Designing Pyrrolidinium-Based Ionic Liquid Electrolytes for Energy Storage: Thermal and Electrical Behaviour of Ternary Mixtures with Lithium Salt and Carbonates
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Differential Scanning Calorimetry
2.3. Thermogravimetric Analysis
2.4. Ionic Conductivity
3. Results and Discussion
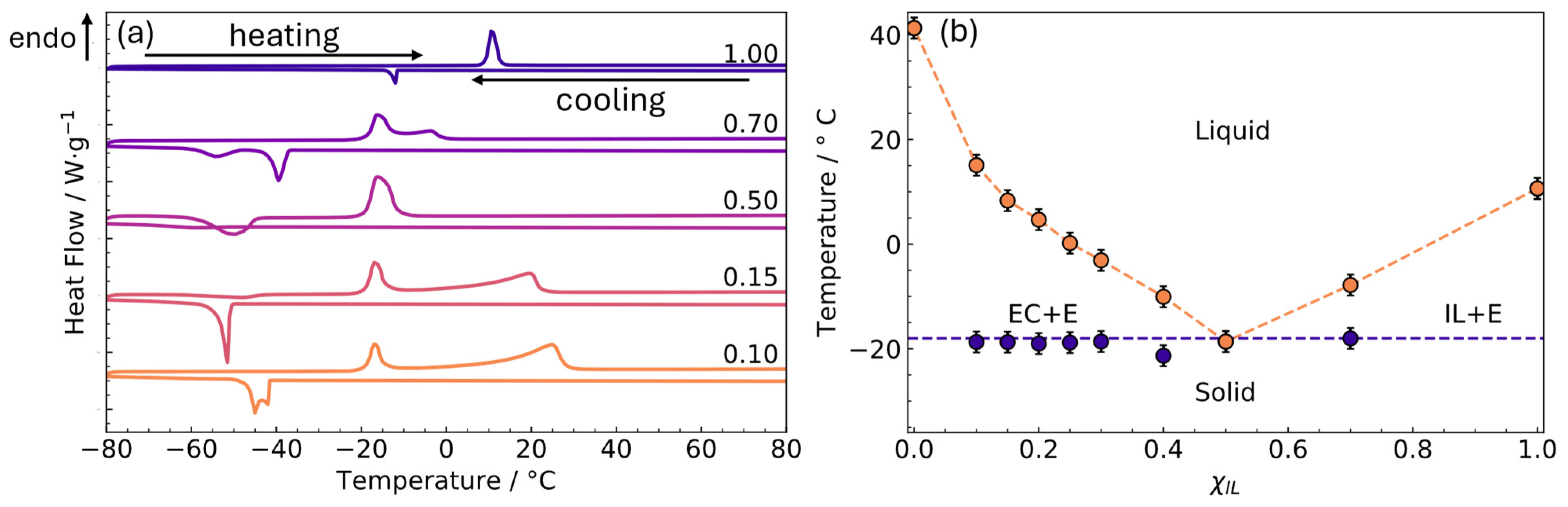
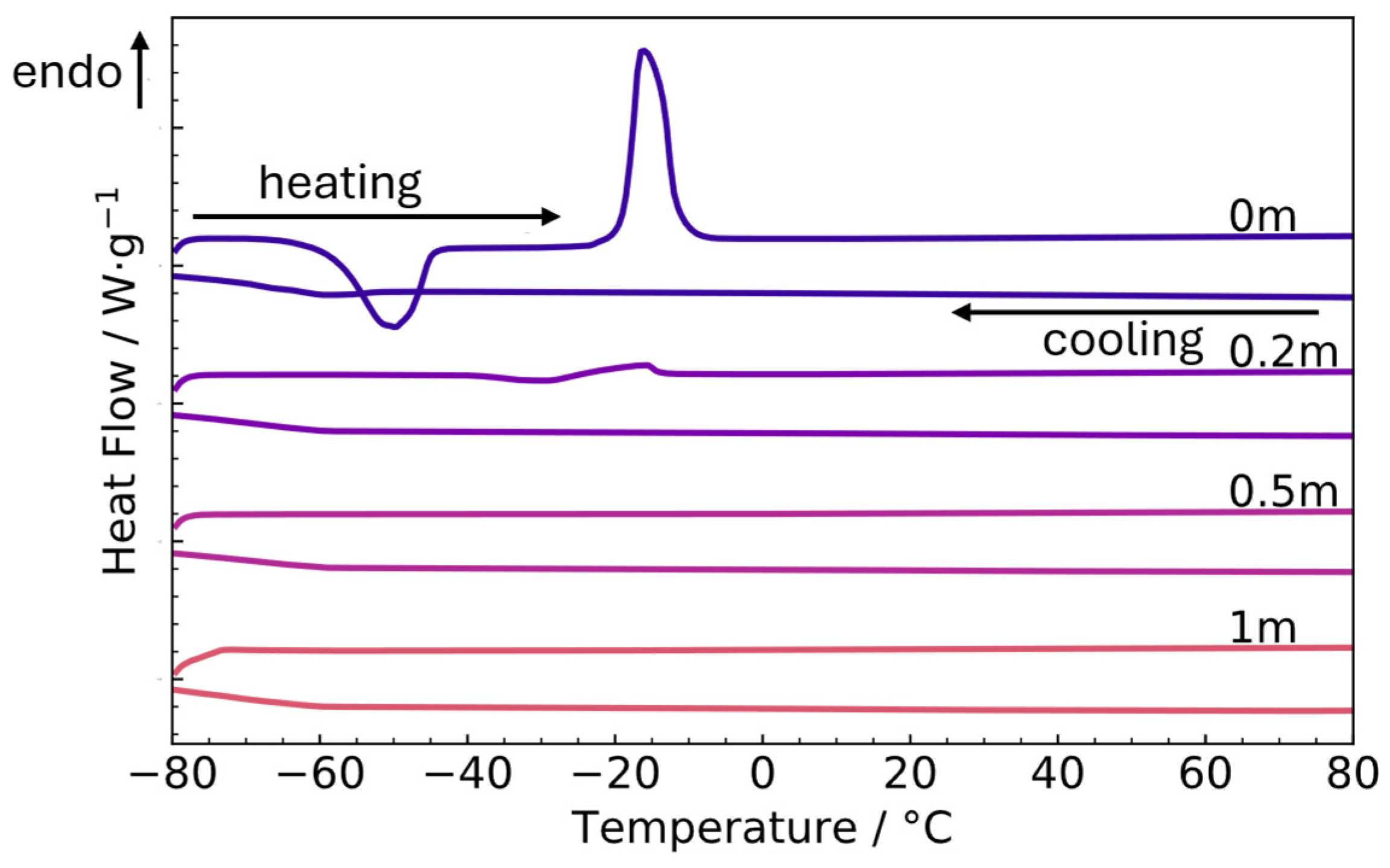
3.1. Differential Scanning Calorimetry (DSC)
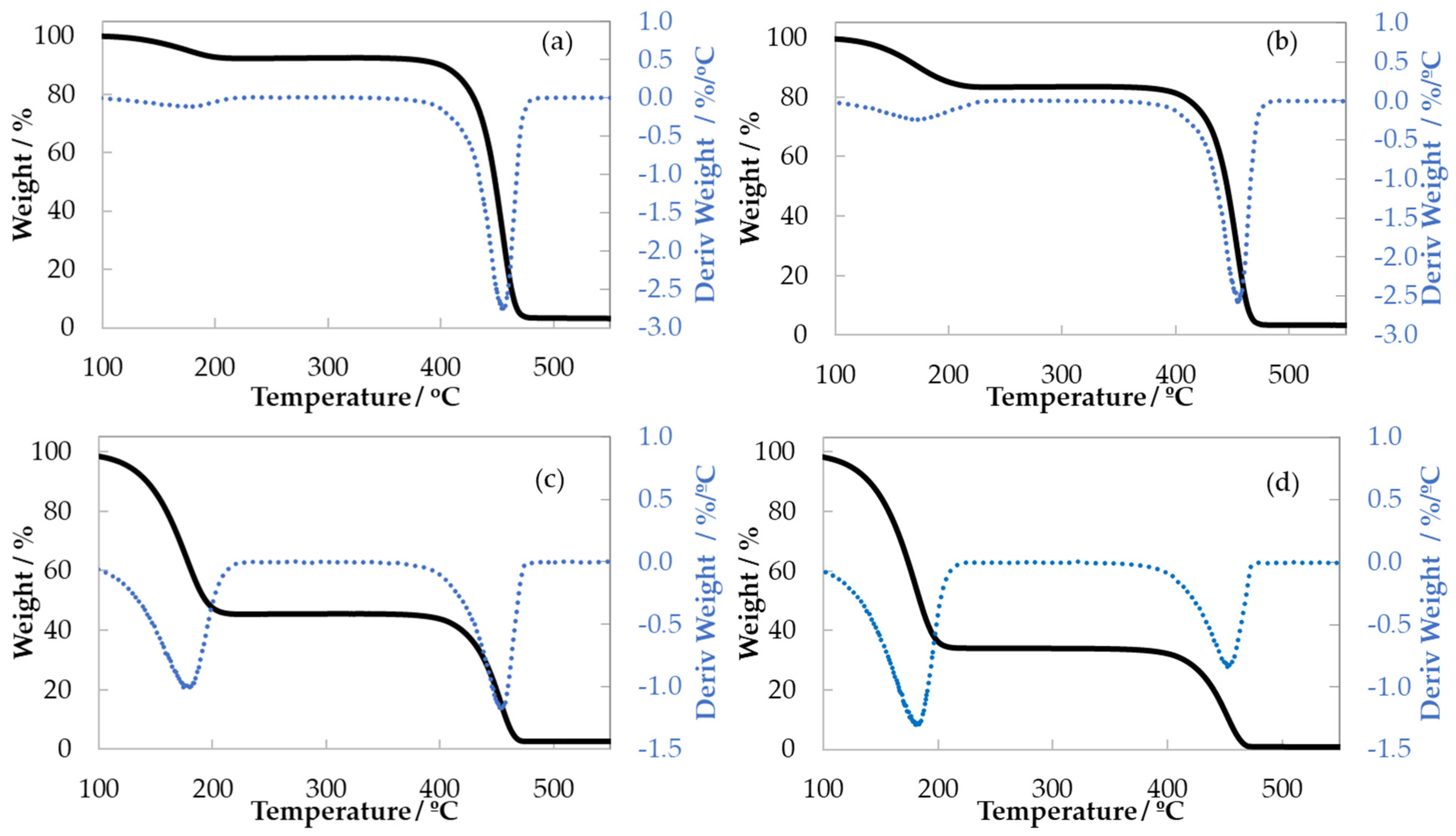
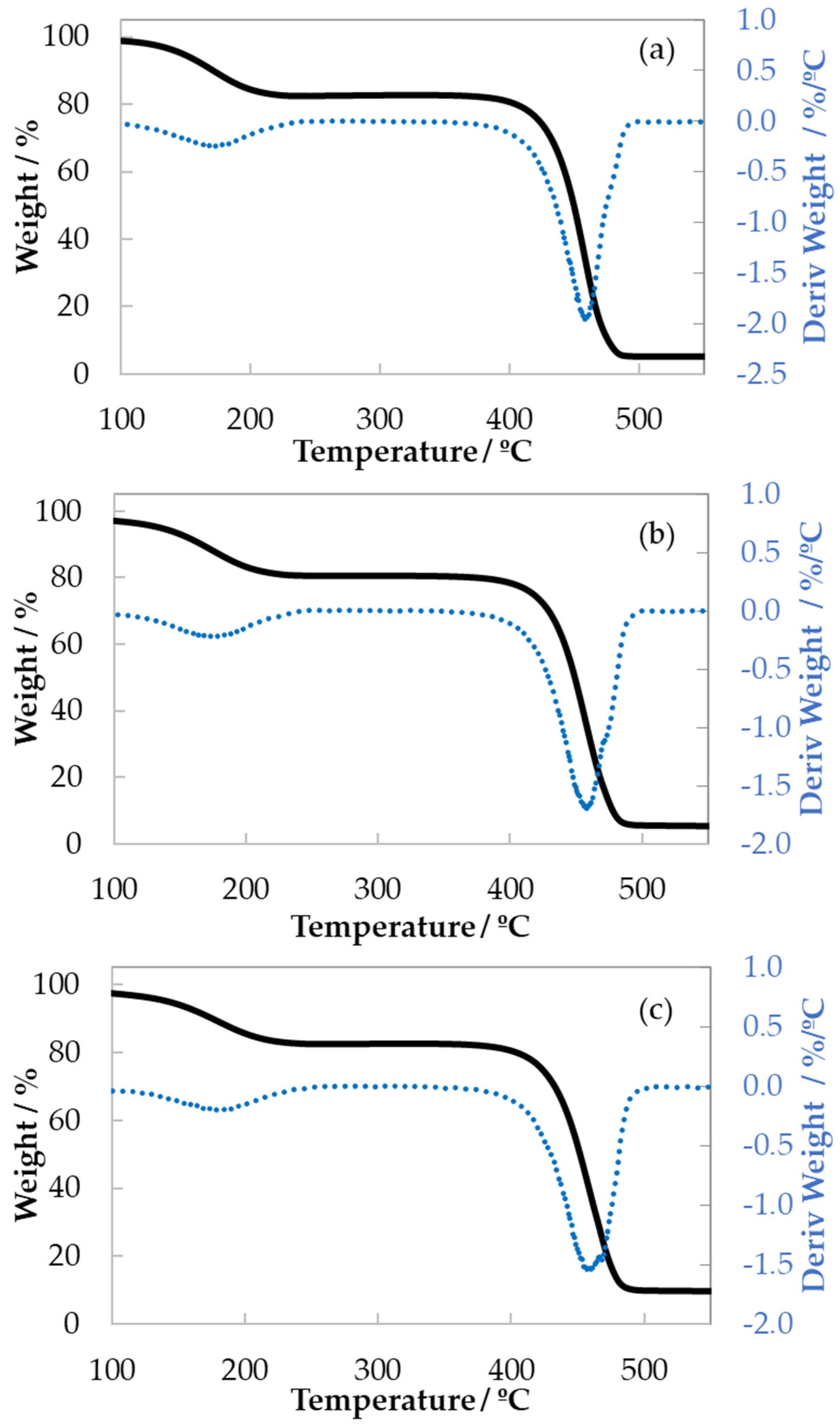
3.2. Thermogravimetric Analysis (TGA)
3.3. Broadband Dielectric Spectroscopy
4. Conclusions
- EC addition provokes the widening of transition peaks in DSC. In addition, melting and crystallisation take place in two steps, which indicates that salt addition and/or EC reduce, or remove, the crystalline behaviour. Binary mixture enthalpies increase with the addition of EC presenting lower values than the corresponding pure EC.
- The binary mixture 0.5 [C3C1Pyrr][TFSI] + 0.5 EC corresponds to the eutectic composition whose melting temperature is the lowest of all the studied concentrations.
- Salt addition to the eutectic composition removes the phase transition at high quantities of salt in the range from −80 to 100 °C.
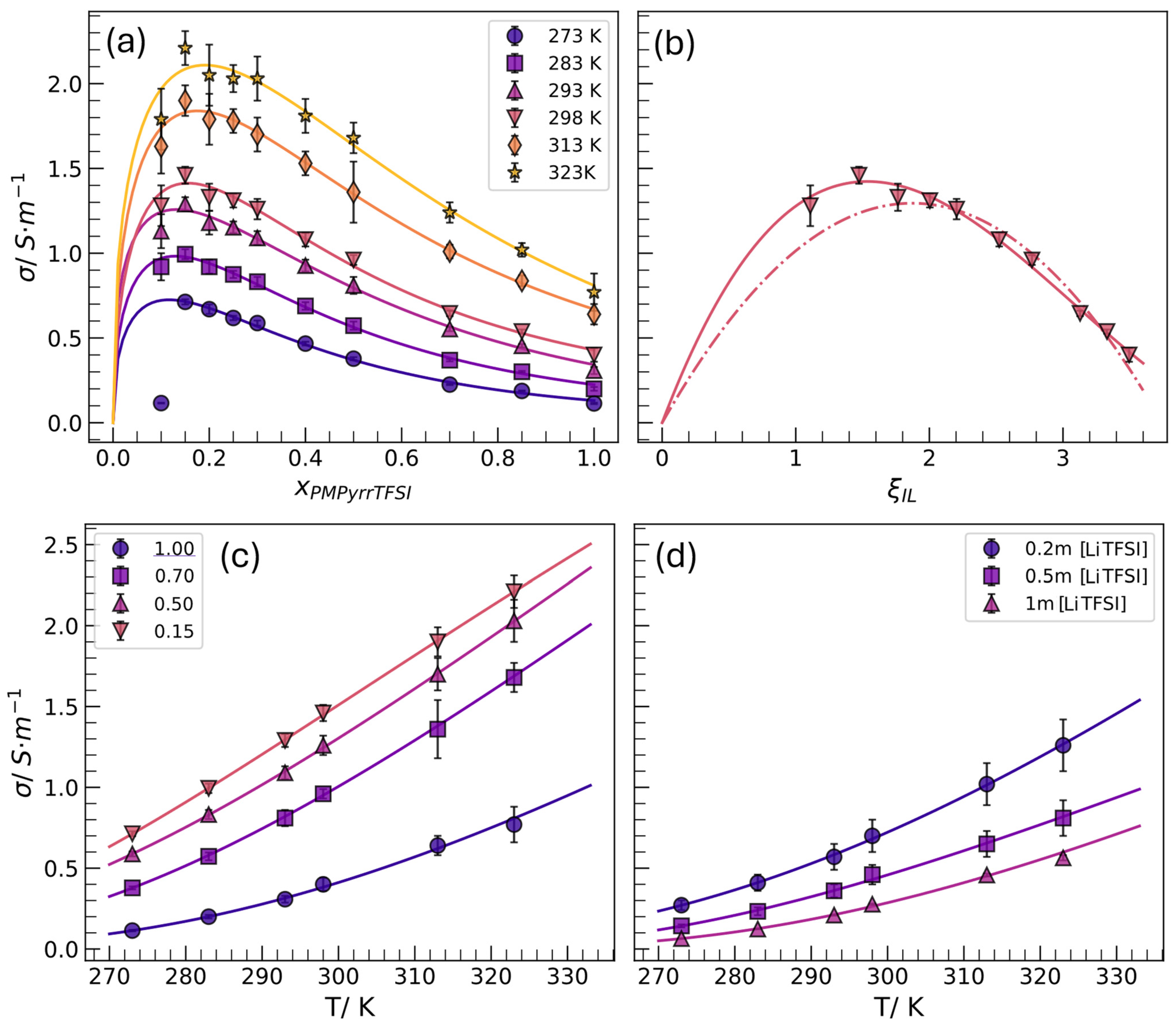
- The values of conductivity of the [C3C1Pyrr][TFSI] + EC mixtures are higher than the pure IL and present a maximum at xIL = 0.15.
- LiTFSI addition to the 0.5 [C3C1Pyrr][TFSI] + 0.5 EC mixture diminishes the conductivity; however, the values at room temperature are higher than the required 0.1 S·m−1 for energy storage applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.C.; Lin, S.M.; Lo, H.A.; Tang, S.Y.; Hsu, Y.C.; Peng, Y.R.; Gu, B.N.; Chen, Y.T.; Shen, Y.C.; Wu, T.S.; et al. Design of Electrolyte Using Deep Eutectic Solvents for High-Performance Rechargeable Nickel-Iodine Batteries. Small 2025, 2025, e2412549. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Tian, C.; Qin, K.; Suo, L. Concentrated Electrolytes for Rechargeable Lithium Metal Batteries. Mater. Futures 2023, 2, 012101. [Google Scholar] [CrossRef]
- Xu, J. High-Entropy Electrolytes in Boosting Battery Performance. Mater. Futures 2023, 2, 047501. [Google Scholar] [CrossRef]
- Ding, M.S.; Xu, K.; Jow, T.R. Phase Diagram of EC-DMC Binary System and Enthalpic Determination of Its Eutectic Composition. J. Therm. Anal. Calorim. 2000, 62, 177–186. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous Liquid Electrolytes for Lithium-Based Rechargeable Batteries. Chem. Rev. 2004, 104, 4303–4417. [Google Scholar] [CrossRef] [PubMed]
- Hess, S.; Wohlfahrt-Mehrens, M.; Wachtler, M. Flammability of Li-Ion Battery Electrolytes: Flash Point and Self-Extinguishing Time Measurements. J. Electrochem. Soc. 2015, 162, A3084–A3097. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, A.R.; Lee, H.J.; Shin, U.; Yoo, Y.; Lim, H.D.; Han, C.; Lee, H.; Kim, Y.J.; Baek, J.; et al. Molecularly Engineered Linear Organic Carbonates as Practically Viable Nonflammable Electrolytes for Safe Li-Ion Batteries. Energy Environ. Sci. 2023, 16, 2924–2933. [Google Scholar] [CrossRef]
- Dupré, N.; Moreau, P.; De Vito, E.; Quazuguel, L.; Boniface, M.; Kren, H.; Bayle-Guillemaud, P.; Guyomard, D. Carbonate and Ionic Liquid Mixes as Electrolytes to Modify Interphases and Improve Cell Safety in Silicon-Based Li-Ion Batteries. Chem. Mater. 2017, 29, 8132–8146. [Google Scholar] [CrossRef]
- Becker, M.; Reber, D.; Aribia, A.; Battaglia, C.; Kühnel, R.-S. The Hydrotropic Effect of Ionic Liquids in Water-in-Salt Electrolytes. Angew. Chem. Int. Ed. 2021, 60, 14100–14108. [Google Scholar] [CrossRef]
- Cabeza, O.; Segade, L.; Domínguez-Pérez, M.; Rilo, E.; García-Garabal, S.; Ausín, D.; Martinelli, A.; López-Lago, E.; Varela, L.M. Tuning Physical Properties and Mesomorphic Structures in Aqueous 1-Ethyl-3-Methylimidazolium Octylsulfate Rigid-Gel with Univalent Salt Doping. J. Chem. Thermodyn. 2017, 112, 267–275. [Google Scholar] [CrossRef]
- Chauhan, S.; Jyoti, J.; Sharma, K.; Kumar, K. A Conductance Study to Analyze the Effect of Organic Solvents on Micellization Behavior of Carbohydrate-Surfactant System at Variable Temperatures. Fluid Phase Equilib. 2014, 375, 286–292. [Google Scholar] [CrossRef]
- Chen, Y.F.; Hu, Y.F.; Yang, Z.Y.; Qi, J.G.; Yin, L.Y.; Zhang, H.R.; Huang, H.Z.; Liu, X.M. Prediction of Density, Viscosity, and Conductivity of the Ternary Aqueous Solutions of Piperidinium-Based Ionic Liquids at Different Temperatures and Atmospheric Pressure Using the Data of Their Binary Subsystems. Fluid Phase Equilib. 2014, 383, 55–71. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, M.; Bai, G.; Zhuo, K.; Yan, C. Conductivities of 1-Alkyl-3-Methylimidazolium Chloride Ionic Liquids in Disaccharide + Water Solutions at 298.15 K. J. Chem. Eng. Data 2016, 61, 3779–3787. [Google Scholar] [CrossRef]
- Choudhury, S.; Mahanta, U.; Prasanna Venkatesh, R.; Banerjee, T. Ionic Liquid Derived Novel Deep Eutectic Solvents as Low Viscous Electrolytes for Energy Storage. J. Mol. Liq. 2022, 366, 120245. [Google Scholar] [CrossRef]
- Papović, S.; Cvjetićanin, N.; Gadžurić, S.; Bešter-Rogač, M.; Vraneš, M. Physicochemical and Electrochemical Characterisation of Imidazolium Based IL + GBL Mixtures as Electrolytes for Lithium-Ion Batteries. Phys. Chem. Chem. Phys. 2017, 19, 28139–28152. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Kim, S.; Ran, F.; Kim, D.S.; Lee, Y.M.; Ryou, M.H. Hybrid Gel Polymer Electrolyte Based on 1-Methyl-1-Propylpyrrolidinium Bis(Trifluoromethanesulfonyl) Imide for Flexible and Shape-Variant Lithium Secondary Batteries. J. Memb. Sci. 2021, 621, 119018. [Google Scholar] [CrossRef]
- Yang, B.; Li, C.; Zhou, J.; Liu, J.; Zhang, Q. Pyrrolidinium-Based Ionic Liquid Electrolyte with Organic Additive and LiTFSI for High-Safety Lithium-Ion Batteries. Electrochim. Acta 2014, 148, 39–45. [Google Scholar] [CrossRef]
- Santiago-Alonso, A.; Sánchez-Pico, J.M.; Emeterio, R.S.; Villanueva, M.; Salgado, J.; Parajó, J.J. Pyrrolidinium-Based Ionic Liquids as Advanced Non-Aqueous Electrolytes for Safer Next Generation Lithium Batteries. Batteries 2024, 10, 319. [Google Scholar] [CrossRef]
- Das, A.; Riyaz, M.; Kobi, S.; Saha, D.; Mukhopadhyay, A. Ethylene Carbonate Free Sulfone-Based Electrolyte for Enabling Superior Performance of High Ni-Containing Li-Transition Metal Oxide Cathodes at High Voltage and High Temperature. Batter. Supercaps 2025, 2025, e202400608. [Google Scholar] [CrossRef]
- Villanueva, M.; Parajó, J.J.; Sánchez, P.B.; García, J.; Salgado, J. Liquid Range Temperature of Ionic Liquids as Potential Working Fluids for Absorption Heat Pumps. J. Chem. Thermodyn. 2015, 91, 127–135. [Google Scholar] [CrossRef]
- Parajó, J.J.; Teijeira, T.; Fernández, J.; Salgado, J.; Villanueva, M. Thermal Stability of Some Imidazolium [NTf2] Ionic Liquids: Isothermal and Dynamic Kinetic Study through Thermogravimetric Procedures. J. Chem. Thermodyn. 2017, 112, 105–113. [Google Scholar] [CrossRef]
- Parajó, J.J.; Villanueva, M.; Otero, I.; Fernández, J.; Salgado, J. Thermal Stability of Aprotic Ionic Liquids as Potential Lubricants. Comparison with Synthetic Oil Bases. J. Chem. Thermodyn. 2018, 116, 185–196. [Google Scholar] [CrossRef]
- Leys, J.; Wübbenhorst, M.; Preethy Menon, C.; Rajesh, R.; Thoen, J.; Glorieux, C.; Nockemann, P.; Thijs, B.; Binnemans, K.; Longuemart, S. Temperature Dependence of the Electrical Conductivity of Imidazolium Ionic Liquids. J. Chem. Phys. 2008, 128, 2827462. [Google Scholar] [CrossRef]
- Hofmann, A.; Migeot, M.; Hanemann, T. Investigation of Binary Mixtures Containing 1-Ethyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Azanide and Ethylene Carbonate. J. Chem. Eng. Data 2016, 61, 114–123. [Google Scholar] [CrossRef]
- Fox, E.T.; Paillard, E.; Borodin, O.; Henderson, W.A. Physicochemical Properties of Binary Ionic Liquid-Aprotic Solvent Electrolyte Mixtures. J. Phys. Chem. C 2013, 117, 78–84. [Google Scholar] [CrossRef]
- Thompson, P.T.; Taylor, R.E.; Wood, R.H. Enthalpy of Fusion and Cryoscopic Constant for Ethylene Carbonate. J. Chem. Thermodyn. 1975, 7, 547–550. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, C.Y.; Wu, J.H.; Kang, X. Fire and Explosion Hazards of Ionic Liquid 1-Methyl-1-Propylpyrrolidinium Bis(Trifluoromethanesulfonyl)Imide. J. Loss Prev. Process Ind. 2019, 60, 233–240. [Google Scholar] [CrossRef]
- Vallet, P.; Parajó, J.J.; Santiago-Alonso, A.; Villanueva, M.; Cabeza, Ó.; Varela, L.M.; Salgado, J. Anomalous Behaviour of the Ionic Conductivity of Nanoconfined IL -Lithium Salt Mixtures. J. Mol. Liq. 2024, 401, 124630. [Google Scholar] [CrossRef]
- Vallet, P.; Parajó, J.J.; Santiago-Alonso, A.; Villanueva, M.; Varela, L.M.; Salgado, J. Thermal Characterization of [C2Im][NO3] and Multivalent Nitrate Salts Mixtures. Crystals 2024, 14, 502. [Google Scholar] [CrossRef]
- Salgado, J.; Parajó, J.J.; Villanueva, M.; Rodríguez, J.R.; Cabeza, O.; Varela, L.M. Liquid Range of Ionic Liquid—Metal Salt Mixtures for Electrochemical Applications. J. Chem. Thermodyn. 2019, 134, 164–174. [Google Scholar] [CrossRef]
- Svoboda, K.; Pohořelý, M.; Hartman, M.; Martinec, J. Pretreatment and Feeding of Biomass for Pressurized Entrained Flow Gasification. Fuel Process. Technol. 2009, 90, 629–635. [Google Scholar] [CrossRef]
- Le, L.T.M.; Vo, T.D.; Ngo, K.H.P.; Okada, S.; Alloin, F.; Garg, A.; Le, P.M.L. Mixing Ionic Liquids and Ethylene Carbonate as Safe Electrolytes for Lithium-Ion Batteries. J. Mol. Liq. 2018, 271, 769–777. [Google Scholar] [CrossRef]
- Martinelli, A.; Matic, A.; Jacobsson, P.; Börjesson, L.; Fernicola, A.; Scrosati, B. Phase Behavior and Ionic Conductivity in Lithium Bis(Trifluoromethanesulfonyl)Imide-Doped Ionic Liquids of the Pyrrolidinium Cation and Bis(Trifluoromethanesulfonyl)Imide Anion. J. Phys. Chem. B 2009, 113, 11247–11251. [Google Scholar] [CrossRef] [PubMed]
- Kerner, M.; Plylahan, N.; Scheers, J.; Johansson, P. Thermal Stability and Decomposition of Lithium Bis(Fluorosulfonyl)Imide (LiFSI) Salts. RSC Adv. 2016, 6, 23327–23334. [Google Scholar] [CrossRef]
- García-Garabal, S.; Domínguez-Pérez, M.; Portela, D.; Varela, L.M.; Cabeza, O. Preliminary Study of New Electrolytes Based on [MPPyr][TFSI] for Lithium Ion Batteries. J. Mol. Liq. 2022, 363, 119758. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Zhang, S.; Zhang, H.; Zhao, Y. Conductivities, Volumes, Fluorescence, and Aggregation Behavior of Ionic Liquids [C4mim][BF4] and [Cnmim]Br (n = 4, 6, 8, 10, 12) in Aqueous Solutions. J. Phys. Chem. B 2007, 111, 6181–6188. [Google Scholar] [CrossRef]
- Dorbritz, S.; Ruth, W.; Kragl, U. Investigation on Aggregate Formation of Ionic Liquids. Adv. Synth. Catal. 2005, 347, 1273–1279. [Google Scholar] [CrossRef]
- Zhang, Q.G.; Sun, S.S.; Pitula, S.; Liu, Q.S.; Welz-Biermann, U.; Zhang, J.J. Electrical Conductivity of Solutions of Ionic Liquids with Methanol, Ethanol, Acetonitrile, and Propylene Carbonate. J. Chem. Eng. Data 2011, 56, 4659–4664. [Google Scholar] [CrossRef]
- Casteel, J.F.; Amis, E.S. Specific Conductance of Concentrated Solutions of Magnesium Salts in Water-Ethanol System. J. Chem. Eng. Data 1972, 17, 55–59. [Google Scholar] [CrossRef]
- Fu, Y.; Cui, X.; Zhang, Y.; Feng, T.; He, J.; Zhang, X.; Bai, X.; Cheng, Q. Measurement and Correlation of the Electrical Conductivity of the Ionic Liquid [BMIM][TFSI] in Binary Organic Solvents. J. Chem. Eng. Data 2018, 63, 1180–1189. [Google Scholar] [CrossRef]
- Varela, L.M.; Carrete, J.; García, M.; Gallego, L.J.; Turmine, M.; Rilo, E.; Cabeza, O. Pseudolattice Theory of Charge Transport in Ionic Solutions: Corresponding States Law for the Electric Conductivity. Fluid Phase Equilib. 2010, 298, 280–286. [Google Scholar] [CrossRef]
- Varela, L.M.; Carrete, J.; García, M.; Rodríguez, J.R.; Gallego, L.J.; Turmine, M.; Cabeza, O. Pseudolattice Theory of Ionic Liquids; Ionic liquids: Theory, Properties, New Approaches; Kokorin, A., Ed.; InTech: London, UK, 2011. [Google Scholar]
| Name | Molecular Mass (g·mol−1) | Structure | Short Name CAS Number | Purity Provenance |
|---|---|---|---|---|
| 1-Methyl-1-propylpyrrolidinium bis(trifluoromethylsulfonyl) imide | 408.4 |  | [C3C1Pyrr][TFSI] 223437-05-6 | 0.99 a Iolitec, Heilbronn, Germany |
| Lithium bis(trifluoromethylsulfonyl) imide | 287.09 | 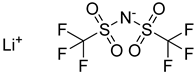 | LiTFSI 90076-65-6 | >0.99 a Sigma Aldrich, San Luis, MI, USA |
| Ethylene carbonate | 88.06 | 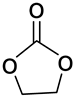 | EC 96-49-1 | >0.99 a Acros Organics, Waltham, MA, USA |
| Sample | ΔHm/J g−1 | ΔHcc; ΔHc/J g−1 | |||
|---|---|---|---|---|---|
| Mixtures (1 − x) EC + x [C3C1Pyrr][TFSI] | |||||
| 1.0 | 9 | −11 | -- | 36 | 33 |
| 0.7 | −18|−8 | −37|−49 | -- | 42 | 35 |
| 0.5 | −19 | 52 | 51 | 33 | |
| 0.4 | −19|−10 | -- | −61 | 63 | 53 |
| 0.3 | −19|−3 | -- | −59 | 73 | 60 |
| 0.25 | −19|0 | -- | −58 | 77 | 60 |
| 0.2 | −19|5 | −59 | −44 | 87 | 13/50 |
| 0.15 | −19|8 | −51 | −60 | 92 | 7/63 |
| 0.1 | −19|15 | −42 | 100 | 85 | |
| Ternary mixtures ([C3C1Pyrr][TFSI]/EC/LiTFSI) | |||||
| 0.2 m | −25 | -- | −39 | 2.6 | 2.6 |
| 0.5 m | -- | -- | -- | -- | -- |
| 1 m | -- | -- | -- | -- | -- |
| Sample | ||||
|---|---|---|---|---|
| Mixtures (1 − x) EC + x [C3C1Pyrr][TFSI] | ||||
| 0.7 | 136/436 | 194/465 | 98/72 | 182/458 |
| 0.5 | 135/435 | 198/468 | 97/66 | 175/455 |
| 0.15 | 144/430 | 196/466 | 89/36 | 178/454 |
| 0.1 | 148/424 | 197/465 | 86/28 | 182/453 |
| Ternary mixtures ([C3C1Pyrr][TFSI]/EC/LiTFSI) | ||||
| 0.2 m | 138/436 | 200/478 | 96/66 | 172/458 |
| 0.5 m | 133/432 | 204/478 | 95/68 | 174/458 |
| 1 m | 138/428 | 210/480 | 95/73 | 176/478 |
| Sample | 273 K | 283 K | 293 K | 298 K | 313 K | 323 K |
|---|---|---|---|---|---|---|
| x | Mixtures (1 − x) EC + x [C3C1Pyrr][TFSI] | |||||
| 1.0 | 0.1138(37) | 0.200(11) | 0.308(21) | 0.397(38) | 0.645(59) | 0,77(11) |
| 0.85 | 0.1878(24) | 0.3010(52) | 0.4542(99) | 0.538(12) | 0.837(32) | 1.023(43) |
| 0.7 | 0.2253(31) | 0.3688(70) | 0.553(13) | 0.645(16) | 1.007(43) | 1.242(61) |
| 0.5 | 0.3783(86) | 0.573(24) | 0.806(48) | 0.960(29) | 1.36(18) | 1.681(86) |
| 0.4 | 0.467(11) | 0.689(22) | 0.927(35) | 1.084(40) | 1.531(75) | 1.809(98) |
| 0.3 | 0.588(15) | 0.832(28) | 1.092(44) | 1.261(63) | 1.704(99) | 2.03(13) |
| 0.25 | 0.618(13) | 0.875(21) | 1.153(34) | 1.306(40) | 1.783(69) | 2.026(83) |
| 0.2 | 0.677(28) | 0.921(42) | 1.180(66) | 1.351(83) | 1.76(15) | 2.02(18) |
| 0.15 | 0.713(15) | 0.994(29) | 1.291(43) | 1.461(50) | 1.903(87) | 2.21(10) |
| 0.1 | 0.1160(21) | 0.924(76) | 1.131(98) | 1.28(12) | 1.63(16) | 1.79(18) |
| Ternary mixtures EC:[C3C1Pyrr][TFSI] (0.5:0.5) + [LiTFSI] | ||||||
| 0.2 m [LiTFSI] | 0.271(30) | 0.406(48) | 0.566(77) | 0.696(96) | 1.02(13) | 1.26(16) |
| 0.5 m [LiTFSI] | 0.1432(94) | 0.233(24) | 0.362(40) | 0.463(58) | 0.648(85) | 0.81(11) |
| 1 m [LiTFSI] | 0.06474(40) | 0.1238(12) | 0.2098(29) | 0.2769(43) | 0.4580(93) | 0.564(16) |
| Temperature/K | σ/S m−1 | xmax | a | b |
|---|---|---|---|---|
| 273 | 0.725 (92) | 0.117 (80) | 0.43 (53) | 0.8 (13) |
| 283 | 0.983 (48) | 0.130 (38) | 0.44 (26) | 0.75 (70) |
| 293 | 1.258 (47) | 0.131 (39) | 0.35 (22) | 0.39 (63) |
| 298 | 1.413 (37) | 0.156 (27) | 0.50 (22) | 0.84 (63) |
| 313 | 1.839 (27) | 0.175 (17) | 0.45 (13) | 0.46 (40) |
| 323 | 2.109 (44) | 0.191 (25) | 0.41 (21) | 0.15 (67) |
| Sample | σ∞/S m−1 | Ea/eV | T0/K |
|---|---|---|---|
| Mixtures (1 − x) EC + x [C3C1Pyrr][TFSI] | |||
| 1 | 12.1 (17) | 0.293 (18) | 203 (13) |
| 0.85 | 20.6 (59) | 0.0352 (51) | 186.0 (74) |
| 0.7 | 19.8 (36) | 0.0310 (30) | 192.6 (46) |
| 0.5 | 19.6 (44) | 0.0279 (38) | 191.1 (67) |
| 0.4 | 19.8 (41) | 0.0282 (37) | 185.6 (68) |
| 0.3 | 20.4 (42) | 0.0285 (40) | 179.7 (76) |
| 0.25 | 13.0 (20) | 0.0202 (25) | 196.1 (57) |
| 0.2 | 13.7 (32) | 0.0223 (42) | 186.9 (97) |
| 0.15 | 11.8 (10) | 0.0179 (14) | 199.1 (34) |
| Ternary mixtures ([C3C1Pyrr][TFSI]/EC/LiTFSI) | |||
| 0.2 m | 30 (14) | 0.041 (10) | 171 (15) |
| 0.5 m | 9.5 (51) | 0.0253 (82) | 203 (14) |
| 1 m | 13.3 (74) | 0.0320 (81) | 203 (10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-Alonso, A.; Sánchez-Pico, J.M.; Emeterio, R.S.; Villanueva, M.; Parajó, J.J.; Salgado, J. Designing Pyrrolidinium-Based Ionic Liquid Electrolytes for Energy Storage: Thermal and Electrical Behaviour of Ternary Mixtures with Lithium Salt and Carbonates. Appl. Sci. 2025, 15, 4354. https://doi.org/10.3390/app15084354
Santiago-Alonso A, Sánchez-Pico JM, Emeterio RS, Villanueva M, Parajó JJ, Salgado J. Designing Pyrrolidinium-Based Ionic Liquid Electrolytes for Energy Storage: Thermal and Electrical Behaviour of Ternary Mixtures with Lithium Salt and Carbonates. Applied Sciences. 2025; 15(8):4354. https://doi.org/10.3390/app15084354
Chicago/Turabian StyleSantiago-Alonso, Antía, José M. Sánchez-Pico, Raquel San Emeterio, María Villanueva, Juan José Parajó, and Josefa Salgado. 2025. "Designing Pyrrolidinium-Based Ionic Liquid Electrolytes for Energy Storage: Thermal and Electrical Behaviour of Ternary Mixtures with Lithium Salt and Carbonates" Applied Sciences 15, no. 8: 4354. https://doi.org/10.3390/app15084354
APA StyleSantiago-Alonso, A., Sánchez-Pico, J. M., Emeterio, R. S., Villanueva, M., Parajó, J. J., & Salgado, J. (2025). Designing Pyrrolidinium-Based Ionic Liquid Electrolytes for Energy Storage: Thermal and Electrical Behaviour of Ternary Mixtures with Lithium Salt and Carbonates. Applied Sciences, 15(8), 4354. https://doi.org/10.3390/app15084354






