The Influence of Different Cooking Techniques on the Biochemical, Microbiological, and Sensorial Profile of Fish-Based Catering Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Selected Fish Products and Applied Culinary Treatments
2.2. Experimental Concept and Sampling
Sample Preparation
2.3. Physico-Chemical Analyses
2.4. Microbiological Analyses
2.5. Sensory Analysis
2.6. Statistical Analysis
3. Results and Discussions
3.1. Evaluation of the Nutrient Content of the Analyzed Fish Dishes
3.1.1. Evaluation of Processing Losses of the Analyzed Products
3.1.2. Evaluation of Macronutrient Content
3.1.3. Evaluation of Water-Soluble and Fat-Soluble Vitamin Content
3.2. Microbiological Analysis
3.2.1. Aerobic Plate Count (APC)
3.2.2. Total Yeast and Mold Count (TYMC)
3.2.3. Coliforms Count
3.2.4. E. coli Determination
3.3. Sensorial Quality Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malinowska, E.; Tarczyńska, A.S.; Urbanowicz, A. Factors Determining the Quality of Catering Services in the Opinion of Consumers and Catering Staff. In Quality Challenges; Dziadkowiec Joanna, M., Niewczas-Dobrowolska, M., Eds.; Wydawnictwo Rys: Dąbrówka, Poland, 2022; pp. 171–197. ISBN 978-83-67287-33-3. [Google Scholar]
- Brănescu, G.; Măzărel, A.; Moja, A.; Lupu, M. Management of Combating Food Fraud in the Field of Public Catering. Ann. Univ. Craiova Agric. Mont. Cadastre Ser. 2023, 53, 31–36. [Google Scholar] [CrossRef]
- Chou, S.F.; Horng, J.S.; Liu, C.H.; Yu, T.Y.; Huang, Y.C.; La, Q.P.; Ng, Y. Comparison of Hotel Fine Dining and Chain Restaurant Consumer Perceived Service Quality and Satisfaction Predictions. J. Hosp. Tour. Insights 2024. ahead of print. [Google Scholar] [CrossRef]
- Li, N.; Cong, L.; Wang, H.; Chen, Y.; Liu, Z.; Li, M.; Yang, D.; Li, H.; Fang, H. Establishing a Nutrition Calculation Model for Catering Food According to the Influencing Factors of Energy and Nutrient Content in Food Processing. Front. Nutr. 2024, 11, 1388645. [Google Scholar] [CrossRef]
- Hurrell, R.F. Food Manufacturing Processes and Their Influence on the Nutritional Quality of Foods. In Nutritional Impact of Food Processing; S. Karger Ag: Basel, Switzerland, 1989; Volume 43, pp. 125–139. ISBN 978-3-8055-4848-9. [Google Scholar]
- Nguyen, L.; Gao, Z.; Anderson, J.L. Regulating Menu Information: What Do Consumers Care and Not Care about at Casual and Fine Dining Restaurants for Seafood Consumption? Food Policy 2022, 110, 102272. [Google Scholar] [CrossRef]
- Nguyen, L.; Gao, Z.; Anderson, J.L. Perception Shifts in Seafood Consumption in the United States. Mar. Policy 2023, 148, 105438. [Google Scholar] [CrossRef]
- Tan, K.; Huang, L.; Tan, K.; Lim, L.; Peng, Y.; Cheong, K.L. Effects of Culinary Treatments on the Lipid Nutritional Quality of Fish and Shellfish. Food Chem. X 2023, 19, 100856. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Lim, L.; Cheong, K.L. Effects of Food Processing on the Lipid Nutritional Quality of Commercially Important Fish and Shellfish. Food Chem. X 2023, 20, 101034. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Rosa, A.; Almeida, A.; Martins, R.; Ribeiro, T.; Pintado, M.; Goncalves, R.F.S.; Pinheiro, A.C.; Fonseca, A.J.M.; Maia, M.R.G.; et al. Omega-3 Fatty Acids from Fish by-Products: Innovative Extraction and Application in Food and Feed. Food Bioprod. Process. 2024, 145, 32–41. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuang, P.; Mao, L.; Chen, X.; Wang, J.; Cheng, L.; Ding, G.; Jiao, J. Current Level of Fish and Omega-3 Fatty Acid Intakes and Risk of Type 2 Diabetes in China. J. Nutr. Biochem. 2019, 74, 108249. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.S.; Carne, A.; Bekhit, A.E.A. Marine Omega-3 (n-3) Phospholipids: A Comprehensive Review of Their Properties, Sources, Bioavailability, and Relation to Brain Health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef]
- Coșansu, S.; Mol, S.; Haskaraca, G. Sous-Vide Cooking: Effects on Seafood Quality and Combination with Other Hurdles. Int. J. Gastron. Food Sci. 2022, 29, 100586. [Google Scholar] [CrossRef]
- Raza, M.L.; Hassan, S.T.; Jamil, S.; Fatima, W.; Fatima, M. Nutritional Interventions in Depression: The Role of Vitamin D and Omega-3 Fatty Acids in Neuropsychiatric Health. Clin. Nutr. 2025, 45, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Golgolipour, S.; Khodanazary, A.; Ghanemi, K. Effects of Different Cooking Methods on Minerals, Vitamins and Nutritional Quality Indices of Grass Carp (Ctenopharyngodon idella). Iran. J. Fish. Sci. 2019, 18, 110–123. [Google Scholar] [CrossRef]
- Orlando, P.; Giardinieri, A.; Lucci, P.; Nartea, A.; Balzano, M.; Pacetti, D.; Frega, N.G.; Silvestri, S.; Tiano, L. Impact of Traditional and Mild Oven Cooking Treatments on Antioxidant Compounds Levels and Oxidative Status of Atlantic Salmon (Salmo salar) Fillets. LWT Food Sci. Technol. 2020, 134, 110011. [Google Scholar] [CrossRef]
- Zhou, Y.; He, A.; Xu, B. Natural Resources, Quantification, Microbial Bioconversion, and Bioactivities of Vitamin B12 for Vegetarian Diet. Food Chem. 2025, 463, 140849. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Walther, B. Natural Vitamin D Content in Animal Products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Scanlon, N.L. Catering Management, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 1-118-09149-3. [Google Scholar]
- Mîșu, G.A.; Canja, C.M.; Lupu, M.; Matei, F. Advances and Drawbacks of Sous-Vide Technique—A Critical Review. Foods 2024, 13, 2217. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems. Requirements. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 22000:2018; Food Safety Management Systems. Requirements for Any Organization in the Food Chain. International Organization for Standardization: Geneva, Switzerland, 2018.
- Rotaru, G.; Sava, N.; Borda, D.; Stanciu, S. Food Quality and Safety Management Systems: A Briief Analysis of the Individual and Integrated Approaches. Sci. Res. Agroaliment. Process. Technol. 2005, XI, 229–236. [Google Scholar]
- ISO 1442:2023; Meat and Meat Products. Determination of moisture content. Reference method. International Organization for Standardization: Geneva, Switzerland, 2023.
- AOAC 923.09-1923; Invert Sugar in Sugars and Syrups. Lane-Eynon General Volumetric Method. Association of Official Analytical Chemists: Rockville, MD, USA, 1923.
- SR ISO 1443:2008; Meat and Meat Products. Determination of total fat content. ASRO—Romanian Standards Association: Bucharest, Romania, 2008.
- ISO 5983-1:2005; Animal Feeding Stuffs. Determination of Nitrogen Content and Calculation of Crude Protein Content. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 8968-4:2016; Milk and Milk Products. Determination of Nitrogen Content. International Organization for Standardization: Geneva, Switzerland, 2016.
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.A.; Kumar, S.; Bhat, H.F. Effect of Processing Technologies on the Digestibility of Egg Proteins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4703–4738. [Google Scholar] [CrossRef]
- ISO 112966-4:2015; Animal and Vegetable Fats and Oils. Gas Chromatography of Fatty Acid Methyl esters. International Organization for Standardization: Geneva, Switzerland, 2015.
- European Commission. Commission Regulation (EC) No 152/2009 of 27 January 2009 laying down the methods of sampling and analysis for the official control of feed. Off. J. Eur. Union 2009, L54, 1–130. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32009R0152 (accessed on 20 January 2025).
- ISO 4833:2013; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2013.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. International Organization for Standardization: Geneva, Switzerland, 2008.
- ISO 4831:2006; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Detection and Enumeration of Coliforms. Most probable number technique. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 6579-1:2017; Microbiology of the Food Chain. Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 11290-1:2017; Microbiology of the Food Chain. Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp. International Organization for Standardization: Geneva, Switzerland, 2017.
- Chan, S.S.; Iversen, S.G.; Skuland, A.V.; Rotabakk, B.T.; Lerfall, J.; Rognsa, G.H.; Roth, B. Using Renowned Chefs for VeriTasteTM as a Sensory Method Analysis in Food Products—A Comparison of Atlantic Salmon Stored in Refrigerated Seawater and on Ice. Int. J. Gastron. Food Sci. 2023, 31, 100635. [Google Scholar] [CrossRef]
- ISO 8589:2007; Sensory Analysis. General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- JASP Team. JASP, version 0.19.3; computer software; JASP Team: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the Number of Clusters in a Data Set via the Gap Statistic. J. R. Stat. Soc. Ser. B Stat. Methodol. 2001, 63, 411–423. [Google Scholar] [CrossRef]
- Ellison, B.; Muth, M.K.; Golan, E. Opportunities and Challenges in Conducting Economic Research on Food Loss and Waste. Appl. Econ. Perspect. Policy 2019, 41, 1–19. [Google Scholar] [CrossRef]
- Lassen, A.; Kall, M.; Hansen, K.; Ovesen, L. A Comparison of the Retention of Vitamins B1, B2 and B6, and Cooking Yield in Pork Loin with Conventional and Enhanced Meal-Service Systems. Eur. Food Res. Technol. 2002, 215, 194–199. [Google Scholar] [CrossRef]
- Roldán, M.; Antequera, T.; Martin, A.; Mayoral, A.I.; Ruiz, J. Effect of Different Temperature–Time Combinations on Physicochemical, Microbiological, Textural and Structural Features of Sous-Vide Cooked Lamb Loins. Meat Sci. 2013, 93, 572–578. [Google Scholar] [CrossRef]
- Alfaifi, B.M.; Ah-Ghamdi, S.; Othman, M.B.; Hobani, A.I.; Suliman, G.M. Advanced Red Meat Cooking Technologies and Their Effect on Engineering and Quality Properties: A Review. Foods 2023, 12, 2564. [Google Scholar] [CrossRef] [PubMed]
- Ángel-Rendón, S.V.; Filomena-Ambrosio, A.; Hernández-Carrión, M.; Llorca, E.; Hernando, I.; Quiles, A.; Sotelo-Díaz, I. Pork Meat Prepared by Different Cooking Methods. A Microstructural, Sensorial and Physicochemical Approach. Meat Sci. 2020, 163, 108089. [Google Scholar] [CrossRef]
- Bhat, Z.F.; Morton, J.D.; Bekhit, A.E.A.; Kumar, S.; Bhat, H.F. Thermal Processing Implications on the Digestibility of Meat, Fish and Seafood Proteins. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4511–4548. [Google Scholar] [CrossRef]
- Inchingolo, R.; Cardenia, V.; Rodriguez-Estrada, M.T. The Effects of Microwave Heating on Edible Oils and Lipid-containing Food. Lipid Technol. 2013, 25, 59–61. [Google Scholar] [CrossRef]
- Leskova, E.; Kubikova, J.; Kovacikova, E.; Kosicka, M.; Porubska, J.; Holcikova, K. Vitamin Losses: Retention during Heat Treatment and Continual Changes Expressed by Mathematical Models. J. Food Compos. Anal. 2006, 19, 252–276. [Google Scholar] [CrossRef]
- Lee, S.; Choi, Y.; Jeong, H.S.; Lee, J.; Sung, J. Effect of Different Cooking Methods on the Content of Vitamins and True Retention in Selected Vegetables. Food Sci. Biotechnol. 2018, 27, 333–342. [Google Scholar] [CrossRef]
- Demir, K.; Tezcan, E.E.; Kesik, S.; Ugur, H.; Yaman, M.; Catak, J. Riboflavin Cooking Losses and Bioaccessibility in Red Meats. J. Culin. Sci. Technol. 2023, 1–15. [Google Scholar] [CrossRef]
- Mogoș, T.V. Alimentația În Bolie de Nutriție Și Metabolism; Editura Didactică și Pedagogică: Bucharest, Romania, 1997; Volume 1, ISBN 973-30-5888-2. [Google Scholar]
- Choe, E.; Huang, R.; Min, D.B. Chemical Reactions and Stability of Riboflavin in Foods. J. Food Sci. 2006, 70, R28–R36. [Google Scholar] [CrossRef]
- Böhm, V.; Kühnert, S.; Rohm, H.; Scholze, G. Improving the Nutritional Quality of Microwave-Vacuum Dried Strawberries: A Preliminary Study. Food Sci. Technol. Int. 2006, 12, 67–75. [Google Scholar] [CrossRef]
- Kala, A.; Prakash, J. Nutritional Composition and Sensory Profile of Microwave and Conventionally Cooked Vegetables. Foodserv. Res. Int. 2005, 15, 1–12. [Google Scholar] [CrossRef]
- Rickman, J.C.; Barrett, D.M.; Bruhn, C.M. Nutritional Comparison of Fresh, Frozen and Canned Fruits and Vegetables. Part 1. Vitamins C and B and Phenolic Compounds. J. Sci. Food Agric. 2007, 87, 930–944. [Google Scholar] [CrossRef]
- Awonorin, S.O.; Bamiro, F.O.; Ayoade, J.A. The Effect of Freezing Rate, Storage and Cooking on Some B-Vitamins in Beef and Pork Roast. Foodserv. Res. Int. 1996, 9, 7–23. [Google Scholar] [CrossRef]
- Kumar, S.; Aalbersberg, B. Nutrient Retention in Foods after Earth-Oven Cooking Compared to Other Forms of Domestic Cooking☆: 2. Vitamins. J. Food Compos. Anal. 2006, 19, 311–320. [Google Scholar] [CrossRef]
- Gentry, T.S.; Roberts, J.S. Formation Kinetics and Application of 5-Hydroxymethylfurfural as a Time–Temperature Indicator of Lethality for Continuous Pasteurization of Apple Cider. Innov. Food Sci. Emerg. Technol. 2004, 5, 324–333. [Google Scholar] [CrossRef]
- Roberts, J.; Walker, L.T.; Anderson, J.C. Assessment of Microwave Blanching as a Preparatory Tool for Home Freezing of Yellow Squash (Cucurbita pepo); Institute of Food Technologists Annual Meeting: Las Vegas, NV, USA, 2004; p. 67C-32. [Google Scholar]
- Diguță, C.F.; Mihai, C.; Toma, R.C.; Cîmpeanu, C.; Matei, F. In Vitro Assessment of Yeasts Strains with Probiotic Attributes for Aquaculture Use. Foods 2022, 12, 124. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2005, L338, 1–26. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32005R2073 (accessed on 20 January 2025).
- Malak, N.M.L.; Abdel-Naeem, H.H.S.; Abdelsalam, A.A.; Ezzat, G.A. A Comparative Study Concerning the Sensory, Physicochemical, Bacteriological, Nutritional Quality, Heavy Metal Content, and Health Risk Assessment of Some Low-Cost Fish Species. Food Control 2025, 169, 111023. [Google Scholar] [CrossRef]
- Cyprian, O.O.; Van Nguyen, M.; Sveinsdottir, K.; Jonsson, A.; Tomasson, T.; Thorkelsson, G.; Arason, S. Influence of Smoking and Packaging Methods on Lipid Stability and Microbial Quality of Capelin (Mallotus villosus) and Sardine (Sardinella gibossa). Food Sci. Nutr. 2015, 3, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Vatavali, K.; Karakosta, L.; Nathanailides, C.; Georgantelis, D.; Kontominas, M.G. Combined Effect of Chitosan and Oregano Essential Oil Dip on the Microbiological, Chemical, and Sensory Attributes of Red Porgy (Pagrus pagrus) Stored in Ice. Food Bioprocess Technol. 2013, 6, 3510–3521. [Google Scholar] [CrossRef]
- Mol, S.; Ozurtan, S.; Cosansu, S. Determination of the Quality and Shelf Life of Sous Vide Packaged Bonito (Sarda sarda, Bloch, 1793) Stored at 4 and 12 C. J. Food Qual. 2011, 35, 137–143. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Ceylan, Z.; Meral, R.; Kilicer, A.; Altay, F. A Novel Strategy for Au in Food Science: Nanoformulation in Dielectric, Sensory Properties, and Microbiological Quality of Fish Meat. Food Biosci. 2021, 41, 101024. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Li, D.; Liu, X.; Luo, Y. Changes in the Microbial Communities of Air-Packaged and Vacuum-Packaged Common Carp (Cyprinus carpio) Stored at 4 °C. Food Microbiol. 2015, 52, 197–204. [Google Scholar] [CrossRef]
- Arkoudelos, J.; Stamatis, N.; Samaras, F. Quality Attributes of Farmed Eel (Anguilla anguilla) Stored under Air, Vacuum and Modified Atmosphere Packaging at 0 °C. Food Microbiol. 2007, 24, 728–735. [Google Scholar] [CrossRef]
- Sebastia, C.; Soriano, J.M.; Iranzo, M.; Rico, H. Microbiological Quality of Sous Vide Cook-Chill Preserved Food at Different Shelf Life. J. Food Process. Preserv. 2010, 34, 964–974. [Google Scholar] [CrossRef]
- Picouet, P.A.; Cofan-Carbo, S.; Vilaseca, H.; Carbone Ballbe, L.; Castells, P. Stability of Sous-Vide Cooked Salmon Loins Processed by High Pressure. Innov. Food Sci. Emerg. Technol. 2011, 12, 26–31. [Google Scholar] [CrossRef]
- Nguvava, J.P. Effects of Post Harvest Handling on Quality and Sensory Attributes of Sardines: A Case Study of Musoma District. Master’s Thesis, Sokoine University of Agriculture, Morogoro, Tanzania, 2013. [Google Scholar]
- Antelm, P.; Juma, I.; Rweyemamu, L.M.P.; Kaale, L.D. Effects of Vacuum Packaging and Chilling Storage on the Microbiological Changes of the Superheated Steam Dried Sardines. Tanzan. J. Sci. 2023, 49, 140–151. [Google Scholar] [CrossRef]
| Applied Treatment | Sample Code | Cooking Method |
|---|---|---|
| None | A0 | None; this sample was tested without applying any thermal treatment. |
| Sous vide cooking—cook–serve (SV-CS) | A1 | The product was placed in a bag (Saporoso, Brașov, Romania) that was then vacuum sealed (SAM Cook, Milanówek, Poland) and cooked at 55 °C for 40 min in a sous vide cooker (Hendi, Ghimbav, Romania). |
| Convective cooking—cook–serve (CC-CS) | A2 | In this cook–serve production system, convection baking (without steam) in an electrical oven (Rational, Luton, UK) was chosen. The product was baked at 170 °C for 25 min. |
| Microwave cooking—cook–serve (Mw-CS) | A3 | The product was cooked in a microwave oven (Galanz, Foshan, China) for this cook–serve production system at 567 W for 15 min. |
| Convective cooking—cook–chill (CC-CC) | A4 | In this cook–chill production system, convection baking (without steam) in an electrical oven (Rational, UK) was also chosen. The product was baked at 180 °C for 25 min. After cooking, the product was cooled in a controlled manner, in two stages: down to 20 °C in the first 60 min and down to 4 °C in the next 60 min, using a rapid blast chiller (Afinox, Campo San Martino, Italy). |
| Convective cooking—cook–freeze (CC-CF) | A5 | In this cook–freeze production system, convection baking (without steam) in an electrical oven (Rational, UK) was also chosen. The product was baked at 180 °C for 25 min. After cooking, the product was cooled and then frozen, in a controlled manner, in three stages: down to 20 °C in the first 60 min, down to 4 °C in the following 60 min, and down to −18 °C in the last 60 min, using a rapid blast chiller (Afinox, Campo San Martino, Italy). |
| Microbiological Analyses | Analysis Methods | |
|---|---|---|
| Quantitative Evaluation * of Microbiological Quality | ||
| 1. | Total Aerobic Microbial Count/Aerobic Plate Count (APC) | Classic method, according to ISO 4833:2013 [32] |
| 2. | Total Yeast and Mold Count TYMC | Classic method, according to ISO 21527-1:2008 [33] |
| 3. | Total Coliform Count | Classic method, according to ISO 4831:2006 [34] |
| Quantitative evaluation * of pathogenic bacteria | ||
| 4. | Staphylococcus aureus count | Rapid TEMPO method (BioMerieux Industry, France). |
| 5. | Bacillus cereus count | |
| 6. | Escherichia coli count | |
| Qualitative evaluation ** of the presence/absence of pathogenic bacteria | ||
| 7. | Salmonella sp. detection | ISO 6579:2017 [35] |
| 8. | Listeria monocytogenes detection | ISO 11290-1:2017 [36] |
| A0 | A1 | A2 | A3 | A4 | A5 | ||
|---|---|---|---|---|---|---|---|
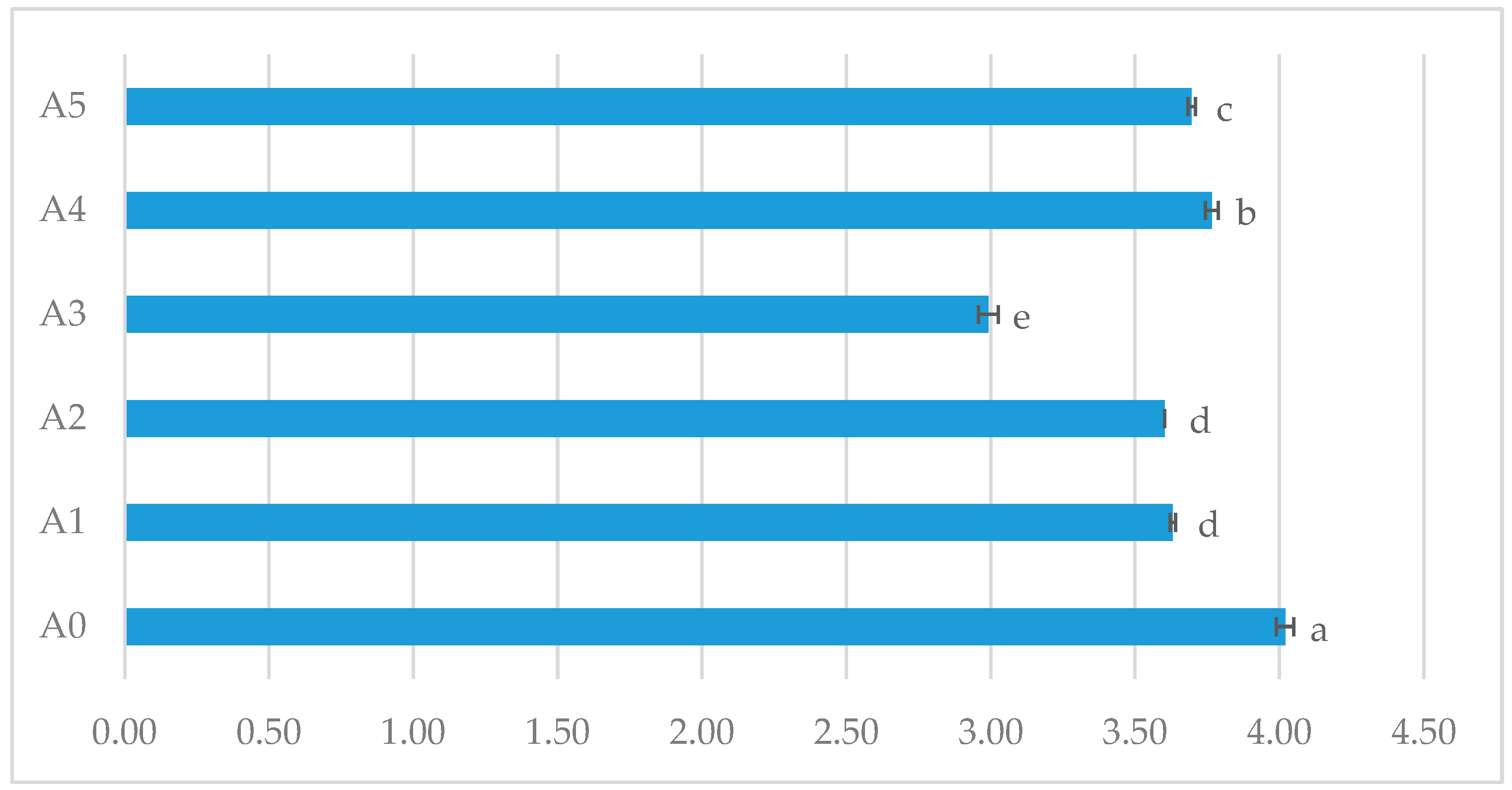
| Content loss, % | - | 14.25 ± 1.57 a | 30.91 ± 2.01 b | 28.55 ± 1.23 b | 35.68 ± 2.32 c | 40.25 ± 1.57 d | |
| Dry substance, % | 30.98 ± 0.02 a | 31.84 ± 0.04 a | 31.47 ± 0.38 a | 31.40 ± 0.08 a | 33.57 ± 0.04 b | 33.86 ± 0.04 b | |
| Proteins, % | 14.32 ± 0.02 a | 15.22 ± 0.09 a | 16.66 ± 0.12 b | 15.90 ± 0.16 a | 16.93 ± 0.21 c | 17.36 ± 0.03 c | |
| Insoluble proteins, % | 4.37 ± 0.01 a | 1.53 ± 0.05 b | 1.53 ± 0.05 b | 1.78 ± 0.02 c | 2.15 ± 0.04 c | 1.58 ± 0.02 b | |
| Digestibility, % | 69.51 a | 89.91 | 89.91 ± 0.03 c | 89.31 ± 0.20 c | 86.48 ± 0.11 b | 90.67 ± 0.21 c | |
| Carbohydrates, % | 0.73 ± 0.02 a | 1.10 ± 0.04 b | 1.05 ± 0.04 b | 1.93 ± 0.05 c | 1.23 ± 0.05 b | 1.25 ± 0.01 b | |
| Lipids, % | 15.01 ± 0.09 a | 14.2 ± 0.08 b | 13.64 ± 0.06 c | 13.93 ± 0.05 c | 14.71 ± 0.09 b | 14.81 ± 0.01 b | |
| Hydro-soluble vitamins | |||||||
| Thiamine | mg | 0.0478 ± 0.01 a | 0.0347 ± 0.01 b | 0.0256 ± 0.01 c | 0.0289 ± 0.01 c | 0.0183 ± 0.01 d | 0.0159 ± 0.01 d |
| TR% | 100 ± 0.01 a | 72.51 ± 0.01 b | 55.32 ± 0.01 c | 63.08 ± 0.01 c | 41.49 ± 0.01 d | 36.45 ± 0.01 d | |
| Riboflavin | mg | 0.0465 ± 0.01 a | 0.0333 ± 0.01 b | 0.0257 ± 0.01 c | 0.0275 ± 0.01 c | 0.0193 ± 0.02 c | 0.02 ± 0.01 c |
| TR% | 100 ± 0.01 a | 71.67 ± 0.01 b | 55.33 ± 0.01 c | 59.20 ± 0.01 c | 41.57 ± 0.01 c | 34.69 ± 0.01 c | |
| Niacin | mg | 5.24 ± 0.06 a | 3.22 ± 0.04 b | 2.05 ± 0.04 c | 2.13 ± 0.01 c | 1.23 ± 0.01 d | 1.14 ± 0.05 d |
| TR% | 100 ± 0.01 a | 61.49 ± 0.04 b | 39.21 ± 0.04 c | 40.74 ± 0.01 c | 23.42 ± 0.01 d | 21.77 ± 0.01 d | |
| Fat-soluble vitamins | |||||||
| Retinol | mg | 0.3411 ± 0.02 a | 0.2123 ± 0.01 b | 0.1251 ± 0.01 c | 0.1148 ± 0.01 c | 0.0941 ± 0.01 c | 0.0917 ± 0.01 c |
| TR% | 100 ± 0.01 a | 62.24 ± 0.01 b | 37.89 ± 0.02 c | 34.10 ± 0.01 c | 29.85 ± 0.01 c | 29.40 ± 0.01 c | |
| α-Tocopherol | mg | 0.62 ± 0.02 a | 0.32 ± 0.02 b | 0.26 ± 0.03 c | 0.22 ± 0.02 c | 0.21 ± 0.02 c | 0.12 ± 0.04 d |
| TR% | 100 ± 0.01 a | 51.61 ± 0.02 b | 43.33 ± 0.01 c | 35.95 ± 0.01 c | 36.65 ± 0.01 c | 21.17 ± 0.01 d | |
| Fatty Acids (%) | A0 | A1 | A2 | A3 |
|---|---|---|---|---|
| C6:0; Caproic | 7.59 ± 0.01 a | 2.18 ± 0.01 b | 2.29 ± 0.01 b | 0.42 ± 0.01 c |
| C8:0; Caprylic | 0.2 ± 0.04 a | 0.21 ± 0.01 a | 0.22 ± 0.01 a | 0.34 ± 0.03 b |
| C10:0; Capric | 0.23 ± 0.01 a | 0.22 ± 0.01 a | 0.22 ± 0.01 a | 0.33 ± 0.01 b |
| C11:0; Undecanoic | n.d. | n.d. | n.d. | n.d. |
| C12:0; Lauric | 2.12 ± 0.04 a | 2.24 ± 0.02 b | 2.49 ± 0.01 c | 3.49 ± 0.02 d |
| C13:0; Tridecanoic | n.d. | n.d. | n.d. | n.d. |
| C14:0; Myristic | 3.18 ± 0.02 a | 3.1 ± 0.03 a | 3.05 ± 0.01 b | 3.14 ± 0.02 a |
| C14:1; Myristoleic | 0.16 ± 0.01 | n.d. | n.d. | n.d. |
| C15:0; Pentadecanoic | 0.22 ± 0.02 a | 0.18 ± 0.01 b | 0.18 ± 0.01 b | 0.18 ± 0.01 b |
| C15:1, cis-10; Pentadecanoic | n.d. | 0.07 ± 0.01 a | 0.07 ± 0.01 a | n.d. |
| C16:0; Palmitic | 12.5 ± 0.87 a | 13.12 ± 0.01 b | 14.18 ± 0.02 c | 14.08 ± 0.02 c |
| C16:1; Palmitoleic | 3.85 ± 0.09 a | 4.08 ± 0.01 b | 4.19 ± 0.01 b | 4.01 ± 0.01 ab |
| C17:0; Heptadecenoic | 0.23 ± 0.01 a | n.d. | n.d. | n.d. |
| C17:1, cis-10; Heptadecenoic | n.d. | n.d. | n.d. | n.d. |
| C18:0; Stearic | 3.93 ± 0.01 a | 4.64 ± 0.01 b | 4.88 ± 0.02 b | 5.48 ± 0.02 c |
| C18:1, cis-9; Oleic | 25.65 ± 0.09 a | 28.82 ± 0.02 b | 30.05 ± 0.02 c | 29.1 ± 0.09 b |
| C18:1 iso, trans-9; Elaidic | 2.17 ± 0.01 a | 2.18 ± 0.01 a | 2.21 ± 0.01 b | 2.18 ± 0.01 a |
| C18:2, cis-9, cis-12; Linoleic (n-6) | 8.27 ± 0.01 a | 9.04 ± 0.01 b | 9.11 ± 0.01 c | 8.7 ± 0.02 b |
| C18:3, cis-9, cis-12, cis-15; Linolenic (n-3) | 2.14 ± 0.01 a | 1.96 ± 0.01 a | 2.02 ± 0.01 a | 1.76 ± 0.01 b |
| C20:0; Arahidic | 0.22 ± 0.01 a | 4.1 ± 0.01 b | 6.62 ± 0.03 c | 4.27 ± 0.03 b |
| C20:1,cis-11; Eicosenoic | 2.63 ± 0.01 a | 2.86 ± 0.01 a | 2.76 ± 0.02 a | 2.24 ± 0.01 b |
| C20:2, cis-11, cis-14; Eicosadienoic (n-6) | 2.16 ± 0.01 a | 3.21 ± 0.01 b | 3.07 ± 0.02 b | 3.22 ± 0.03 b |
| S.F.A. | 30.42 ± 0.77 a | 29.99 ± 0.05 a | 34.13 ± 0.05 b | 31.73 ± 0.03 a |
| M.U.F.A. | 34.46 ± 0.16 a | 37.94 ± 0.03 b | 39.21 ± 0.02 c | 37.53 ± 0.08 b |
| P.U.F.A. | 12.57 ± 0.02 a | 14.21 ± 0.02 b | 14.2 ± 0.03 b | 13.68 ± 0.16 c |
| U.F.A/S.F.A | 1.55 ± 0.03 a | 1.74 ± 0.01 b | 1.56 ± 0.01 a | 1.61 ± 0.01 a |
| P.U.F.A./S.F.A | 0.41 ± 0.01 a | 0.47 ± 0.01 a | 0.42 ± 0.01 a | 0.43 ± 0.01 a |
| n-6/n-3 | 4.87 ± 0.01 a | 6.25 ± 0.01 b | 6.03 ± 0.01 b | 6.77 ± 0.11 c |
| Sample | APC (CFU/g ± SD) | TYMC (CFU/g ± SD) | Coliforms (CFU/g ± SD) | E. coli (CFU/g ± SD) | Salmonella sp. (CFU/25 g) | Listeria sp. (CFU/25 g) | |
|---|---|---|---|---|---|---|---|
| A0 | (1.037 ± 0.071) × 104 a | (2.69 ± 0.11) × 102 a | (2.6 ± 0.11) × 101 a | 7 ± 0.58 a | nd | nd | |
| A1 | (4.27 ± 0.10) × 103 b | (6.14 ± 0.04) × 101b | (1.67 ± 0.09) × 101 b | 4.67 ± 0.94 b | nd | nd | |
| A2 | (4 ± 0.01) × 103 b | (6.1 ± 0.32) × 101 b | (1.1 ± 0.11) × 101 b | 3 ± 0.58 b | nd | nd | |
| A3 | (9.83 ± 0.76) × 102 c | (4.7 ± 0.15) × 101 c | 3 ± 0.15 c | nd | nd | nd | |
| A4 | (5.83 ± 0.29) × 103 d | (6.2 ± 0.67) × 101 b | 4 ± 0.8 c | nd | nd | nd | |
| A4-a (day 1) | (5.67 ± 0.29) × 103 d | (7 ± 0.25) × 101 d | 2 ± 0.58 c | nd | nd | nd | |
| A4-b (day 2) | (6.50 ± 0.5) × 103 e | (7 ± 0.38) × 101 d | 1 ± 0.58 c | nd | nd | nd | |
| A4-c (day 3) | (6.67 ± 0.58) × 103 e | (8.2 ± 0.29) × 101 e | 1 ± 0.58 c | nd | nd | nd | |
| A4-d (day 4) | (7 ± 0.5) × 103 f | (8.7 ± 0.58) × 101 e | 1 ± 0.58 c | nd | nd | nd | |
| A4-e (day 5) | (6.83 ± 0.29) × 103 e | (1.07 ± 0.06) × 102 f | 1 ± 0.58 c | nd | nd | nd | |
| A5 | (4.97 ± 0.15) × 103 b | (2.4 ± 0.2) × 101 g | 3 ± 1.15 c | nd | nd | nd | |
| A5-a (day 10) | (4.63 ± 0.12) × 103 b | (2.3 ± 0.16) × 101 g | 2 ± 0.58 c | nd | nd | nd | |
| A5-b (day 20) | (4.5 ± 0.01) × 103 b | (2.1 ± 0.16) × 101 g | 3 ± 1.15 c | nd | nd | nd | |
| A5-c (day 30) | (4.17 ± 0.29) × 103 b | (2.1 ± 0.16) × 101 g | 2 ± 0.58 c | nd | nd | nd | |
| A5-d (day 40) | (4.5 ± 0.01) × 103 b | (2.2 ± 0.2) × 101 g | 1 ± 0.58 c | nd | nd | nd | |
| A5-c (day 50) | (5.13 ± 0.23) × 103 d | (2.0 ± 0.2) × 101 g | 1 ± 0.58 c | nd | nd | nd | |
| Treatment | Sample | Exterior Appearance | Interior Appearance | Consistency | Odor | Taste | Overall Acceptability | Total Points |
|---|---|---|---|---|---|---|---|---|
| Sous vide cook–serve (SV-CS) | A1 | 7.73 ± 0.44 b | 7.77 ± 0.50 bc | 8.30 ± 0.69 a | 8.37 ± 0.56 b | 8.43 ± 0.48 b | 8.27 ± 0.29 b | 8.15 ± 0.31 b |
| Convective cooking cook–serve (CC-CS) | A2 | 8.20 ± 0.48 a | 8.23 ± 0.76 a | 8.30 ± 0.59 a | 8.50 ± 0.62 a | 8.73 ± 0.51 a | 8.50 ± 0.61 a | 8.41 ± 0.40 a |
| Microwave cook–serve (Mw-CS) | A3 | 7.40 ± 0.51 cd | 7.70 ± 0.46 c | 8.07 ± 0.51 b | 8.07 ± 0.56 e | 8.00 ± 0.64 d | 8.20 ± 0.33 b | 7.91 ± 0.45 c |
| Convective cooking cook–chill (CC-CC) | A4 | 7.67 ± 0.55 bc | 7.47 ± 0.85 d | 8.07 ± 0.71 c | 8.13 ± 0.47 d | 8.13 ± 0.35 c | 7.87 ± 0.65 c | 7.89 ± 0.29 c |
| Convective cooking cook–freeze (CC-CF) | A5 | 7.20 ± 0.65 d | 7.77 ± 0.88 c | 7.97 ± 0.62 d | 7.97 ± 0.51 f | 7.7 ± 0.67 d | 7.87 ± 0.59 c | 7.75 ± 0.71 c |
| Fine dining of A1 | OA1 | 7.07 ± 0.21 d | 7.23 ± 0.32 e | 8.33 ± 0.50 a | 8.37 ± 0.51 b | 8.37 ± 0.62 b | 8.23 ± 0.44 b | 7.93 ± 0.63 c |
| Fine dining of A2 | OA2 | 8.37 ± 0.34 a | 8.27 ± 0.38 a | 8.30 ± 0.48 a | 8.57 ± 0.50 a | 8.73 ± 0.56 a | 8.57 ± 0.33 a | 8.47 ± 0.57 a |
| Fine dining of A3 | OA3 | 7.73 ± 0.41 bc | 7.90 ± 0.46 b | 8.07 ± 0.44 c | 8.27 ± 0.61 c | 8.20 ± 0.39 c | 8.23 ± 0.62 b | 8.07 ± 0.43 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Măzărel, A.; Rădoi-Encea, R.-Ș.; Pădureanu, V.; Maier, A.; Matei, F. The Influence of Different Cooking Techniques on the Biochemical, Microbiological, and Sensorial Profile of Fish-Based Catering Products. Appl. Sci. 2025, 15, 4417. https://doi.org/10.3390/app15084417
Măzărel A, Rădoi-Encea R-Ș, Pădureanu V, Maier A, Matei F. The Influence of Different Cooking Techniques on the Biochemical, Microbiological, and Sensorial Profile of Fish-Based Catering Products. Applied Sciences. 2025; 15(8):4417. https://doi.org/10.3390/app15084417
Chicago/Turabian StyleMăzărel, Adrian, Raluca-Ștefania Rădoi-Encea, Vasile Pădureanu, Alina Maier, and Florentina Matei. 2025. "The Influence of Different Cooking Techniques on the Biochemical, Microbiological, and Sensorial Profile of Fish-Based Catering Products" Applied Sciences 15, no. 8: 4417. https://doi.org/10.3390/app15084417
APA StyleMăzărel, A., Rădoi-Encea, R.-Ș., Pădureanu, V., Maier, A., & Matei, F. (2025). The Influence of Different Cooking Techniques on the Biochemical, Microbiological, and Sensorial Profile of Fish-Based Catering Products. Applied Sciences, 15(8), 4417. https://doi.org/10.3390/app15084417







