mRNA Vaccine Delivery via Intramuscular Electroporation Induces Protective Antiviral Immune Responses in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. In Vitro mRNA Transcription
2.3. Mouse Immunization Studies
2.4. Bioluminescence Imaging
2.5. Cytometric Bead Array (CBA)
2.6. RNA Extraction and RT-qPCR
2.7. Virus and Cell Line
2.8. SARS-CoV-2 Infection
2.9. Plaque Assay
2.10. Plaque Reduction Neutralization Test (PRNT)
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Flow Cytometry
2.13. Statistical Analyses
3. Results
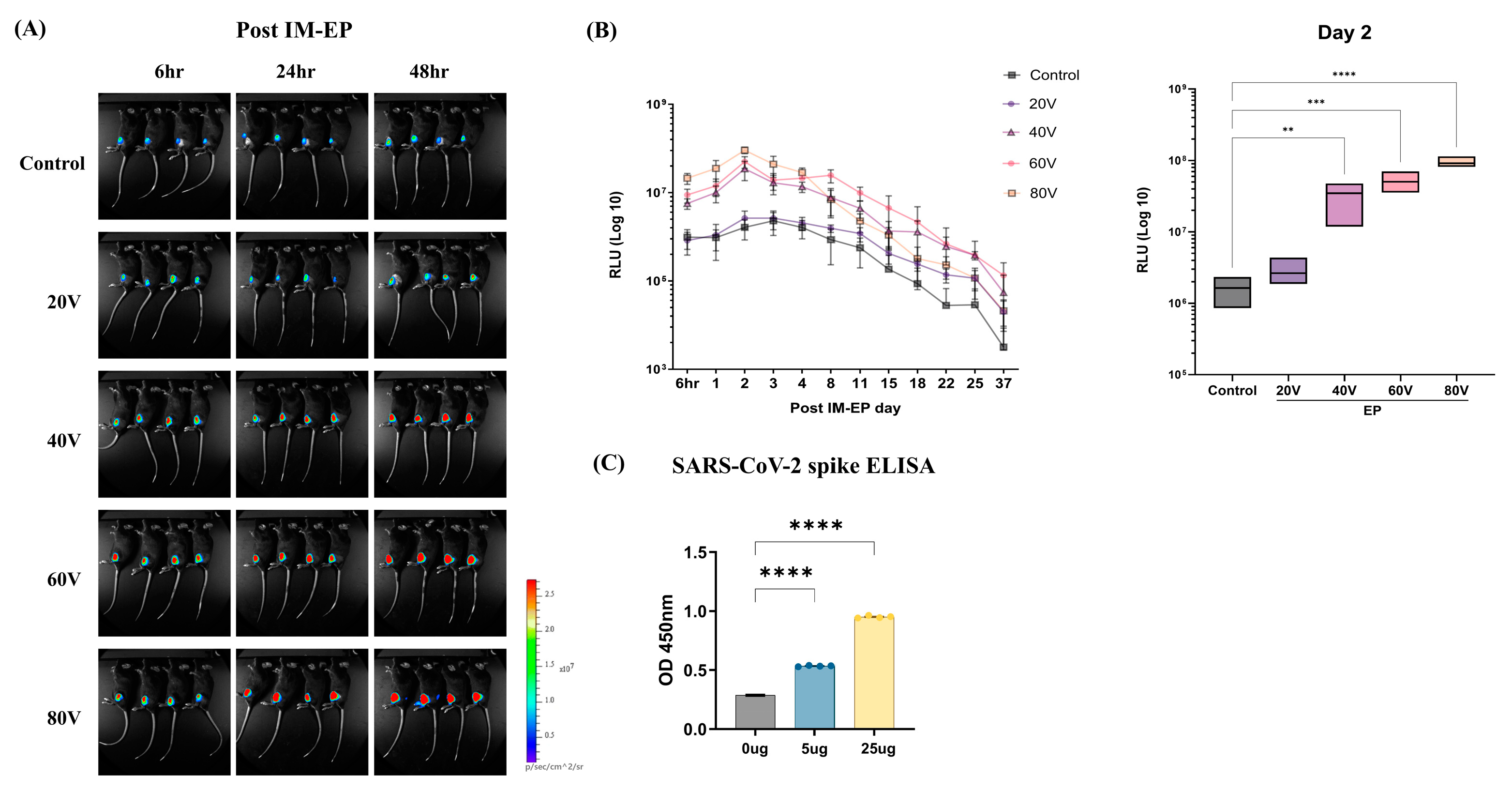
3.1. Optimization of mRNA Vaccine Delivery by Electroporation
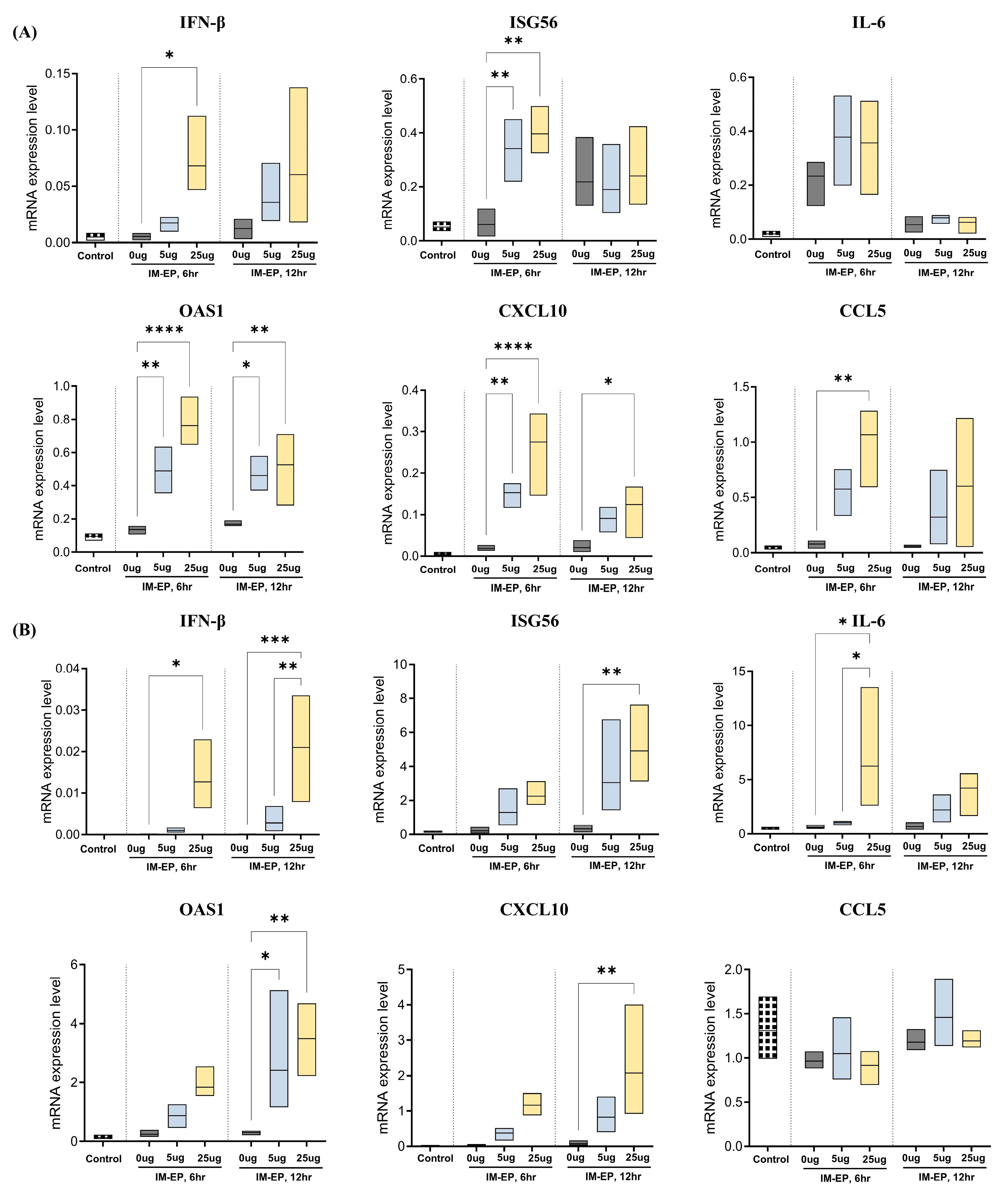
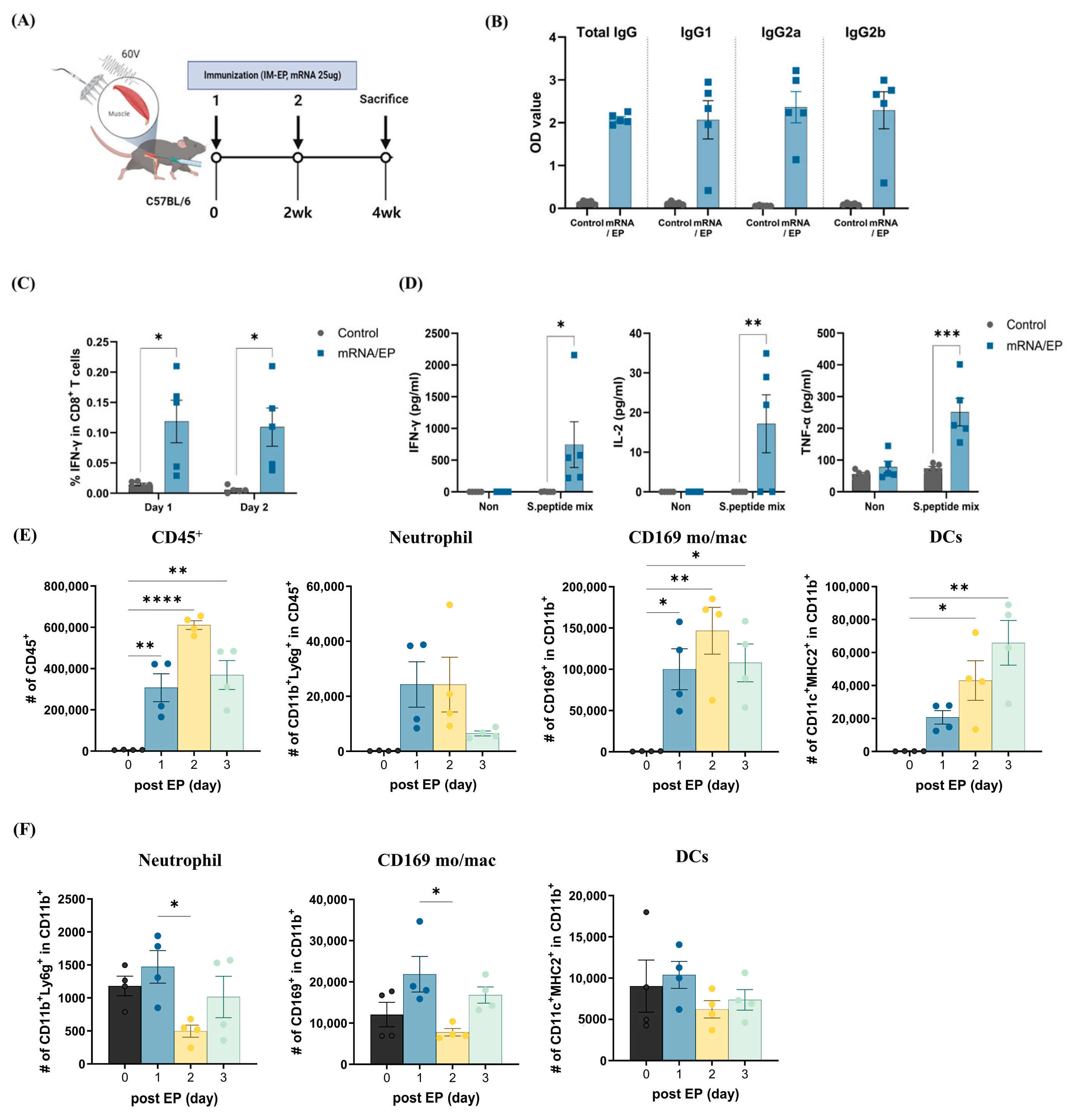
3.2. Naked mRNA Vaccination via IM-EP Effectively Induces Antigen-Specific Immune Responses in Mice
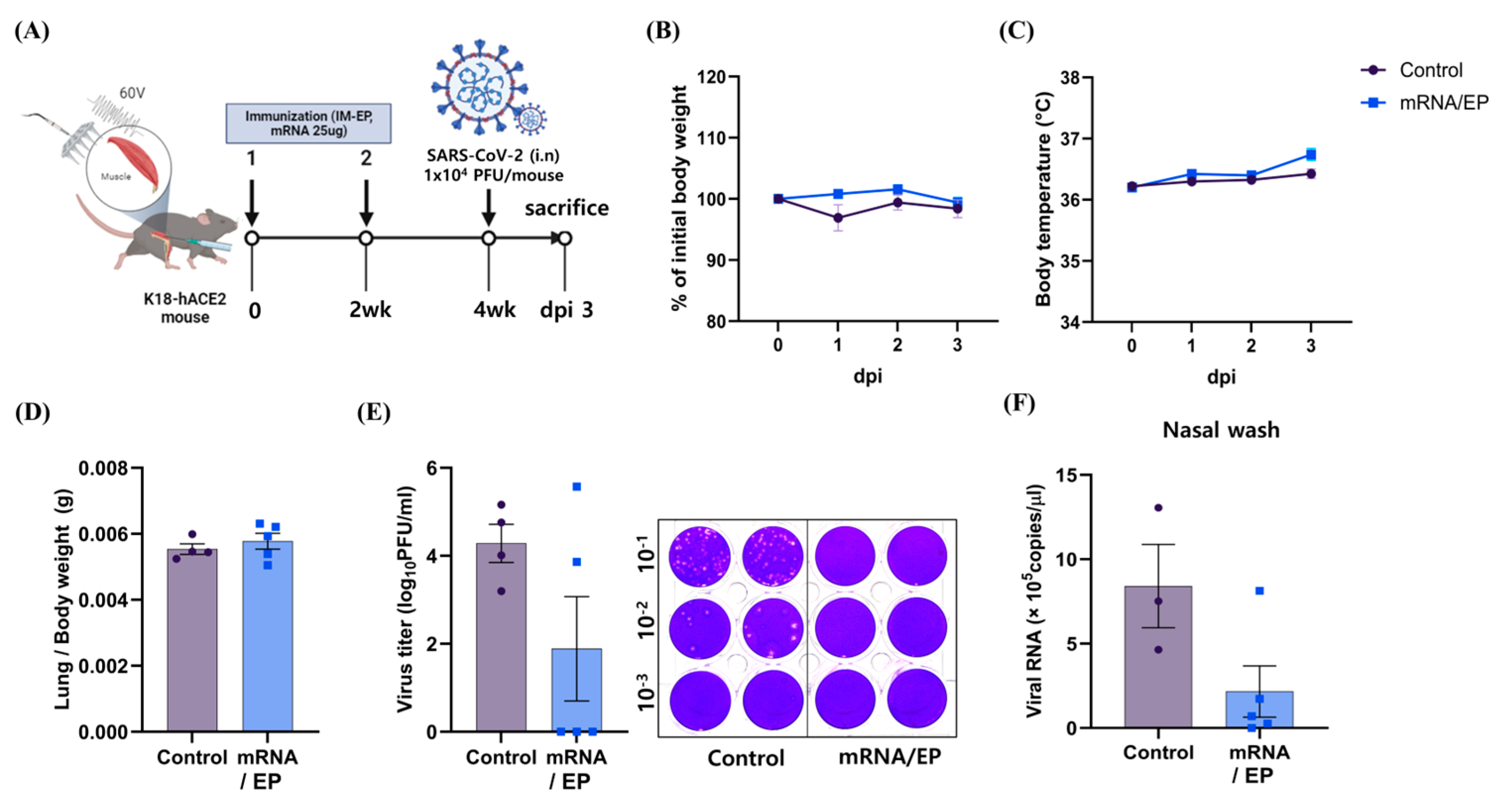
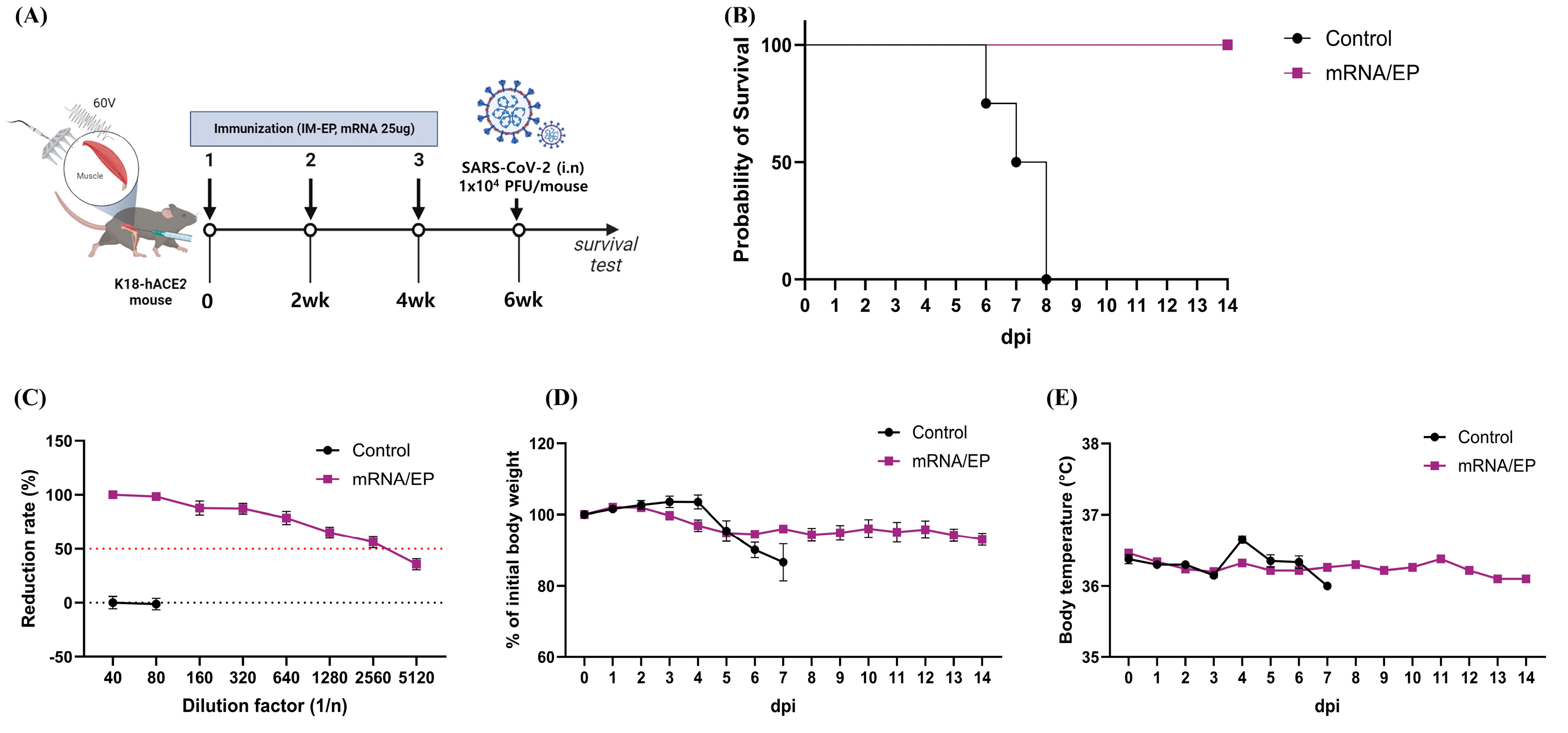
3.3. IM-EP-Mediated Naked mRNA Vaccination Reduces SARS-CoV-2 Load in K18-hACE2 Tg Mice
3.4. Naked mRNA Vaccine Protects K18-hACE2 Mice from Lethal Infection of SARS-CoV-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APCs | Antigen-presenting cells |
| BSL3 | Biosafety level 3 |
| CBA | Cytometric bead array |
| CTL | Cytotoxic T lymphocytes |
| COVID-19 | Coronavirus disease 2019 |
| DCs | Dendritic cells |
| DMEM | Dulbecco’s modified eagle medium |
| DPI | Days post infection |
| FBS | Fetal bovine serum |
| Fluc | Firefly luciferase |
| IM-EP | Intramuscular electroporation |
| ID-EP | Intradermal electroporation |
| ISG56 | IFN-stimulated gene 56 |
| LNP | Lipid nanoparticle |
| mRNA | Messenger RNA |
| PFU | Plaque-forming units |
| PRNT | Plaque reduction neutralization test |
| RBD | Receptor-binding domain |
| ROI | Region of interest |
| Th1 | Type 1 helper T cells |
| TNF-α | Tumor necrosis factor alpha |
References
- Semple, S.C.; Leone, R.; Barbosa, C.J.; Tam, Y.K.; Lin, P.J.C. Lipid Nanoparticle Delivery Systems to Enable mRNA-Based Therapeutics. Pharmaceutics 2022, 14, 398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tang, T.; Chen, Y.; Huang, X.; Liang, T. mRNA vaccines in disease prevention and treatment. Signal Transduct. Target. Ther. 2023, 8, 365. [Google Scholar] [CrossRef] [PubMed]
- Kiaie, S.H.; Majidi Zolbanin, N.; Ahmadi, A.; Bagherifar, R.; Valizadeh, H.; Kashanchi, F.; Jafari, R. Recent advances in mRNA-LNP therapeutics: Immunological and pharmacological aspects. J. Nanobiotechnol. 2022, 20, 276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Sim, W.K.; Shen, T.L.; Lim, S.K. Engineered EVs with pathogen proteins: Promising vaccine alternatives to LNP-mRNA vaccines. J. Biomed. Sci. 2024, 31, 9. [Google Scholar] [CrossRef]
- Heidecker, B.; Dagan, N.; Balicer, R.; Eriksson, U.; Rosano, G.; Coats, A.; Tschöpe, C.; Kelle, S.; Poland, G.A.; Frustaci, A.; et al. Myocarditis following COVID-19 vaccine: Incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur. J. Heart Fail. 2022, 24, 2000–2018. [Google Scholar] [CrossRef]
- Kapoor, M.; Spillane, J.; Englezou, C.; Sarri-Gonzalez, S.; Bell, R.; Rossor, A.; Manji, H.; Reilly, M.M.; Lunn, M.P.; Carr, A. Thromboembolic risk with IVIg: Incidence and risk factors in patients with inflammatory neuropathy. Neurology 2020, 94, e635–e638. [Google Scholar] [CrossRef]
- Muir, K.-L.; Kallam, A.; Koepsell, S.A.; Gundabolu, K. Thrombotic thrombocytopenia after Ad26.COV2.S vaccination. N. Engl. J. Med. 2021, 384, 1964–1965. [Google Scholar] [CrossRef]
- Fahrni, M.L.; Ismail, I.A.; Refi, D.M.; Almeman, A.; Yaakob, N.C.; Saman, K.M.; Mansor, N.F.; Noordin, N.; Babar, Z.U. Management of COVID-19 vaccines cold chain logistics: A scoping review. J. Pharm. Policy Pract. 2022, 15, 16. [Google Scholar] [CrossRef]
- Broderick, K.E.; Humeau, L.M. Electroporation-enhanced delivery of nucleic acid vaccines. Expert Rev. Vaccines 2015, 14, 195–204. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Laddy, D.J.; Yan, J.; Kutzler, M.; Kobasa, D.; Kobinger, G.P.; Khan, A.S.; Greenhouse, J.; Sardesai, N.Y.; Draghia-Akli, R.; Weiner, D.B. Heterosubtypic protection against pathogenic human and avian influenza viruses via in vivo electroporation of synthetic consensus DNA antigens. PLoS ONE 2008, 3, e2517. [Google Scholar] [CrossRef] [PubMed]
- Todorova, B.; Adam, L.; Culina, S.; Boisgard, R.; Martinon, F.; Cosma, A.; Ustav, M.; Kortulewski, T.; Le Grand, R.; Chapon, C. Electroporation as a vaccine delivery system and a natural adjuvant to intradermal administration of plasmid DNA in macaques. Sci. Rep. 2017, 7, 4122. [Google Scholar] [CrossRef]
- Lin, F.; Shen, X.; McCoy, J.R.; Mendoza, J.M.; Yan, J.; Kemmerrer, S.V.; Khan, A.S.; Weiner, D.B.; Broderick, K.E.; Sardesai, N.Y. A novel prototype device for electroporation-enhanced DNA vaccine delivery simultaneously to both skin and muscle. Vaccine 2011, 29, 6771–6780. [Google Scholar] [CrossRef] [PubMed]
- Han, K.T.; Sin, J.I. DNA vaccines targeting human papillomavirus-associated diseases: Progresses in animal and clinical studies. Clin. Exp. Vaccine Res. 2013, 2, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Hirao, L.A.; Draghia-Akli, R.; Prigge, J.T.; Yang, M.; Satishchandran, A.; Wu, L.; Hammarlund, E.; Khan, A.S.; Babas, T.; Rhodes, L.; et al. Multivalent smallpox DNA vaccine delivered by intradermal electroporation drives protective immunity in nonhuman primates against lethal monkeypox challenge. J. Infect. Dis. 2011, 203, 95–102. [Google Scholar] [CrossRef]
- Golzio, M.; Rols, M.P.; Teissie, J. In vitro and in vivo electric field-mediated permeabilization, gene transfer, and expression. Methods 2004, 33, 126–135. [Google Scholar] [CrossRef]
- Golzio, M.; Mazzolini, L.; Moller, P.; Rols, M.P.; Teissie, J. Inhibition of gene expression in mice muscle by in vivo electrically mediated siRNA delivery. Gene Ther. 2005, 12, 246–251. [Google Scholar] [CrossRef]
- Van Nuffel, A.M.; Corthals, J.; Neyns, B.; Heirman, C.; Thielemans, K.; Bonehill, A. Immunotherapy of cancer with dendritic cells loaded with tumor antigens and activated through mRNA electroporation. Methods Mol. Biol. 2010, 629, 405–452. [Google Scholar] [CrossRef]
- Cu, Y.; Broderick, K.E.; Banerjee, K.; Hickman, J.; Otten, G.; Barnett, S.; Kichaev, G.; Sardesai, N.Y.; Ulmer, J.B.; Geall, A. Enhanced Delivery and Potency of Self-Amplifying mRNA Vaccines by Electroporation In Situ. Vaccines 2013, 1, 367–383. [Google Scholar] [CrossRef]
- Paz, S.; Vilasco, M.; Werden, S.J.; Arguello, M.; Joseph-Pillai, D.; Zhao, T.; Nguyen, T.L.; Sun, Q.; Meurs, E.F.; Lin, R.; et al. A functional C-terminal TRAF3-binding site in MAVS participates in positive and negative regulation of the IFN antiviral response. Cell Res. 2011, 21, 895–910. [Google Scholar] [CrossRef]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Seth, S.; Matsui, Y.; Fosnaugh, K.; Liu, Y.; Vaish, N.; Adami, R.; Harvie, P.; Johns, R.; Severson, G.; Brown, T.; et al. RNAi-based therapeutics targeting survivin and PLK1 for treatment of bladder cancer. Mol. Ther. 2011, 19, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Gebremeskel, S.; Nelson, A.; Walker, B.; Oliphant, T.; Lobert, L.; Mahoney, D.; Johnston, B. Natural killer T cell immunotherapy combined with oncolytic vesicular stomatitis virus or reovirus treatments differentially increases survival in mouse models of ovarian and breast cancer metastasis. J. Immunother. Cancer 2021, 9, e002096. [Google Scholar] [CrossRef]
- Yang, M.S.; Park, M.J.; Lee, J.; Oh, B.; Kang, K.W.; Kim, Y.; Lee, S.M.; Lim, J.O.; Jung, T.Y.; Park, J.H.; et al. Non-invasive administration of AAV to target lung parenchymal cells and develop SARS-CoV-2-susceptible mice. Mol. Ther. 2022, 30, 1994–2004. [Google Scholar] [CrossRef]
- Hong, S.H.; Oh, H.; Park, Y.W.; Kwak, H.W.; Oh, E.Y.; Park, H.J.; Kang, K.W.; Kim, G.; Koo, B.S.; Hwang, E.H.; et al. Immunization with RBD-P2 and N protects against SARS-CoV-2 in nonhuman primates. Sci. Adv. 2021, 7, eabg7156. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Hassett, K.J.; Benenato, K.E.; Jacquinet, E.; Lee, A.; Woods, A.; Yuzhakov, O.; Himansu, S.; Deterling, J.; Geilich, B.M.; Ketova, T.; et al. Optimization of Lipid Nanoparticles for Intramuscular Administration of mRNA Vaccines. Mol. Ther. Nucleic Acids 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Shoji, M.; Katayama, K.; Tachibana, M.; Tomita, K.; Sakurai, F.; Kawabata, K.; Mizuguchi, H. Intramuscular DNA immunization with in vivo electroporation induces antigen-specific cellular and humoral immune responses in both systemic and gut-mucosal compartments. Vaccine 2012, 30, 7278–7285. [Google Scholar] [CrossRef]
- Gary, E.N.; Weiner, D.B. DNA vaccines: Prime time is now. Curr. Opin. Immunol. 2020, 65, 21–27. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Parums, D.V. Editorial: SARS-CoV-2 mRNA vaccines and the possible mechanism of vaccine-induced immune thrombotic thrombocytopenia (VITT). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e932899. [Google Scholar] [CrossRef]
- Verma, A.K.; Lavine, K.J.; Lin, C.-Y. Myocarditis after COVID-19 mRNA vaccination. N. Engl. J. Med. 2021, 385, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Shiomichi, Y.; Takeuchi, S.; Akamine, S.; Yoneda, R.; Yoshizawa, S. IgA vasculitis following COVID-19 vaccination. Mod. Rheumatol. Case Rep. 2023, 7, 122–126. [Google Scholar] [CrossRef]
- Flemming, A. mRNA vaccine shows promise in autoimmunity. Nat. Rev. Immunol. 2021, 21, 72. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Wang, P.; Li, X.M.; Shuai, Z.W.; Ye, D.Q.; Pan, H.F. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 2022, 165, 386–401. [Google Scholar] [CrossRef]
- Ndeupen, S.; Qin, Z.; Jacobsen, S.; Bouteau, A.; Estanbouli, H.; Igyártó, B.Z. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience 2021, 24, 103479. [Google Scholar] [CrossRef]
- Khobragade, A.; Bhate, S.; Ramaiah, V.; Deshpande, S.; Giri, K.; Phophle, H.; Supe, P.; Godara, I.; Revanna, R.; Nagarkar, R.; et al. Efficacy, safety, and immunogenicity of the DNA SARS-CoV-2 vaccine (ZyCoV-D): The interim efficacy results of a phase 3, randomised, double-blind, placebo-controlled study in India. Lancet 2022, 399, 1313–1321. [Google Scholar] [CrossRef]
- Abbasi, S.; Matsui-Masai, M.; Yasui, F.; Hayashi, A.; Tockary, T.A.; Mochida, Y.; Akinaga, S.; Kohara, M.; Kataoka, K.; Uchida, S. Carrier-free mRNA vaccine induces robust immunity against SARS-CoV-2 in mice and non-human primates without systemic reactogenicity. Mol. Ther. 2024, 32, 1266–1283. [Google Scholar] [CrossRef] [PubMed]
- Ekrami, N.; Ahmadian, M.; Nourshahi, M.; Shakouri, G.H. Wet-cupping induces anti-inflammatory action in response to vigorous exercise among martial arts athletes: A pilot study. Complement. Ther. Med. 2021, 56, 102611. [Google Scholar] [CrossRef] [PubMed]
| Title 1 | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| IFNβ | ATGGTGGTCCGAGCAGAGAT | CCACCACTCATTCTGAGGCA |
| ISG56 [20] | CTCTGAAAGTGGAGCCAGAAAAC | AAATCTTGGCGATAGGCTACGA |
| IL-6 [21] | AGAATTGCCATTGCACA | CTCCCAACAGACCTGTCTATA |
| OAS1 [22] | CTTTGATGTCCTGGGTCATGT | GCTCCGTGAAGCAGGTAGAG |
| CXCL10 | GCAACTGCATCCATATCGATGACG | GATTCCGGATTCAGACATCTCTGC |
| CCL5 [23] | CTCACCATATGGCTCGGACA | ACAAACACGACTGCAAGATTGG |
| Actin | TCCAGCCTTCCTTCTTGGGT | GCACTGTGTTGGCATAGAGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.-H.; Kim, Y.; Kim, M.; Lee, Y.J.; Seo, Y.; Jin, H.; Lee, S.-M. mRNA Vaccine Delivery via Intramuscular Electroporation Induces Protective Antiviral Immune Responses in Mice. Appl. Sci. 2025, 15, 4428. https://doi.org/10.3390/app15084428
Park S-H, Kim Y, Kim M, Lee YJ, Seo Y, Jin H, Lee S-M. mRNA Vaccine Delivery via Intramuscular Electroporation Induces Protective Antiviral Immune Responses in Mice. Applied Sciences. 2025; 15(8):4428. https://doi.org/10.3390/app15084428
Chicago/Turabian StylePark, So-Hyun, Yeonhwa Kim, Mina Kim, Yong Jin Lee, Yeji Seo, Hao Jin, and Sang-Myeong Lee. 2025. "mRNA Vaccine Delivery via Intramuscular Electroporation Induces Protective Antiviral Immune Responses in Mice" Applied Sciences 15, no. 8: 4428. https://doi.org/10.3390/app15084428
APA StylePark, S.-H., Kim, Y., Kim, M., Lee, Y. J., Seo, Y., Jin, H., & Lee, S.-M. (2025). mRNA Vaccine Delivery via Intramuscular Electroporation Induces Protective Antiviral Immune Responses in Mice. Applied Sciences, 15(8), 4428. https://doi.org/10.3390/app15084428






