Abstract
Wet deposition of atmospheric polycyclic aromatic hydrocarbons (PAHs) is considered an important source of these potentially toxic compounds in soils. In addition to affecting soil quality, they might be taken up by higher plants, potentially causing phytotoxicity or being accumulated in various organs. Plants are exposed to atmospheric PAHs via the aerial parts and via the soil-root system. The primary aim of this study was to present an experimental setup which can be properly used to quantify PAH accumulation investigating both potential pathways. Rocket (Eruca sativa Mill.) was selected as the model species. The test was conducted following the No. 227 OECD Vegetative Vigor Test. Plants were sprayed with the extract of particles generated during the operation of a diesel-powered vehicle simulating the air–aerial parts–root pathway, while the same extract was used to treat the soil simulating the soil–root–aerial parts pathway. In the soil–root–stem–leaf pathway, the total PAH concentration was 108 μg/kg in the soil, 143 μg/kg in the roots, 92.3 μg/kg in the stems, and 62.5 μg/kg in the leaves. Results showed that higher molecular weight PAHs were mostly accumulated in the roots, but their transfer to above-ground parts cannot be excluded. This study supports the importance of wet deposition in transferring atmospheric PAHs to soils.
1. Introduction
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous persistent organic pollutants. In rural areas, main anthropogenic sources include biomass burning [1] and traffic-related emissions [2]. While biomass burning for heating purposes deteriorates air quality in the cold seasons, traffic is a year-round pollution source, impacting plants in the vegetation period.
It is generally assumed that foliar uptake is the major PAH exposure pathway for higher plants [3]. However, PAHs from the atmosphere can be transported to the soil, providing an additional exposure route for plants [4]. Wang et al. [5] used source appointment calculations to reveal that PAHs sedimented in Chongming wetland (China) came mainly from long-distance atmospheric transportation. While the uptake of PAHs from the soil or the atmosphere is often discussed, these studies mostly address exposure routes separately. Relatively few test systems exist which have been designed to follow PAH uptake simultaneously from the atmosphere and the soil. Wang et al., for example, used a combination of soil-cultured and hydroponic plants to identifying the contributions of root and foliage gaseous/particle uptakes of organic pollutants in indoor environments [6]. As such, one of the main aims of this study was to present a test system where both pathways can be followed and evaluated. The main question was to quantify how atmospheric PAHs move in the soil-plant system after wet deposition, with special regard to heavier compounds.
The accumulation of PAHs in the soil has been widely addressed, as a significant proportion of PAHs remain in the soil, depending on the type of the compound [7]. Being lipophilic molecules, PAHs generally tend to bind strongly to soil particles [8]; soil will then provide a sink to these compounds.
The behavior of low molecular weight (LMW) and high molecular weight (HMW) PAHs differs: 4–6-ring PAHs are most likely deposited close to emission sources, while the lower molecular weight compounds are more capable of long-range transport [9]. Also, after reaching the ground, LMW PAHs will be degraded or returned to the atmosphere due to volatilization or microbial biodegradation [10], while HMW PAHs will remain in the soil bound to particles [11].
As PAHs are poorly soluble in water, once deposited in the soil, their downwards migration is limited. They mostly accumulate in the upper layers—according to Clément et al., the upper 10 cm layer is the most affected [12]. However, in the case of highly polluted soils, they might also occur in the deeper soil layers [13]. Slow downwards migration has been experimentally confirmed in the study of Gateuille et al., based on migration behavior of 137Cs and 210Pbxs [14].
In Europe, PAH content of arable soil moves within a very wide range. Levels of 16 EPA PAHs were measured in Polish arable soils with concentrations between 80 and 7264 µg/kg, although 75% of soil samples showed concentrations below 695 µg/kg [15]. Škrbić et al. reported total concentration of 16 PAHs in the range of 55 and 4584 µg/kg in agricultural soils in Serbia [16]. The no-contamination benchmark of 200 μg/kg was suggested by Maliszewska-Kordybach [17].
Zhang et al. showed a close relationship between PAHs in dustfall and surface soil [18]. In the study of Jia et al., soil absorption contributed to 9.4% of total PAH uptake in tested vegetables, demonstrating that atmospheric dustfall serves as an important source of increasing soil PAH concentrations [19]. Once uptaken, compounds are assumed to be transported within the plant through the plant vascular system, or by diffusion through the cells [20].
Aerial tissues of winter wheat samples were analyzed in the study by Tian et al. [21]. Samples were collected in areas affected by coal combustion and PAH concentrations were measured. The results showed that HMW (6-ring) PAHs originated from atmosphere, while LMW (3–4-ring) PAHs were taken up by the roots. Wang et al. demonstrated that the molecular weight-specific uptake of PAHs from rhizosphere soils was highly dependent on the growth stage of winter wheat [22].
Xiong et al. investigated the relationships between bioconcentration factors and the physicochemical properties of PAHs and found that solubility and the octanol–water partition coefficient were strongly correlated with the soil-to-root bioconcentration factors, while vapor pressure and the octanol–air partition coefficient proved to be good predictors for the air-to-leaf accumulation of PAHs [23].
In addition to direct deposition of particles on soil or plant surfaces, both gas- and particle-phase PAHs can be washed out by rain or snow, which is defined as wet deposition [24]. Wet deposition provides an important pathway for PAH removal from the atmosphere; however, it depends on the amount of precipitation, showing seasonal patterns [25]. The process has been proven efficient for all particle-associated PAHs [26].
While most studies assess dry deposition or bulk deposition [27], the direct effects of wet deposition have been rarely addressed. In our study, wet deposition was mimicked using the adopted version of the No. 227 OECD Guideline for the Testing of Chemicals: Terrestrial Plant Test: Vegetative Vigor Test (hereinafter referred to as No. 227 OECD Guideline) [28]. This standard was developed for herbicide regulation to evaluate the risk of general chemicals, biocides and crop protection products [29]. The original protocol has been slightly modified to test the potential phytotoxic effects of the water-soluble components of airborne particle-bound contaminants [30] and to assess their bioaccumulation [31]. The present study first intends to describe the treatment protocol used to simulate wet deposition. It is also aimed at quantifying the transfer of atmospheric PAHs via wet deposition both to soil and plant surfaces, under controlled conditions prescribed by the guideline. The major goal was to follow PAH accumulation in different compartments of the test plants, getting precise data on how PAHs move from the aerial parts to the roots following foliar uptake, and to what extent the uptake from the soil will contribute to the PAH content in the aerial parts.
Rocket (Eruca sativa Mill., family Brassicaceae) was selected as a model species. Different Brassicaceae species have been reported as sensitive and easy-to-use test species, showing good PAH accumulation capacity [32,33,34].
2. Materials and Methods
A graphical summary of the experimental protocol is given in Figure 1.
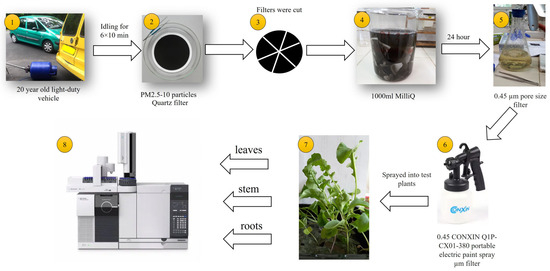
Figure 1.
Graphical summary of the experimental protocol.
2.1. Sample Preparation
A diesel emission sample was obtained operating a 20-year-old light-duty vehicle (EURO3 passenger car) at idling for 6 × 10 min. PM2.5–10 particles were collected on quartz filters (Whatman QMA Ø 150 mm, Sigma-Aldrich, Burlington, MA, USA) using a KÁLMÁN PM2.5 sampler (flow rate 32 m3/h). The instrument was placed about 1 m from the tailpipe. Six consecutive samples were taken, and the filters were immediately changed after each 10 min interval. After sampling, each filter was wrapped unfolded in aluminum foil and placed in a zip-sealed plastic bag. These filters were processed immediately. Using the 6 filters, a composite sample was made by cutting all filters into small pieces and placing them in a beaker with 1000 mL of high-purity water. Extraction took 24 h at room temperature, and during that time, the beaker was stirred several times. The extract was filtered through a 0.45 µm pore size filter (GN-6 Membrane, 0.45 µm Hydrophilic mixed cellulose esters) and stored at −20 °C until use.
2.2. Treatment of Test Plants
Rocket seeds were purchased from Garafarm Ltd., Kecskemét, Hungary. Test plants were cultivated and treated as described in the bioaccumulation study by Teke et al. [31], based on the protocol given in the No. 227 OECD Guideline. Shortly, seeds were sown in pots Ø 15 cm. In the experiment, commercial soil was used (N (m/m%): min 0.3; P2O5 (m/m%): min 0.1; K2O (m/m%): min 0.3; dark brown in color; bulk density: 0.8 kg/dm3; dry matter content: 45% m/m; organic matter content: 40% m/m; total water-soluble salt content: max. 2.0; and pH in a 10% aqueous suspension: 7.0 ± 0.5).
Five–five pots were set in all treatment groups as well as in the control. After emergence, five–five plants were left in each pot; therefore, all treatment groups included twenty-five test plants. Between the treatments, pots were placed randomly and were repositioned every day to minimize variability.
Cultivation and subsequent testing were conducted in a glasshouse, and the environmental conditions were as described in the Guideline (temperature: 22 °C ± 10 °C; humidity: 70% ± 25%; photoperiod: minimum 16 h light; and light intensity: 350 ± 50 µE/m2/s). The environmental parameters were monitored using HOBO MX1104 Wireless system (Tempcon Co., Ford, UK).
Treatment started when the plants reached the 4-true leaf stage, according to the Guideline. Two test groups were set: In the first one, the above-ground parts of the plants were sprayed with the extract (1.5 mL/plant) (air–aerial parts–roots pathway). During spraying, the soil was covered to avoid contamination. In the second test group, only the soil was sprayed with the extract [35]. Spraying was performed close to the shoot of the individual plants (1.5 mL/plant). During the treatment, the aerial parts of the plants were covered.
Three consecutive treatments were applied: on Day 1, Day 8, and Day 15.
2.3. Analysis of PAHs
Gas chromatography (GC-MS) method was used to determine PAH content in aerosol extract according to MSZ 1484-6:2003 standard [36] (MSZ 1484-6:2003: Testing of waters. Determination of polycyclic aromatic hydrocarbons (PAH) content by gas chromatography–mass spectrometry, LOD: 0.005 µg/L per component) and plant samples analysis based on MSZ EN 15527:2009 [37] (Characterization of waste. Determination of polycyclic aromatic hydrocarbons (PAH) in waste using gas chromatography–mass spectrometry (GC/MS, LOD: 0.1 µg/kg per component). In our study, 19 PAHs were analyzed: Naphthalene (Nap), 2-Methyl-naphthalene (Methy-Nap), 1-Methyl-naphthalene (Me-Nap), Acenaphthylene (Acy), Acenaphthene (Ace), Fluorene (Flu), Phenanthrene (Phen), Anthracene (Ant), Fluoranthene (Flt), Pyrene (Pyr), Benzo[a]anthracene (B(a)a), Chrysene (Cry), Benzo[b]fluoranthene (B(b)f), Benzo[k]fluoranthene (B(k)f), Benzo[a]pyrene (B(a)p), Benzo[e]pyrene (B(e)p), Indeno(l,2,3-cd)pyrene (IP), Dibenzo[a,h]anthracene (D(a,h)a), and Benzo(g,h,i)perylene (B(g,h,i,)p).
Different plant parts were separately grinded in a ceramic mortar with 10 g anhydrous sodium sulphate. An amount of 10 mL acetone was added, and the samples were spiked with 100 µL of 0.01 µg/mL deuterated PAH surrogate mixture containing Naphtalene-d8, Acenaphthene-d10, Phenanthrene-d10, Chryzene-d12, Benzo[a]pyrene-d12, and Perylene-d12 (Restek Corporation, Bellefonte, PA, USA) and extracted with 20 mL n-hexane 3 times with ultrasonic extraction for 20 min. In a dry nitrogen stream, the extract was concentrated to 1 mL, and silica gel and alumina oxide clean-up was performed.
For the GC-MS analysis, HP-6890 gas chromatograph was coupled with an HP-5973 (Agilent Technologies, Palo-Alto, CA, USA) quadrupole mass spectrometer (low-resolution single MS).
From a standard PAH mixture, a five-point calibration for each of the target chemical compound was established, detected over a concentration range of 0.5–5.0 µg/L. All data were corrected for the average value of the blanks. Each compound and surrogate standards relative response factors was calculated for every calibration level using the following equation
where Rfi = response factor of analyte i;
Ai = peak response of compound i;
Cs = concentration of internal standard (µg/g, or µg/L);
As = peak response Internal Standard; and
Ci = concentration of analyte or surrogate (µg/g, or µg/L).
The limit of detection (LOD) values for PAHs in plant sample were as follows: Nap 0.0100 µg/g; Methy-Nap 0.0120 µg/g; Me-Nap 0.0110 µg/g; Acy 0.0105 µg/g; Ace 0.0120 µg/g; Flu 0.0110 µg/g, Phen 0.0100 µg/g, Ant 0.0110 µg/g, Flt 0.0100 µg/g, Pyr 0.0110 µg/g, B(a)a 0.0110 µg/g, Cry 0.0130 µg/g, B(b)f 0.0110 µg/g, B(k)f 0.0110, B(e)p 0.0130 µg/g, B(a)p 0.0125 µg/g, IP 0.0140 µg/g, D(a,h)a 0.0150 µg/g, B(g,h,i,)p 0.0140 µg/g. In aerosol extract, the LOD vales were as follows: Nap 0.00027 µg/L, Methy-Nap 0.00028 µg/L, Me-Nap 0.00027 µg/L, Acy 0.00025 µg/L, Ace 0.00024 µg/L, Flu 0.00021 µg/L, Phen 0.00020 µg/L, Ant 0.00017 µg/L, Flt 0.00015 µg/L, Pyr 0.00021 µg/L µg/L, B(a)a 0.00018 µg/L, Cry 0.00022 µg/L, B(b)f 0.00021 µg/L, B(k)f 0.00023 µg/L, B(e)p 0.00024 µg/L, B(a)p 0.00026 µg/L, IP 0.00030 µg/L, D(a,h)a 0.00028 µg/L, B(g,h,i,)p 0.00025 µg/L.
In plant samples, the average analyte recovery for spiked PAH samples were as follows: Nap 102.6%, Methy-Nap 93.6%, Me-Nap 98.3%, Acy 98.8%, Ace 100.5%, Flu 104.9%, Phen 104.4%, Ant 106.4%, Flt 92.7%, Pyr 90.3%, B(a)a 92.4%, Cry 88.7%, B(b)f 91.8%, B(k)f 88.3%, B(e)p 96.7%, B(a)p 90.6%, IP 98.7%, D(a,h)a 91.0%, B(g,h,i,)p 90.3%. In aerosol extract te recoveries were Nap 103.2%, Methy-Nap 106.4%, Me-Nap 111.2%, Acy 108.8%, Ace 101.9%, Flu 108.7%, Phen 93.7%, Ant 111.1%, Flt 107.3%, Pyr 102.4%, B(a)a 99.8%, Cry 99.8%, B(b)f 105.0%, B(k)f 107.6%, B(e)p 102.2%, B(a)p 99.2%, IP 105.5%, D(a,h)a 104.9%, B(g,h,i,)p 102.5%.
Analytical determinations were performed by the courtesy of the Laboratory of the ELGOSCAR-2000 Environmental Technology and Water Management Ltd., accredited by the National Accreditation Authority (complies with the criteria of Standard MSZ EN ISO/IEC 17025:2018 [38]), registration number NAH-1-1278/2015.
2.4. Statistical Analysis
Statistical analyses were performed using the R 4.0.0 program (https://cran.r-project.org/bin/windows/base/old/4.0.0/, accessed on 21 September 2024) with the Rcmdr package, as described previously [31]. The differences in concentrations of accumulated polycyclic aromatic hydrocarbons (PAHs) with different ring numbers in plants were compared using a paired Student’s t-test. The level of statistical significance was set at p ≤ 0.05.
3. Results
Altogether 19 PAHs were detected in the extract. Table 1 shows the concentrations of accumulated PAHs in different plant compartments, as well as in the soil, in case of direct treatment of the soil with the extract. They included the 16 priority PAHs enlisted by US EPA [39] and the so-called car PAHs, such as Benzo[a]anthracene, Chrysene, Benzo[b]fluoranthene, Benzo[k]fluoranthene, Benzo[a]pyrene, Dibenzo[a,h]anthracene, Indeno[1,2,3-cd]pyrene, and Benzo[g,h,i]perylene [40].

Table 1.
Concentration of PAHs in the aerosol extract given in μg/L and in the samples given in μg/kg. The limit of PAH detection (LOD) was 0.1 µg/kg dry plant material. US EPA PAHs are given in bold. S: soil treated; A: aerial parts treated.
In the extract, the prevalence of LMW PAHs could be observed. In addition to naphthalenes, the dominant PAHs were the 3-ring Flu (1.472 μg/L) and Phen (0.448 μg/L). Most studies report the dominance of these species in atmospheric samples (reviewed by [41]) as well as in bulk deposition [42]. Phen was the dominant PAH in a study assessing distribution of traffic-related contaminants in urban topsoils [43]. Phen was also characteristic in different diesel blends reported by de Souza et al. [44].
The dominant PAH in the treated soil was 5-ring B(b)f (11.7 μg/kg). Qi et al. also measured the highest share of B(b)f in urban sites (39.94%) [45]. The 4-ring Pyr and Cry were also detected in high concentrations, 9.2 μg/kg and 8.3 μg/kg. These PAHs can be regarded as characteristic in urban soils [46].
In the case of the soil–root–aerial parts pathway, the distribution of 2–6 ring PAHs in different compartments is shown in Figure 2. Figure 3 gives the ratio of different PAH isomers for the aerial parts–roots pathway.
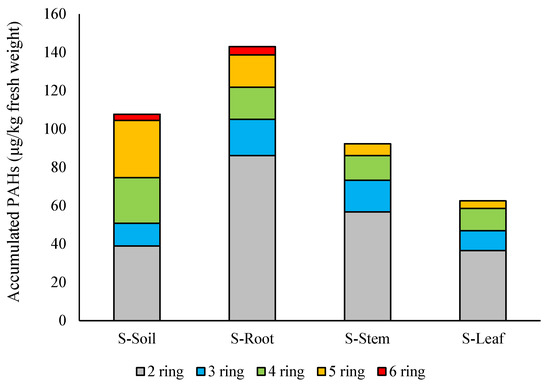
Figure 2.
Concentration of PAHs with different molecular weight in soil and treated plant compartments, simulating the soil–root–aerial parts pathway.
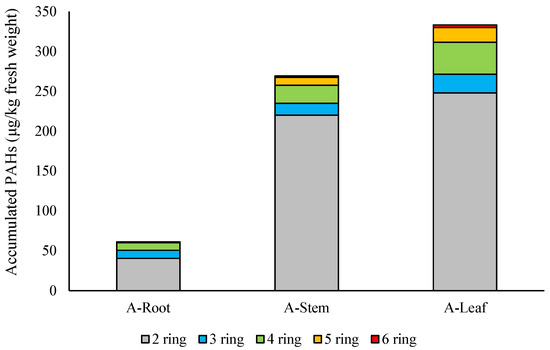
Figure 3.
Concentration of PAHs with different molecular weight in plant compartments, simulating the air–aerial parts–roots pathway.
4. Discussion
In terms of accumulation and possible transport of the sum of the 19 PAHs, a clear tendency could be observed both in the soil–root–stem–leaf pathway and in the leaf–stem–root pathway (Figure 4). These pathways are discussed separately.
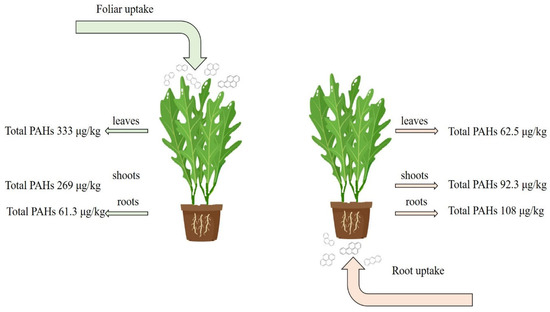
Figure 4.
Total PAHs accumulation in different compartments.
4.1. Soil-to-Aerial Parts Pathway
After the three treatments—on Day 1, Day 8, and Day 15—total PAH concentration in the treated soil was 108 μg/kg, indicating that wet deposition can be an important source of soil PAH contents. The concentration of ∑PAHs was 143 μg/kg in the roots, 92.3 μg/kg in the stems, and finally, 62.5 μg/kg in the leaves.
In an experiment conducted on the uptake of pyrene and phenanthrene by ryegrass, Kang et al. concluded that PAHs first adsorb to plant cell walls, followed by the gradual diffusion into subcellular fractions of tissues [47]. Potential toxicity of PAHs entering the cells can be reduced by metabolic processes, including transformation, conjugation, and compartmentalization [48].
Several studies are available that examined PAH uptake from polluted soils and subsequent partition between different organs of the plant. Alves et al. used fluorescence microscopy [49] to detect the location of selected PAHs in alfalfa plants. The main translocation mechanisms described in the study involved passive diffusion of PAHs into vascular tissues.
Assessing the soil–root–aerial parts pathway, results in one hand show bioconcentration between the root–soil compartments and a diminishing tendency in the root–stem–leaves compartments. This trend corresponds well with the published data. While early studies assumed that root uptake was the main uptake pathway of persistent organic pollutants (POPs) [50], some recent findings demonstrated that the transport of PAHs from the roots to the above-ground parts of plants is relatively low [51,52].
Some studies, however, were not able to determine such clear pathways. Jia et al., for example, cultivated vegetables, including Shanghai green cabbage (Brassica chinensis var.) and Chinese cabbage (Brassica rapa var.), in experimentally treated soils. They found that the average concentrations of 16 PAHs were 204.7 μg/kg in the soils, 83.0 μg/kg in the roots, and higher concentrations in the vegetable leaves, at 93.9 μg/kg [53].
In the case of soil treatment, uptake and even bioconcentration could be experienced for LMW PAHs (having 2 and 3 rings) and in the case of one 4-ring PAH, Flt. Sushkova et al. [54] assessed the remediation potential of reed (Phragmites australis) in heavily contaminated soils and found effective accumulation of 3- and 4-ring PAHs. Imam et al. found mostly LMW PAHs accumulated in leafy vegetables that were grown in polluted soils [55]. Zhang et al. used Chinese cabbage (Brassica chinensis L.), a close relative of rocket, in a pot study when uptake of PAHs with different rings was assessed. The study discussed that uptake of PAHs from experimentally spiked soil showed a negative correlation with the increase in the molecular weight of the PAHs, being 76.55% for LWM PAHs, but only 6.05% for HMW PAHs [56]. Higher bioconcentration factor (BCF) values were reported by Cao et al. for LMW PAHs when accumulation in carrot (Daucus carota L.) and cabbage (Brassica pekinensis L.) was examined [57]. Jiao et al. used a pot study to follow migration of PAH congeners in the soil-ryegrass exposure system. It was demonstrated that PAHs with higher molecular weights (molecular weight ≥ B(a)a) mostly adsorbed to soil particles, which reduced their bioavailability and uptake [58].
Statistical evaluation shows that significant differences were found between 3-ring PAH concentrations in roots and leaves (t = 3.6327, df = 4, p-value = 0.02211), as well as between 4-ring PAH concentrations in roots and leaves (t = 3.8903, df = 3, p-value = 0.03012). When 5-ring PAH concentrations were compared, differences were significant between root and stem contents (t = 4.5771, df = 4, p-value = 0.01021), as well as between root and leaves (t = 6.041, df = 4, p-value = 0.003787).
The concentration of 6-ring PAHs was very similar in the soil and in the roots—IP 1.2 μg/kg and 1.5 μg/kg and B(g,h,i,)p 2.1 μg/kg and 2.8 μg/kg. They were not detected in the above-ground parts of the test plants (Figure 2). These two PAHs were also excluded from maize tissues when uptake of PAHs in wastewater-irrigated soils was assessed [59]. Paraíba et al. concluded that HMW PAHs were less able to be transported via transpiration stream and showed low accumulative potential in corn grains [60]. A similar tendency was reported by Jia et al. [53]. In experimentally treated soils, the ratio of HMW PAHs was relatively high (5-ring PAHs amounted to 20% and 6-ring PAHs to 85%), but they were not detected in vegetables cultivated in these model soils.
Available studies, however, are still contradictory. Most studies agree that high-weight PAHs show strong adsorption upon the root epidermis, which works against translocation to shoots [61,62]. POPs are generally assumed to bind tightly to the root epidermis [63]. Wei et al. compared PAH distribution in soil, rhizosphere soil, and the roots of different crop species, including one Brassicaceae, broccoli (Brassica oleracea L. var. botrytis L.). The study suggested that uptake can be prevented by root exudates and the root peel [64].
On the other hand, Meudec et al. [65] detected HMW PAHs in shoot tissues of Salicornia fragilis (a halophytic plant) which was artificially exposed to petroleum-polluted sediments. In an exceptional case, significant bioconcentration of the 6-ring B(g,h,i,)p was found in maize in Eastern China [59].
The uptake of PAHs in the root zone is suggested to depend on lipophilicity (log Kow, the water–octanol partition coefficient), and as such, HMW molecules are adsorbed on the roots but not absorbed by them [66].
4.2. Atmosphere-to-Aerial Parts Pathway
Considering the leaf–stem–root pathway (the above-ground parts were treated with the extract), the highest concentration was found in the leaves (333 μg/kg), a similarly high concentration in the stems (269 μg/kg), and a low concentration in the roots (61.3 μg/kg), showing a diminishing tendency in the leaves–stem–roots compartments.
Assessing the atmospheric uptake of HMW PAHs (air–leaves–stem–root pathway), a gradual decrease in individual PAHs was experienced, similarly to the concentration of 19 PAHs. These decreases showed bigger differences in the case of HMW PAHs (Figure 3). Statistically significant differences were found between 4-ring PAH concentrations in leaves and roots (t = 4.5244, df = 3, p-value = 0.02019), as well as in stems and roots (t = 8.175, df = 3, p-value = 0.003829). When 5-ring PAH concentrations were compared, differences were significant between leaves and roots (t = 3.2911, df = 4, p-value = 0.03019) as well as between stems and roots (t = 8.242, df = 4, p-value = 0.001182).
In the leaves, the 2-ring Nap showed the highest concentration, 234 μg/kg. Of 3-ring PAHs, Phen was the dominant species, and its concentration was 8.6 μg/kg. In the study by Zhao et al., it was the most abundant PAH in Salix matsudana leaves, showing good consistency with atmospheric concentrations [67]. Comparable results were given by Yakovleva et al., reporting that Phen was the most abundant PAH in plants and atmospheric wet deposition, assessing the soil-plant systems in northern taiga biocenoeses [68].
In the leaves and stem compartments, 6-ring PAHs were detected. The concentration of IP was 1 μg/kg in the leaves and 0.5 μg/kg in the stems, while the concentration of B(g,h,i,)p was 2.1 μg/kg in the leaves and 1.3 μg/kg in the stems. They were not translocated to the roots, however. Similarly, 5-ring PAHs could be detected in the leaf and stem compartments (Figure 3), but except for B(b)f, 5-ring PAHs were not translocated to the roots. The concentration of B(b)f shows a clear diminishing tendency: 8.4 μg/kg in the leaves, 3.2 μg/kg in the stems, and 1.1 μg/kg in the roots. These results also show that bioaccumulation of HMW PAHs cannot be neglected. High PAH concentrations in internal leaf tissues can be attributed to the impaired translocation and relative persistence of these hydrophobic compounds [69,70].
4.3. Comparative Assessment
Comparing the accumulated concentrations of ∑PAHs in different parts of rocket, it should be noted that studies addressing actual field contamination report values in a very broad range. In our test, the concentration of ∑19 PAHs in the leaves (edible parts) of rocket was 62.5 μg/kg in the case of the soil–aerial parts pathway and 333 μg/kg in case of the air–aerial parts pathway. In European context, Soceanu et al. [71] measured the concentration of 15 PAHs in cabbage collected both in urban and rural areas in Romania. In urban samples, the measured value was 10 μg/kg, while in the rural samples it was 8.1 μg/kg. Jánská et al. [72] reported the accumulation of total PAHs in cabbage samples collected from Southern Moravia (Czech Republic) in the range of 12.34 and 78.09 μg/kg. A German study detected PAH contamination at 120 μg/kg in kale [73]. Nevertheless, Chinese studies have found higher PAH concentrations, most likely due to heavier contamination levels. Zhang et al. [34], e.g., reported average concentration of ∑PAHs reaching as high as 1052 ± 73 μg/kg in Brassica chinensis samples collected in Xi’an city (northwestern China). In the study of Mo et al. [74], the average concentration of ∑PAHs in the same species was 950 μg/kg, and 1790 μg kg−1 in B. juncea (mustard).
Rocket, however, is consumed in very small quantities and only occasionally, which makes human health risk assessment rather irrelevant. Soil PAH level, on the other hand, can give a more reliable estimation. After the treatment, the concentration of B(a)p was 3.6 μg/kg, which was well below the 0.02 mg/kg BaP level considered acceptable for the purpose of crop production [4].
4.4. Contribution of Wet Deposition to PAH Transfer
Wet deposition is an important process in the transfer of atmospheric PAHs both to the soil and onto plant surfaces. According to Golomb et al., the PAH content of wet deposition depends on regional PAH emitting sources, with Flt and Phen being the most abundant in the majority of the samples [75]. Several studies have identified traffic as the main contributor to the PAH content of wet deposition [76,77]. The 3-ring Phen was also a dominant compound in the extract used for this study. Its concentration (0.448 μg/L) is comparable with reported quantities in real-world wet depositions (reviewed by Birgül et al. [24]).
Wet deposition was found to show seasonal patterns: in the study of Lee and Lee [78], dry deposition of PAHs was higher in winter, while wet deposition was higher in summer. Wang et al. [25] also detected higher wet deposition in summer. However, the same study also concluded that the total removal of atmospheric PAHs via wet deposition is generally lower than that of dry deposition, considering the relatively shorter durations of precipitations.
5. Conclusions
The No. 227 OECD Guideline was modified in our study to suggest treatment mechanisms simulating wet deposition. Treatment implied spraying the aerosol extract on these surfaces; therefore, air–aerial parts–root and soil–root–aerial parts exposure pathways could be separately investigated. As the soil was directly sprayed with the sample under laboratory conditions, other pollution sources could be excluded. Measuring PAH accumulation in different parts of Eruca sativa, the results clearly show that wet deposition can be an important source of soil PAH concentrations. PAHs deposited on the soil will accumulate in the roots and gradually move to the above-ground parts of the plant. This will also result in the transfer of PAHs with higher molecular weights, although in much lower concentrations. Considering accumulation in the leaves—which are the edible parts of leafy vegetables—direct atmospheric deposition via precipitation is the main pathway. Rocket can accumulate PAHs with even higher molecular weights in considerable quantities. The main benefit of the proposed treatment protocol is that wet deposition-associated PAH accumulation can be investigated in other crop species, which might have different accumulation capacities.
Author Contributions
Conceptualization, K.H. and N.K.; methodology and investigation, K.H., B.E.-V., S.T. and G.T.; data curation, K.H.; visualization, K.H. and S.T.; writing—original draft preparation, N.K.; supervision, project administration, funding acquisition, N.K. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support was provided by the NKFIH-872 project ‘Establishment of a National Multidisciplinary Laboratory for Climate Change’.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors thank the ELGOSCAR-2000 Environmental Technology and Water Management Ltd. (Head Office: 164 Soroksari u. H-1095 Budapest, Laboratory: H-8184 Balatonfuzfo) for conducting analytical measurements and the HUN-REN–PE Air Chemistry Research Group (University of Pannonia) for providing the samples.
Conflicts of Interest
Author Gábor Teke was employed by the company ELGOSCAR-2000 Environmental Technology and Water Management Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
PM—particulate matter; PAH—polycyclic aromatic hydrocarbon; LMW—low molecular weight; HMW—high molecular weight; Naphthalene—Nap; 2-Methyl-naphthalene (Methy-Nap); 1-Methyl-naphthalene (Me-Nap); Acenaphthylene—Acy; Acenaphthene—Ace; Fluorene—Flu; Phenanthrene—Phen; Anthracene—Ant; Fluoranthene—Flt; Pyrene—Pyr; Benzo[a]anthracene—B(a)a; Chrysene—Cry; Benzo[b]fluoranthene—B(b)f; Benzo[k]fluoranthene—B(k)f; Benzo[e]pyrene—B(e)p; Benzo[a]pyrene—B(a)p; Indeno[1,2,3-cd]pyrene—IP; Dibenzo[a,h]anthracene—D(a,h)a; Benzo[g,h,i]perylene—B(g,h,i,)p; persistent organic pollutant—POP.
References
- Lhotka, R.; Pokorná, P.; Zíková, N. Long-term trends in PAH concentrations and sources at rural background site in Central Europe. Atmosphere 2019, 10, 687. [Google Scholar] [CrossRef]
- Fachinger, F.; Drewnick, F.; Borrmann, S. How villages contribute to their local air quality—The influence of traffic-and biomass combustion-related emissions assessed by mobile mappings of PM and its components. Atmos. Environ. 2021, 263, 118648. [Google Scholar] [CrossRef]
- Tao, S.; Cui, Y.H.; Xu, F.L.; Li, B.G.; Cao, J.; Liu, W.X.; Schmitt, G.; Wang, X.J.; Shen, W.R.; Qing, B.P.; et al. Polycyclic aromatic hydrocarbons (PAHs) in agricultural soil and vegetables from Tianjin. Sci. Total Environ. 2004, 320, 11–24. [Google Scholar] [CrossRef]
- Kulhánek, A.; Trapp, S.; Sismilich, M.; Janků, J.; Zimová, M. Crop-specific human exposure assessment for polycyclic aromatic hydrocarbons in Czech soils. Sci. Total Environ. 2005, 339, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Z.; Yang, Y.; Li, T.; Liu, M. Distribution of PAHs in tissues of wetland plants and the surrounding sediments in the Chongming wetland, Shanghai, China. Chemosphere 2012, 89, 221–227. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Xu, Y.; Rodgers, T.F.; Ablimit, M.; Li, J.; Tan, F. Identifying the contributions of root and foliage gaseous/particle uptakes to indoor plants for phthalates, OPFRs and PAHs. Sci. Total Environ. 2023, 883, 163644. [Google Scholar] [CrossRef] [PubMed]
- Sehili, A.M.; Lammel, G. Global fate and distribution of polycyclic aromatic hydrocarbons emitted from Europe and Russia. Atmos. Environ. 2007, 41, 8301–8315. [Google Scholar] [CrossRef]
- Yang, L.; Jin, M.; Tong, C.; Xie, S. Study of dynamic sorption and desorption of polycyclic aromatic hydrocarbons in silty-clay soil. J. Hazard. Mater. 2013, 244, 77–85. [Google Scholar] [CrossRef]
- Nadal, M.; Schuhmacher, M.; Domingo, J.L. Levels of PAHs in soil and vegetation samples from Tarragona County Spain. Environ. Pollut. 2004, 132, 1–11. [Google Scholar] [CrossRef]
- Al-Nasir, F.; Hijazin, T.J.; Al-Alawi, M.M.; Jiries, A.; Al-Madanat, O.Y.; Mayyas, A.; Al-Dalain, S.A.; Al-Dmour, R.; Alahmad, A.; Batarseh, M.I. Accumulation, source identification, and cancer risk assessment of polycyclic aromatic hydrocarbons (PAHs) in different Jordanian vegetables. Toxics 2022, 10, 643. [Google Scholar] [CrossRef]
- Biache, C.; Mansuy-Huaulta, L.; Faure, P. Impact of oxidation and biodegradation on the most commonly used polycyclic aromatic hydrocarbon (PAH) diagnostic ratios: Implications for the source identifications. J. Hazard. Mater. 2014, 267, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Clément, N.; Muresan, B.; Hedde, M.; François, D. PAH dynamics in roadside environments: Influence on the consistency of diagnostic ratio values and ecosystem contamination assessments. Sci. Total Environ. 2015, 538, 997–1009. [Google Scholar] [CrossRef]
- Liao, X.; Ma, D.; Yan, X.; Yang, L. Distribution pattern of polycyclic aromatic hydrocarbons in particle-size fractions of coking plant soils from different depth. Environ. Geochem. Health 2012, 35, 271–282. [Google Scholar] [CrossRef]
- Gateuille, D.; Evrard, O.; Lefevre, I.; Moreau-Guigon, E.; Alliot, F.; Chevreuil, M.; Mouchel, J.M. Combining measurements and modelling to quantify the contribution of atmospheric fallout, local industry and road traffic to PAH stocks in contrasting catchments. Environ. Pollut. 2013, 189, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Maliszewska-Kordybach, B.; Smreczak, B.; Klimkowicz-Pawlas, A.; Terelak, H. Monitoring of the total content of polycyclic aromatic hydrocarbons (PAHs) in arable soils in Poland. Chemosphere 2008, 73, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Škrbić, B.D.; Antić, I.; Živančev, J.; Vágvölgyi, C. Comprehensive characterization of PAHs profile in Serbian soils for conventional and organic production: Potential sources and risk assessment. Environ. Geochem. Health 2021, 43, 4201–4218. [Google Scholar] [CrossRef]
- Maliszewska-Kordybach, B. Polycyclic aromatic hydrocarbons in agricultural soils in Poland: Preliminary proposals for criteria to evaluate the level of soil contamination. Appl. Geochem. 1996, 11, 121–127. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, D.; Xiong, G.; Duan, Y.; Cai, C.; Wang, X.; Li, J.; Tao, S.; Liu, W. Structural equation modeling of PAHs in ambient air, dust fall, soil, and cabbage in vegetable bases of Northern China. Environ. Pollut. 2018, 239, 13–20. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Zhang, J.J.; Chen, Z. Atmospheric deposition and vegetable uptake of polycyclic aromatic hydrocarbons (PAHs) based on experimental and computational simulations. Atmos. Environ. 2019, 204, 135–141. [Google Scholar] [CrossRef]
- Watts, A.W.; Ballestero, T.P.; Gardner, K.H. Soil and atmospheric inputs to PAH concentrations in salt marsh plants. Water Air Soil Pollut. 2008, 189, 253–263. [Google Scholar] [CrossRef]
- Tian, K.; Bao, H.Y.; Liu, X.P.; Wu, F.Y. Accumulation and distribution of PAHs in winter wheat from areas influenced by coal combustion in China. Environ. Sci. Pollut. Res. 2018, 25, 23780–23790. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, H.; Bao, H.; Li, J.; Li, J.; Xing, W.; Hong, H.; Wu, F. Dynamic distribution and accumulation of PAHs in winter wheat during whole plant growth: Field investigation. Ecotoxicol. Environ. Saf. 2020, 202, 110886. [Google Scholar] [CrossRef]
- Xiong, G.N.; Zhang, Y.H.; Duan, Y.H.; Cai, C.Y.; Wang, X.; Li, J.Y.; Tao, S.; Liu, W. Uptake of PAHs by cabbage root and leaf in vegetable plots near a large coking manufacturer and associations with PAHs in cabbage core. Environ. Sci. Pollut. Res. 2017, 24, 18953–18965. [Google Scholar] [CrossRef]
- Birgül, A.; Tasdemir, Y.; Cindoruk, S.S. Atmospheric wet and dry deposition of polycyclic aromatic hydrocarbons (PAHs) determined using a modified sampler. Atmos. Res. 2011, 101, 341–353. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, M.; Li, Y.; Liu, Y.; Li, S.; Ge, R. Dry and wet deposition of polycyclic aromatic hydrocarbons and comparison with typical media in urban system of Shanghai, China. Atmos. Environ. 2016, 144, 175–181. [Google Scholar] [CrossRef]
- Škrdlíková, L.; Landlová, L.; Klánová, J.; Lammel, G. Wet deposition and scavenging efficiency of gaseous and particulate phase polycyclic aromatic compounds at a central European suburban site. Atmos. Environ. 2011, 45, 4305–4312. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, H.; Zhang, Y.; Xiong, G.; Zhang, Q.; Li, Y.; Tao, S.; Liu, W. Evaluation of PAHs in edible parts of vegetables and their human health risks in Jinzhong City, Shanxi Province, China: A multimedia modeling approach. Sci. Total Environ. 2021, 773, 145076. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 227: Terrestrial Plant Test: Vegetative Vigour Test. In OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006. [Google Scholar] [CrossRef]
- Boutin, C.; Aya, K.L.; Carpenter, D.; Thomas, P.J.; Rowland, O. Phytotoxicity testing for herbicide regulation: Shortcomings in relation to biodiversity and ecosystem services in agrarian systems. Sci. Total Environ. 2012, 41, 79–92. [Google Scholar] [CrossRef]
- Kováts, N.; Horváth, E.; Eck-Varanka, B.; Csajbók, E.; Hoffer, A. Adapting the Vegetative Vigour Terrestrial Plant Test for assessing ecotoxicity of aerosol samples. Environ. Sci. Pollut. Res. 2017, 24, 15291–15298. [Google Scholar] [CrossRef]
- Teke, G.; Hubai, K.; Diósi, D.; Kováts, N. Assessment of Foliar Uptake and Accumulation of Airborne Polyaromatic Hydrocarbons Under Laboratory Conditions. Bull. Environ. Contam. Toxicol. 2020, 104, 444–448. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Zhang, J.; Jin, X.; Chen, Z. Characterization of polycyclic aromatic hydrocarbons (PAHs) in vegetables near industrial areas of Shanghai, China: Sources, exposure, and cancer risk. Environ. Pollut. 2018, 241, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, F.; Zhang, J.; Zhou, M.; Li, F.; Liu, X. Accumulation characteristics and potential risk of PAHs in vegetable system grow in home garden under straw burning condition in Jilin, Northeast China. Ecotoxicol. Environ. Saf. 2018, 162, 647–654. [Google Scholar] [CrossRef]
- Zhang, J.; Fan, S.; Du, X.; Yang, J.; Wang, W.; Hou, H. Accumulation, Allocation, and Risk Assessment of Polycyclic Aromatic Hydrocarbons (PAHs) in Soil-Brassica chinensis System. PLoS ONE 2015, 10, e0115863. [Google Scholar] [CrossRef] [PubMed]
- Miglietta, M.L.; Rametta, G.; Manzo, S.; Salluzzo, A.; Rimauro, J.; Di Francia, G. Methodological issues about techniques for the spiking of standard OECD soil with nanoparticles: Evidence of different behaviours. J. Nanopart. Res. 2015, 17, 312. [Google Scholar] [CrossRef]
- MSZ 1484-6:2003; Testing of Waters. Part 6: Determination of Polycyclic Aromatic Hydrocarbons (PAH) Content by Gas Chromatographic-Mass Spectrometry. Hungarian Standard Association: Budapest, Hungary, 2003.
- MSZ EN 15527:2009; Characterization of Waste. Determination of Polycyclic Aromatic Hydrocarbons (PAH) in Waste Using Gas Chromatography Mass Spectrometry (GC/MS). Hungarian Standard Association: Budapest, Hungary, 2009.
- SO/IEC 17025:2018; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2018.
- Keith, L.H. The Source of U.S. EPA's Sixteen PAH Priority Pollutants. Polycycl. Aromat. Compd. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Srogi, K. Monitoring of environmental exposure to polycyclic aromatic hydrocarbons: A review. Environ. Chem. Lett. 2007, 5, 169–195. [Google Scholar] [CrossRef]
- Dat, N.D.; Chang, M.B. Review on characteristics of PAHs in atmosphere, anthropogenic sources and control technologies. Sci. Total Environ. 2017, 609, 682–693. [Google Scholar] [CrossRef]
- Ollivon, D.; Blanchoud, H.; Motelay-Massei, A.; Garban, B. Atmospheric deposition of PAHs to an urban site, Paris, France. Atmos. Environ. 2002, 36, 2891–2900. [Google Scholar] [CrossRef]
- Nikolaeva, O.; Rozanova, M.; Karpukhin, M. Distribution of traffic-related contaminants in urban topsoils across a highway in Moscow. J. Soils Sediments 2017, 17, 1045–1053. [Google Scholar] [CrossRef]
- De Souza, C.V.; Corrêa, S.M. Polycyclic aromatic hydrocarbons in diesel emission, diesel fuel and lubricant oil. Fuel 2016, 185, 925–931. [Google Scholar] [CrossRef]
- Qi, A.; Wang, P.; Lv, J.; Zhao, T.; Huang, Q.; Wang, Y.; Zhang, X.; Wang, M.; Xiao, Y.; Yang, L.; et al. Distributions of PAHs, NPAHs, OPAHs, BrPAHs, and ClPAHs in air, bulk deposition, soil, and water in the Shandong Peninsula, China: Urban-rural gradient, interface exchange, and long-range transport. Ecotoxicol. Environ. Saf. 2023, 265, 115494. [Google Scholar] [CrossRef]
- Wang, X.S. Polycyclic aromatic hydrocarbons (PAHs) in particle-size fractions of urban topsoils. Environ. Earth Sci. 2013, 70, 2855–2864. [Google Scholar] [CrossRef]
- Kang, F.; Chen, D.; Gao, Y.; Zhang, Y. Distribution of polycyclic aromatic hydrocarbons in subcellular root tissues of ryegrass (Lolium multiflorum Lam.). BMC Plant Biol. 2010, 10, 210. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Semenkov, I.; Klink, G.; Tarigholizadeh, S.; Sushkova, S. Phylogenetic analysis of hyperaccumulator plant species for heavy metals and polycyclic aromatic hydrocarbons. Environ. Geochem. Health 2021, 43, 1629–1654. [Google Scholar] [CrossRef] [PubMed]
- Alves, W.S.; Manoel, E.A.; Santos, N.S.; Nunes, R.O.; Domiciano, G.C.; Soares, M.R. Detection of polycyclic aromatic hydrocarbons (PAHs) in Medicago sativa L. by fluorescence microscopy. Micron 2017, 95, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Huelster, A.; Mueller, J.F.; Marschner, H. Soil-plant transfer of polychlorinated dibenzo-p-dioxins and dibenzofurans to vegetables of the cucumber family (Cucurbitaceae). Environ. Sci. Technol. 1994, 28, 1110–1115. [Google Scholar] [CrossRef]
- Wild, E.; Dent, J.; Thomas, G.O.; Jones, K.C. Visualizing the air-to-leaf transfer and within-leaf movement and distribution of phenanthrene: Further studies utilizing two-photon excitation microscopy. Environ. Sci. Technol. 2006, 40, 907–916. [Google Scholar] [CrossRef]
- Wieczorek, J.K.; Wieczorek, Z.J. Phytotoxicity and accumulation of anthracene applied to the foliage and sandy substrate in lettuce and radish plants. Ecotoxicol. Environ. Saf. 2007, 66, 369–377. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Jin, X.; Zeng, Y.; Deng, L.; Wang, X.; Chen, Z. Uptake, translocation, and risk assessment of PAHs in contaminated soil-air-vegetable systems based on a field simulation experiment. Environ. Pollut. 2021, 271, 116361. [Google Scholar] [CrossRef]
- Sushkova, S.; Minkina, T.; Tarigholizadeh, S.; Antonenko, E.; Konstantinova, E.; Gülser, C.; Dudnikova, T.; Barbashev, A.; Kızılkaya, R. PAHs accumulation in soil-plant system of Phragmites australis Cav. in soil under long-term chemical contamination. Eurasian J. Soil Sci. 2020, 9, 242–253. [Google Scholar] [CrossRef]
- Inam, E.; Ibanga, F.; Essien, J. Bioaccumulation and cancer risk of polycyclic aromatic hydrocarbons in leafy vegetables grown in soils within automobile repair complex and environ in Uyo, Nigeria. Environ. Monit. Assess. 2016, 188, 681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, G.; Liao, X. Negative role of biochars in the dissipation and vegetable uptake of polycyclic aromatic hydrocarbons (PAHs) in an agricultural soil: Cautions for application of biochars to remediate PAHs-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 213, 112075. [Google Scholar] [CrossRef]
- Cao, C.; Wu, Y.Y.; Lv, Z.Y.; Wang, J.W.; Wang, C.W.; Zhang, H.; Wang, J.J.; Chen, H. Uptake of polycyclic aromatic hydrocarbons (PAHs) from PAH–contaminated soils to carrots and Chinese cabbages under the greenhouse and field conditions. Chemosphere 2024, 360, 142405. [Google Scholar] [CrossRef]
- Jiao, S.; Hou, X.; Zhao, G.; Feng, Y.; Zhang, S.; Zhang, H.; Liu, J.; Jiang, G. Migration of polycyclic aromatic hydrocarbons in the rhizosphere micro-interface of soil-ryegrass (Lolium perenne L.) system. Sci. Total Environ. 2023, 903, 166299. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, H.; Lu, Y.; Yu, X.; Wang, J.; Sun, S.; Liu, M.; Li, D.; Li, Y.F.; Zhang, D. Uptake and translocation of polycyclic aromatic hydrocarbons (PAHs) and heavy metals by maize from soil irrigated with wastewater. Sci. Rep. 2017, 7, 12165. [Google Scholar] [CrossRef] [PubMed]
- Paraíba, L.C.; Queiroz, S.C.N.; Maia, A.D.H.N.; Ferracini, V.L. Bioconcentration factor estimates of polycyclic aromatic hydrocarbons in grains of corn plants cultivated in soils treated with sewage sludge. Sci. Total Environ. 2010, 408, 3270–3276. [Google Scholar] [CrossRef] [PubMed]
- Kipopoulou, A.; Manoli, E.; Samara, C. Bioconcentration of polycyclic aromatic hydrocarbons in vegetables grown in an industrial area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef]
- Bogolte, B.T.; Ehlers, G.A.C.; Braun, R.; Loibner, A.P. Estimation of PAH bioavailability to Lepidium sativum using sequential supercritical fluid extraction–a case study with industrial contaminated soils. Eur. J. Soil Biol. 2007, 43, 242–250. [Google Scholar] [CrossRef]
- Moeckel, C.; Nizzetto, L.; Strandberg, B.; Lindroth, A.; Jones, K.C. Air-boreal forest transfer and processing of polychlorinated biphenyls. Environ. Sci. Technol. 2009, 43, 5282–5289. [Google Scholar] [CrossRef]
- Wei, B.; Liu, C.; Bao, J.; Wang, Y.; Hu, J.; Qi, M.; Jin, J.; Wei, Y. Uptake and distributions of polycyclic aromatic hydrocarbons in cultivated plants around an E-waste disposal site in Southern China. Environ. Sci. Pollut. Res. 2021, 28, 2696–2706. [Google Scholar] [CrossRef]
- Meudec, A.; Dussauze, J.; Deslandes, E.; Poupart, N. Evidence for bioaccumulation of PAHs within internal shoot tissues by a halophytic plant artificially exposed to petroleum-polluted sediments. Chemosphere 2006, 65, 474–481. [Google Scholar] [CrossRef]
- Fismes, J.; Perrin-Ganier, C.; Empereur-Bissonnet, P.; Morel, J.L. Soil to-root transfer and translocation of polycyclic aromatic hydrocarbons by vegetables grown on industrial contaminated soils. J. Environ. Qual. 2002, 31, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; He, M.; Shang, H.; Yu, H.; Wang, H.; Li, H.; Piao, J.; Quinto, M.; Li, D. Biomonitoring polycyclic aromatic hydrocarbons by Salix matsudana leaves: A comparison with the relevant air content and evaluation of environmental parameter effects. Atmos. Environ. 2018, 181, 47–53. [Google Scholar] [CrossRef]
- Yakovleva, E.V.; Beznosikov, V.A.; Kondratenok, B.M.; Gabov, D.N. Bioaccumulation of polycyclic aromatic hydrocarbons in the soil-plant systems of the northern-taiga biocenoses. Eurasian Soil Sci. 2012, 45, 309–320. [Google Scholar] [CrossRef]
- Desalme, D.; Binet, P.; Epron, D.; Bernard, N.; Gilbert, D.; Toussaint, M.-L.; Plain, C.; Chiapusio, G. Atmospheric phenanthrene pollution modulates carbon allocation in red clover (Trifolium pratense L.). Environ. Pollut. 2011, 159, 2759–2765. [Google Scholar] [CrossRef]
- Desalme, D.; Binet, P.; Bernard, N.; Gilbert, D.; Toussaint, M.L.; Chiapusio, G. Atmospheric phenanthrene transfer and effects on two grassland species and their root symbionts: A microcosm study. Environ. Exp. Bot. 2011, 71, 146–151. [Google Scholar] [CrossRef]
- Soceanu, A.; Dobrinas, S.; Stanciu, G.; Popescu, V. Polycyclic aromatic hydrocarbons in vegetables grown in urban and rural areas. Environ. Eng. Manag. J. 2014, 13, 2311–2315. [Google Scholar]
- Jánská, M.; Hajšlová, J.; Tomaniová, M.; Kocourek, V.; Vávrová, M. Polycyclic Aromatic Hydrocarbons in Fruits and Vegetables Grown in the Czech Republic. Bull. Environ. Contam. Toxicol. 2006, 77, 492–499. [Google Scholar] [CrossRef]
- Wennrich, L.; Popp, P.; Zeibig, M. Polycyclic aromatic hydrocarbon burden in fruit and vegetable species cultivated in allotments in an industrial area. Int. J. Environ. Anal. Chem. 2002, 82, 677–690. [Google Scholar] [CrossRef]
- Mo, C.H.; Cai, Q.Y.; Tang, S.R.; Zeng, Q.Y.; Wu, Q.T. Polycyclic aromatic hydrocarbons and phthalic acid esters in vegetables from nine farms of the Pearl River Delta, South China. Arch. Environ. Contam. Toxicol. 2009, 56, 181–189. [Google Scholar] [CrossRef]
- Golomb, D.; Barry, E.; Fisher, G.; Varanusupakul, P.; Koleda, M.; Rooney, T. Atmospheric deposition of polycyclic aromatic hydrocarbons near New England coastal waters. Atmos. Environ. 2001, 35, 6245–6258. [Google Scholar] [CrossRef]
- Pekey, B.; Karakaş, D.; Ayberk, S. Atmospheric deposition of polycyclic aromatic hydrocarbons to Izmit Bay, Turkey. Chemosphere 2007, 67, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Liang, B.; Chen, L.; Zhu, Y.; Gao, M.; Chen, J.; Wang, F.; Chen, Y.; Tian, M. Atmospheric wet and dry depositions of polycyclic aromatic compounds in a megacity of Southwest China. Environ. Res. 2022, 204, 112151. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Lee, C.B. Development of an improved dry and wet deposition collector and the atmospheric deposition of PAHs onto Ulsan Bay, Korea. Atmos. Environ. 2004, 38, 863–871. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).