low-cost advantage compared to traditional electrolytic water splitting materials for hydrogen production. Additionally, their safety is relatively low, and hydrogen leakage can easily occur during the hydrogen production process via hydrolysis, posing safety hazards [13,16,17]. Regarding hydrogen production via hydrolysis using aluminum-based materials, aluminum, as a safe, inexpensive, lightweight metal material with a high specific surface area, and also as an electrochemically active element, can rapidly react with water upon contact during the hydrolysis reaction to release hydrogen [18]. However, during the reaction between aluminum and water, a dense byproduct of Al(OH)3 forms on the surface of the aluminum-based material [16,19,20]. This dense oxide film, encapsulating the material’s surface as Al(OH)3, severely hinders further contact between the fresh internal metal and water for hydrolysis reactions [21,22]. Consequently, further treatment of this byproduct is required, significantly reducing the efficiency and continuity of hydrogen production via hydrolysis. Magnesium-based materials are widely used in research on hydrogen production via hydrolysis due to their low cost, abundant resources, and high catalytic activity [23,24,25]. However, traditional magnesium-based materials for hydrogen production via hydrolysis face similar issues to those encountered with aluminum-based materials [24,26,27]. The byproduct Mg(OH)2 formed during hydrolysis acts as a passivation layer, further hindering the internal metal from undergoing further hydrolysis reactions, thereby leading to a deterioration in hydrolysis performance [13,28,29]. To address the aforementioned issues, extensive research has been conducted on ball milling modification, aqueous solution regulation, and the introduction of noble metal catalysts [30]. However, these methods all require cumbersome preparation processes for the reactants, significantly increasing the cost of hydrogen production via hydrolysis. Therefore, there is a need to explore new strategies for hydrogen production via hydrolysis to achieve a low-cost, high-efficiency, high-purity, and high-safety reaction process [31]. Yinon Yavor et al. studied the laws of hydrogen production by the hydrolysis of nanoscale and microscale aluminum powders within a temperature range of 80–200 °C in a high-pressure reaction vessel. The hydrogen yield increases with the rise in temperature and the decrease in particle size. The conversion rate can be maximized by increasing the reaction temperature [32]. Xiao et al. significantly improved the hydrolysis kinetic performance by ball-milling magnesium with low-melting-point metals. Mg-10In% has a relatively low corrosion potential and a high corrosion current density, thus exhibiting good hydrogen production performance through hydrolysis [33]. S. Al Bacha et al. prepared the intermetallic compound Mg17Al12 in the Mg-Al alloy through alloying. They utilized the potential difference between Mg and Mg17Al12 to accelerate the corrosion rate of hydrogen evolution of the Mg-Al alloy in a 3.5 wt.% NaCl aqueous solution [34]. Magnesium metal and its hydrides can spontaneously react with water under ambient temperature and pressure conditions to produce hydrogen gas. The reaction equations are as follows:
Herein, a novel hydrogen production method for pure Mg and its alloys utilizes high-temperature water vapor. High-temperature hydrolysis significantly boosts the hydrogen production rate and conversion efficiency. Introducing low-melting-point alloy phases such as Mg17Al12 and Mg7Zn3 accelerates hydrolysis kinetics, thereby reducing the critical reaction temperature and enhancing hydrogen production performance. The chemical equations for the reactions of pure Mg, Mg-Al alloys, and Mg-Zn alloys with water vapor in a high-temperature environment are as follows:
This study investigates the high-temperature hydrolysis reaction of pure Mg and low-melting-point Mg-Al and Mg-Zn alloys. Magnesium, aluminum, and zinc are abundant and cost-effective metals. The research systematically examines how temperature, alloy composition, and low-melting-point alloy content influence the hydrolysis-based hydrogen production of Mg-based metals. It reveals the significant impact of the Mg(OH)2 passivation layer on hydrogen production efficiency. The results demonstrate that adjusting alloy composition and content can lower the reaction temperature and enhance efficiency. Both Al and Zn improve hydrogen production, with Zn exhibiting superior performance. This work paves the way for designing Mg-based materials for high-yield “green” hydrogen production and portable hydrogen devices.
Aluminum and zinc were added to the Mg matrix through alloying to form Mg-10Al, Mg-20Al, Mg-30Al, Mg-10Zn, Mg-20Zn, and Mg-30Zn, respectively, introducing the low-melting-point second phases Mg17Al12 and Mg7Zn3. Firstly, compared to pure magnesium, there exists a significant difference in melting points between the Mg matrix and the Mg17Al12 and Mg7Zn3 secondary phases in Mg-Al and Mg-Zn alloys. Consequently, under high-temperature reaction conditions, the low-melting-point Mg17Al12 and Mg7Zn3 phases react first, providing accessible channels for water vapor to enter and facilitate the subsequent hydrolysis reaction of the Mg matrix. The reaction of Mg17Al12 and Mg7Zn3 phases subsequently provides more effective channels for water vapor to penetrate into the interior of the magnesium matrix. Secondly, due to the differences in electrochemical activity between the magnesium matrix and the Mg17Al12 and Mg7Zn3 secondary phases, the formation of micro-galvanic cells promotes the occurrence of the hydrolysis reaction. Lastly, the incorporation of aluminum and zinc to form low-melting-point secondary phases enhances the hydrogen production performance of Mg-based metals via hydrolysis, and these secondary phases themselves can also serve as raw materials for hydrogen production through hydrolysis.
The preparation processes for pure Mg and Mg-Al, Mg-Zn alloys are straightforward, and no further specialized treatment is required for these reactants in the hydrolysis reaction. In Mg-Al and Mg-Zn alloys, the Mg17Al12 and Mg7Zn3 phases, which are secondary phases with different morphologies, have melting points lower than the Mg matrix. Therefore, under various reaction temperature conditions, they can react before the Mg matrix melts, thereby providing numerous voids within the Mg matrix. This allows water vapor to rapidly penetrate into the interior of the magnesium matrix to undergo rapid hydrolysis for hydrogen production. This effectively prevents the formation of a dense Mg (OH)2 passivation layer on the surface, which can hinder the efficiency of hydrogen production via hydrolysis. Consequently, the reaction rate of hydrogen production via hydrolysis is significantly enhanced, while simultaneously achieving a low-cost hydrogen production process via hydrolysis.
2. Experimental Procedure
2.1. Material Preparation
In this study, Mg-Al and Mg-Zn alloys with different compositions were investigated. Raw materials, as-cast plates from Beijing Kairui, needed no special treatment. Instead of complex processing, alloy plates underwent simple turning and milling. Cast ingots were milled by a CNC machining center to obtain 2–3 mm metal shavings for reactions. Table 1 shows their compositions. The addition of metallic Al and Zn elements can lower the alloy melting point; based on phase diagram analysis, the melting point of Mg-10Al is approximately 600 °C. It is worth noting that the decrease in the melting point of the original hydrogen-producing alloy material is not the key factor. Instead, the critical reaction temperature observed in the experiment is primarily influenced by the distribution state, morphology, and content of the Mg17Al12 and Mg7Zn3 alloy phases. For reference, pure Mg has a melting point of 650 °C, while the melting points of the Mg17Al12 and Mg7Zn3 phases are approximately 400 °C and 340 °C, respectively.

Table 1.
Composition mass ratios of pure magnesium, magnesium–aluminum, and magnesium–zinc alloys.
2.2. Characterization
X-ray diffraction (XRD) analysis was conducted on Mg and the reactant materials of Mg-10/20/30Al and Mg-10/20/30Zn alloys with varying compositions and contents, with a scanning range of 10–90°. The microstructures and the distribution of precipitated phases in Mg, Mg-10/20/30Al, and Mg-10/20/30Zn alloys were investigated using scanning electron microscopy (SEM). Simultaneously, energy dispersive spectroscopy (EDS) combined with X-ray diffraction (XRD) was employed to determine and analyze the phase composition. The Mg-10/20/30Al and Mg-10/20/30Zn alloys were progressively polished using 240-, 280-, 360-, 400-, 600-, 800-, 1000-, 1200-, 1500-, 2000-, and 2500-grit abrasive papers on a metallographic grinding machine until the surface was free of visible scratches. Diamond polishing pastes with grit sizes of 3.5, 1.5, 1.0, and 0.5 μm were selected as abrasives, and a polishing machine was used to polish the entire surface. The hydrolysis reaction products obtained under high-temperature conditions were white powders. X-ray diffraction (XRD) analysis was conducted on the reaction products of Mg, Mg-10/20/30Al, and Mg-10/20/30Zn alloys with varying compositions and contents at different reaction temperatures, with a scanning range of 10–90°. Scanning electron microscopy (SEM) was used to observe the microstructures of the reaction products of Mg, Mg-10/20/30Al, and Mg-10/20/30Zn alloys at different reaction temperatures. Energy dispersive spectroscopy (EDS) was employed to investigate the elemental distribution in the reaction products of these alloys under various reaction temperature conditions.
2.3. Hydrolysis Test
Hydrolysis Experiment: The hydrogen production performance of as-cast Mg, Mg-10/20/30Al, and Mg-10/20/30Zn alloys at different reaction temperatures was tested using a self-made hydrogen production reactor with a hydrolysis setup, with the hydrogen volume measured at atmospheric pressure. Before each experiment, 10 g of the original material is weighed and used for the reaction. Each group of experiments is repeated 4 times. The hydrogen production setup mainly consists of a steam generator, a reactor, a data recorder, and a terminal display controller. Specifically, the internal volume of the reaction kettle is 2 L. The heating rate is set at 5 °C per minute and, before steam injection, the internal pressure was 10 Pa; after injection, it increased to 0.8 MPa. The data recording equipment primarily includes a hydrogen flowmeter, capable of detecting both instantaneous and cumulative hydrogen flow rates; a hydrogen purity analyzer for detecting the purity of the produced hydrogen; and a thermocouple for real-time monitoring of temperature changes inside the reactor. The reactor primarily serves as a container for the Mg/H2O hydrogen production reaction, where superheated steam generated by a steam generator and a steam heater is introduced into the reactor to participate in the hydrogen production reaction. The outer wall of the reactor is uniformly wrapped with resistance wires and employs variable frequency heating technology. Accurate and controllable temperatures within the range of 100–700 °C can be achieved through a control box connected to the reactor and multiple temperature sensors installed inside the reactor for measuring temperature at various points. At its top, the reactor is equipped with steam inlet piping and hydrogen outlet piping. At the other end of the hydrogen outlet piping, there is a hydrogen condensation, purification, and refinement unit, followed by a hydrogen purity analyzer and a flowmeter for real-time data detection and collection. The steam generation device is located outside the reaction kettle. The reaction kettle itself is equipped with a steam inlet pipeline. In each experimental process, the reaction kettle device is first heated up to the set temperature, and then the steam is introduced by connecting the external steam pipeline to the steam inlet pipeline of the reaction kettle itself. Before each experiment, the tightness of the pipeline is checked, and it is ensured that there is no blockage of other products inside the pipeline. The H2O-H2 mixed gas generated by the magnesium metal hydrolysis reaction passes through a condenser and a drying oven in sequence to obtain high-purity hydrogen. For the hydrogen separation and purification device, as well as the data collection part, all the pipeline connections upstream of the condenser are made of 316 L stainless steel seamless pipes, and the pipeline connections after the condenser are all made of pressure-resistant pneumatic quick-connect pipes. The steam generation device is outside the reaction kettle with a steam inlet pipeline. Before each experiment, the pipeline tightness is checked. The kettle is heated to the set temperature, then steam is introduced. The H2O-H2 gas from magnesium hydrolysis passes through a condenser and drying oven for high-purity hydrogen. For separation, purification, and data collection, 316 L stainless steel seamless pipes are used upstream of the condenser, and pressure-resistant pneumatic quick-connect pipes are used downstream. The purified hydrogen (T < 30 °C) has its purity analyzed by a hydrogen purity analyzer, and its flow rate detected by a flowmeter with data acquisition software for real-time and cumulative data. To ensure the accuracy of hydrogen production data, the entire hydrogen production setup’s airtightness is repeatedly checked before conducting the experiment. Figure 1 depicts the flowchart of the magnesium-based metal hydrolysis hydrogen production reaction.
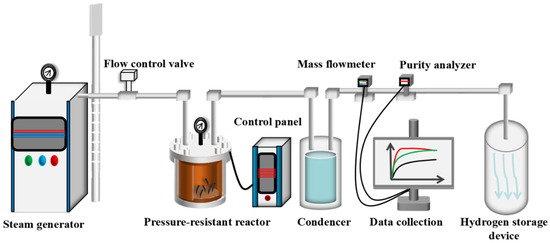
Figure 1.
Schematic diagram of hydrogen production plant by high temperature hydrolysis of Mg, Mg-Al and Mg-Zn.
3. Results and Discussion
3.1. Phase Composition and Microstructure Characteristics of As-Melt Alloys
Figure 2a presents the XRD pattern of as-cast pure Mg. Figure 2b shows the XRD patterns of as-cast Mg-10Al, Mg-20Al, and Mg-30Al alloys. Figure 2c displays the XRD patterns of as-cast Mg-10Zn, Mg-20Zn, and Mg-30Zn alloys. For as-cast pure Mg, only the Bragg peaks of Mg were observed. For Mg-10Al, Mg-20Al, and Mg-30Al, the Bragg peaks of both Mg and Mg17Al12 were observed. For Mg-10Zn, Mg-20Zn, and Mg-30Zn, the Bragg peaks of both Mg and Mg7Zn3 were observed. The presence of weak MgO diffraction peaks in pure Mg may be attributed to slight oxidation during the experimental operation, specifically when the Mg metal was briefly exposed to air during turning. No other impurities or oxides, such as MgO, were observed in the XRD patterns of Mg-Al and Mg-Zn alloys, indicating that both materials were not oxidized during the initial preparation process. No shift in the Bragg peaks of Mg was observed in the XRD patterns of the aforementioned materials.
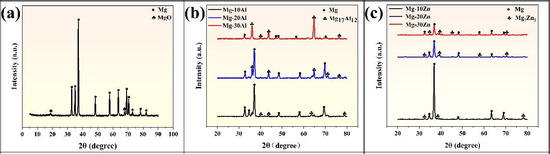
Figure 2.
The XRD pattern of the initial material of (a) pure-Mg. XRD patterns of the initial materials of (b) Mg-10Al, Mg-20Al, and Mg-30Al alloys. XRD patterns of the initial materials of (c) Mg-10Zn, Mg-20Zn, and Mg-30Zn alloys.
Figure 3 displays the SEM and EDS images of as-cast pure Mg. As observed in Figure 3, the machined magnesium milling surface exhibits a stepped appearance accompanied by locally torn gaps. This phenomenon arises due to the poor plasticity of metallic magnesium during the machining process. Additionally, trace amounts of oxygen elements are observed in the EDS images, consistent with the previous XRD patterns. The formation of a trace amount of MgO passivation film on the Mg matrix surface due to slight oxidation does not significantly affect the hydrogen production performance of magnesium-based metal hydrolysis under high-temperature conditions.

Figure 3.
The SEM and EDS spectra of the initial material of pure-Mg.
As observed in Figure 4a–c, the as-cast Mg-10Al, Mg-20Al, and Mg-30Al binary alloys primarily consist of a Mg matrix and a secondarily precipitated Mg17Al12 microstructure distributed within it. The corresponding microstructural details of the secondarily precipitated Mg17Al12 are shown in the locally enlarged images. With increasing aluminum content, when the Al content is only 10%, the secondarily precipitated Mg17Al12 is dispersed in a dot-like and partially lamellar form within the magnesium matrix. When the Al content rises to 20%, the secondarily precipitated Mg17Al12 exhibits a continuous lamellar distribution, with a small amount also observed in a dot-like form. When the aluminum content increases to 30%, the secondarily precipitated Mg17Al12 forms a continuous lamellar network distribution, nearly covering the entire surface of the magnesium matrix, making it difficult to observe dot-like dispersed Mg17Al12 phases at this point. The EDS spectra of magnesium and aluminum elements reflect the distribution of the Mg17Al12 phase. Combined with the XRD patterns shown in Figure 2b, it further confirms that the materials were not oxidized during the initial machining process, and no MgO was formed. With increasing aluminum content, there is a clear distinction between the dot-like, lamellar, and lamellar network secondarily precipitated Mg17Al12 phases and the magnesium matrix phase.
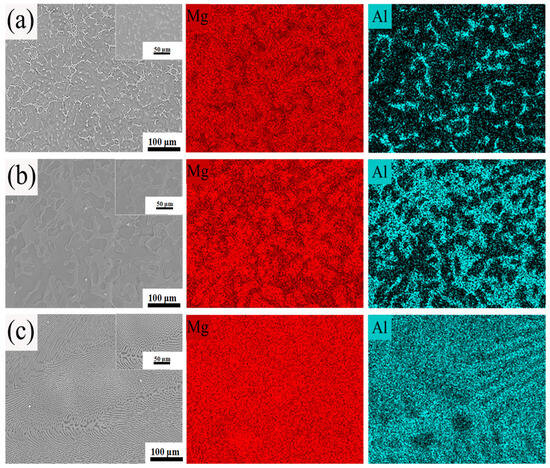
Figure 4.
The SEM and EDS images of the initial material of (a) Mg-10Al, (b) Mg-20Al, and (c) Mg-30Al.
As observed in Figure 5a–c, the as-cast Mg-10Zn, Mg-20Zn, and Mg-30Zn binary alloys primarily consist of a magnesium matrix and a secondarily precipitated Mg7Zn3 microstructure distributed within it. With increasing zinc content, changes are observed. When the zinc content is 10%, the secondarily precipitated Mg7Zn3 is dispersed in a dot-like and discontinuous strip-like form within the magnesium matrix. When the zinc content rises to 20%, the secondarily precipitated Mg7Zn3 exhibits a distribution of a few dot-like forms and partially continuous strip-like forms within the Mg matrix. When the zinc content increases to 30%, the secondarily precipitated Mg7Zn3 is distributed in a very small number of dot-like forms, continuous strip-like forms, and lamellar forms within the magnesium matrix. Since the solid solubility of zinc in the magnesium matrix is lower than that of aluminum in the magnesium matrix, there are significant differences in the distribution of the secondarily precipitated Mg7Zn3 compared to Mg17Al12 within the magnesium matrix. Figure 5 also shows the EDS images of the secondarily precipitated regions in the as-cast Mg-10Zn, Mg-20Zn, and Mg-30Zn alloys. The EDS spectra of magnesium and zinc elements reflect the distribution of the Mg7Zn3 phase. With increasing zinc content, there is a clear distinction between the dot-like, strip-like, and lamellar secondarily precipitated Mg7Zn3 phases and the magnesium matrix phase. Combined with the XRD patterns shown in Figure 2c, it further confirms that the materials were not oxidized during the initial machining process, and no MgO was formed.
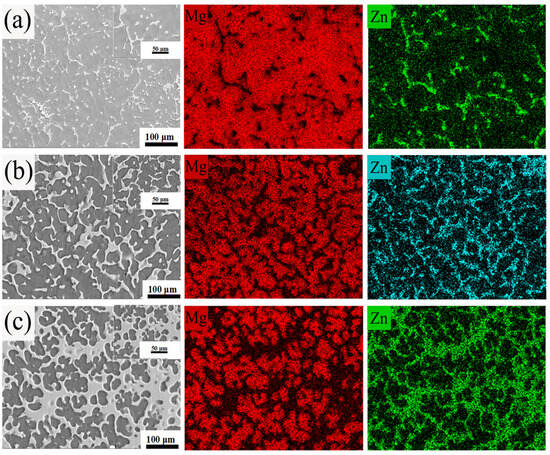
Figure 5.
The SEM and EDS images of the initial material of (a) Mg-10Zn, (b) Mg-20Zn, and (c) Mg-30Zn.
3.2. Effects of Alloy Type and Temperature on Hydrolysis Hydrogen Generation
Based on the hydrolysis reaction of magnesium-based metals with water for hydrogen production, the theoretical hydrogen yield for pure magnesium is approximately 1.0058 L per gram (L/g) at 0 °C and 1 atmosphere (atm, standard conditions). For Mg-10Al, Mg-20Al, and Mg-30Al alloys, the theoretical hydrogen yields are calculated to be approximately 1.0414 L/g, 1.0773 L/g, and 1.1124 L/g, respectively. In contrast, Mg-10Zn, Mg-20Zn, and Mg-30Zn alloys have theoretical hydrogen yields of approximately 0.9414 L/g, 0.8057 L/g, and 0.7478 L/g, respectively.
Figure 6 illustrates the hydrolysis hydrogen production kinetics curves of pure magnesium under various temperature conditions. The results indicate a strong dependence of the hydrolysis kinetics and hydrogen production rate of magnesium-based metallic materials on temperature. As depicted in Figure 6a–c, these figures present the hydrogen conversion rates, hydrolysis hydrogen production kinetics curves, and temperature variation curves during hydrogen production for pure magnesium reacting with water vapor at 510 °C, 520 °C, 560 °C, and 600 °C. Enhancing the temperature of the hydrolysis reaction significantly improves the hydrogen production performance of pure magnesium. Figure 6a shows the hydrogen conversion rates of pure magnesium under different reaction temperature conditions. Except for the condition at 510 °C where hydrolysis did not occur, complete hydrolysis was achieved at 520 °C, 560 °C, and 600 °C, with final hydrogen conversion rates reaching up to 94%. As shown in Figure 6b, the hydrogen production curves of pure magnesium reacting with water vapor under different reaction temperatures are presented. An increase in reaction temperature significantly enhances the hydrolysis kinetics during the initial stage, thereby greatly accelerating the hydrogen production rate. The maximum instantaneous hydrogen production rate rapidly reaches its peak and then gradually decreases. Specifically, the maximum hydrogen production rates during the initial stage for pure magnesium at 510 °C, 520 °C, 560 °C, and 600 °C increase from 0 L/min to 19.122 L/min, 23.636 L/min, and 24.396 L/min, respectively (note: the value for 510 °C should be 0 as no hydrolysis occurred, but it is included here for completeness of the temperature range mentioned). In Figure 6c, the temperature variations during the hydrolysis hydrogen production process of pure magnesium under different reaction temperature conditions are presented. At 510 °C, due to the inability of pure magnesium to undergo hydrolysis, the temperature change is insignificant. When the reaction temperature is ≥520 °C, significant temperature changes are observed. The higher the initial temperature, the more rapid and intense the hydrolysis reaction, leading to increased heat release and a shorter time to reach the maximum temperature. Once the temperature peaks, the hydrolysis reaction ceases immediately, and the temperature then drops linearly. Table 2 presents the induction times and complete reaction times for pure magnesium under reaction conditions at 510 °C, 520 °C, 560 °C, and 600 °C.
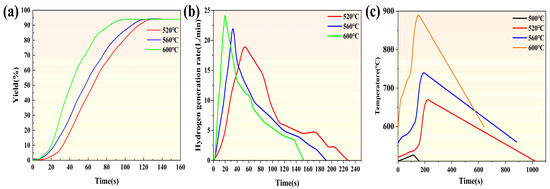
Figure 6.
The hydrogen generation yield curves of (a) pure-Mg; hydrogen generation rate curves of (b) pure-Mg; and the temperature variation curves of (c) pure-Mg.

Table 2.
Hydrogen generation performance of the Mg samples in water vapor.
The results indicate that an increase in temperature can significantly enhance hydrogen production kinetics. As the temperature rises, the reaction becomes more intense, leading to a substantial reduction in both the induction time and the overall reaction time. Consequently, the performance of hydrogen production through hydrolysis is significantly improved.
Figure 7 illustrates the hydrolysis hydrogen production kinetics curves of Mg-10Al, Mg-20Al, and Mg-30Al under various temperature conditions, respectively. The results indicate a strong dependence of the hydrolysis kinetics and hydrogen conversion rates of magnesium-based metallic materials on the composition, content, and temperature of the low-melting alloy phases. Specifically, Figure 7(a1–a3) show the hydrogen conversion rate curves of Mg-10Al, Mg-20Al, and Mg-30Al under different temperature conditions. When the reaction temperature is 420 °C, hydrolysis does not occur for Mg-10Al, resulting in a conversion rate of 0%. Furthermore, Mg-10Al undergoes complete hydrolysis under reaction conditions at 430 °C, 460 °C, 490 °C, 520 °C, and 550 °C, with a hydrogen conversion rate of 89%. Additionally, Mg-20Al and Mg-30Al also undergo complete hydrolysis under reaction conditions at 420 °C, 430 °C, 460 °C, 490 °C, 520 °C, and 550 °C, with hydrogen conversion rates of 92% and 97%, respectively. Figure 7(b1–b3) depict the hydrolysis hydrogen production kinetics curves of Mg-10Al, Mg-20Al, and Mg-30Al, respectively, under various temperature conditions. When Mg-10Al is subjected to a reaction temperature of 420 °C, no hydrolysis reaction occurs, thus no data are generated for this condition. It is also evident that an increase in temperature has a significant impact on the hydrogen production rate. As the temperature rises, Mg-10Al, Mg-20Al, and Mg-30Al all exhibit faster initial instantaneous hydrogen production rates with higher rate values, which is consistent with the effect of temperature on the hydrogen production performance of pure magnesium mentioned above. Furthermore, with the increase in the aluminum content, a transition is observed from Mg-10Al not reacting at all at 420 °C to Mg-20Al and Mg-30Al being able to undergo complete hydrolysis for hydrogen production at the same temperature. Compared with pure magnesium in Figure 6, the incorporation of aluminum in Mg-based alloys directly reduces the critical reaction temperature from 520 °C for pure magnesium to 430 °C. Furthermore, as the aluminum content increases, the reaction temperature decreases from 430 °C for Mg-10Al to 420 °C. The results indicate that with the incorporation and increase in aluminum content, the required temperature for hydrogen production through hydrolysis of magnesium-based metals can be effectively lowered, significantly enhancing the hydrogen production rate and improving the performance of hydrolysis hydrogen production. Figure 7(c1–c3) depict the temperature changes during the hydrolysis reactions of Mg-10Al, Mg-20Al, and Mg-30Al, respectively. At 420 °C, Mg-10Al does not undergo hydrolysis, resulting in insignificant temperature changes. However, when the reaction temperature is ≥420 °C, significant temperature changes are observed. The higher the initial temperature, the more rapid and intense the hydrolysis reaction, releasing more heat and resulting in a shorter time to reach the maximum temperature. Once the temperature peaks, the hydrolysis reaction immediately ceases, and the temperature drops linearly. Mg-20Al and Mg-30Al can undergo complete reactions within the temperature range of 420 °C to 550 °C, with temperature trends similar to Mg-10Al. Table 3 presents the induction time and complete reaction time for pure Mg, Mg-10Al, Mg-20Al, and Mg-30Al under reaction conditions at 420 °C, 430 °C, 460 °C, 490 °C, 520 °C, and 550 °C, respectively. The results indicate that increased aluminum content and temperature significantly enhance the hydrolysis hydrogen production kinetics. As aluminum content and temperature rise, the reaction becomes more intense, with greatly reduced induction and reaction times, leading to a significant improvement in hydrogen production performance through hydrolysis.
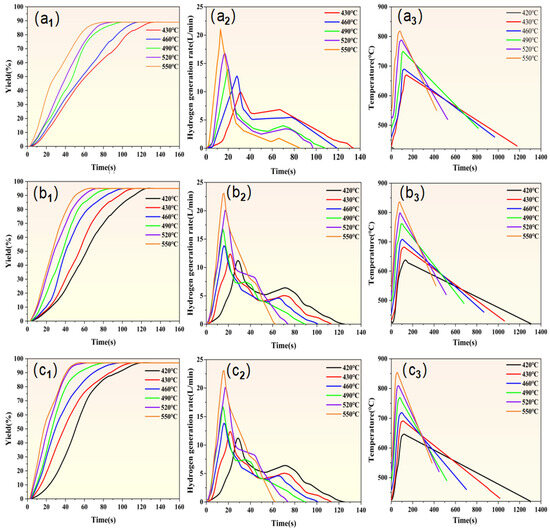
Figure 7.
The hydrogen generation yield curves of (a1) Mg-10Al, (b1) Mg-20Al, and (c1) Mg-30Al; hydrogen generation rate curves of (a2) Mg-10Al, (b2) Mg-20Al, and (c2) Mg-30Al; and the temperature variation curves of (a3) Mg-10Al, (b3) Mg-20Al, and (c3) Mg-30Al.

Table 3.
Hydrogen generation performance of the Mg-Al alloy samples in water vapor.
Figure 8 presents the hydrolysis hydrogen production kinetics curves of Mg-10Zn, Mg-20Zn, and Mg-30Zn under different temperature conditions. The results also indicate that the hydrolysis kinetics and hydrogen production conversion rate of Mg-based metallic materials are highly dependent on the composition, content, and temperature of the low-melting alloy phases. Specifically, Figure 8(a1–a3) show the hydrogen production conversion rate curves of Mg-10Zn, Mg-20Zn, and Mg-30Zn, respectively, under various temperature environments. When the reaction temperature is 300 °C, Mg-10Zn does not undergo hydrolysis, resulting in a conversion rate of 0%. Furthermore, Mg-10Zn undergoes complete hydrolysis at reaction temperatures of 330 °C, 360 °C, 390 °C, 420 °C, and 450 °C, with a hydrogen production conversion rate of 91%. Additionally, Mg-20Zn and Mg-30Zn also undergo complete hydrolysis at reaction temperatures ranging from 300 °C to 450 °C (including 330 °C, 360 °C, 390 °C, 420 °C), with hydrogen conversion rates of 94% and 98%, respectively. Mg-20Zn and Mg-30Zn undergo complete hydrolysis at reaction temperatures of 300 °C, 330 °C, 360 °C, 390 °C, 420 °C, and 450 °C, with hydrogen conversion rates of 94% and 98%, respectively. As shown in Figure 8(b1–b3), the hydrolysis hydrogen production kinetics curves for Mg-10Zn, Mg-20Zn, and Mg-30Zn under different temperature conditions are presented, respectively. When Mg-10Zn is subjected to a reaction temperature of 300 °C, no hydrolysis reaction occurs, resulting in no data being generated for this condition. It is also clearly observable that an increase in temperature has a significant impact on the hydrogen production rate. Specifically, as the temperature rises, Mg-10Zn, Mg-20Zn, and Mg-30Zn all exhibit faster initial instantaneous hydrogen production rates with higher rate values, which is consistent with the aforementioned influence on the hydrogen production performance of pure magnesium. Furthermore, with the increasing zinc content, there is a transition from Mg-10Zn, which exhibits no reaction at all at a temperature of 300 °C, to Mg-20Zn and Mg-30Zn, which can undergo complete hydrolysis for hydrogen production at the same temperature of 300 °C. When compared with pure magnesium, as shown in Figure 4, the incorporation of zinc directly reduces the critical reaction temperature from 520 °C for pure magnesium to 330 °C for Mg-based alloys. Additionally, as the zinc content further increases, the reaction temperature decreases from 330 °C for Mg-10Zn to 300 °C for Mg-20Zn. The results indicate that with the incorporation and increase in zinc content, the temperature required for hydrogen production through hydrolysis of Mg-based metals can be effectively lowered, significantly enhancing the hydrogen production rate and improving the performance of hydrogen production through hydrolysis. This is attributed to the formation of Mg7Zn3 as a secondary precipitate phase with the magnesium matrix upon the addition of zinc, even though the solid solubility of zinc in the magnesium matrix is only 6.2%, which is lower than the solid solubility of aluminum in the magnesium matrix at 12.7%. The secondary precipitate phase formed is significantly less than that of Mg17Al12, but the melting point of the Mg7Zn3 precipitate phase is much lower than that of pure magnesium (Mg7Zn3 < Mg17Al12 < Mg). Therefore, it is evident that the required reaction temperature for Mg-Zn alloys is much lower than that for Mg-Al alloys and even lower than that for pure magnesium. Similarly, with the increase in zinc content, more Mg7Zn3 precipitate phases with low melting points are formed in the magnesium matrix. These precipitates can react prior to the hydrolysis of the magnesium matrix, providing more channels for the subsequent hydrolysis of fresh internal metal. This facilitates the timely diffusion of water vapor into the interior and ensures sufficient contact with the fresh metal for the completion of the hydrolysis reaction. Effectively, this avoids the hindrance of the MgO passivation layer on the material surface, which could otherwise impede the full contact between water vapor and internal metal. Consequently, the temperature required for hydrolysis can be significantly reduced, and the hydrogen production performance of magnesium-based metals through hydrolysis is notably improved. Figure 8(c1–c3) depict the temperature variations during the hydrolysis reactions of Mg-10Zn, Mg-20Zn, and Mg-30Zn, respectively. At 300 °C, Mg-10Zn fails to undergo hydrolysis, resulting in insignificant temperature changes. When the reaction temperature reaches or exceeds 330 °C, significant temperature variations are observed, with higher initial temperatures leading to more rapid and intense hydrolysis reactions, releasing more heat and reaching the maximum temperature sooner. Once the temperature peaks, the hydrolysis reaction ceases immediately, and the temperature drops linearly. Mg-20Zn and Mg-30Zn can completely react within the temperature range of 300 °C to 450 °C, following a similar temperature trend as Mg-10Al. Table 4 presents the induction times and complete reaction times for Mg-10Zn, Mg-20Zn, and Mg-30Zn under reaction conditions of 300 °C, 330 °C, 360 °C (corrected from 660 °C for consistency), 390 °C, 420 °C, and 450 °C. The results indicate that increasing zinc content and temperature significantly enhance hydrogen production kinetics. As zinc content and temperature rise, the reactions become more intense, with significantly shortened induction times and overall reaction durations, leading to a notable improvement in hydrogen production performance through hydrolysis.
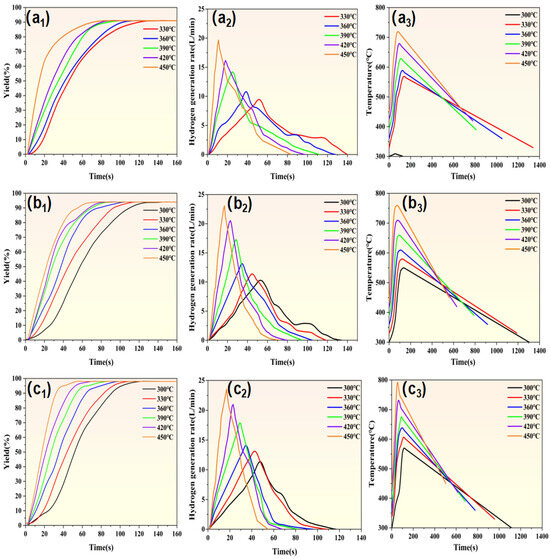
Figure 8.
The hydrogen generation yield curves of (a1) Mg-10Zn, (b1) Mg-20Zn, and (c1) Mg-30Zn; hydrogen generation rate curves of (a2) Mg-10Zn, (b2) Mg-20Zn, and (c2) Mg-30Zn; and the temperature variation curves of (a3) Mg-10Zn, (b3) Mg-20Zn, and (c3) Mg-30Al.

Table 4.
Hydrogen generation performance of the Mg-Zn alloy samples in water vapor.
3.3. Microstructure of Hydrolytic Alloys
Solid products were collected after the complete reaction of pure Mg, Mg-Al alloys (10/20/30 wt.% Al), and Mg-Zn alloys (10/20/30 wt.% Zn) with water vapor under various temperature conditions. Figure 9 presents the X-ray diffraction (XRD) patterns of the hydrolysis products of these materials at high temperatures. Specifically, Figure 9a shows the XRD pattern of the reaction products after the complete hydrolysis of pure Mg, where only MgO diffraction peaks are observed. Figure 9b displays the XRD patterns of the reaction products after the complete hydrolysis of Mg-10Al, Mg-20Al, and Mg-30Al, where both MgO and Al2MgO4 diffraction peaks are present. As the aluminum content increases from 10% to 20% and 30%, the MgO diffraction peak gradually weakens, while the Al2MgO4 diffraction peak gradually intensifies, albeit remaining relatively weak compared to the MgO peak. This is due to the increase in the Mg17Al12 secondary phase with rising aluminum content. Consequently, after complete hydrolysis, the MgO diffraction peak decreases, while the Al2MgO4 diffraction peak becomes more prominent. Figure 9c presents the X-ray diffraction (XRD) patterns of the reaction products after the complete hydrolysis of pure Mg-10Zn, Mg-20Zn, and Mg-30Zn. It is observed that both MgO and ZnO diffraction peaks are present. As the zinc content increases, the MgO diffraction peak gradually weakens, while the ZnO diffraction peak gradually intensifies, albeit remaining relatively weak compared to the MgO peak. This is attributed to the increase in the Mg7Zn3 phase with rising zinc content. Consequently, after complete hydrolysis, the MgO diffraction peak decreases, while the ZnO diffraction peak becomes more prominent. Among the reaction products of Mg, Mg-Al, and Mg-Zn alloys, no characteristic peaks of metallic magnesium (Mg) or Mg (OH)2 were observed. The primary reason may be that after complete hydrolysis, magnesium initially forms Mg (OH)2 as a byproduct, which gradually converts to MgO under high-temperature conditions. This also indicates that Mg, Mg-Al, and Mg-Zn alloys can undergo complete hydrolysis to produce hydrogen at the reaction temperatures set in this study.
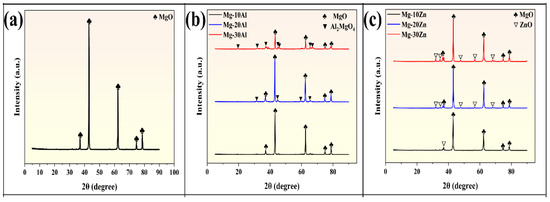
Figure 9.
The XRD pattern of the reaction products of (a) pure-Mg; XRD patterns of the reaction products of (b) Mg-10Al, Mg-20Al, and Mg-30Al alloys; XRD patterns of the reaction products of (c) Mg-10Zn, Mg-20Zn, and Mg-30Zn alloys.
As shown in Figure 10, the SEM and EDS images depict the products of pure magnesium after complete hydrolysis at high temperatures. It is evident that the morphology of the reaction products consists of small, aggregated square-like particles. The EDS spectrum reveals the presence of only magnesium and oxygen elements, further confirming that the hydrolysis product of pure magnesium under high-temperature conditions is solely MgO, with no remaining metallic magnesium.
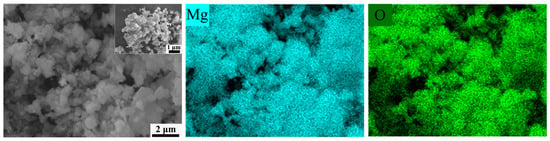
Figure 10.
The SEM and EDS images of the reaction products of pure-Mg.
Figure 11a–c present the SEM and EDS images of the hydrolysis products of Mg-10Al, Mg-20Al, and Mg-30Al alloys at high temperatures, respectively. It is clearly observed that the morphology of the reaction products consists of small, aggregated square-like particles accompanied by a small amount of interconnected fluffy products. As the aluminum content increases, a notable increase in the number of square-like particles is observed, as shown in Figure 11a–c, while the fluffy products decrease accordingly. Most of the fluffy MgO appears between the small square-like MgO particles. As the reaction progresses, the surface oxide film gradually cracks, allowing the internal molten magnesium metal to eject along the cracks or gaps in the oxide film and react with water vapor to form fluffy MgO products. The primary reason for this is that at lower aluminum contents, the internal metal does not have sufficient channels for water vapor to penetrate and react simultaneously with the external metal. Therefore, as the reaction continues, the internal molten magnesium metal can only wait for the surface oxide film to crack, allowing it to eject along the cracks or gaps and react with water vapor to form fluffy MgO products. With the increase in aluminum content, the precipitated Mg17Al12 phase increases accordingly, providing more effective channels for water vapor to penetrate into the magnesium matrix. This enables almost simultaneous reactions on both the internal and external surfaces. Furthermore, as the aluminum content increases, the reaction rate accelerates, and the reaction becomes more intense. Therefore, Mg-Al alloys with high aluminum content can undergo almost simultaneous complete hydrolysis reactions on both the external and internal metals within the shortest time. After the high-temperature reaction, the EDS images show the presence of magnesium, aluminum, and oxygen elements, which is consistent with the XRD pattern in Figure 9b, further confirming that the hydrolysis products of pure magnesium under high-temperature conditions are MgO and Al2MgO4.
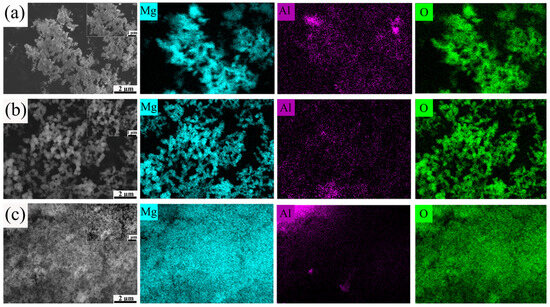
Figure 11.
The SEM and EDS images of the reaction products of (a) Mg-10Al, (b) Mg-20Al, and (c) Mg-30Al.
Figure 12a–c present the SEM and EDS images of the reaction products of Mg-10Zn, Mg-20Zn, and Mg-30Zn, respectively, after complete hydrolysis. It is clearly observed that the morphology of the reaction products consists of small, aggregated square-like particles accompanied by a small amount of interconnected fluffy products. As the zinc content increases, a notable increase in the number of square-like particles is observed, as shown in Figure 12, while the fluffy products decrease accordingly. Most of the fluffy MgO appears between the small square-like MgO particles. As the reaction progresses, the surface oxide film gradually cracks, allowing the internal molten magnesium metal to eject along the cracks or gaps in the oxide film and react with water vapor to form fluffy MgO products. The primary reason for this observation is that, at lower zinc contents, the internal metal does not have sufficient channels for water vapor to penetrate and react simultaneously with the external metal. Therefore, as the reaction continues, the internal molten magnesium metal can only wait for the surface oxide film to crack, allowing it to eject along the cracks or gaps and react with water vapor to form fluffy MgO products. However, as the zinc content increases, the precipitated Mg7Zn3 phase increases accordingly, providing more effective channels for water vapor to penetrate into the magnesium matrix. This enables almost simultaneous reactions on both the internal and external surfaces. Furthermore, as the zinc content increases, the reaction rate accelerates, and the reaction becomes more intense. Therefore, Mg-Zn alloys with high zinc content can undergo almost simultaneous complete hydrolysis reactions on both the external and internal metals within the shortest time. After the high-temperature reaction, the EDS images show the presence of magnesium, zinc, and oxygen elements, which is consistent with the XRD pattern in Figure 9c, further confirming that the hydrolysis products of pure magnesium under high-temperature conditions are MgO and ZnO.
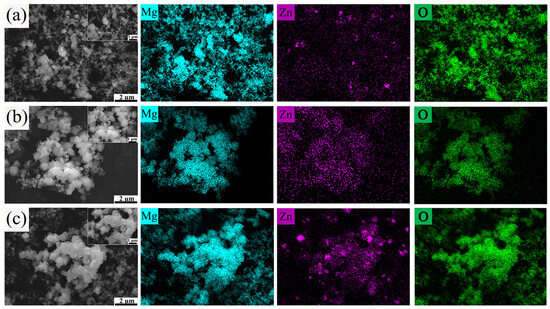
Figure 12.
The SEM and EDS images of the reaction products of (a) Mg-10Zn, (b) Mg-20Zn, and (c) Mg-30Zn.
Ultimately, a comparative investigation was carried out on the hydrogen production performance via hydrolysis of pure Mg, Mg-30wt.%Al, and Mg-30wt.%Zn alloys, as shown in Table 5. Regarding the critical temperature requisite for the reaction, the critical reaction temperature of pure Mg is the highest, attaining 520 °C. That of Mg-30wt.%Al is at an intermediate state, being 420 °C, while the critical reaction temperature of Mg-30wt.%Zn is the lowest, merely 300 °C, which represents a significant reduction of 220 °C compared to pure Mg. From the perspective of the final hydrogen conversion rate, the conversion rate of Mg-30wt.%Zn is as high as 98%. The final conversion rate of Mg-30wt.%Al is slightly lower, at 97%, whereas that of pure Mg is only 94%. In terms of the complete reaction time and induction time, under the operating condition of its critical reaction temperature of 520 °C, the complete reaction time and induction time of pure Mg are 123 s and 360 s, respectively. For Mg-30wt.%Al, under the operating condition of its critical reaction temperature of 420 °C, both the reaction time and induction time are decreased, dropping to 117 s and 270 s, respectively. For Mg-30wt.%Zn, under the operating condition of its critical reaction temperature of 300 °C, the reaction time and induction time are also reduced, being 115 s and 225 s, respectively. Although the variation range of the reaction time is not remarkable, the downward trend of the induction time is extremely prominent. The decrease in the critical reaction temperature, the enhancement of the hydrogen production conversion rate, and the shortening of the reaction time and induction time are all intuitive manifestations of the superiority or inferiority of the performance of hydrogen production via hydrolysis. Herein, compared with pure Mg, both Mg-30wt.%Al and Mg-30wt.%Zn exhibit more excellent hydrogen production performance via hydrolysis, among which Mg-30wt.%Zn has the most outstanding hydrogen production performance via hydrolysis.

Table 5.
Comparison of the hydrogen production performance through hydrolysis of pure Mg, Mg-30wt.%Al alloy, and Mg-30wt.%Zn alloy.
4. Conclusions
This study investigates the effects of reaction temperature, low-melting-point alloy phases Mg17Al12 and Mg7Zn3, and their concentrations on the hydrolytic hydrogen production performance of magnesium-based metals. Looking forward, we will continue to explore the impact of other low-melting-point alloy phases on the hydrolytic hydrogen behavior of magnesium-based metals. By incorporating low-melting-point alloy phases and elevating the reaction temperature, we aim to replace the high-cost and complex material preparation and aqueous solution modification processes required for hydrolytic hydrogen production from magnesium-based metals under ambient conditions. The main conclusions are as follows:
- Compared to the magnesium-based metal hydrolysis hydrogen production under ambient conditions, a high-temperature environment significantly reduces the adverse effects of hydrolysis by-products Mg(OH)2 and partial MgO passivation films on the hydrogen production performance of magnesium-based metals. Under such conditions, the required induction time can be minimized to 96 s, and the complete reaction time is only 55 s. For Mg-Al and Mg-Zn alloys with the same elemental content, the hydrogen production performance of hydrolysis exhibits superior characteristics with increasing temperature.
- The incorporation of low-melting-point alloy phases Mg17Al12 and Mg7Zn3 effectively provides a multitude of channels for water vapor to penetrate into the magnesium-based metal, significantly enhancing the hydrogen production performance compared to pure magnesium. This not only substantially reduces the required reaction temperature but also effectively improves the kinetics of hydrolytic hydrogen production. Specifically, the reaction temperature for Mg-Al alloys is reduced from 510 °C for pure Mg to 420 °C, while the reaction temperature for Mg-Zn alloys is markedly decreased to 300 °C.
- In the Mg-Al alloy system, with an increase in aluminum content, the induction and reaction times are longest for the Mg-10Al alloy and shortest for the Mg-30Al alloy under identical reaction temperatures. Similarly, in the Mg-Zn alloy system, with an increase in zinc content, the induction and reaction times are longest for the Mg-10Zn alloy and shortest for the Mg-30Zn alloy under the same reaction temperature conditions.
The results demonstrate that enhancing the hydrolytic hydrogen production performance of magnesium-based metals and alloys is feasible by elevating the reaction temperature and incorporating low-melting-point alloy phases.
Author Contributions
J.R.: investigation, formal analysis, visualization, data curation, writing—original draft. Z.Z.: investigation, formal analysis, visualization, data curation. Z.W.: investigation, formal analysis, visualization, data curation. S.Z.: investigation, formal analysis, visualization, data curation. Z.H.: review and editing, funding acquisition, supervision. L.M.: review and editing, data curation, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The work received financial backing from the Key R&D and Transformation Plan of Science and Technology Department of Qinghai Province (No. 2022-GX-156).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Zhigang Zeng was employed by the company China National Nuclear Corporation 272 Uranium Industry Co, Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Al Bacha, S.; Thienpont, A.; Zakhour, M.; Nakhl, M.; Bobet, J.-L. Clean hydrogen production by the hydrolysis of magnesium-based material: Effect of the hydrolysis solution. J. Clean. Prod. 2021, 282, 124498. [Google Scholar] [CrossRef]
- Tan, Z.; Ouyang, L.; Huang, J.; Liu, J.; Wang, H.; Shao, H.; Zhu, M. Hydrogen generation via hydrolysis of Mg2Si. J. Alloy. Compd. 2019, 770, 108–115. [Google Scholar] [CrossRef]
- Alsabawi, K.; Gray, E.; Webb, C. The effect of ball-milling gas environment on the sorption kinetics of MgH2 with/without additives for hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 2976–2980. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Huang, Z.; Fang, F.; Hu, J.; Yang, Y.; Gao, M.; Pan, H.; Liu, Y. Ultrafast hydrogenation of magnesium enabled by tetragonal ZrO2 hierarchical nanoparticles. Mater. Today Nano 2022, 18, 100200. [Google Scholar] [CrossRef]
- Weber, G.; Sciora, E.; Guichard, J.; Bouyer, F.; Bezverkhyy, I.; Bernard, F.; Lecoq, H.; Besnard, R.; Bellat, J.-P. New insight on the lithium hydride–water vapor reaction system. Int. J. Hydrogen Energy 2018, 43, 22557–22567. [Google Scholar] [CrossRef]
- Xie, X.; Ni, C.; Wang, B.; Zhang, Y.; Zhao, X.; Liu, L.; Wang, B.; Du, W. Recent advances in hydrogen generation process via hydrolysis of Mg-based materials: A short review. J. Alloy. Compd. 2020, 816, 152634. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, J.; Wang, H.; Yang, F.; Wu, Z.; Zhang, Z. Study on high hydrogen yield for large-scale hydrogen fuel storage and transportation based on liquid organic hydrogen carrier reactor. Fuel 2022, 321, 124095. [Google Scholar] [CrossRef]
- Lindemer, M.D.; Advani, S.G.; Prasad, A.K. Hydrogen production via the heterogeneous hydrolysis of Zn vapor under a temperature gradient: Modeling and efficiency analysis. Int. J. Hydrogen Energy 2016, 41, 10557–10567. [Google Scholar] [CrossRef]
- Purnima, P.; Jayanti, S. Water neutrality and waste heat management in ethanol reformer—HTPEMFC integrated system for on-board hydrogen generation. Appl. Energy 2017, 199, 169–179. [Google Scholar] [CrossRef]
- Gong, B.; Huang, Q.; Xia, G.; Habibullah; Wu, J.; Guo, C.; Wang, Y.; Yan, Y.; Chen, Y.; Wu, C. Challenges and breakthroughs of Mg-based materials for hydrogen generation by hydrolysis. Int. J. Hydrogen Energy 2025, 105, 1008–1025. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Wu, Y. ChemInform Abstract: Mg-Based Nanocomposites with Improved Hydrogen Storage Performances. ChemInform 2014, 45, 14262–14274. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Y.; Yang, Y.; Hu, R.; Yang, G.; Feng, L.; Suo, G. Microstructure evolution and controlled hydrolytic hydrogen generation strategy of Mg-rich Mg-Ni-La ternary alloys. Energy 2019, 188, 116081. [Google Scholar] [CrossRef]
- Hammad, A.; Ning, F.; Zou, S.; Liu, Y.; Tian, B.; He, C.; Chai, Z.; Wen, Q.; He, L.; Zhou, X. Aluminum hydrolysis for hydrogen generation enhanced by sodium hydride. Int. J. Hydrogen Energy 2024, 77, 138–148. [Google Scholar] [CrossRef]
- Garcia, G.; Arriola, E.; Chen, W.-H.; De Luna, M.D. A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability. Energy 2021, 217, 119384. [Google Scholar] [CrossRef]
- Laurinavichene, T.V.; Kosourov, S.N.; Ghirardi, M.L.; Seibert, M.; Tsygankov, A.A. Prolongation of H2 photoproduction by immobilized, sulfur-limited Chlamydomonas reinhardtii cultures. J. Biotechnol. 2008, 134, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Edalati, K.; Arita, M.; Horita, Z. Hydrolytic Hydrogen Production on Al–Sn–Zn Alloys Processed by High-Pressure Torsion. Materials 2018, 11, 1209. [Google Scholar] [CrossRef]
- Idriss, H. Hydrogen production from water: Past and present. Curr. Opin. Chem. Eng. 2020, 29, 74–82. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A review on hydrogen generation from the hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Eom, K.; Kim, M.; Oh, S.; Cho, E.; Kwon, H. Design of ternary Al–Sn–Fe alloy for fast on-board hydrogen production, and its application to PEM fuel cell. Int. J. Hydrogen Energy 2011, 36, 11825–11831. [Google Scholar] [CrossRef]
- Burton, N.; Padilla, R.; Rose, A.; Habibullah, H. Increasing the efficiency of hydrogen production from solar powered water electrolysis. Renew. Sustain. Energy Rev. 2021, 135, 110255. [Google Scholar] [CrossRef]
- Chen, X.; Chen, R.; Yu, K.; Ding, X.; Li, X.; Ding, H.; Su, Y.; Guo, J. Effect of Ce substitution on hydrogen absorption/ desorption of Laves phase-related BCC solid solution Ti33V37Mn30 alloy. J. Alloy. Compd. 2019, 783, 617–624. [Google Scholar] [CrossRef]
- Rafi, M.; Kolupula, A.P.; Vadali, V.S.S.; Varam, S. An overview of hydrogen generation by hydrolysis of Al and its alloys with the addition of carbon-based materials. Int. J. Hydrogen Energy 2023, 48, 21345–21359. [Google Scholar] [CrossRef]
- Endrődi, B.; Smulders, V.; Simic, N.; Wildlock, M.; Mul, G.; Mei, B.; Cornell, A. In situ formed vanadium-oxide cathode coatings for selective hydrogen production. Appl. Catal. B Environ. 2019, 244, 233–239. [Google Scholar] [CrossRef]
- Shang, H.; Zhang, Y.; Li, Y.; Qi, Y.; Guo, S.; Zhao, D. Effects of adding over-stoichiometrical Ti and substituting Fe with Mn partly on structure and hydrogen storage performances of TiFe alloy. Renew. Energy 2019, 135, 1481–1498. [Google Scholar] [CrossRef]
- Zhang, K.; Bao, W.; Chang, L.; Wang, H. A review of recent researches on Bunsen reaction for hydrogen production via S–I water and H2S splitting cycles. J. Energy Chem. 2019, 33, 46–58. [Google Scholar] [CrossRef]
- Dixit, V.; Huot, J. Investigation of the microstructure, crystal structure and hydrogenation kinetics of Ti-V-Cr alloy with Zr addition. J. Alloys Compd. 2019, 785, 1115–1120. [Google Scholar] [CrossRef]
- Guo, L.; Gu, X.; Kang, K.; Wu, Y.; Cheng, J.; Liu, P.; Wang, T.; Su, H. Porous nitrogen-doped carbon-immobilized bimetallic nanoparticles as highly efficient catalysts for hydrogen generation from hydrolysis of ammonia borane. J. Mater. Chem. A 2015, 3, 22807–22815. [Google Scholar] [CrossRef]
- Castro, F.J.; Bobet, J.-L.; Urretavizcaya, G. Reprocessing different Mg-alloy wastes for hydrogen production by hydrolysis. Int. J. Hydrogen Energy 2025, 99, 808–818. [Google Scholar] [CrossRef]
- Naseem, K.; Qin, F.; Suo, G.; Ahmed, S.; Hanif, M.; Gilani, N. Recent progress of mechanically activated Mg-based materials to promote hydrogen generation via hydrolysis. Fuel 2025, 391, 134783. [Google Scholar] [CrossRef]
- Davies, J.; du Preez, S.P.; Bessarabov, D.G. On-Demand Hydrogen Generation by the Hydrolysis of Ball-Milled Aluminum–Bismuth–Zinc Composites. Materials 2022, 15, 1197. [Google Scholar] [CrossRef]
- Yuksel, Y.E.; Ozturk, M.; Dincer, I. Thermodynamic analysis and assessment of a novel integrated geothermal energy-based system for hydrogen production and storage. Int. J. Hydrogen Energy 2018, 43, 4233–4243. [Google Scholar] [CrossRef]
- Yavor, Y.; Goroshin, S.; Bergthorson, J.M.; Frost, D.L. Comparative reactivity of industrial metal powders with water for hydrogen production. Int. J. Hydrogen Energy 2015, 40, 1026–1036. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, Y.; Yang, R.; Li, J. Hydrogen generation from hydrolysis of activated magnesium/low-melting-point metals alloys. Int. J. Hydrogen Energy 2019, 44, 1366–1373. [Google Scholar] [CrossRef]
- Al Bacha, S.; Aubert, I.; Devos, O.; Zakhour, M.; Nakhl, M.; Bobet, J.L. Corrosion of pure and milled Mg17Al12 in “model” seawater solution. Int. J. Hydrogen Energy 2020, 45, 15805–15813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).