Abstract
Pancreatic cancer (PaCa) is among the most aggressive malignancies of the digestive system. Inflammation plays a critical role in tumor growth and dissemination, with soluble cytokines serving as messengers that facilitate interactions between immune and cancer cells. The release of cell-free mitochondrial DNA (cf-mtDNA) into the bloodstream has been identified as a potent proinflammatory trigger, acting as a mitochondrial-derived damage-associated molecular pattern (mtDAMP). Whether a relationship exists between circulating cf-mtDNA (ccf-mtDNA) unloading and inflammation in PaCa remains unclear. In this study, we quantified ccf-mtDNA levels in plasma/serum samples from PaCa patients and healthy controls and examined their association with inflammatory markers. Analyses were conducted on 14 participants: 3 controls (mean age: 52.0 ± 16.0 years, 67% women) and 11 PaCa patients (mean age: 69.1 ± 10.0 years, 27% women). Circulating levels of ccf-mtDNA in PaCa patients did not show differences compared to controls (p = 0.06). In contrast, concentrations of interleukin (IL)-8, IL-17, and interferon-gamma were significantly higher in PaCa patients. Stratification of PaCa patients based on the median ccf-mtDNA concentration revealed significantly higher levels of IL-4, IL-9, monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein 1-beta in those with ccf-mtDNA levels above the median (p < 0.05). Significant positive associations were also observed between levels of ccf-mtDNA and IL-8, fibroblast growth factor, and MCP-1. These results suggest a potential association between elevated ccf-mtDNA levels and increased concentrations of proinflammatory cytokines, especially in PaCa patients with an unfavorable prognosis. Further research with larger cohorts is required to validate these findings and assess the prognostic value of these biomarkers.
1. Introduction
Pancreatic cancer (PaCa) is one of the most lethal malignancies of the digestive tract, owing to its often late-stage diagnosis, the absence of effective therapies, and the unpredictable nature of disease progression [1,2]. Both local and systemic inflammation have been implicated as factors that contribute to promoting PaCa growth and dissemination [3]. The relationship between inflammation and PaCa carcinogenesis is mediated by the infiltration of inflammatory cells into the tumor microenvironment (TME) [4]. In the PaCa TME, M1 macrophages, dendritic cells (DCs), and effector CD4+ and CD8+ T lymphocytes are less abundant than myeloid-derived suppressor cells (MDSCs), M2-polarized macrophages, and regulatory T lymphocytes (Treg) [5]. This immunological microenvironment favors tumor growth, and the TME may acquire additional immunosuppressive properties. In this setting, cancer cell survival may be supported by a proinflammatory environment, potentially leading to the establishment of a dynamic and metabolically symbiotic TME [4]. Soluble inflammatory cytokines serve as key messengers for facilitating interactions between immune and cancer cells [3]. Proinflammatory mediators such as interleukin (IL)-1, IL-6, tumor necrosis factor-alpha (TNF-α), anti-inflammatory IL-10, and transforming growth factor-beta (TGF-β) are among the major inflammatory factors in this immunomodulatory network [3].
The extracellular release of mitochondrial-derived damage-associated molecular patterns (mtDAMPs), including circulating cell-free mitochondrial DNA (ccf-mtDNA), has been described across various pathological conditions, including cancer, and is associated with systemic sterile inflammation [6,7,8,9]. Despite the evolutionary compartmentalization of DNA within the nucleus and mitochondria, the release and subsequent accumulation of both nuclear and mtDNA in the cytosol has been documented in pathological conditions. In particular, the cyclic GMP–AMP synthase–stimulator of the interferon genes (cGAS–STING) pathway mediates the release of mtDNA into the cytosol, where it triggers ferroptosis [6,7,8]. In this context, cytosolic and extracellular mtDNA becomes a potent stimulator of inflammation, regulating biological processes integral to cell proliferation and survival [6,7,9]. The inflammation induced by mtDNA release contributes to the amplification of tissue and organ damage [8,10,11]. By acting as an mtDAMP, cf-mtDNA promotes neutrophil migration and degranulation at sites of injury, thereby contributing to cellular damage and the initiation of local inflammatory responses [11]. Interestingly, Gao et al. [8] have demonstrated that M1 pancreatic resident macrophages secrete extracellular vesicles (EVs) containing mitochondria, which exhibit proinflammatory properties and can enter pancreatic β cells. These mitochondria-derived EVs induce lipid peroxidation and mitochondrial damage [8].
Recently, the detection and quantification of ccf-mtDNA in biological fluids of cancer patients have garnered significant interest as a potential approach for identifying diagnostic and prognostic biomarkers. For instance, lower ccf-mtDNA levels have been reported in the plasma of women with breast cancer compared with healthy controls [12]. In contrast, elevated ccf-mtDNA levels have been observed in the serum of patients with bladder, prostate, renal cell, and testicular cancers [13,14]. Increased ccf-mtDNA levels have also been described in women with epithelial ovarian cancer relative to peers with benign ovarian conditions and healthy controls [15]. However, no studies have investigated whether the sterile inflammatory milieu of PaCa is linked to the release of cellular proinflammatory components, particularly ccf-mtDNA, or whether the nature of such components contributes to distinct inflammatory profiles in PaCa patients. To address this gap in knowledge, we quantified ccf-mtDNA levels in PaCa patients and examined their association with patient inflammatory profiles.
2. Materials and Methods
2.1. Study Design and Participants
The study was conducted as a cross-sectional, case-control investigation within the framework of the single-center EXOPanc study (Role of EXOsomes in Pancreatic Cancer Progression), carried out at the S. Orsola–Malpighi Hospital in Bologna, Italy. The study protocol was approved by the Area Vasta Emilia Centro (AVEC) Ethics Committee (Protocol no. 262/2022/Sper/AOUBo; approval date 4 April 2022). All participants were enrolled at the time of pancreatic cancer (PaCa) diagnosis between 2022 and 2023 and provided written informed consent prior to inclusion.
The patient cohort consisted of men and women aged 59 to 85 years, all diagnosed with pancreatic adenocarcinoma G3 (histologic grading system) [16]. Healthy controls were recruited from the general population during the same time period and had no current or previous history of malignancy. All participants were anonymized using alphanumeric codes (PC01 to PC11 for PaCa patients, and CTR01 to CTR03 for healthy controls). The study was conducted in accordance with the ethical standards established in the Declaration of Helsinki.
2.2. Blood Collection and Processing
Blood samples were collected at the time of diagnosis using commercially available collection tubes. Serum samples were obtained from patients PC01, PC02, and PC07–PC11, as well as from healthy controls CTR01–CTR03. Following collection, blood samples were allowed to clot at room temperature for 30 min. Samples were then centrifuged at 1100× g for 15 min at 4 °C, and the resulting supernatant (serum) was aliquoted and stored at –80 °C until analysis. For patients PC03–PC06, blood was collected in EDTA tubes, and plasma was separated accordingly. These samples were centrifuged at 3000× g for 10 min at 4 °C. The resulting plasma was collected in 0.5-mL aliquots and stored at –80 °C until analysis. The use of either serum or plasma in this study was determined based on sample availability.
2.3. Quantification of Circulating Cell-Free Mitochondrial DNA by Droplet Digital PCR
The ccf-mtDNA was quantified in plasma or serum samples using droplet digital PCR (ddPCR), as previously described [17]. Briefly, each 20 μL reaction mixture contained 10 μL of 2× ddPCR Supermix for Probes (No dUTP) (Bio-Rad Laboratories, Hercules, CA, USA), 0.9 μM of the forward primer (mtDNA-85F), 0.9 μM of the reverse primer (mtDNA-85R), 0.1 μM of the hydrolysis probe (FAM-mtDNA-85P) (Table 1), and 1 μL of the biological sample, serving as the source of the mtDNA template.

Table 1.
Primer and probe sequences for the droplet digital PCR assay.
The reaction mixture (20 µL) was used to generate droplets using the QX200 Droplet Generator (Bio-Rad Laboratories, Hercules, CA, USA). PCR amplification of the target mtDNA within the emulsified droplets was carried out under the following thermal cycling conditions: 95 °C for 10 min; 40 cycles of 94 °C, 30 s each; 60 °C for 1 min; 98 °C for 10 min; 4 °C on hold. A ramp rate of 2 °C/s was applied between all steps.
Following amplification, fluorescence was measured using a QX200 Droplet Reader (Bio-Rad Laboratories), detecting on average 10,000–20,000 droplets per sample. Each droplet was analyzed individually using FAM fluorescence detection, allowing for the absolute quantification of target mtDNA based on the ratio of PCR-positive to PCR-negative droplets. Data acquisition and analysis were performed using Bio-Rad QX Manager 1.2 Standard Edition software. The concentration of mtDNA in the original sample (copies/µL) was determined by applying a fluorescence threshold for positive droplets, established based on droplet clustering across all experimental samples and compared to a negative control consisting of a serum- and plasma-free sample.
2.4. Quantification of Circulating Inflammatory Markers
A panel of inflammatory biomolecules was quantified in duplicate using the Bio-Plex Pro™ Human Cytokine 27-Plex Assay Kit (#M500KCAF0Y, Bio-Rad Laboratories) on a Bio-Plex® System equipped with Luminex xMAP® Technology (Bio-Rad Laboratories). The inflammatory markers included in the panel are listed in Table 2. Data acquisition was performed using the Bio-Plex Manager Software version 6.1 (Bio-Rad Laboratories) under default instrument settings. To ensure the accuracy and reliability of the results, standard curves were optimized for each analyte, and data quality was further refined by identifying and excluding outliers.

Table 2.
List of inflammatory biomolecules assayed by multiplex immunoassay.
2.5. Statistical Analysis
Data are presented as mean ± standard deviation (SD). Normality was assessed by the Kolmogorov–Smirnov test. For variables exhibiting a normal distribution, comparisons between groups (controls vs. PaCa, or groups stratified by median ccf-mtDNA concentration) were performed using the unpaired Student’s t-test. For non-normally distributed variables, the non-parametric Mann–Whitney U test was applied.
To explore the relationship between ccf-mtDNA concentrations and levels of inflammatory biomolecules, Spearman’s rank correlation analysis was conducted. Statistical significance was set at an alpha level of 5% (p < 0.05). All analyses were performed using GraphPad Prism version 6.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Characteristics of Study Participants
The main characteristics of the study participants are summarized in Table 3. The study sample consisted of three healthy controls (mean age: 52.0 ± 16.0 years; 67% women) and 11 patients diagnosed with PaCa (mean age: 69.1 ± 10.0 years; 27% women). Among the PaCa patients, four were diagnosed with stage II/III of the disease, while the remaining seven presented with stage IV of the disease and distant metastases, mainly to the liver. In most cases (64%), the primary tumor was located in the head of the pancreas, with a mean lesion diameter of 35.7 mm. As mentioned earlier, blood samples were collected at the time of diagnosis, prior to the initiation of chemotherapy or any tumor cytoreductive procedures.

Table 3.
Main characteristics of study participants (n = 14).
3.2. Circulating Cell-Free Mitochondrial DNA and Inflammatory Biomolecules in Pancreatic Cancer Patients and Controls
PaCa patients exhibited a trend toward higher circulating levels of ccf-mtDNA compared with controls (Figure 1A). However, this difference did not reach statistical significance (p = 0.06). The absence of a statistically significant difference in ccf-mtDNA levels between the two groups may be attributed to the highly heterogeneous distribution of ccf-mtDNA concentrations within the PaCa patient cohort (Figure 1B). In contrast, circulating levels of interleukin (IL)-8, IL-17, and interferon-gamma (IFN-γ) were significantly higher in serum or plasma from PaCa patients relative to controls (Figure 1C–E).
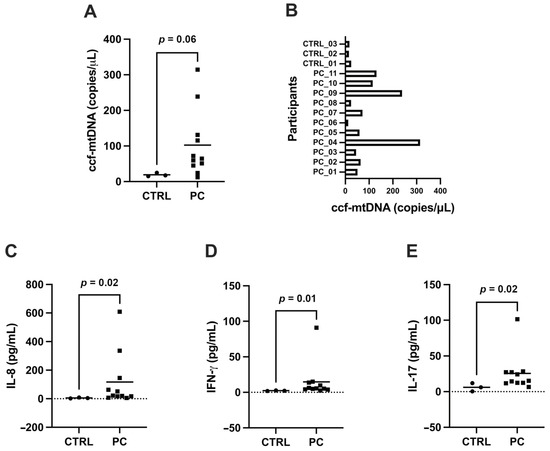
Figure 1.
Circulating cell-free mitochondrial DNA (ccf-mtDNA) and inflammatory biomolecules. (A) Levels of ccf-mtDNA and (B) interleukin-8 (IL-8), (C) IL-17, and (D) interferon-gamma (IFN-γ) in samples of PaCa patients (PC) and controls (CTRL); (E) Levels of ccf-mtDNA in the cohort of PaCa patients.
3.3. Cytokine Signatures According to Cell-Free Mitochondrial DNA Circulating Levels
To explore the relationship between ccf-mtDNA and inflammatory biomolecules, PaCa patients were stratified into two groups based on the median level of ccf-mtDNA (i.e., below and above the median value).
Levels of anti-inflammatory IL-4 and those of proinflammatory IL-9, monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein 1-beta (MIP-1β) were significantly higher in participants with ccf-mtDNA concentrations above the median value (Figure 2A–D). No significant differences were observed for the remaining inflammatory markers between the two groups (Supplementary Figure S1).
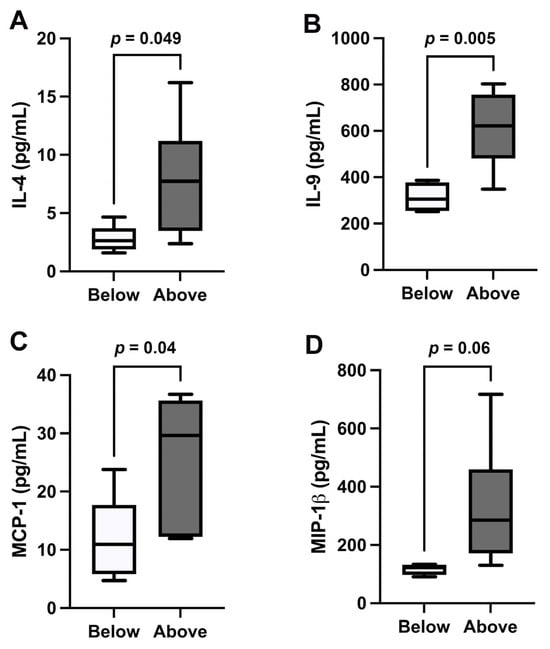
Figure 2.
Cytokine levels in patients with pancreatic cancer. (A) Levels of interleukin-4 (IL-4), (B) IL-9, (C) monocyte chemoattractant protein-1 (MCP-1), and (D) macrophage inflammatory protein 1-beta (MIP-1β) in samples of patients with pancreatic cancer. “Above” indicates above the median value of circulating cell-free mitochondrial DNA (ccf-mtDNA) in the cohort of patients with pancreatic cancer; “Below” indicates below the median value of ccf-mtDNA in the cohort of patients with pancreatic cancer.
To further explore the relationship between ccf-mtDNA and inflammatory biomolecules in PaCa patients, Spearman’s correlation analysis was performed. The results revealed a significant positive correlation between ccf-mtDNA levels and IL-8, fibroblast growth factor (FGF), and MCP-1 (Table 4). Scatter plots are shown in Supplementary Figure S2.

Table 4.
Correlation analysis between circulating levels of mitochondrial DNA and inflammatory markers.
4. Discussion
To the best of our knowledge, this is the first study in which ccf-mtDNA was quantified and correlated with inflammatory markers in blood samples from patients with PaCa. Levels of ccf-mtDNA tended to be higher in PaCa patients compared with healthy controls. However, the high heterogeneity of values in PaCa patients, likely due to interindividual variability stemming from biological (e.g., response to stress, age, metabolic differences) and clinical factors (e.g., tumor stage, comorbid conditions), as well as the small sample size, prevented the difference from reaching statistical significance. In contrast, we observed significantly higher circulating levels of IL-8, IL-17, and IFN-γ in PaCa patients compared with controls.
PaCa is characterized by a poor five-year survival rate, due to its aggressive nature, rapid development of distant metastases, and limited improvements in surgical and medical treatments over recent decades [18]. Therefore, recent research has focused on identifying markers for early diagnosis and prognostication [19,20,21,22,23]. By exploring the potential of ccf-mtDNA as a marker of tumor progression, we found that blood samples from patients with stage IV PaCa and distant metastasis (PC04 and PC09) exhibited the highest levels of ccf-mtDNA, while PC06, who had stage II cancer without distant metastasis, showed the lowest ccf-mtDNA levels. This suggests that ccf-mtDNA levels may increase in advanced stages of the disease. However, a larger cohort is needed to confirm the role of ccf-mtDNA as a prognostic marker in PaCa.
The TME has gained significant attention as a contributor to the development and progression of PaCa [24]. Studies have identified a heterogeneous array of TME components in epithelial cancers, including fibroblasts, extracellular matrix, immune and inflammatory cells, blood and lymphatic vessels, and nerves, all of which influence the malignancy phenotype [25,26]. The immune system plays a key role in the TME, contributing to cancer development and progression. Furthermore, while treatments such as chemotherapy, radiotherapy, and targeted therapy effectively eliminate cancer cells, they can also induce immunogenic cell death and inflammation as “byproducts” of cancer treatment [27,28]. Cells undergoing death release molecules into the surrounding environment, alerting the immune system and inducing inflammation [29,30,31]. These molecules, known as DAMPs, include a variety of chemically unrelated mediators such as high mobility group box 1 (HMGB1), S100 proteins, hyaluronan, heat shock proteins, ATP, and calreticulin. These biomolecules are normally retained inside the cell, but are released following stress or cell death, allowing the host to sense and respond to damage via specific DAMP receptors [32]. Moreover, mtDNA has recently been recognized as a DAMP. Although defective mitochondria and damaged mtDNA are degraded by mitophagy, mtDNA can escape this process and act as a DAMP to regulate inflammatory responses by activating IFN genes, toll-like receptor 9 (TLR9), and the nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 (NLRP3) inflammasome [33]. Thus, mtDNA integrity may be compromised by oxidative stress due to its proximity to the electron transport chain, a major source of reactive oxygen species, and the absence of protective histones [6,9,34]. This loss of integrity facilitates the release of mtDNA into the cytoplasm and extracellular space, where it can trigger proinflammatory responses [6,9,34].
An interesting aspect of ccf-mtDNA concerns its fragmentation profiles. Recent studies on the fragmentomic features of cell-free DNA (cf-DNA) (e.g., fragment size, end motifs, breakpoint motifs, nucleosome footprints) have shown their potential as biomarkers in lung cancer [35], colorectal cancer [36], and hepatocellular carcinoma [37]. Cristiano et al. [38] reported that cancer patients exhibit a distinct genome-wide fragmentation profile of cf-DNA compared with healthy individuals. Liu et al. [39] demonstrated that ccf-mtDNA is characterized by shorter fragments compared with circulating cell-free nuclear DNA, due to differences in protein binding patterns [40]. Given the polyploidy of mtDNA and sequence variability across individuals and tissues, it becomes clear why the fragment size distribution of ccf-mtDNA varies among individuals. Avital et al. [41] found that cancer patients release significantly shorter fragments of ccf-mtDNA and that tumor-specific mutations were more strongly associated with shorter ccf-mtDNA fragments, indicating that aberrant fragmentomic features may be the source of tumor-derived ccf-mtDNA fragments.
Moreover, recent research has highlighted the role of acute psychological stress in inducing the unloading of ccf-mtDNA into the bloodstream, suggesting that the release of mtDNA may serve as a marker of the body’s response to stress [42,43,44]. Cancer diagnosis, particularly PaCa, may induce varying degrees of psychological stress depending on an individual’s resilience, which could partly explain the substantial interindividual variability in ccf-mtDNA levels observed in our PaCa cohort.
Notably, we found that circulating ccf-mtDNA levels in PaCa patients were correlated with IL-8 and FGF basic, both of which are angiogenic growth factors [45,46], as well as MCP-1, a molecule that recruits macrophages to the TME and promotes angiogenesis in various cancers [47]. Elevated IL-8 levels are also associated with sarcopenia and cancer cachexia, and have been shown to serve as prognostic markers for PaCa [48]. Our analyses also revealed an interesting relationship between ccf-mtDNA and anti-inflammatory IL-4, which has been found at elevated levels in PaCa patients and is associated with poorer prognosis [49]. IL-4 has been shown to enhance malignant PaCa cell phenotypes, promoting epithelial–mesenchymal transition, eventually leading to increased cell proliferation and migration [50]. While the role of IL-9 in PaCa remains unclear, anti-IL-9 antibody treatment has been shown to inhibit PaCa growth in mice [51]. A recent study correlated serum cytokine levels in PaCa patients before and after treatment with FOLFIRINOX (5- fluorouracil with leucovorin, oxaliplatin, and irinotecan) [52]. The authors found that anti-inflammatory cytokines, such as IL-1ra, IL-7, and MIP-1β, were higher in responding patients, while proinflammatory cytokines, such as IL-18 and IL-1β, were elevated in patients with early disease progression. However, only two out of 11 patients in our cohort received treatment. Thus, although being aware that a limited number of patients were analyzed, we observed elevated ccf-mtDNA levels along with an increase in inflammatory markers in 4 out of 11 patients, of whom 3 had an unfavorable prognosis.
While our study provides valuable insights into the potential role of ccf-mtDNA and inflammation in PaCa, several limitations must be acknowledged. First, the study is cross-sectional in design, which precludes causal inferences. Second, the study did not incorporate deep-sequencing to elucidate the signaling pathways associated with ccf-mtDNA release and develop mechanistic hypotheses. Third, the small sample size and the exploratory nature of the study prevented stratification according to disease stage and adjustment for potential confounders such as age, sex, and proinflammatory pancreatitis. The cross-sectional nature of the study also limited our ability to explore the relationship between ccf-mtDNA levels and patient survival. Lastly, multiple comparisons were performed, and, although the False Discovery Rate correction using the Benjamini–Hochberg method was applied, the possibility remains that some significant findings could have arisen by chance, while comparisons that did not reach statistical significance may have lacked statistical power.
5. Conclusions
This exploratory study identified an association between elevated levels of ccf-mtDNA and proinflammatory cytokines in patients with PaCa and an unfavorable prognosis. Larger, longitudinal studies are needed to validate these preliminary findings and to further investigate the prognostic potential of these markers. Future research should also include a cohort of participants with pancreatitis, a key contributor to PaCa due to its role as precancerous lesion, for which ccf-mtDNA may be tested as a predictive biomarker. Finally, the application of deep-sequencing techniques is crucial, as they would provide insights into the signaling pathways associated with ccf-mtDNA release and elucidate the mechanisms underlying its secretion.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15084410/s1; Supplementary Figure S1: Inflammatory markers with no significant differences between individuals with cell-free mitochondrial DNA (ccf-mtDNA) levels below or above the median value in the cohort of patients with pancreatic cancer; Supplementary Figure S2: Scatter plots of Spearman’s correlation analysis between circulating levels of mitochondrial DNA and inflammatory markers.
Author Contributions
Conceptualization, G.G., F.G., A.P. and C.B.; data curation, G.G., F.G. and A.P.; funding acquisition, E.M. and C.B.; investigation, G.G., F.G. and A.P.; resources, L.H.U.E. and C.R.; supervision, E.M. and C.B.; writing—original draft, G.G., F.G. and A.P.; writing—review and editing, L.H.U.E., E.M. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Università Cattolica del Sacro Cuore [D1.2023 and D1.2024] and the nonprofit research foundation “Centro Studi Achille e Linda Lorenzon” [N/A]. This work was also partially funded by the Italian Ministry of Health [Ricerca Corrente 2025 and Progetto della rete degli IRCCS Aging R.C.R. 2024] and AIRC [IG 2021—ID. 25795] and Next Generation EU PRIN [2022YNENP3]. The authors also acknowledge co-funding from Next Generation EU, in the context of the National Recovery and Resilience Plan, Investment PE8—Project Age-It: “Ageing Well in an Ageing Society”. This resource was co-financed by the Next Generation EU [DM 1557 11.10.2022]. The views and opinions expressed are only those of the authors and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Area Vasta Emilia Centro—AVEC Ethical Committee (Protocol no. 262/2022/Sper/AOUBo, approval date 4 April 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BHQ1 | Black Hole Quencher-1 |
| ccf-mtDNA | Circulating cell-free mitochondrial DNA |
| CCL | C-C motif chemokine ligand |
| cf-mtDNA | Cell-free mitochondrial DNA |
| cGAS–STING | Cyclic GMP-AMP synthase–stimulator of interferon genes |
| CTR | Control participant |
| DAMP | Damage-associated molecular pattern |
| DCs | Dendritic cells |
| ddPCR | Droplet digital PCR |
| EVs | Extracellular vesicles |
| EXOPanc | role of EXOsomes in Pancreatic cancer progression |
| F | Forward |
| FAM | 6-carboxyfluorescein |
| FGF | Fibroblast growth factor |
| FOLFIRINOX | 5- fluorouracil with leucovorin, oxaliplatin, and irinotecan |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte macrophage colony-stimulating factor |
| HMGB1 | High mobility group box 1 |
| IL | Interleukin |
| IL-1ra | Interleukin 1 receptor agonist |
| IP | Interferon-induced protein |
| MCP-1 | Monocyte chemoattractant protein 1 |
| MDSCs | Myeloid-derived suppressor cells |
| MIP | Macrophage inflammatory protein |
| mtDAMP | Mitochondrial damage-associated molecular pattern |
| NLRP3 | Nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 |
| PaCa | Pancreatic cancer |
| PC | Participant with pancreatic cancer |
| PDGF-BB | Platelet derived growth factor BB |
| R | Reverse |
| SD | Standard deviation |
| TGF-β | Transforming growth factor beta |
| TLR9 | Toll-like receptor 9 |
| TME | Tumor microenvironment |
| TNF-α | Tumor necrosis factor-alpha |
| Treg | Regulatory T-lymphocytes |
| VEGF | Vascular endothelial growth factor |
References
- Bisht, S.; Feldmann, G. Novel targets in pancreatic cancer therapy—Current status and ongoing translational efforts. Oncol. Res. Treat. 2018, 41, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Deplanque, G.; Demartines, N. Pancreatic cancer: Are more chemotherapy and surgery needed? Lancet 2017, 389, 985–986. [Google Scholar] [CrossRef] [PubMed]
- Yako, Y.Y.; Kruger, D.; Smith, M.; Brand, M. Cytokines as biomarkers of pancreatic ductal adenocarcinoma: A systematic review. PLoS ONE 2016, 11, e0154016. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Brehm, C.U.; Gress, T.M.; Buchholz, M.; Alashkar Alhamwe, B.; von Strandmann, E.P.; Slater, E.P.; Bartsch, J.W.; Bauer, C.; Lauth, M. The immune microenvironment in pancreatic cancer. Int. J. Mol. Sci. 2020, 21, 7307. [Google Scholar] [CrossRef]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and pancreatic cancer: Focus on metabolism, cytokines, and immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef]
- West, A.P.; Khoury-Hanold, W.; Staron, M.; Tal, M.C.; Pineda, C.M.; Lang, S.M.; Bestwick, M.; Duguay, B.A.; Raimundo, N.; MacDuff, D.A.; et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature 2015, 520, 553–557. [Google Scholar] [CrossRef]
- Gao, Y.; Mi, N.; Wu, W.; Zhao, Y.; Fan, F.; Liao, W.; Ming, Y.; Guan, W.; Bai, C. Transfer of inflammatory mitochondria via extracellular vesicles from M1 macrophages induces ferroptosis of pancreatic beta cells in acute pancreatitis. J. Extracell. Vesicles 2024, 13, e12410. [Google Scholar] [CrossRef]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef]
- Simmons, J.D.; Lee, Y.L.; Mulekar, S.; Kuck, J.L.; Brevard, S.B.; Gonzalez, R.P.; Gillespie, M.N.; Richards, W.O. Elevated levels of plasma mitochondrial DNA DAMPs are linked to clinical outcome in severely injured human subjects. Ann. Surg. 2013, 258, 591–596; discussion 596–598. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.; Radpour, R.; Barekati, Z.; Asadollahi, R.; Bitzer, J.; Wight, E.; Burki, N.; Diesch, C.; Holzgreve, W.; Zhong, X.Y. Levels of plasma circulating cell free nuclear and mitochondrial DNA as potential biomarkers for breast tumors. Mol. Cancer 2009, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, J.; Albers, P.; Muller, S.C.; von Ruecker, A.; Bastian, P.J. Circulating mitochondrial DNA in the serum of patients with testicular germ cell cancer as a novel noninvasive diagnostic biomarker. BJU Int. 2009, 104, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, J.; Muller, D.C.; Muller, S.C.; Hauser, S.; Heukamp, L.C.; von Ruecker, A.; Bastian, P.J.; Walgenbach-Brunagel, G. Circulating mitochondrial DNA in serum: A universal diagnostic biomarker for patients with urological malignancies. Urol. Oncol. 2012, 30, 509–515. [Google Scholar] [CrossRef]
- Zachariah, R.R.; Schmid, S.; Buerki, N.; Radpour, R.; Holzgreve, W.; Zhong, X. Levels of circulating cell-free nuclear and mitochondrial DNA in benign and malignant ovarian tumors. Obstet. Gynecol. 2008, 112, 843–850. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Podlesniy, P.; Trullas, R. Biomarkers in cerebrospinal fluid: Analysis of cell-free circulating mitochondrial DNA by digital PCR. Methods Mol. Biol. 2018, 1768, 111–126. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Kleeff, J.; Michl, P.; Costello, E.; Greenhalf, W.; Palmer, D.H. Therapeutic developments in pancreatic cancer: Current and future perspectives. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 333–348. [Google Scholar] [CrossRef]
- Fahrmann, J.F.; Bantis, L.E.; Capello, M.; Scelo, G.; Dennison, J.B.; Patel, N.; Murage, E.; Vykoukal, J.; Kundnani, D.L.; Foretova, L.; et al. A plasma-derived protein-metabolite multiplexed panel for early-stage pancreatic cancer. J. Natl. Cancer Inst. 2019, 111, 372–379. [Google Scholar] [CrossRef]
- Girolimetti, G.; Pelisenco, I.A.; Eusebi, L.H.; Ricci, C.; Cavina, B.; Kurelac, I.; Verri, T.; Calcagnile, M.; Alifano, P.; Salvi, A.; et al. Dysregulation of a subset of circulating and vesicle-associated miRNA in pancreatic cancer. Noncoding RNA 2024, 10, 29. [Google Scholar] [CrossRef]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Ishige, F.; Hoshino, I.; Iwatate, Y.; Chiba, S.; Arimitsu, H.; Yanagibashi, H.; Nagase, H.; Takayama, W. MIR1246 in body fluids as a biomarker for pancreatic cancer. Sci. Rep. 2020, 10, 8723. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Chen, R.; Crispin, D.A.; May, D.; Stevens, T.; McIntosh, M.W.; Bronner, M.P.; Ziogas, A.; Anton-Culver, H.; Brentnall, T.A. Protein alterations associated with pancreatic cancer and chronic pancreatitis found in human plasma using global quantitative proteomics profiling. J. Proteome Res. 2011, 10, 2359–2376. [Google Scholar] [CrossRef]
- Farrow, B.; Albo, D.; Berger, D.H. The role of the tumor microenvironment in the progression of pancreatic cancer. J. Surg. Res. 2008, 149, 319–328. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Wallach, D.; Kovalenko, A.; Kang, T.B. ‘Necrosome’-induced inflammation: Must cells die for it? Trends Immunol. 2011, 32, 505–509. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Midwood, K.S. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef]
- Rubartelli, A.; Lotze, M.T. Inside, outside, upside down: Damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007, 28, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Huebener, P.; Schwabe, R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 2016, 35, 5931–5941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Hu, Q.; Wu, J.; Wang, G.; Hong, Z.; Ren, J.; Lab for Trauma and Surgical Infections. Mitochondrial DNA in liver inflammation and oxidative stress. Life Sci. 2019, 236, 116464. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef]
- Mathios, D.; Johansen, J.S.; Cristiano, S.; Medina, J.E.; Phallen, J.; Larsen, K.R.; Bruhm, D.C.; Niknafs, N.; Ferreira, L.; Adleff, V.; et al. Detection and characterization of lung cancer using cell-free DNA fragmentomes. Nat. Commun. 2021, 12, 5060. [Google Scholar] [CrossRef]
- Ma, X.; Chen, Y.; Tang, W.; Bao, H.; Mo, S.; Liu, R.; Wu, S.; Bao, H.; Li, Y.; Zhang, L.; et al. Multi-dimensional fragmentomic assay for ultrasensitive early detection of colorectal advanced adenoma and adenocarcinoma. J. Hematol. Oncol. 2021, 14, 175. [Google Scholar] [CrossRef]
- Jin, C.; Liu, X.; Zheng, W.; Su, L.; Liu, Y.; Guo, X.; Gu, X.; Li, H.; Xu, B.; Wang, G.; et al. Characterization of fragment sizes, copy number aberrations and 4-mer end motifs in cell-free DNA of hepatocellular carcinoma for enhanced liquid biopsy-based cancer detection. Mol. Oncol. 2021, 15, 2377–2389. [Google Scholar] [CrossRef]
- Cristiano, S.; Leal, A.; Phallen, J.; Fiksel, J.; Adleff, V.; Bruhm, D.C.; Jensen, S.O.; Medina, J.E.; Hruban, C.; White, J.R.; et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature 2019, 570, 385–389. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, F.; Wang, S.; Jiao, H.; Dang, M.; Zhou, K.; Guo, W.; Guo, S.; Zhang, H.; Song, W.; et al. Aberrant fragmentomic features of circulating cell-free mitochondrial DNA as novel biomarkers for multi-cancer detection. EMBO Mol. Med. 2024, 16, 3169–3183. [Google Scholar] [CrossRef]
- Lo, Y.M.; Chan, K.C.; Sun, H.; Chen, E.Z.; Jiang, P.; Lun, F.M.; Zheng, Y.W.; Leung, T.Y.; Lau, T.K.; Cantor, C.R.; et al. Maternal plasma DNA sequencing reveals the genome-wide genetic and mutational profile of the fetus. Sci. Transl. Med0 2010, 2, 61ra91. [Google Scholar] [CrossRef]
- Avital, G.; Buchshtav, M.; Zhidkov, I.; Tuval Feder, J.; Dadon, S.; Rubin, E.; Glass, D.; Spector, T.D.; Mishmar, D. Mitochondrial DNA heteroplasmy in diabetes and normal adults: Role of acquired and inherited mutational patterns in twins. Hum. Mol. Genet. 2012, 21, 4214–4224. [Google Scholar] [CrossRef] [PubMed]
- Trumpff, C.; Marsland, A.L.; Sloan, R.P.; Kaufman, B.A.; Picard, M. Predictors of ccf-mtDNA reactivity to acute psychological stress identified using machine learning classifiers: A proof-of-concept. Psychoneuroendocrinology 2019, 107, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Systematic Review. Psychosom. Med. 2018, 80, 141–153. [Google Scholar] [CrossRef]
- Ferrara, N.; Houck, K.; Jakeman, L.; Leung, D.W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr. Rev. 1992, 13, 18–32. [Google Scholar] [CrossRef]
- Koch, A.E.; Polverini, P.J.; Kunkel, S.L.; Harlow, L.A.; DiPietro, L.A.; Elner, V.M.; Elner, S.G.; Strieter, R.M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Pirnia, F.; Breuleux, M.; Schneider, E.; Hochmeister, M.; Bates, S.E.; Marti, A.; Hotz, M.A.; Betticher, D.C.; Borner, M.M. Uncertain identity of doxorubicin-resistant MCF-7 cell lines expressing mutated p53. J. Natl. Cancer Inst. 2000, 92, 1535–1536. [Google Scholar] [CrossRef]
- Hou, Y.C.; Wang, C.J.; Chao, Y.J.; Chen, H.Y.; Wang, H.C.; Tung, H.L.; Lin, J.T.; Shan, Y.S. Elevated serum interleukin-8 level correlates with cancer-related cachexia and sarcopenia: An indicator for pancreatic cancer outcomes. J. Clin. Med. 2018, 7, 502. [Google Scholar] [CrossRef]
- Piro, G.; Simionato, F.; Carbone, C.; Frizziero, M.; Malleo, G.; Zanini, S.; Casolino, R.; Santoro, R.; Mina, M.M.; Zecchetto, C.; et al. A circulating T(H)2 cytokines profile predicts survival in patients with resectable pancreatic adenocarcinoma. Oncoimmunology 2017, 6, e1322242. [Google Scholar] [CrossRef]
- Liu, C.Y.; Xu, J.Y.; Shi, X.Y.; Huang, W.; Ruan, T.Y.; Xie, P.; Ding, J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013, 93, 844–854. [Google Scholar] [CrossRef]
- Lu, D.; Qin, Q.; Lei, R.; Hu, B.; Qin, S. Targeted blockade of interleukin 9 inhibits tumor growth in murine model of pancreatic cancer. Adv. Clin. Exp. Med. 2019, 28, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- van der Sijde, F.; Dik, W.A.; Mustafa, D.A.M.; Vietsch, E.E.; Besselink, M.G.; Debets, R.; Koerkamp, B.G.; Haberkorn, B.C.M.; Homs, M.Y.V.; Janssen, Q.P.; et al. Serum cytokine levels are associated with tumor progression during FOLFIRINOX chemotherapy and overall survival in pancreatic cancer patients. Front. Immunol. 2022, 13, 898498. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).