Isotopic Analysis (δ13C, δ15N, and δ34S) of Modern Terrestrial, Marine, and Freshwater Ecosystems in Greece: Filling the Knowledge Gap for Better Understanding of Sulfur Isotope Imprints—Providing Insights for the Paleo Diet, Paleomobility, and Paleoecology Reconstructions
Abstract
Featured Application
Abstract
1. Introduction
1.1. δ34S in Terrestrial and Aquatic Ecosystems
- The lithology;
- The chemical form of sulfur in organic and synthetic fertilizers and S application over time;
- The soil conditions (mineralization, adsorption/desorption), the soil type (i.e., salinity), and the distance from the coast region (sea spray effect);
- The dissolved sulfate contents of water;
- The atmospheric deposition of sulfuric gases that originate from pollution;
- The cultivation type and the cultivation history of the soil.
Sulfur Isotopic Ratios in Archaeology
- The association between the deposition of sea salt aerosols and the distance from the coast to δ34S values is not linear. At distances greater than 150 km from the coast, the deposition rate of sea salt aerosols decreases significantly, causing δ34S values in human collagen to drop rapidly [21]. At distances greater than 200 km, where sea salt aerosol depositions are low, δ34S values in human collagen remain unaffected by sea salt deposition, and other factors influence δ34S values [27].
- Northwestern Europe receives the highest rate of sea salt aerosols due to strong winds from the North Atlantic that transport sea salt to these regions [28].
- The circum-Mediterranean region exhibits moderately high δ34S values, influenced by marine sea salt deposition and Sahara dust aerosols. The δ34S values in the Sahara range from 12 to 16‰ [29], with particularly high values observed in locations like Crete, where strong sea surface winds contribute to these elevated sulfur values [27].
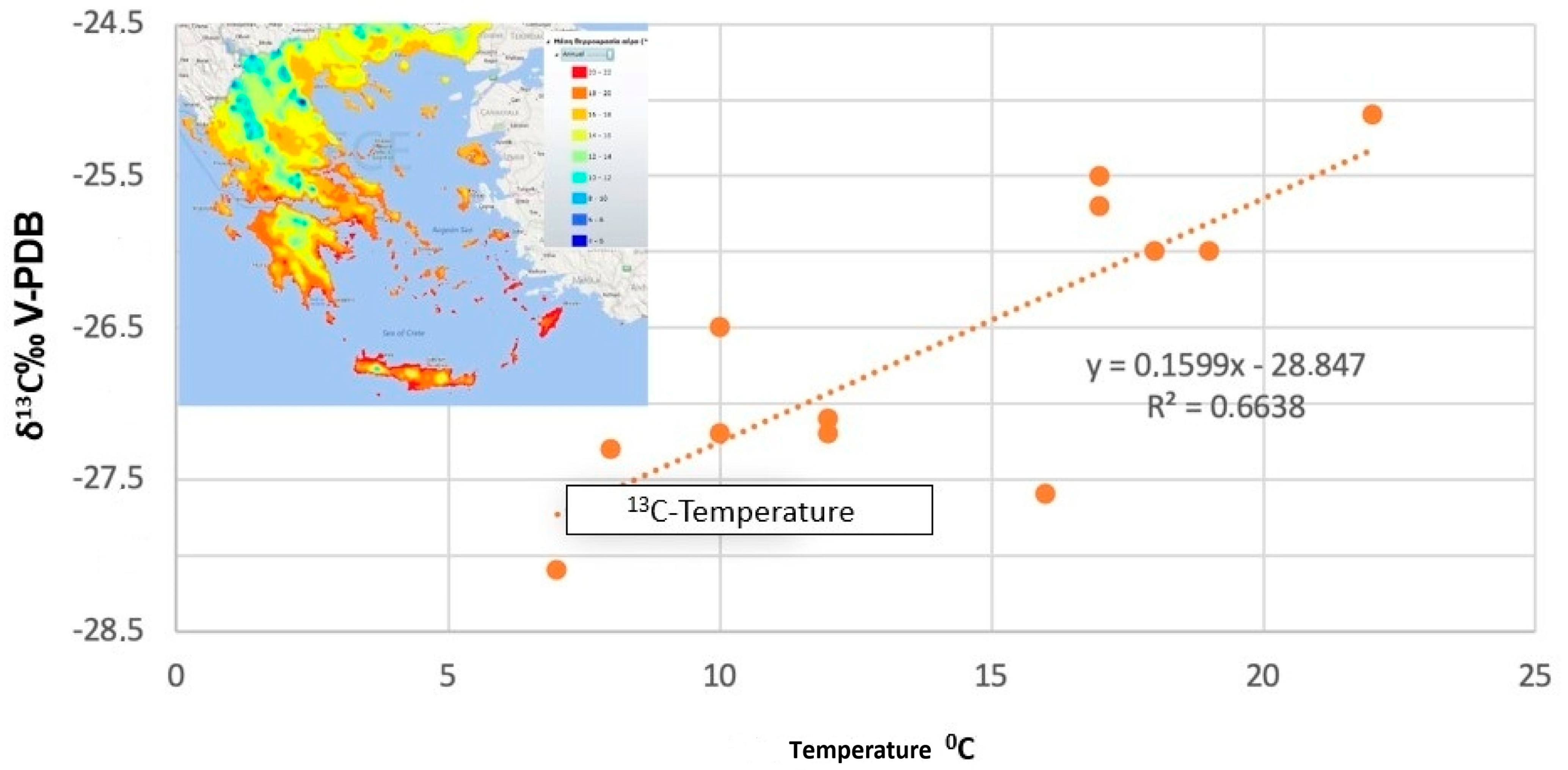
1.2. Carbon and Nitrogen Isotopes
2. Materials and Methods
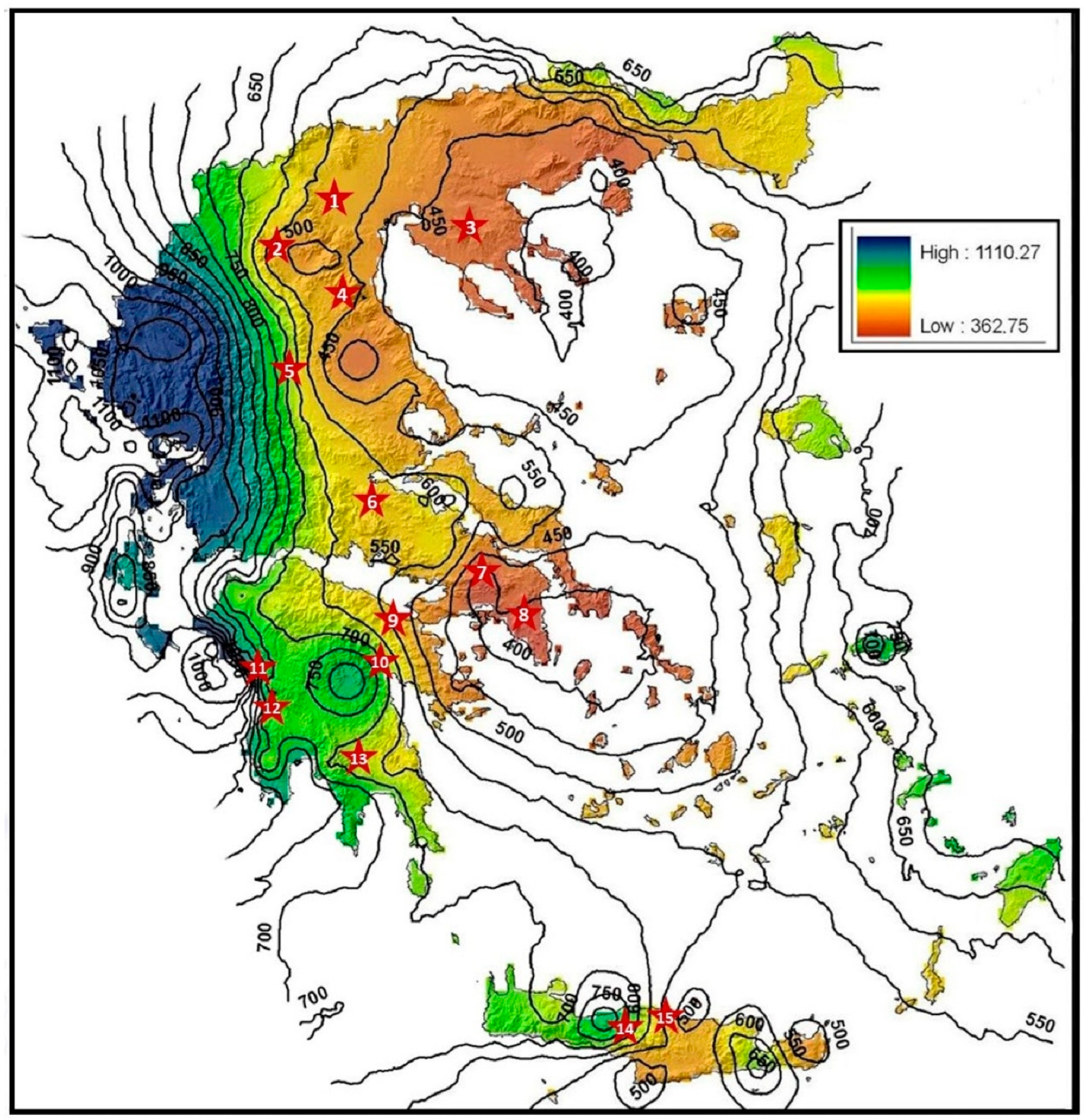
| Location | Lat | Long | Species Cultivation Practices | δ15N (‰), -Air | δ13C (‰), V-PDB | δ34S (‰), V-CDT |
|---|---|---|---|---|---|---|
| Ilia, South Gr | 37.84 | 21.28 | Tomato, Solanum lycopersicum. MF | 3.1 | −28.1 | −1.6 |
| 3.9 | −28.0 | −2.0 | ||||
| 4.3 | −28.7 | 2.4 | ||||
| 4.3 | −28.9 | −2.1 | ||||
| 4.4 | −28.7 | −2.1 | ||||
| 4.9 | −29.6 | 2.1 | ||||
| Median | 4.3 | −28.7 | −1.9 | |||
| SD | 0.6 | 0.5 | 2.6 | |||
| Kiato, South Gr | 38.01 | 22.75 | Orange, Citrus sinensis. MF | 1.8 | −25.6 | 4.6 |
| 2.3 | −24.1 | 4.7 | ||||
| 2.7 | −24.8 | 4.7 | ||||
| 2.9 | −25.1 | 4.8 | ||||
| 2.8 | −25.1 | 4.7 | ||||
| 2.2 | −25.4 | 4.8 | ||||
| Median | 2.5 | −25.1 | 4.7 | |||
| SD | 0.4 | 0.5 | 0.1 | |||
| Sparta, South Gr | 37.08 | 22.43 | Orange, Citrus sinensis. MF | 5.1 | −25.4 | 5.1 |
| 4.5 | −24.7 | 5.2 | ||||
| 4.9 | −25.1 | 5.1 | ||||
| Median | 4.9 | −25.1 | 5.1 | |||
| SD | 0.3 | 0.4 | 0.1 | |||
| Kiato, South Gr | 38.01 | 22.75 | Peach, Prunus persica. MF | 1.2 | −25.0 | 4.2 |
| 1.2 | −25.4 | 4.4 | ||||
| 1.8 | −25.6 | 3.9 | ||||
| 1.6 | −25.4 | 2.6 | ||||
| 0.1 | −25.9 | 2.3 | ||||
| 1.2 | −25.2 | 2.8 | ||||
| Median | 1.2 | −25.4 | 3.3 | |||
| SD | 0.6 | 0.3 | 0.9 | |||
| Kiato, South Gr | 38.01 | 22.75 | Citrus, Rutaceae family. OF | 12.1 | −25.9 | 5.1 |
| Basilicum, Ocimum basilicum. NF | 5.2 | −26.7 | 7.2 | |||
| Nemea, South Gr | 37.82 | 22.66 | Peach, Prunus persica. MF | 2.4 | −25.7 | 3.1 |
| Naousa- North Gr | 40.63 | 22.07 | Peach, Prunus persica. MF | −0.2 | −26.5 | 3.1 |
| −2.1 | −26.4 | 3.6 | ||||
| −1.6 | −26.6 | 2.0 | ||||
| −1.2 | −26.5 | 0.9 | ||||
| −1.5 | −26.6 | 2.2 | ||||
| −1.8 | −26.4 | 2.8 | ||||
| Median | −1.5 | −26.5 | 2.5 | |||
| SD | 0.7 | 0.1 | 1.0 | |||
| Messinia, South Gr | 37.04 | 22.11 | Olive, Olea Europea. MF | −0.4 | −29.5 | 1.4 |
| 2.5 | −29.4 | 1.5 | ||||
| 3.4 | −28.1 | 1.4 | ||||
| 3.7 | −28.2 | 1.6 | ||||
| 3.9 | −28.2 | 1.5 | ||||
| 4.3 | −27.8 | 1.4 | ||||
| 4.8 | −26.0 | 1.6 | ||||
| 3.7 | −28.2 | 1.5 | ||||
| 1.7 | 1.2 | 0.9 | ||||
| Nemea, South Gr | 37.82 | 22.66 | 3.5 | −27.6 | 1.4 | |
| 3.7 | −27.6 | 1.5 | ||||
| Median | 3.6 | −27.6 | 1.5 | |||
| SD | 0.1 | 0.0 | 0.1 | |||
| Iraklio, Crete | 35.34 | 25.14 | Olive, Olea Europea. MF | 4.1 | −26.0 | 1.6 |
| Olympus, North Gr | 40.08 | 22.35 | Wild, Olea Europea. NF | 3.2 | −27.1 | 1.5 |
| Fhiotida, Between Nort and South Gr | 38.90 | 22.53 | Wheat, Triticum dicoccum. NF | 2.8 | −23.2 | 4.4 |
| 2.8 | −23.8 | 4.8 | ||||
| 0.2 | −24.4 | 1.4 | ||||
| 0.1 | −24.1 | 2.5 | ||||
| 2.7 | −24.1 | 0.2 | ||||
| 0.4 | −24.3 | 0.2 | ||||
| Median | 1.6 | −24.1 | 2.0 | |||
| SD | 1.4 | 0.4 | 2.0 | |||
| Barley, Hordeum. NF | 3.2 | −25.3 | 5.2 | |||
| 0.6 | −26.1 | 5.6 | ||||
| 1.2 | −26.2 | 5.9 | ||||
| 3.0 | −25.7 | 6.7 | ||||
| Median | 2.1 | −26.0 | 5.8 | |||
| SD | 1.3 | 0.4 | 0.6 | |||
| Peas, Pisum. MF | 0.8 | −24.8 | 3.8 | |||
| 0.8 | −24.9 | 3.1 | ||||
| −1.6 | −25.4 | 3.0 | ||||
| −0.2 | −25.7 | 1.3 | ||||
| Median | 0.3 | −25.2 | 3.1 | |||
| SD | 1.1 | 0.4 | 1.0 | |||
| Beans, Fadae. MF | 0.9 | −25.5 | 5.9 | |||
| −2.7 | −26.9 | 5.9 | ||||
| −0.3 | −25.7 | 4.8 | ||||
| 0.5 | −25.6 | 5.1 | ||||
| −0.7 | −25.1 | 4.2 | ||||
| −1.9 | −26.1 | 4.6 | ||||
| Median | −0.5 | −25.7 | 5.0 | |||
| SD | 1.4 | 0.6 | 0.7 | |||
| Lentil, Lens. NF | 1.7 | −26.8 | −2.0 | |||
| 1.1 | −26.5 | −2.0 | ||||
| 0.6 | −26.2 | −2.0 | ||||
| Median | 1.1 | −26.5 | −2.0 | |||
| SD | 0.6 | 0.3 | 0.0 | |||
| Kozani, North Gr | 40.18 | 21.47 | Maize, Zea mays. MF | 2.4 | −12 | 5.5 |
| 1.5 | −10.9 | 12.7 | ||||
| 1.1 | −11.1 | 3.6 | ||||
| 0.1 | −12.7 | 5.1 | ||||
| Median | 1.3 | −11.6 | 5.3 | |||
| SD | 1.0 | 0.8 | 3.6 | |||
| Kozani, North Gr | 40.18 | 21.47 | Walnut, Juglans regia. NF | 1.2 | −27.3 | 6.6 |
| 1.2 | −27.3 | 6.3 | ||||
| Median | 1.2 | −27.3 | 6.4 | |||
| SD | 0.0 | 0.0 | 0.2 | |||
| Kozani, North Gr | 40.18 | 21.47 | Saffron, Crocus. NF | 1.2 | −26.4 | 2.5 |
| Olympus, North Gr | 40.08 | 22.35 | Wild thyme, Thymus. NF | 0.7 | −28.2 | 4.2 |
| Wild Oregano, Origanum vulgare. NF | −3.1 | −27.3 | 7.3 | |||
| Wild Salvia, Salvia officinalis NF | −1.1 | −28.1 | 6.2 | |||
| Red zasberry, Rubus idaeus. MF | −3.2 | −26.3 | 3.1 | |||
| Blueberry, Vaccinium corymbosum. MF | −0.5 | −26.5 | 2.0 | |||
| Myrtilus, Myrtillus genus. MF | −4.0 | −29.2 | 5.1 | |||
| Parnitha, South Gr | 37.92 | 23.86 | Wild thyme, Thymus. NF | 1.1 | −27.8 | 0.5 |
| Levande, Lavandula angustifolia. NF | 0.1 | −27.2 | 4.3 | |||
| Chamomile, Chamaemelum nobile. NF | 4.2 | −26.4 | 2.5 | |||
| Rethymno (Psiloritis), Crete | 35.34 | 25.14 | Marjoram, Origanum majorana. NF | 1.3 | −27.5 | 5.1 |
| Probably from Athens area, South Gr | 37.92 | 23.86 | Lettuce, Lactuca. MF | 3.0 | −24.2 | 1.9 |
| 4.0 | −26.6 | 2.0 | ||||
| 4.0 | −25.1 | 1.1 | ||||
| 1.8 | −25.4 | 2.6 | ||||
| 2.0 | −26.4 | 2.1 | ||||
| 30 | −26.1 | 1.8 | ||||
| Median | 3.0 | −25.7 | 2.0 | |||
| SD | 0.9 | 0.9 | 0.5 |
| Location | Lat | Long | Species | δ15N (‰) -Air-N2 | δ13C (‰) -VPDB | δ34S (‰) -VCDT |
|---|---|---|---|---|---|---|
| Chalkidiki, North Gr | 40.51 | 23.63 | Sheep, Ovisaries, F | 6.5 | −20.7 | 7.6 |
| 5.4 | −21.8 | 6.8 | ||||
| 4.0 | −21.9 | 7.1 | ||||
| 6.5 | −22.0 | 6.1 | ||||
| 5.5 | −22.7 | 6.3 | ||||
| 5.3 | −20.2 | 7.6 | ||||
| 5.1 | −21.9 | 7.1 | ||||
| 4.2 | −20.9 | 7.4 | ||||
| 4.0 | −20.6 | 6.9 | ||||
| 4.2 | −20.8 | 7.8 | ||||
| 5.5 | −21.1 | 8.1 | ||||
| 6.9 | −21.5 | 7.3 | ||||
| 5.8 | −21.2 | 6.9 | ||||
| 4.1 | −20.8 | 6.8 | ||||
| 4.7 | −21.1 | 7.6 | ||||
| 6.8 | −22.2 | 4.7 | ||||
| 6.6 | −19.5 | 6.1 | ||||
| 7.8 | −21.4 | 6.9 | ||||
| 5.1 | −20.2 | 6.5 | ||||
| Median | 5.4 | −21.2 | 7.0 | |||
| SD | 1.1 | 0.8 | 0.8 | |||
| Chalkidiki, North Gr | 40.51 | 23.63 | Cattle, Bos taurus, F | 5.5 | −21.7 | 8.3 |
| 4.2 | −20.4 | 6.5 | ||||
| 4.9 | −20.4 | 4.0 | ||||
| 4.7 | −21.3 | 4.6 | ||||
| 5.1 | −21.1 | 7.0 | ||||
| Median | 4.9 | −21.1 | 6.5 | |||
| SD | 0.5 | 0.6 | 1.8 | |||
| Sparta, South Gr | 37.08 | 22.43 | Sheep, Ovisaries, D | 7.1 | −20.7 | 9.1 |
| 5.1 | −21.1 | 8.5 | ||||
| 3.1 | −22.5 | 9.1 | ||||
| 3.6 | −23.6 | 9.1 | ||||
| 5.1 | −21.9 | 8.9 | ||||
| Median | 5.1 | −21.9 | 9.1 | |||
| SD | 1.6 | 1.2 | 0.3 | |||
| Sparta, South Gr | 37.08 | 22.43 | Cattle, Bos taurus, D | 3.9 | −20.7 | 7.9 |
| 4.6 | −21.4 | 6.3 | ||||
| Median | 4.3 | −21.0 | 7.1 | |||
| SD | 0.5 | 0.5 | 1.1 | |||
| Crete, Rethymno | 35.34 | 25.14 | Sheep, Ovisaries, D | 8.3 | −22.6 | 9.6 |
| 6.0 | −21.8 | 9.0 | ||||
| 6.2 | −20.9 | 9.6 | ||||
| 7.5 | −22.3 | 9.6 | ||||
| 7.3 | −21.9 | 9.7 | ||||
| Median | 7.3 | −21.9 | 9.6 | |||
| SD | 0.9 | 0.6 | 0.3 | |||
| Crete, Rethymno | 35.34 | 25.14 | Cattle, Bos taurus, F | 6.5 | −20.1 | 8.2 |
| 5.6 | −21.6 | 9.1 | ||||
| 6.0 | −21.5 | 6.8 | ||||
| 6.3 | −21.0 | 4.6 | ||||
| 5.9 | −20.8 | 7.8 | ||||
| Median | 6.1 | −21.1 | 7.8 | |||
| SD | 0.4 | 0.6 | 1.7 | |||
| Super market (probably Attika), South Gr | 37.92 | 23.86 | Pig, Sus scrofa, D | 5.3 | −18.9 | 8.3 |
| 5.2 | −20.0 | 7.5 | ||||
| 4.6 | −18.9 | 7.9 | ||||
| 5.2 | −19.8 | 7.3 | ||||
| Median | 5.2 | −19.4 | 7.7 | |||
| SD | 0.3 | 0.6 | 0.5 | |||
| Karditsa, North Gr | 39.37 | 21.93 | Pig, Sus scrofa, D | 3.6 | −17.6 | 10.9 |
| 4.9 | −20.1 | 8.0 | ||||
| Median | 4.3 | −19.0 | 9.5 | |||
| SD | 0.9 | 1.7 | 2.1 |
| Fish | δ13C, ‰. V-PDB | δ15N, ‰. Air | δ 34S, ‰. V-CDT |
|---|---|---|---|
| Carp, Cyprinus carpio, Edessa, North Greece | −18.7 | 8.0 | 5.4 |
| Carp, Cyprinus carpio, Edessa, North Greece | −19.8 | 7.5 | 4.6 |
| Carp, Cyprinus carpio, Edessa, North Greece | −20.5 | 13.0 | 14.0 |
| Sole, Solea Vulgaris | −18.3 | 12.7 | 17.8 |
| Sea bass, Dicentrarchus lavrax | −18.2 | 11.2 | 14.5 |
| Grouper, Epinephelus aeneus | −18.9 | 13.4 | 13.8 |
| Macherel, Chubmackerel Scomper japonicus | −17.8 | 10.5 | 17.0 |
| Gilt-head bream, Sparus Aurata | −13.1 | 6.3 | 18.5 |
| Red mullet, Mullus barbatus | −19.5 | 9.6 | 18.0 |
3. Results and Discussion
3.1. Plants Isotopic Values (C-N-S), Comparison Between Regions
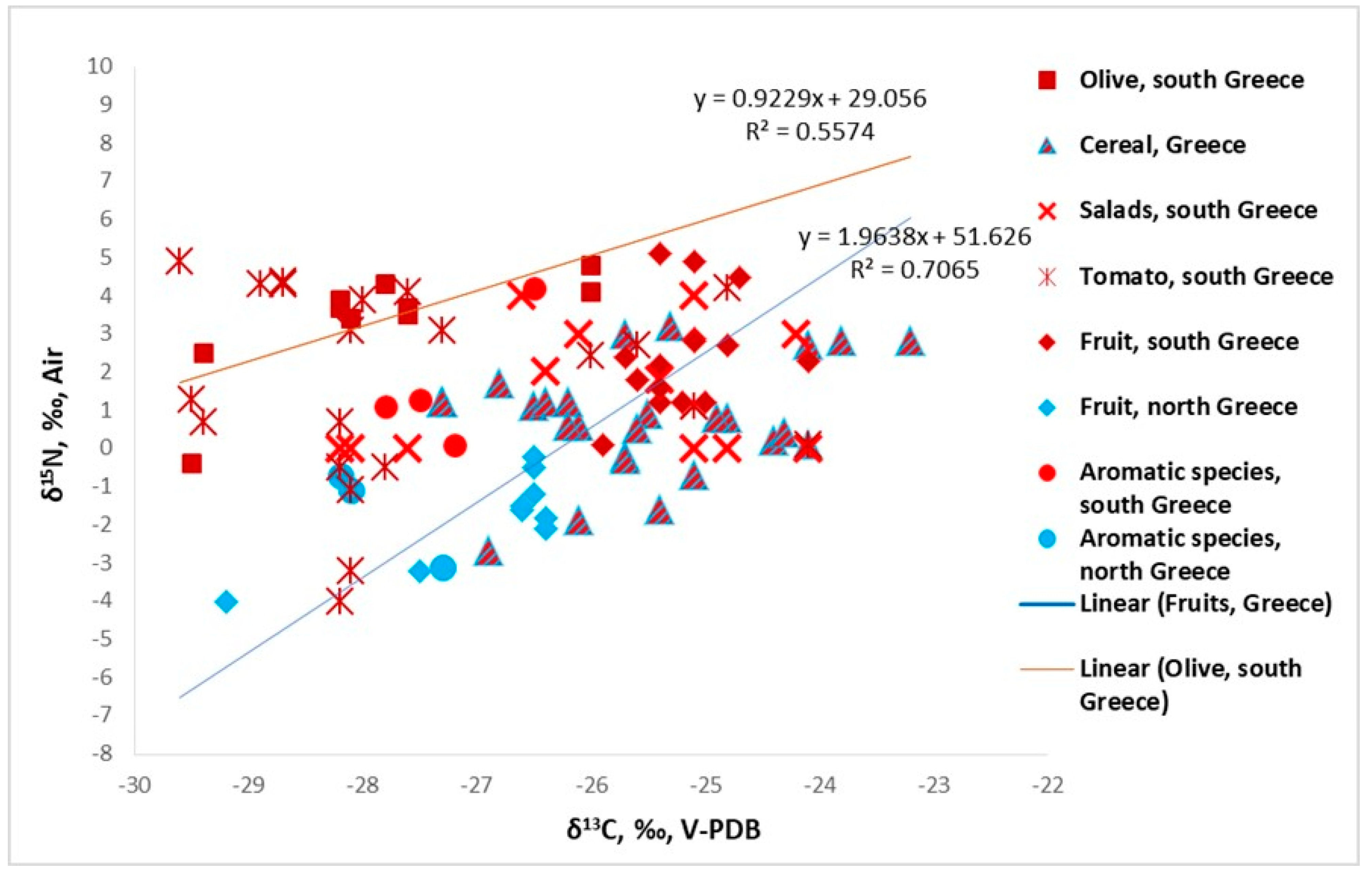
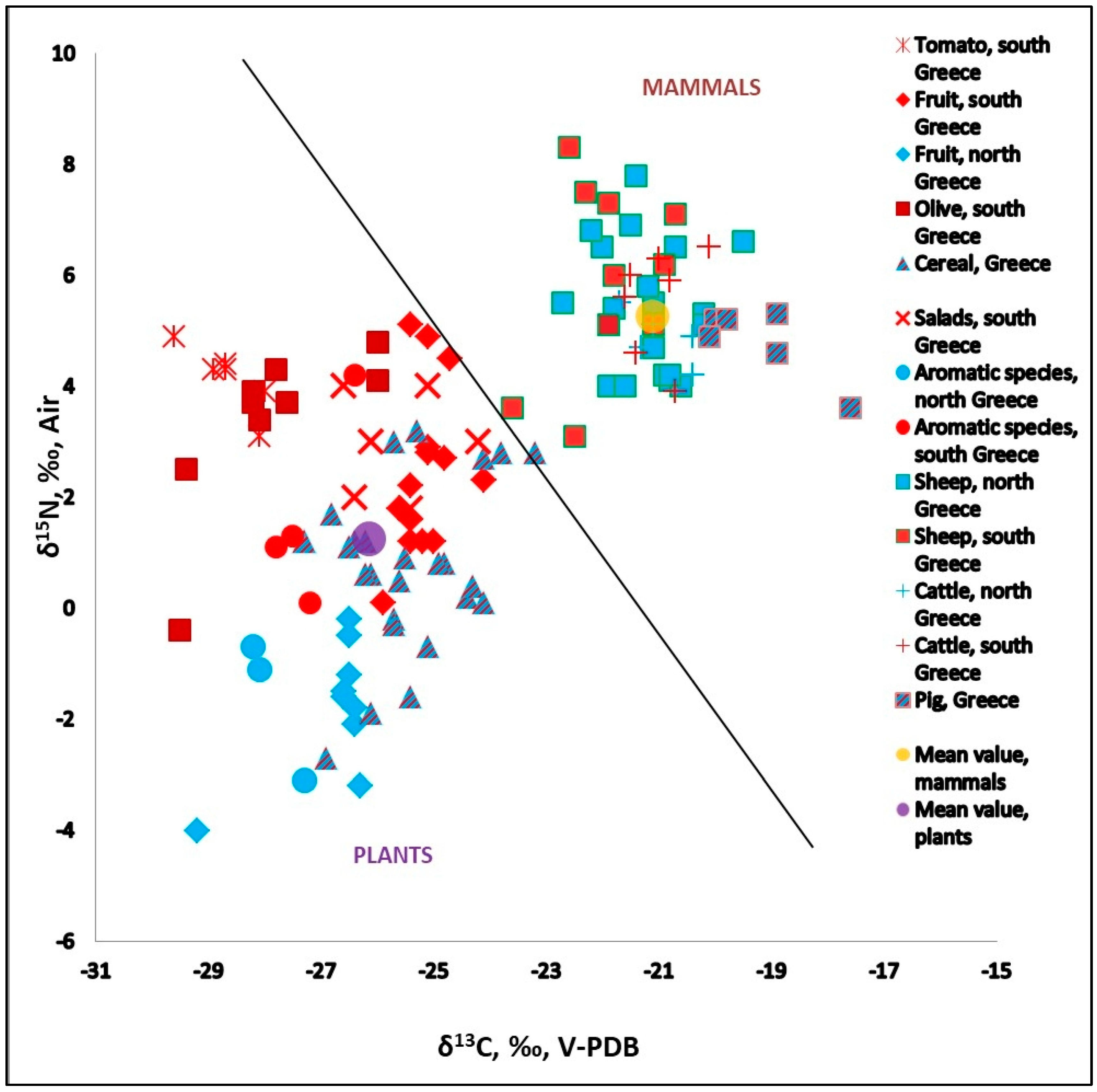
3.1.1. δ13C of Plants
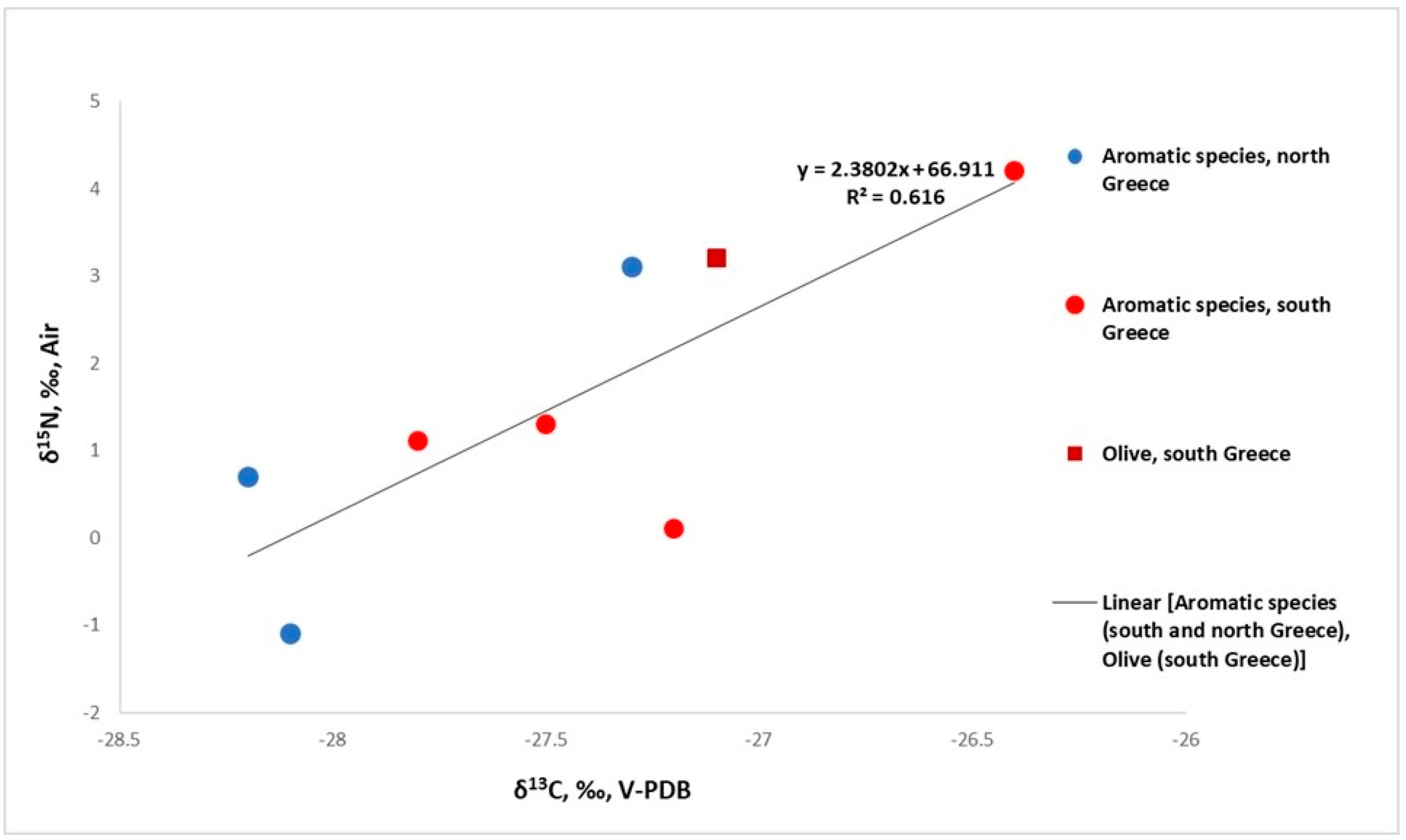
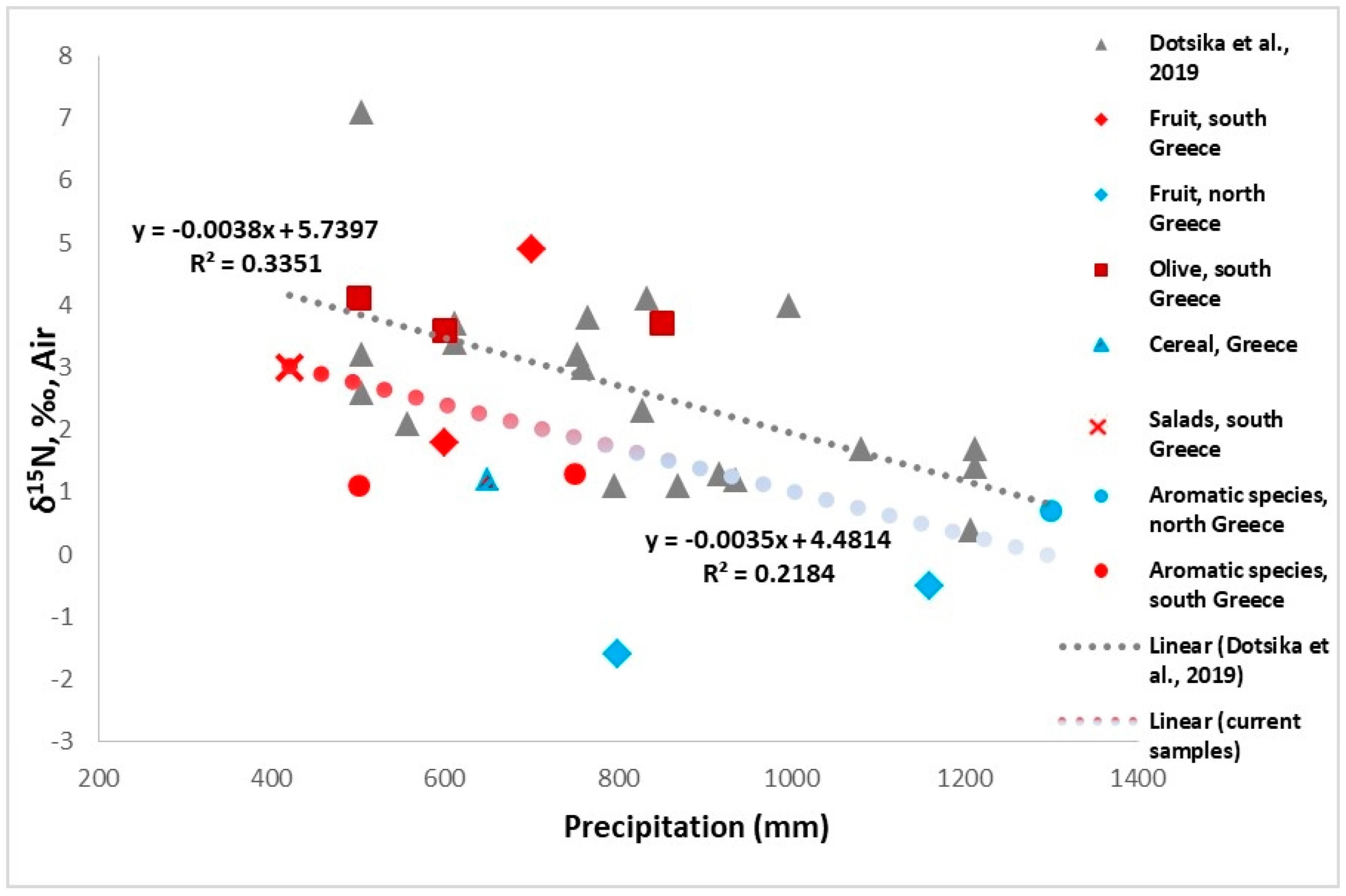
3.1.2. δ15N of Plants
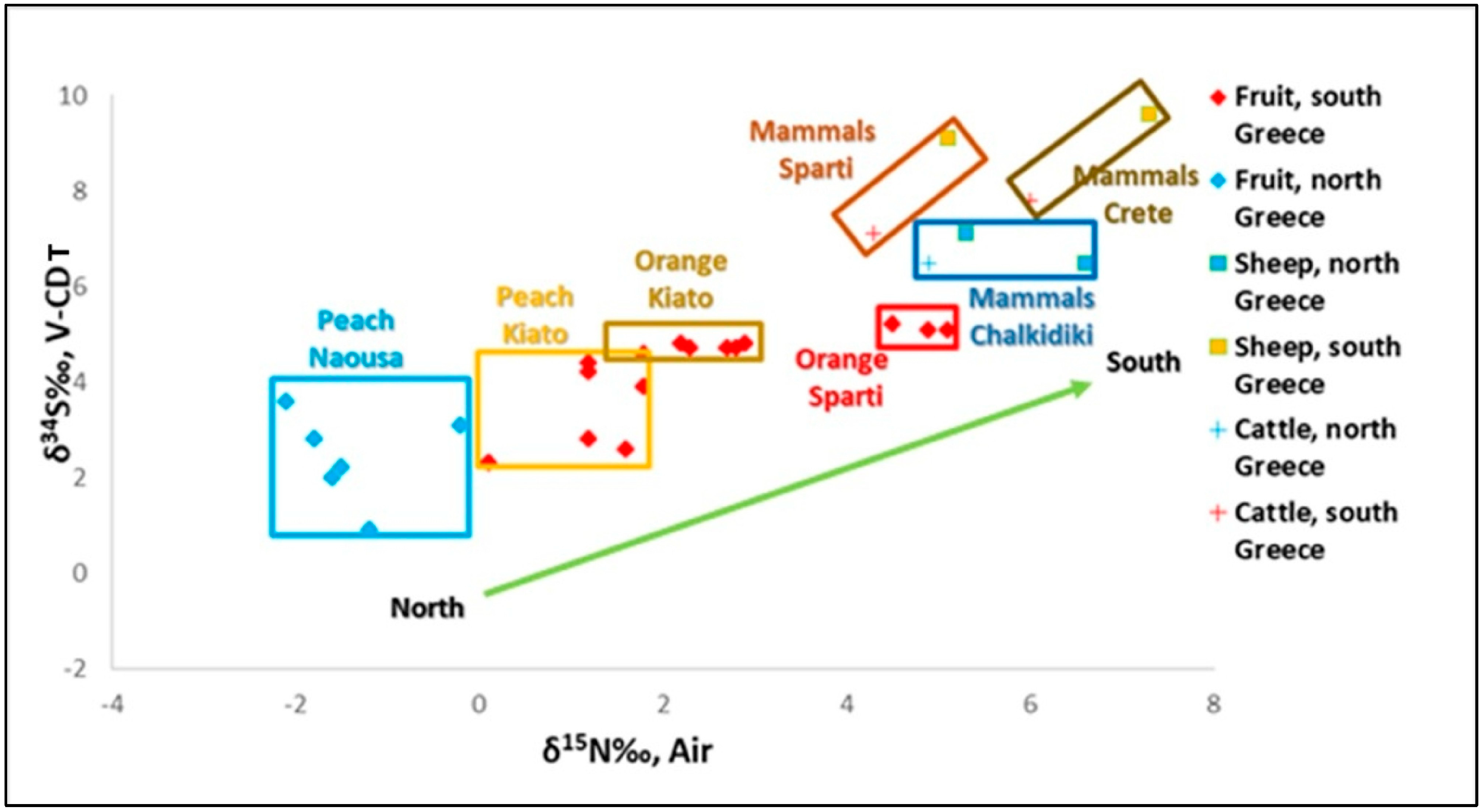
3.1.3. δ34S of Plants
3.1.4. δ13C, δ15N, and δ34S of Marine Species
3.2. Isotopic Analysis in Modern Mammals
3.2.1. δ13C and δ15N Values of Various Mammals
3.2.2. δ34S Values of Various Mammals
4. Sulfur Isotope Analysis in Modern Terrestrial, Marine, and Freshwater Environments: Contribution of the Paleo Diet and Paleo Mobility Studies
| Conversion Data to Modern Hair Keratin (MKE) and to Modern Diet Equivalent (MDE) | δ13C (‰) ‰, V-PDB | δ15N (‰) ‰, V-AIR | δ34S ‰, V-CDT | Source |
|---|---|---|---|---|
| Convert modern bone collagen to modern (2010) hair keratin (MKE) | −1.4 | −0.9 | [51] | |
| Convert ancient atmospheric δ13C to modern (2010) atmospheric equivalent δ13C | −1.9 | - | [90] | |
| Convert ancient bone collagen to Modern Hair Keratin (MKE), including conversion to modern CO2, δ13C | −3.3 | −0.9 | [92] | |
| Convert modern keratin equivalent (MKE) to Modern Diet Equivalent (MDE) | −2.5 | −5.15 | [50,51] | |
| Convert modern bone collagen to modern hair keratin (MKE) | +2.5 * +0.4 to +4.0 ** | * [34] ** [8] | ||
| Convert modern bone collagen to Modern Diet Equivalent (MDE) | +1.5 * −0.5 to +0.5 ** | * [34] ** [8] | ||
| Convert modern keratin to Modern Diet Equivalent (MDE) | −2 * −5 to −2 ** +1 (C3-plant diet) to – 4 (C4-plant diet) *** | * [34] ** [8] *** [35] |
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cernusak, L.A.; Ubierna, N.; Winter, K.; Holtum, J.A.M.; Marshall, J.D.; Farquhar, G.D. Environmental and physiological determinants of carbon isotope discrimination in terrestrial plants. New Phytol. 2013, 200, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Tcherkez, G.; Tea, I. 32S/34S isotope fractionation in plant sulphur metabolism. New Phytol. 2013, 200, 44–53. [Google Scholar] [CrossRef]
- Chung, I.M.; Lee, T.J.; Oh, Y.-T.; Ghimire, B.K.; Jang, I.B.; Kim, S.H. Ginseng authenticity testing by measuring carbon, nitrogen, and sulfur stable isotope compositions that differ based on cultivation land and organic fertilizer type. J. Ginseng Res. 2017, 41, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.D. Physiological mechanisms influencing plant nitrogen isotope composition. Trends Plant Sci. 2001, 6, 121–126. [Google Scholar] [CrossRef]
- Barbour, M.M. Stable oxygen isotope composition of plant tissue: A review. Plant Biol. 2007, 34, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Giannioti, Z.; Ogrinc, N.; Suman, M.; Camin, F.; Bontempo, L. Isotope ratio mass spectrometry (IRMS) methods for distinguishing organic from conventional food products: A review. TrAC Trends Anal. Chem. 2024, 170, 117476. [Google Scholar] [CrossRef]
- Trust, B.A.; Fry, B. Stable sulphur isotopes in plants: A review. Plant Cell Environ. 1992, 15, 1105–1110. [Google Scholar] [CrossRef]
- Tanz, N.; Schmidt, H.-L. δ34S-Value Measurements in Food Origin Assignments and Sulfur Isotope Fractionations in Plants and Animals. J. Agric. Food Chem. 2010, 58, 3139–3146. [Google Scholar] [CrossRef]
- Chalk, C.P.; Inácio, C.T.; Chen, D. Tracing S dynamics in agro-ecosystems using 34S. Soil Biol. Biochem. 2017, 114, 295–308. [Google Scholar] [CrossRef]
- Claypool, G.E.; Holser, W.T.; Kaplan, I.R.; Sakai, H.; Zak, I. The age curves of sulfur and oxygen isotopes in marine sulfate and their mutual interpretation. Chem. Geol. 1980, 28, 199–260. [Google Scholar] [CrossRef]
- Krouse, H.R.; Mayer, B. Sulphur and Oxygen Isotopes in Sulphate. In Environmental Tracers in Subsurface Hydrology; Cook, P.G., Herczeg, A.L., Eds.; Springer: Boston, MA, USA, 2000; pp. 195–231. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Nielsen, H.; Pilot, J.; Grinenko, L.N.; Grinenko, V.A.; Lein, A.Y.; Smith, J.W.; Pankina, R.G. Lithospheric Sources of Sulphur. In Stable Isotopes: Natural and Anthropogenic Sulphur in the Environment; Krouse, H.R., Grinenko, V.A., Eds.; Wiley: Hoboken, NJ, USA, 1991; pp. 65–132. [Google Scholar]
- Vitòria, L.; Otero, N.; Soler, A.; Canals, À. Fertilizer Characterization: Isotopic Data (N, S, O, C, and Sr). Environ. Sci. Technol. 2004, 38, 3254–3262. [Google Scholar] [CrossRef]
- Ault, W.U.; Kulp, J.L. Isotopic geochemistry of sulphur. Geochim. Cosm. Act. 1959, 16, 201–235. [Google Scholar] [CrossRef]
- Thode, H.G.; Monster, J.; Dunford, H.B. Sulphur isotope geochemistry. Geochim. Cosmochim. Acta 1961, 25, 159174. [Google Scholar] [CrossRef]
- Rees, M.C.; Minson, D.J. Fertilizer sulphur as a factor affecting voluntary intake, digestibility and retention time of pangola grass (Digitaria decumbens) by sheep. Br. J. Nutr. 1978, 39, 5–11. [Google Scholar] [CrossRef]
- Böttcher, F.; Barth, S.; Peinke, J. Small and large scale fluctuations in atmospheric wind speeds. Stoch. Environ. Res. Risk Assess. 2007, 21, 299–308. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Coker, R.D.; Barrie, L.A. Origin of sulphur in Canadian Arctic haze from isotope measurements. Nature 1991, 349, 142–145. [Google Scholar] [CrossRef]
- Baryakhtar, I.V.; Ivanov, B.A. Vector field dynamic. Phys. Lett. A 1983, 98, 222–226. [Google Scholar] [CrossRef]
- Nehlich, O. The application of sulphur isotope analyses in archaeological research: A review. Earth-Sci. Rev. 2015, 142, 1–17. [Google Scholar] [CrossRef]
- Deegan, L.; Garritt, R. Evidence for spatial variability in estuarine food webs. Mar. Ecol. Prog. Ser. 1997, 147, 31–47. [Google Scholar] [CrossRef]
- Leakey, C.D.; Attrill, M.J.; Jennings, S.; Fitzsimons, M.F. Stable isotopes in juvenile marine fishes and their invertebrate prey from the Thames Estuary, UK, and adjacent coastal regions. Estuar. Coast. Shelf Sci. 2008, 77, 513–522. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Amrani, A.; Said-Ahmad, W.; Shaked, Y.; Kiene, R.P. Sulfur isotope homogeneity of oceanic DMSP and DMS. Earth Atmos. Planet. Sci. 2013, 110, 18413–18418. [Google Scholar] [CrossRef] [PubMed]
- Thode, H.G. Sulphur isotopes in nature and the environment: An overview. In Stable Isotopes: Natural and Anthropogenic Sulphur in the Environment; Krouse, H.R., Grinenko, V.A., Eds.; Wiley: Hoboken, NJ, USA, 1991. [Google Scholar]
- Bataille, C.P.; Jaouen, K.; Milano, S.; Trost, M.; Steinbrenner, S.; Crubézy, É.; Colleter, R. Triple sulfur-oxygen-strontium isotopes probabilistic geographic assignment of archaeological remains using a novel sulfur isoscape of western Europe. PLoS ONE 2021, 16, e0250383. [Google Scholar] [CrossRef]
- Mahowald, N.M.; Muhs, D.R.; Levis, S.; Rasch, P.J.; Yoshioka, M.; Zender, C.S.; Luo, C. Change in atmospheric mineral aerosols in response to climate: Last glacial period, preindustrial, modern, and doubled carbon dioxide climates. J. Geophys. Res. Atmos. 2006, 111. [Google Scholar] [CrossRef]
- Drake, N.A.; Eckardt, F.D.; White, K.H. Sources of sulphur in gypsiferous sediments and crusts and pathways of gypsum redistribution in southern Tunisia. Earth Surf. Process. Landf. 2004, 29, 1459–1471. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D.G.; Taubald, H. Preservation of bone collagen sulphur isotopic compositions in an early Holocene river-bank archaeological site. Palaeogeogr. Palaeoclim. Palaeoecol. 2011, 310, 32–38. [Google Scholar] [CrossRef]
- Nehlich, O.; Fuller, B.T.; Jay, M.; Mora, A.; Nicholson, R.A.; Smith, C.I.; Richards, M.P. Application of sulphur isotope ratios to examine weaning patterns and freshwater fish consumption in Roman Oxfordshire, UK. Geochim. Cosmochim. Acta 2011, 75, 4963–4977. [Google Scholar] [CrossRef]
- Drucker, D.G.; Valentin, F.; Thevenet, C.; Mordant, D.; Cottiaux, R.; Delsate, D.; Van Neer, W. Aquatic resources in human diet in the Late Mesolithic in Northern France and Luxembourg: Insights from carbon, nitrogen and sulphur isotope ratios. Archaeol. Anthr. Sci. 2018, 10, 351–368. [Google Scholar] [CrossRef]
- Rodiouchkina, K.; Rodushkin, I.; Goderis, S.; Vanhaecke, F. A comprehensive evaluation of sulfur isotopic analysis (δ34S and δ33S) using multi-collector ICP-MS with characterization of reference materials of geological and biological origin. Anal. Chim. Acta 2022, 1240, 340744. [Google Scholar] [CrossRef]
- Webb, E.C.; Lewis, J.; Shain, A.; Kastrisianaki-Guyton, E.; Honch, N.V.; Stewart, A.; Miller, B.; Tarlton, J.; Evershed, R.P. The influence of varying proportions of terrestrial and marine dietary protein on the stable carbon-isotope compositions of pig tissues from a controlled feeding experiment. Sci. Technol. Archaeol. Res. 2017, 3, 28–44. [Google Scholar] [CrossRef]
- Richards, M.P.; Fuller, B.T.; Sponheimer, M.; Robinson, T.; Ayliffe, L. Sulphur isotopes in palaeodietary studies: A review and results from a controlled feeding experiment. Int. J. Osteoarchaeol. 2003, 13, 37–45. [Google Scholar] [CrossRef]
- Ehleringer, J.R. Interpreting Stable Isotope Ratios in Plants and Plant-Based Foods. Ιn Food Forensics; Carter, J.F., Chesson, L.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 46–62. [Google Scholar]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Smith, B.N.; Epstein, S. Two categories of 13C/12C ratios for higher plants. Plant Physiol. 1971, 47, 380–384. [Google Scholar] [CrossRef]
- Walker, P.L.; Deniro, M.J. Stable nitrogen and carbon isotope ratios in bone collagen as indices of prehistoric dietary dependence on marine and terrestrial resources in Southern California. Am. J. Phys. Anthr. 1986, 71, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.P.; Schulting, R.J. Touch not the fish: The Mesolithic-Neolithic change of diet and its significance. Antiquity 2006, 80, 444–456. [Google Scholar] [CrossRef]
- Krueger, H.W.; Sullivan, C.H. Models for carbon isotope fractionation between diet and bone. In Stable Isotopes in Nutrition; Turnlund, J.R., Johnson, P.E., Eds.; ACS Symposium Series: Washington, DC, USA, 1984; Volume 258, pp. 205–220. [Google Scholar]
- Lee-Thorp, J.A. On isotopes and old bones. Archaeometry 2008, 50, 925–950. [Google Scholar] [CrossRef]
- Ambrose, S.H.; Norr, L. Experimental evidence for the relationship of the carbon isotope ratios of whole diet and dietary protein to those of bone collagen and carbonate. In Prehistoric Human Bone: Archaeology at The Molecular Level; Lambert, J., Grupe, G., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 1–37. [Google Scholar]
- Ambrose, S.H. Preparation and characterization of bone and tooth collagen for isotopic analysis. J. Archaeol. Sci. 1990, 17, 431–451. [Google Scholar] [CrossRef]
- Tieszen, L.; Fagre, T. Effect of Diet Quality and Composition on the Isotopic Composition of Respiratory CO2, Bone Collagen, Bioapatite, and Soft Tissues. In Prehistoric Human Bone; Lambert, J., Grupe, G., Eds.; Springer Nature: Dordrecht, The Netherlands, 1993; pp. 157–171. [Google Scholar] [CrossRef]
- Fernández, R.D.; Arenas, R. The Late Devonian Variscan suture of the Iberian Massif: A correlation of high-pressure belts in NW and SW Iberia. Tectonophysics 2015, 654, 96–100. [Google Scholar] [CrossRef]
- Chartrand, M.; Gilles, S.-J. Forensic Attribution of CBRNE Materials: (A Chemical Fingerprint Database 2015); Government of Canada: Ottawa, ON, Canada, 2015; Available online: http://cradpdf.drdc-rddc.gc.ca/PDFS/unc200/p801299_A1b.pdf (accessed on 12 May 2024).
- Dotsika, E.; Diamantopoulos, G. Influence of Climate on Stable Nitrogen Isotopic Values of Contemporary Greek Samples: Implications for Isotopic Studies of Human Remains from Neolithic to Late Bronze Age Greece. Geosciences 2019, 9, 217. [Google Scholar] [CrossRef]
- Heaton, T.H.E. The 15N/14N ratios of plants in South Africa and Namibia: Relationship to climate and coastal/saline environments. Oecologia 1987, 74, 236–246. [Google Scholar] [CrossRef]
- O’Connell, T.C.; Hedges, R.E. Investigations into the effect of diet on modern human hair isotopic values. Am. J. Phys. Anthr. 1999, 108, 409–425. [Google Scholar] [CrossRef]
- O’Connell, T.C.; Hedges, R.E.M.; Healey, M.A.; Simpson, A.H.R.W. Isotopic Comparison of Hair, Nail and Bone: Modern Analyses. J. Archaeol. Sci. 2001, 28, 1247–1255. [Google Scholar] [CrossRef]
- Adams, J.; Brickley, M.; Buteux, S.; Adams, T.; Richards, M.P. Martin’s Uncovered: Investigations in the Churchyard of St Martin’s-in-the-Bull Ring. Birmingham, 2001; Oxbow Books: Oxford, UK, 2006; pp. 147–151. [Google Scholar]
- Longin, R. New Method of Collagen Extraction for Radiocarbon Dating. Nature 1971, 230, 241–242. [Google Scholar] [CrossRef]
- Tykot, R.H. Stable Isotopes and Diet: You Are What to Eat. In Proceedings of the International School of Physics “Enrico Fermi”; Martini, M., Milazzo, M., Piacentini, M., Eds.; IOS Press: Amsterdam, The Netherlands, 2004; pp. 433–443. [Google Scholar]
- Nehlich, O.; Richards, M.P. Establishing collagen quality criteria for sulphur isotope analysis of archaeological bone collagen. Archaeol. Anthropol. Sci. 2009, 1, 59–75. [Google Scholar] [CrossRef]
- Rainfall Map of Greece. Available online: https://www.geogreece.gr/rain.php (accessed on 5 September 2024).
- Szpak, P.; Longstaffe, F.J.; Macdonald, R.; Millaire, J.-F.; White, C.D.; Richards, M.P. Plant sulfur isotopic compositions are altered by marine fertilizers. Archaeol Anthropol. Sci. 2019, 11, 2989–2999. [Google Scholar] [CrossRef]
- Dotsika, E.; Michael, D.E.; Iliadis, E.; Karalis, P.; Diamantopoulos, G. Isotopic reconstruction of diet in Medieval Thebes (Greece). J. Archaeol. Sci. Rep. 2018, 22, 482–491. [Google Scholar] [CrossRef]
- Winde, V.; Böttcher, M.E.; Voss, M.; Mahler, A. Bladder wrack (Fucus vesiculosus) as a multi-isotope bio-monitor in an urbanized fjord of the western Baltic Sea. Isot. Environ. Health Stud. 2017, 53, 563–579. [Google Scholar] [CrossRef]
- Buša, L.; Bērtiņš, M.; Vīksna, A.; Legzdiņa, L.; Kobzarevs, D. Evaluation of carbon, nitrogen, and oxygen isotope ratio measurement data for characterization of organically and conventionally cultivated spring barley (Hordeum vulgare L.) grain. Grain 2021, 193, 1364–1372. [Google Scholar] [CrossRef]
- Siqueira-Neto, M.; Popin, G.V.; Piccolo, M.C.; Corbeels, M.; Scopel, E.; Camargo, P.B.; Bernoux, M. Impacts of land use and cropland management on soil organic matter and greenhouse gas emissions in the Brazilian Cerrado. Eur. J. Soil Sci. 2021, 72, 1431–1446. [Google Scholar] [CrossRef]
- Bateman, A.S.; Kelly, S.D. Fertilizer nitrogen isotope signatures. Isot. Environ. Health Stud. 2007, 43, 237–247. [Google Scholar] [CrossRef]
- Šturm, M.; Lojen, S. Nitrogen isotopic signature of vegetables from the Slovenian market and its suitability as an indicator of organic production. Isot. Environ. Health Stud. 2011, 47, 214–220. [Google Scholar] [CrossRef]
- Singh, R.; Patel, S.-T.; Tiwari, A.-K.; Singh, G.-S. Assessment of flood recession farming for livelihood provision, food security and environmental sustainability in the Ganga River Basin. Curr. Res. Environ. Sustain. 2021, 3, 100038. [Google Scholar] [CrossRef]
- Ray, L.I.P.; Swetha, K.; Singh, A.; Singh, N. Water productivity of major pulses—A review. Agric. Water Manag. 2023, 281, 108249. [Google Scholar] [CrossRef]
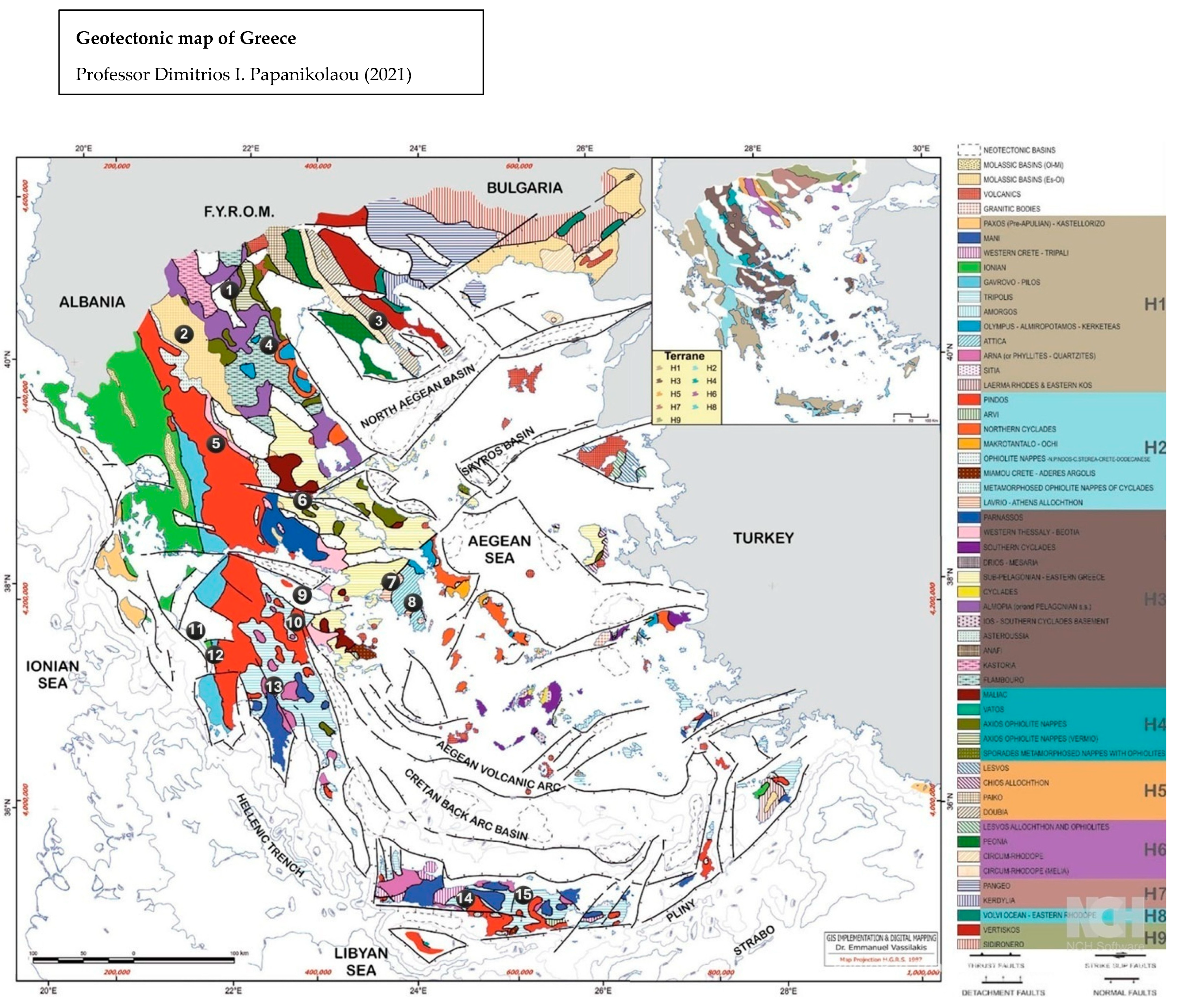
- Papanikolaou, D. The Geology of Greece; Regional Geology Reviews Series; Springer: Dordrecht, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Economidis, S.P.; Banarescu, P. The distribution and origins of freshwater fishes in the Balkan Peninsula, Especially in Greece. Hydrobiology 1991, 76, 257–284. [Google Scholar] [CrossRef]
- Garcia-Guixé, E.; Subirà, M.E.; Marlasca, R.; Richards, M.P. δ13C and δ15N in ancient and recent fish bones from the Mediterranean Sea. J. Nord. Archaeol. Sci. 2010, 17, 83–92. [Google Scholar]
- Vika, E.; Theodoropoulou, T. Re-investigating fish consumption in Greek antiquity: Results from δ13C and δ15N analysis from fish bone collagen. J. Archaeol. Sci. 2012, 39, 1618–1627. [Google Scholar] [CrossRef]
- Papathanassiou, A.; Sherry, C.F.; Richards, P.M. Archaeodiet in the Greek World: Dietary Reconstruction from Stable Isotope Analysis; ASCSA Publications: Athens, Greece, 2015; 224p. [Google Scholar]
- Richards, M.; Karavanić, I.; Pettitt, P.; Miracle, P. Isotope and faunal evidence for high levels of freshwater fish consumption by Late Glacial humans at the Late Upper Palaeolithic site of Šandalja II, Istria, Croatia. J. Archaeol. Sci. 2015, 61, 204–212. [Google Scholar] [CrossRef]
- Panagiotopoulou, E. Reconstructing Diet, Tracing Mobility: Ιsotopic Approach to Social Change During the Transition from the Bronze to the Early Iron Age in Thessaly, Greece. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2018. [Google Scholar]
- Vika, E. Strangers in the grave? Investigating local provenance in a Greek Bronze Age mass burial using δ34S analysis. J. Archaeol. Sci. 2009, 36, 2024–2028. [Google Scholar] [CrossRef]
- Bocherens, H.; Drucker, D. Trophic level isotopic enrichment of carbon and nitrogen in bone collagen: Case studies from recent and ancient terrestrial ecosystems. Int. J. Osteoarchaeol. 2003, 13, 46–53. [Google Scholar] [CrossRef]
- Jim, S.; Ambrose, S.H.; Evershed, R.P. Stable carbon isotopic evidence for differences in the dietary origin of bone cholesterol, collagen and apatite: Implications for their use in palaeodietary reconstruction. Geochim. Cosmochim. Acta 2003, 68, 61–72. [Google Scholar] [CrossRef]
- Warinner, C.; Tuross, N. Alkaline cooking and stable isotope tissue-diet spacing in swine: Archaeological implications. J. Archaeol. Sci. 2009, 36, 1690–1697. [Google Scholar] [CrossRef]
- Fernandes, R.; Nadeau, M.-J.; Grootes, P.M. Macronutrient-based model for dietary carbon routing in bone collagen and bioapatite. Archaeol. Anthropol. Sci. 2012, 4, 291–301. [Google Scholar] [CrossRef]
- Froehle, A.W.; Kellner, C.M.; Schoeninger, M.J. FOCUS: Effect of diet and protein source on carbon stable isotope ratios in collagen: Follow up to Warinner and Tuross (2009). J. Archaeol. Sci. 2010, 37, 2662–2670. [Google Scholar] [CrossRef]
- Howland, M.R.; Corr, L.T.; Young, S.M.M.; Jones, V.; Jim, S.; Van Der Merwe, N.J.; Mitchell, A.D.; Evershed, R.P. Expression of the dietary isotope signal in the compound-specific δ13C values of pig bone lipids and amino acids. Int. J. Osteoarchaeol. 2003, 13, 54–65. [Google Scholar] [CrossRef]
- Richards, M.P.; Fuller, B.T.; Hedges, R.E.M. Sulphur isotopic variation in ancient bone collagen from Europe: Implications for human palaeodiet, residence mobility, and modern pollutant studies. Earth Planet. Sci. Lett. 2001, 1919, 185–190. [Google Scholar] [CrossRef]
- Richards, M.; Smith, C.; Nehlich, O.; Grimes, V.; Weston, D.; Mittnik, A.; Krause, J.; Dobney, K.; Tzedakis, Y.; Martlew, H. Finding Mycenaeans in Minoan Crete? Isotope and DNA analysis of human mobility in Bronze Age Crete. PLoS ONE 2022, 17, e0272144. [Google Scholar] [CrossRef]
- Dotsika, E.; Poutoukis, D.; Raco, B.; Psomiadis, D. Stable isotope composition of Hellenic bottled waters. J. Geochem. Explor. 2010, 107, 299–304. [Google Scholar] [CrossRef]
- Gamaletsos, P.; Godelitsas, A.; Dotsika, E.; Tzamos, E.; Göttlicher, J.; Filippidis, A. Geological sources of As in the environment of Greece: A review. In Threats to the Quality of Groundwater Resources: Prevention and Control. The Handbook of Environmental Chemistry; Scozzari, A., Dotsika, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 77–113. [Google Scholar] [CrossRef]
- Siron, C.R.; Rhys, D.; Thompson, J.F.; Baker, T.; Veligrakis, T.; Camacho, A.; Dalampiras, L. Structural Controls on Porphyry Au-Cu and Au-Rich Polymetallic Carbonate-Hosted Replacement Deposits of the Kassandra Mining District, Northern Greece. Econ. Geol. 2018, 113, 309–345. [Google Scholar] [CrossRef]
- Höss, A.; Haase, K.M.; Keith, M.; Klemd, R.; Melfos, V.; Gerlach, L.; Pelloth, F.; Falkenberg, J.J.; Voudouris, P.; Strauss, H.; et al. Magmatic and hydrothermal evolution of the Skouries Au-Cu porphyry deposit, northern Greece. Ore Geol. Rev. 2024, 173, 106233. [Google Scholar] [CrossRef]
- Peterson, B.J.; Howarth, R.W.; Garritt, R.H. Multiple Stable Isotopes Used to Trace the Flow of Organic Matter in Estuarine Food Webs. Science 1985, 227, 1361–1363. [Google Scholar] [CrossRef]
- Anagnostopoulou, F. Investigation of Climatic Variable’s Spatial Distribution in Greece. Postgraduate Thesis, NTUA, Athens, Greece, 2006. Available online: https://www.itia.ntua.gr/en/docinfo/872 (accessed on 15 May 2024).
- Weber, Y.; De Jonge, C.; Rijpstra, W.I.C.; Hopmans, E.C.; Stadnitskaia, A.; Schubert, C.J.; Lehmann, M.F.; Damsté, J.S.S.; Niemann, H. Identification and carbon isotope composition of a novel branched GDGT isomer in lake sediments: Evidence for lacustrine branched GDGT production. Geochim. Cosmochim. Acta 2015, 154, 118–129. [Google Scholar] [CrossRef]
- Frydas, D.; Keupp, H.; Bellas, S. Stratigraphical investigations based on calcareous and siliceous phytoplankton assemblages from the Upper Cenozoic deposits of Messara Basin, Crete, Greece. Z. Dtsch. Ges. Geowiss. 2008, 159, 415–438. [Google Scholar] [CrossRef]
- Graven, H.; Allison, C.E.; Etheridge, D.M.; Hammer, S.; Keeling, R.F.; Levin, I.; Meijer, H.A.J.; Rubino, M.; Tans, P.P.; Trudinger, C.M.; et al. Compiled records of carbon isotopes in atmospheric CO2 for historical simulations in CMIP6. Geosci. Model Dev. 2017, 10, 4405–4417. [Google Scholar] [CrossRef]
- Bellas, S.M.; Keupp, H. Contribution to the late neogene stratigraphy of the ancient gortys area (southern central Crete, Greece). Bull. Geol. Soc. Greece 2017, 43, 579. [Google Scholar] [CrossRef]
- Bird, Μ.Ι.; Crabtree, A.S.; Haig, J.; Ulm, S.; Wurster, C.M. A global carbon and nitrogen isotope perspective on modern and ancient human diet. Anthropology 2021, 118, e2024642118. [Google Scholar] [CrossRef] [PubMed]




| Fish/Faunal | Age | δ13C. ‰. V-PDB | δ15N. ‰. V-Air | δ34S. ‰. V-CD | Authors |
|---|---|---|---|---|---|
| Pandora, Pagellus erythrinus (bones) | Recent | −13.1 to −14.1 | 10.3 to 10.1 | [68] | |
| Moray eel, Muraena Helena (bones) | Recent | −13.7 | 10.8 | ||
| Barracuda, Sphyraena sphyraena (bones) | Recent | −13.7 to −14 | 7.9 to 7.8 | ||
| Dusky grouper, Epinephelus marginatus (bones) | Recent | −8.4 to −8.3 | 11.4 to 10.7 | ||
| Marine fish (bones) | Ancient | −10.1 to −19.2 | 11.6 to 6.1 | [69] | |
| Freshwater fish (bones) | Ancient | −11.9 to −20.8 | 10.9 to 4.9 | ||
| Marine fish (bones) | Ancient | −11 to −14 | 19 to 7 | [71] | |
| Marine fish (bass, bones) | Ancient | −11.4 to −10.3 | 11 to 8.44 | [69] | |
| Faunal (bones) | Ancient | −18.3 to −20.5 | 7 to 4.2 | 9.9 το 14.7 | [73] |
| Faunal (bones) | Ancient | −18.5 to −20.1 | 8.2 to 2.2 | 8.2 to 2.1 | [71] |
| Faunal (bones) | Ancient | −19.9 to −20.4 | 3.9 to 5.7 | 15 to 18.2 | [52] |
| Species | Age | δ34S, ‰. V-CD | sd | Authors |
|---|---|---|---|---|
| All herbivores mammal samples | Recent | 7.50 | 1.47 | This work |
| All herbivores mammal samples | Old, from Greece | 12.45 | 4.53 | [72,73] |
| All herbivores mammal samples | Classical site, Thebes | 13.30 | 2.18 | [73] |
| All herbivores mammal samples | Early Iron Age, Thessaly | 7.90 | 2.50 | [80] |
| All herbivores mammal samples | Minoan, Chamalevri | 17.7 | 1.72 | [80] |
| Cattle, from Crete (one sample) | Minoan, Chamalevri | 18.20 | [80] | |
| All herbivores mammal samples | Earl Minoan to Roman, Crete | 11.60 | 2,45 | [81] |
| Sheep, Ovi saries | Middle to Late Minoan, Chamalevri | 12.60 | 2.72 | |
| Sheep, Ovi saries | Middle Minoan, Apodoulou | 10.40 | 0.63 | |
| Cattle, from Crete | Recent | 9.60 | 2.16 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karalis, P.; Dotsika, E.; Poutouki, A.-E.; Diamantopoulos, G.; Gkelou, L.; Kyropoulou, D.; Bellas, S.; Gamaletsos, P.N. Isotopic Analysis (δ13C, δ15N, and δ34S) of Modern Terrestrial, Marine, and Freshwater Ecosystems in Greece: Filling the Knowledge Gap for Better Understanding of Sulfur Isotope Imprints—Providing Insights for the Paleo Diet, Paleomobility, and Paleoecology Reconstructions. Appl. Sci. 2025, 15, 4351. https://doi.org/10.3390/app15084351
Karalis P, Dotsika E, Poutouki A-E, Diamantopoulos G, Gkelou L, Kyropoulou D, Bellas S, Gamaletsos PN. Isotopic Analysis (δ13C, δ15N, and δ34S) of Modern Terrestrial, Marine, and Freshwater Ecosystems in Greece: Filling the Knowledge Gap for Better Understanding of Sulfur Isotope Imprints—Providing Insights for the Paleo Diet, Paleomobility, and Paleoecology Reconstructions. Applied Sciences. 2025; 15(8):4351. https://doi.org/10.3390/app15084351
Chicago/Turabian StyleKaralis, Petros, Elissavet Dotsika, Anastasia-Electra Poutouki, Giorgos Diamantopoulos, Liana Gkelou, Dafni Kyropoulou, Spyridon Bellas, and Platon N. Gamaletsos. 2025. "Isotopic Analysis (δ13C, δ15N, and δ34S) of Modern Terrestrial, Marine, and Freshwater Ecosystems in Greece: Filling the Knowledge Gap for Better Understanding of Sulfur Isotope Imprints—Providing Insights for the Paleo Diet, Paleomobility, and Paleoecology Reconstructions" Applied Sciences 15, no. 8: 4351. https://doi.org/10.3390/app15084351
APA StyleKaralis, P., Dotsika, E., Poutouki, A.-E., Diamantopoulos, G., Gkelou, L., Kyropoulou, D., Bellas, S., & Gamaletsos, P. N. (2025). Isotopic Analysis (δ13C, δ15N, and δ34S) of Modern Terrestrial, Marine, and Freshwater Ecosystems in Greece: Filling the Knowledge Gap for Better Understanding of Sulfur Isotope Imprints—Providing Insights for the Paleo Diet, Paleomobility, and Paleoecology Reconstructions. Applied Sciences, 15(8), 4351. https://doi.org/10.3390/app15084351









