Abstract
Among free-living amoebae (FLA), Acanthamoeba polyphaga is an important causal agent of Acanthamoeba keratitis (AK), a severe and potentially sight-threatening condition. The present study evaluated the “in vitro” efficiency of Melaleuca alternifolia Cheel (tea tree) (tea tree oil—TTO) and Eucalyptus globulus Labill. (Eucalyptus essential oil—EEO) essential oils against an Acanthamoeba strain isolated from human keratitis. The Minimum Inhibitory Concentration (MIC) of the EOs and the Fractional Inhibitory Concentration (FIC) Index were used to evaluate the decrease in viable cells of Acanthamoeba over time and at different concentrations of EOs, used alone or in association. A relevant amoebicidal effect emerged during the first hours of exposure for both compounds, and TTO was the most effective. The TTO/EEO association clearly indicated a synergistic effect in all tests, and at 2 days post-treatment, no viable A. polyphaga cells were observed at all tested concentrations. In conclusion, the potential therapeutic use of EOs represents a promising therapeutic strategy for the treatment of AK.
1. Introduction
Acanthamoeba spp. are free-living amoebas (FLA), which are ubiquitous in both natural and anthropized environments. Acanthamoeba spp. are divided into 23 different genotypes (T1–T23), the most predominant of which is T4 [1,2]. FLA have been isolated from sewage and sewage-related environments [3], coastal marine waters [4], natural hot springs [5], drinking water [6], and air conditioning systems in hospital environments [7]. Swimming pools and recreational waters are among the environments where the greatest amount of human exposure to free-living amoebae occurs, and among the major genera of the medically important free-living amoebae, Acanthamoeba is the genus most involved in human infections [8].
Acanthamoeba spp. are responsible for keratitis (Acanthamoeba keratitis, AK), granulomatous amoebic encephalitis (GAE), lung infections, and skin infections in immunocompromised subjects. Epidemiological studies demonstrate an annual increase in Acanthamoeba infections, particularly AK infections [9].
The main disease caused by Acanthamoeba, AK, is a serious, painful eye infection that, if not diagnosed and treated early, can cause corneal perforation and fusion [10]. GAE is a rare but almost always fatal central nervous system infection affecting debilitated or immunocompromised individuals [11], although cases have been reported in apparently healthy patients. This rare central nervous system disease is highly fatal, with a mortality rate greater than 90%, leading to death within 1–2 months of symptom onset due to increased intracranial pressure. Between 1990 and 2020, 75 cases of Acanthamoeba spp. GAE have been described worldwide [12]. Other rare pathological conditions are cutaneous acanthamoebiasis (CA) [13] and Acanthamoeba pneumonia (AP), mainly observed in patients with AIDS and in patients with a low immune response, respectively. From 1990 to 2020, 19 cases of AP or similar lung infections were related to Acanthamoeba spp., mainly in the USA, but similar events have also been observed in Europe and Eastern countries [14].
As previously mentioned, the number of infections caused by Acanthamoeba is very high, resulting in an incidence of 23,561 annual cases, as reported in a recent study that collected data from 20 countries [15]. Risk factors for AK vary between developing and developed countries. In the latter, most AK cases (86%) are related to contact lens use (inadequate disinfection of the lens or insufficient efficacy of the solution), while in developing countries, trauma remains the main risk factor (27%). The current AK treatment protocol consists of topical agents (chlorhexidine, polyhexamethylene, and antifungal drugs); however, due to the ability to form cysts, the biological stage of the pathogen is particularly difficult to treat due to unfavourable environmental conditions and the emergence of drug-resistant parasites; alternative approaches with tolerable side effects are urgently needed. Despite the significant progress that has been made in identifying new therapeutic agents that are active against Acanthamoeba infections [16], to date, there are no highly effective and low-toxicity anti-amoebic drugs available. Recently, in fields including antiparasitics, the interest of researchers has focused on alternative therapeutics from herbal sources. Many natural compounds are widely studied for numerous biological activities, including the ability to antagonize pathogens of public health concern, including protozoan disease agents such as amoebae [17,18]. Of particular interest are various plant extracts, including essential oils (EOs) and mixtures of volatile organic compounds, which have attracted increasing interest over time due to their biological properties [19,20,21,22,23]. Notably, Melaleuca alternifolia and Eucalyptus globulus EOs are increasingly used in traditional medicine due to various medical implications such as antibacterial, anti-inflammatory, and antifungal effects. In our previous study, M. alternifolia Cheel (tea tree) (tea tree oil—TTO) and E. globulus Labill. (Eucalyptus essential oil—EEO) essential oils showed the ability to combat antibiotic-resistant bacteria even when organized in biofilms, which is a promising result for these two important public health problems [24].
To broaden the knowledge on the biological activity of these two essential oils, the aim of the present study was to evaluate “in vitro” the anti-acanthamoebic potential of natural extracts obtained from these two plants. In particular, the decrease in viable cells of an Acanthamoeba strain isolated from a person affected by AK was evaluated over time and with different concentrations of EOs used alone or in association.
2. Materials and Methods
2.1. Amoeba Strain
The amoeba strain used in this study was isolated in a clinical setting by corneal scrapes from a patient with keratitis. The strain was grown under axenic conditions as monolayers in 25 cm2 tissue culture flasks (Sarstedt, Nümbrecht, Germany) in Peptone Yeast Extract Glucose (PYG) medium containing 0.75% (w/v) proteose peptone, 0.75% (w/v) yeast extract, and 1.5% (w/v) glucose (Oxoid, Milan, Italy) at 30 °C, and monitored by examination under an inverted microscope.
For all experiments, five-day-old PYG cultures of protozoa, grown to confluence, were suspended by tapping the flask, washed three times in sterile phosphate-buffered saline solution (PBS), centrifuged at 2000 rpm, and resuspended in the same media.
2.2. Genotypic Characterization of Amoeba Strain
For the extraction of nuclear DNA, trophozoites were harvested by centrifugation at 2500 rpm for 5 min. After centrifugation, the supernatant was aspirated, and the pellet was resuspended twice in 25 mL of phosphate-buffered saline. Subsequently, the pellet was resuspended in lysis buffer (10 mM Tris HCl, pH 8.5, 5 mM EDTA, 200 mM NaCl, 0.2% SDS) and incubated at 60 °C for 5 min. Then, 2.5 µL of proteinase K and 5 mL of RNASE (1 mg/mL) were added and incubated at 60 °C for 1 h. After incubation, 250 µL of NaCl (5 M) was added and incubated on ice for 5 min then centrifuged at 2500 rpm for 15 min. The supernatant was transferred into a new tube and an equivalent volume of isopropanol was added. Following incubation at −20 °C for 60 min, the DNA pellet was washed with 500 μL of ethanol (70%). Ethanol was carefully removed using a micropipette, and after the pellet was dried, 200 μL tris–EDTA (TE) buffer (pH 8.0) was added (all reagents were purchased from Sigma-Aldrich, Milan, Italy).
DNA was subjected to PCR aiming for the specific recognition of 18S rDNA from amoebae of the genus Acanthamoeba using the primers JDP1 (5′-GGC CCA GAT CGT TTA CCG TGA A-3′) and JDP2 (5′-TCT CAC AAG CTG CTA GGG GAG TCA-3′).
The following DNA thermal cycler was performed for 42 cycles: 95 °C for 7 min, then 95 °C for 1 min, 55 °C for 1 min, and 72 °C for 2 min, followed by 40 cycles of 72 °C for 10 min, as described earlier [25].
The DNA extraction product was evaluated by electrophoresis on a 1% agarose gel, to determine the presence of DNA.
The QIAquick PCR Purification Kit (Qiagen, Milan, Italy) was employed to purify positive amplicons from PCR, to recover approximately 95% of clean DNA up to 10 kb (as per the manufacturer’s instructions). After purification, the amplicons were sequenced by the ABI PRISM 3130XL Genetic Analyzer (Applied Biosystem, Milan, Italy) using amplification primers. The BLAST (version 1.30) program of the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov), available online, was used to perform sequence alignment and analysis.
A matrix-assisted laser desorption ionization (MALDI) time-of-flight mass spectrometer (TOF/MS) (bioMérieux, Craponne, France) was employed to obtain further confirmation of the strain’s affiliation to the Acanthamoeba genus [26]. The MALDI-TOF MS compares spectra produced by intact cells against a database of unique, conserved peaks that are used to identify the species.
2.3. Essential Oils
M. alternifolia Cheel (tea tree) (TTO) and E. globulus Labill. (EEO) essential oils (EOs), isolated by hydro-distillation and characterized in a previous investigation, were chosen and employed in this study due to their strong biological activity, which is particularly evident when the two compounds are used in association [24].
2.4. EOs Minimum Inhibitory Concentration (MIC)
The MIC of each EO was determined by the broth microdilution method using 96-well microplates.
Each well was aliquoted with 95 µL of PYG and 5 µL of trophozoites up to a final inoculum concentration of 106 cells/mL. A volume of 100 µL of each EO serial dilution was added to obtain concentrations ranging from 512 to 0.125 μg/mL. The plates were then incubated at 30 °C for 24 h. After incubation, cell viability was checked with a Bürker chamber under an inverted microscope using the trypan blue exclusion test.
The negative control wells consisted of amoeba cells in PYG without EOs [27].
2.5. Determination of the Fractional Inhibitory Concentration (FIC) Index
Using the Fractional Inhibitory Concentration (FIC) Index, the effect of EO/EO association on amoeba cells was tested. The potential synergistic amoebicidal activity of TTO and EEO was determined based on the FIC index method [28], and the results are expressed as follows: synergy (FIC ≤ 0.5), addition (0.5 ≤ FIC ≥ 1), indifference (1 ≤ FIC ≥ 4), and antagonism (FIC > 4).
2.6. Amoebicidal Activity of EOs
One hundred microliters of five-day-old PYG cultures of protozoa suspended in sterile PBS was seeded in each well of a microtiter plate (approximately 104 cells/well) [29] and incubated for 24 h at 30 °C. After incubation, the non-adherent cells were removed by washing the wells with 100 μL of Page’s Amoeba Saline Solution (PAS) (Oxoid, Milan, Italy). TTO and EEO, used alone and in association, were added to the wells at the MIC and at the best synergistic concentration, as detected by the FIC Index assay. The amoeba cells were exposed to EOs and to EO/EO synergic association at 30 °C for 24 h. At predetermined times (1 h and 24 h), amoeba cells were counted using a Burker chamber, differentiating between dead and viable cells using trypan blue. At the end of each incubation period, an appropriate aliquot of trypan blue was added to each well to facilitate cell counting, resulting in a final concentration of 4%, and incubated for at least 10 min. Using an inverted microscope at 20X magnification, the cells were counted, distinguishing between dead cells, stained blue due to loss of membrane integrity and subsequent dye absorption, and viable cells, appearing white with intact membranes. Trophozoites not exposed to EO activity were used as a control.
2.7. Statistical Analysis
Statistical significance was determined by t-tests and ANOVA using the statistical program GraphPad Prism 9.2.0 (San Diego, CA, USA). Analysis was performed using Bonferroni’s post hoc test. p-values were considered significant at ≤0.05. The experiment was performed in three replicates to check the reproducibility of the results.
3. Results
3.1. Genotypic Characterization of Amoeba Strain
By BLAST analysis of the amplified gene products, >98% identity was obtained with the reference sequences of the Acanthamoeba18S rRNA gene. BLAST and MALDI-TOF MS analysis confirmed that the amoeba strain used in this study, isolated in a clinical context, belongs to the genus Acanthamoeba, and it was identified as A. polyphaga, belonging to the T4 genotype.
3.2. Minimum Inhibitory Concentration (MIC) and Fractional Inhibitory Concentration (FIC) Index of TTO and EEO
The Minimum Inhibitory Concentration (MIC) of each EO and the Fractional Inhibitory Concentration (FIC) Index are shown in Table 1.

Table 1.
Minimum Inhibitory Concentration (MIC) of M. alternifolia Cheel (tea tree) and E. globulus Labill. (µg/mL) against A. polyphaga and Fractional Inhibitory (FIC) Index value.
TTO displays the best activity, with an MIC value of 16 µg/mL, while for EEO the MIC value was 32 µg/mL. The associated EO/EO also showed anti-amoebal and synergistic activity (FIC = 0.5), with a reduction in the concentrations of both active compounds of 75%.
3.3. Amoebicidal Activity of M. alternifolia (Tea Tree) (TTO)
As reported in Figure 1a, a powerful amoebicidal effect from the first hour of exposure emerged for TTO at all concentrations tested (p < 0.0001), especially for the concentrations 1xMIC and 2xMIC.
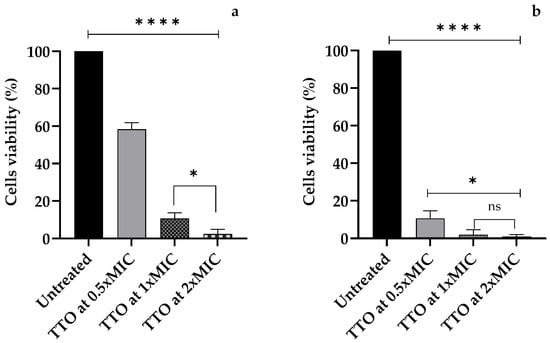
Figure 1.
The cell viability of A. polyphaga trophozoites after (a) 1 h and (b) 24 h of exposure to M. alternifolia Cheel (tea tree) (TTO) essential oil at concentrations of 0.5xMIC, 1xMIC, and 2xMIC. Each bar represents the mean ± SD of the three determinations (error bar = S.D.; n = 3). p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) were considered significant during t-tests and ANOVA with the Bonferroni correction. ns stands for not statistically significant.
After 24 h of exposure, a very high amount of amoebicidal activity was observed, up to the disappearance of viable cells upon treatment with a TTO concentration of 2xMIC, with a significant difference compared to the 0.5xMIC TTO concentration (p = 0.016) (Figure 1b).
3.4. Amoebicidal Activity of E. globulus Labill. (EEO)
A gradual increase in amoebicidal activity from the first hour of exposure emerged for all the EEO concentrations tested (p = 0.0007 for 0.5xMIC, p < 0.0001 for 1xMIC and 2xMIC) compared to the untreated sample. Cells treated with an EEO concentration of 2xMIC showed a decrease of 57.33% (compared to untreated cells) and a significant difference compared to the EEO concentrations of 0.5xMIC (p < 0.0001) and 1xMIC (p = 0.0002) (Figure 2a).
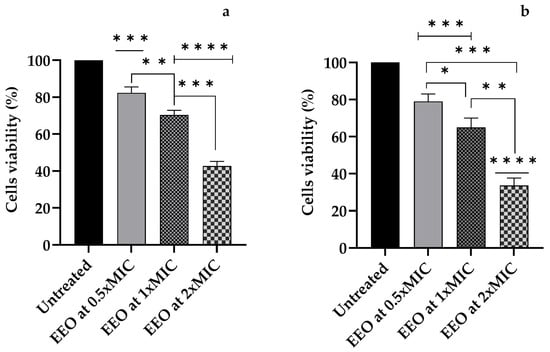
Figure 2.
The cell viability of A. polyphaga trophozoites after (a) 1 h and (b) 24 h of exposure to E. globulus Labill. (EEO) essential oil at concentrations of 0.5xMIC, 1xMIC, and 2xMIC. Each bar represents the mean ± SD of the three determinations (error bar = S.D.; n = 3). p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) were considered significant during t-tests and ANOVA with the Bonferroni correction. ns stands for not statistically significant.
At 24 h post-treatment we observed a decrease in A. polyphaga cells compared to the untreated control of 57.3% for 0.5xMIC (p = 0.0008), 1xMIC (p = 0.0003), and 1xMIC (p = 0.0001) (Figure 2b).
3.5. Amoebicidal Activity of Associated TTO/EEO
After 1 h of experimentation, the TTO/EEO association sample displayed a decrease in the cell viability of A. polyphaga (p < 0.0001) compared to the untreated sample. A very high amount of amoebicidal activity was observed for 1xMIC and 2xMIC (p = 0.0001 and p < 0.0001 compared to 0.5xMIC, respectively); cells treated with the highest concentration of TTO/EEO were no longer viable (Figure 3a).
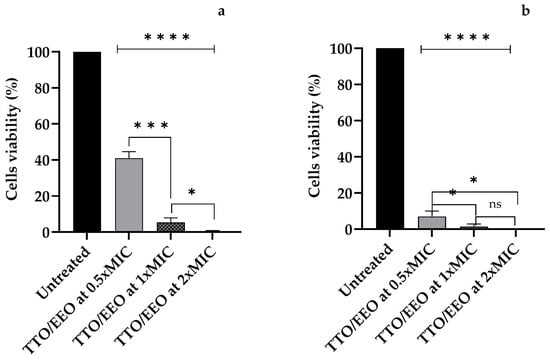
Figure 3.
The cell viability of A. polyphaga trophozoites after (a) 1 h and (b) 24 h of exposure to the associated M. alternifolia Cheel (tea tree) (TTO)/E. globulus Labill. (EEO) essential oil at concentrations of 0.5xMIC, 1xMIC, and 2xMIC. Each bar represents the mean ± SD of the three determinations (error bar = S.D.; n = 3). p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) were considered significant during t-tests and ANOVA with the Bonferroni correction. ns stands for not statistically significant.
After 24 h of TTO/EEO exposure, the amount of viable cells of A. polyphaga in the sample treated with the 0.5xMIC concentration was only 7.0%, and the amount in the sample treated with the 1xMIC concentration was 1.3%. No viable cells were observed following treatment with the association at the 2xMIC concentration (Figure 3b).
Figure 4 shows an example of the ability of the TTO/EEO association to negatively affect the survival of amoebae, which leads to the rapid destruction of almost all A. polyphaga trophozoites and morphological changes in the residual cells.
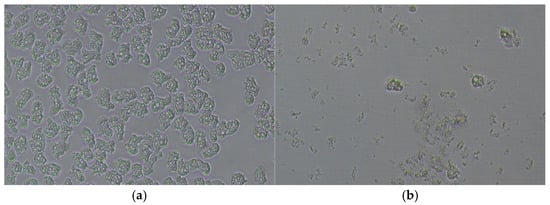
Figure 4.
The rapid destruction of A. polyphaga trophozoites and the morphological changes in the residual cells observed by an inverted microscope (a) in the control and (b) after 1 h of contact with TTO/EEO at the 1xMIC concentration.
In the Supplementary Materials section, the morphological changes and reduction in viable count of A. polyphaga trophozoites at TTO/EEO concentrations of 0.5xMIC and 2xMIC are shown in Figures S1 and S2, respectively.
4. Discussion
Although AK is less common than other forms of infectious keratitis, its incidence has increased in recent decades, as reported in a study conducted between 2009 and 2015 on 224 patients diagnosed with Acanthamoeba keratitis in the Netherlands [30]. AK represents approximately 2% of all corneal infections worldwide [31], and contact lens use is one of the most common risk factors, particularly in developed countries, affecting 1–33 users per million each year [32]. The type of contact lens used also plays a role, with a more than three-fold increased risk for daily reusable lens users versus daily disposable lens users [33]. Daily reusable lens solutions can become contaminated at the point of use through contact with water or dirty hands [34], becoming a risk factor related to maintaining the sterility of the solution. Non-lens users can also develop this condition if exposed to environmental risks like contaminated tap water, swimming pools, hot tubs, and soil or dust [35,36].
As for the therapies currently used for the treatment of infections caused by Acanthamoeba, these still pose important challenges, such as toxicity to human cells and resistance to the drugs used. A. polyphaga is an opportunistic protozoan pathogen that is very difficult to eradicate. Therapy must be started as quickly as possible because if the parasite reaches the corneal stroma the therapeutic approach becomes more difficult, partly due to the ability of the protozoan to encyst. Cysts are in fact much more resistant to pharmacological treatment than trophozoites and require longer therapy cycles with sometimes uncertain outcomes. Therapy can, therefore, become long and demanding, and its management requires great experience because it is not always easy to evaluate the response to treatment, and complications can be very serious and difficult to manage. Resistance to therapy can develop during treatment, as well as drug-induced toxicity, with the latter leading to an initial worsening of both the inflammatory response and symptoms. New generations of drugs must be developed to help treat acanthamoebiasis, in order to reduce both the recurrence of infection and the adverse reactions caused by the current therapies. Natural compounds like plant extracts and bacterial metabolites are very interesting and promising sources of future drugs [37,38,39,40]. Hadas et al. [41] showed that extracts from Passiflora spp. have amoebostatic and amoebicidal properties in concentrations from 4 to 12 mg/mL. A preliminary investigation into Eryngium alpinum L. extracts revealed remarkable amoebicidal action against trophozoites, which reached the highest antiamoebicidal effect after two days of treatment at the concentrations of 5 mg/mL, 2.5 mg/mL, and 0.5 mg/mL [42]. In recent years, various researchers have investigated the effectiveness of nanoparticle-conjugated drugs and/or naturally occurring plant compounds against Acanthamoeba. In addition to significant growth inhibition, these natural compounds and nanoconjugates do not exhibit in vitro cytotoxic effects against human cells [43]. The combined use of several natural compounds allows the reduction of their concentrations and, consequently, the decrease of toxic effects during therapy [44,45].
Other applied nanotechnologies have been shown to enhance the anti-Acanthamoeba activity in the encapsulated nanoparticles, opening the way for new therapeutic options [46,47].
Amoebicidal activity has been confirmed for the natural compounds used in the present investigation, which show remarkable effectiveness when combined. The TTO/EEO association clearly indicated a synergistic effect in all tests, and at 24 h post-treatment, no viable A. polyphaga cells were observed for the 2xMIC concentration. To further expand the knowledge about the well-known antagonistic potential of EOs [19], TTO and EEO were chosen for this preliminary study. In our previous investigation, M. alternifolia Cheel (tea tree) and E. globulus Labill. essential oils showed the ability to combat antibiotic-resistant strains even when organized in biofilms, with the results showing promise regarding these two important public health problems [24]. Lastly, the role of “trojan horse” played by this protozoan is well known. Water (Legionella, Aeromonas hydrophila) and food-borne (Listeria monocytogenes, Salmonella enterica serovar Enteritidis, Yersinia enterocolitica) pathogens are examples of this endosymbiotic relationship, which is capable of implementing environmental spread and resistance [48,49,50].
This endosymbiotic relationship is beneficial, allowing both partners to survive and take advantage of the utilization of nutrients (excreted catabolites, remains of dead bacteria, etc.), the development of new characteristics, and adaptation to new environments [51]. These host/parasite interactions indeed seem to be of considerable importance, increasing the parasites’ potential virulence and their resistance to biocides and antibiotics [52,53,54,55,56,57].
Therefore, based on all these concerns and given the challenges associated with the current AK treatment protocols, new and alternative biocides must be investigated for future clinical and environmental purposes.
The data emerging from the present study suggest that both of the studied EOs are interesting natural compounds endowed with amoebicidal activity. TTO proved to be the most effective compound, with significant activity after only 1 h of exposure, especially for the concentrations 1xMIC and 2xMIC. EEO was less effective, and a reduction in viable amoeba cells of 62% was observed after 24 h for the highest concentration used (2xMIC). The synergistic interaction of TTO and EEO employed in the present investigation once again highlights the fact that the adequate association of these two EOs can improve the biological activity of the single compounds. This increased amoebicidal activity confirms that the biological activity of these EOs is not given exclusively by the action of their main compound, but is also due to the synergism established between all their different phytochemical active components. This is consistent with other studies confirming the activity of EOs against A. castellani [27,58] and A. polyphaga [59]. Other studies also agree that among the EOs with amoebicidal activity, tea tree EO is the most effective against different strains of Acanthamoeba, both individually [60] and in synergistic combination with compounds like dimethyl sulfoxide, which is used as a disinfectant [61], and EO blends [44], similarly to what emerged in the present investigation.
5. Conclusions
All research data on the biological characteristics of natural substances encourage the scientific community to discover new therapeutics for possible pharmacological applications. The development of alternative therapies extracted from natural products represents an important step in trying to solve global health challenges, as stated by the results of this study. Therefore, the use of EOs, alone or in association with therapeutics, as alternative biocides in contact lens solution preservatives or for other potential applications in the prevention of AK infections may represent a potential future strategy. Although EOs have demonstrated amoebicidal activity, further studies are needed to evaluate their other properties of clinical interest, as seen in other studies. Their cytotoxicity, especially against human epithelial cells, will be explored, along with their other pharmacological properties, to better define the future impact of essential oils on human public health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15084198/s1, Figure S1. Rapid destruction of A. polyphaga trophozoites and morphological changes in residual cells observed by inverted microscope, after 1 h of contact with TTO/EEO at 0.5xMIC concentration (b) with respect to control (a). Scale bar is same for all images. Figure S2. Rapid destruction of A. polyphaga trophozoites and morphological changes in residual cells observed by inverted microscope, after 1 h of contact with TTO/EEO at 2xMIC concentration (b) with respect to control (a). Scale bar is same for all images.
Author Contributions
Conceptualization, P.M. and R.I.; methodology, P.M. and C.S.; validation, R.I. and C.S.; investigation, R.I. and P.M.; resources, M.M. and P.M.; data curation, R.I. and M.M.; writing—original draft preparation, R.I. and M.M.; writing—review and editing, P.M., R.I. and M.M.; visualization, R.I. and C.S.; supervision, P.M. and C.S.; project administration, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Corsaro, D. Update on Acanthamoeba phylogeny. Parasitol. Res. 2020, 119, 3327–3338, Erratum in Parasitol. Res. 2021, 120, 1927–1928. [Google Scholar] [CrossRef] [PubMed]
- Putaporntip, C.; Kuamsab, N.; Nuprasert, W.; Rojrung, R.; Pattanawong, U.; Tia, T.; Yanmanee, S.; Jongwutiwes, S. Analysis of Acanthamoeba genotypes from public freshwater sources in Thailand reveals a new genotype, T23 Acanthamoeba bangkokensis sp. nov. Sci. Rep. 2021, 11, 17290. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.C.B.; Chaúque, B.J.M.; Benitez, G.B.; Rott, M.B. Global prevalence of potentially pathogenic free-living amoebae in sewage and sewage-related environments-systematic review with meta-analysis. Parasitol. Res. 2024, 123, 148. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhee, B.; Williams, N.L.R.; Siboni, N.; Rodgers, K.; Willcox, M.; Henriquez, F.L.; Seymour, J.R.; Potts, J.; Johnson, C.; Scanes, P.; et al. Identification and quantification of Acanthamoeba spp. within seawater at four coastal lagoons on the east coast of Australia. Sci. Total Environ. 2023, 901, 165862. [Google Scholar] [CrossRef]
- Fabros, M.R.L.; Diesta, X.R.S.; Oronan, J.A.; Verdejo, K.S.; Garcia, J.S.M.; Sophia Romey, M.; Milanez, G.J. Current report on the prevalence of free-living amoebae (FLA) in natural hot springs: A systematic review. J. Water Health 2021, 19, 563–574. [Google Scholar] [CrossRef]
- Hoffmann, R.; Michel, R. Distribution of free-living amoebae (FLA) during preparation and supply of drinking water. Int. J. Hyg. Environ. Health 2001, 203, 215–219. [Google Scholar] [CrossRef]
- Wopereis, D.B.; Bazzo, M.L.; de Macedo, J.P.; Casara, F.; Golfeto, L.; Venancio, E.; de Oliveira, J.G.; Rott, M.B.; Caumo, K.S. Free-living amoebae and their relationship to air quality in hospital environments: Characterization of Acanthamoeba spp. obtained from air-conditioning systems. Parasitology 2020, 147, 782–790. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; Dos Santos, D.L.; Anvari, D.; Rott, M.B. Prevalence of free-living amoebae in swimming pools and recreational waters, a systematic review and meta-analysis. Parasitol. Res. 2022, 121, 3033–3050. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological characteristics and pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 1147077. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Haston, J.C.; O’Laughlin, K.; Matteson, K.; Roy, S.; Qvarnstrom, Y.; Ali, I.K.M.; Cope, J.R. The Epidemiology and Clinical Features of Non-Keratitis Acanthamoeba Infections in the United States, 1956–2020. Open Forum Infect. Dis. 2023, 10, ofac682. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp. Parasitol. 2020, 208, 107788. [Google Scholar] [CrossRef] [PubMed]
- Torno, M.S., Jr.; Babapour, R.; Gurevitch, A.; Witt, M.D. Cutaneous acanthamoebiasis in AIDS. J. Am. Acad. Dermatol. 2000, 42 Pt 2, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Łanocha-Arendarczyk, N.; Kosik-Bogacka, D. Immunopathogenicity of Acanthamoeba spp. in the brain and lungs. Int. J. Mol. Sci. 2021, 22, 1261. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Wei, Z.; Cao, K.; Zhang, Z.; Liang, Q. The global epidemiology and clinical diagnosis of Acanthamoeba keratitis. J. Infect. Public Health 2023, 16, 841–852. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Siddiqui, R.; Khan, N.A. Drug Discovery against Acanthamoeba Infections: Present Knowledge and Unmet Needs. Pathogens 2020, 9, 405. [Google Scholar] [CrossRef]
- Dickson, A.; Cooper, E.; Fakae, L.B.; Wang, B.; Chan, K.L.A.; Elsheikha, H.M. In Vitro Growth- and Encystation-Inhibitory Efficacies of Matcha Green Tea and Epigallocatechin Gallate Against Acanthameoba Castellanii. Pathogens 2020, 9, 763. [Google Scholar] [CrossRef]
- Fakae, L.B.; Stevenson, C.W.; Zhu, X.Q.; Elsheikha, H.M. In vitro activity of Camellia sinensis (green tea) against trophozoites and cysts of Acanthamoeba castellanii. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 59–72. [Google Scholar] [CrossRef]
- Deans, S.G.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans: Microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement. Altern. Med. 2010, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef] [PubMed]
- George, D.R.; Smith, T.J.; Shiel, R.S.; Sparagano, O.A.E.; Guy, J.H. Mode of action and variability in efficacy of plant essential oils showing toxicity against the poultry red mite, Dermanyssus gallinae. Vet. Parasitol. 2009, 161, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Mariani, M.; Benvenuti, S.; Truzzi, E.; Messi, P. Effects of Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. essential oils on antibiotic-resistant bacterial biofilms. Molecules 2023, 28, 1671. [Google Scholar] [CrossRef]
- Casero, R.D.; Mongi, F.; Laconte, L.; Rivero, F.; Sastre, D.; Teherán, A.; Herrera, G.; Ramírez, J.D. Molecular and morphological characterization of Acanthamoeba isolated from corneal scrapes and contact lens wearers in Argentina. Infect. Genet. Evol. 2017, 54, 170–175. [Google Scholar] [CrossRef]
- Megha, K.; Sharma, M.; Gupta, A.; Sehgal, R.; Khurana, S. Protein profiling of Acanthamoeba species using MALDI-TOF MS for specific identification of Acanthamoeba genotype. Parasitol. Res. 2018, 117, 729–736. [Google Scholar] [CrossRef]
- Sama-Ae, I.; Sangkanu, S.; Siyadatpanah, A.; Norouzi, R.; Chuprom, J.; Mitsuwan, W.; Surinkaew, S.; Boonhok, R.; Paul, A.K.; Mahboob, T.; et al. Targeting Acanthamoeba proteins interaction with flavonoids of propolis extract by in vitro and in silico studies for promising therapeutic effects. F1000Research 2022, 11, 1274. [Google Scholar] [CrossRef]
- Hemaiswaryaa, S.; Kruthiventib, A.K.; Doblea, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Saoudi, S.; Sifaoui, I.; Chammem, N.; Reyes-Batlle, M.; López-Arencibia, A.; Pacheco-Fernández, I.; Pino, V.; Hamdi, M.; Jiménez, I.A.; Bazzocchi, I.L.; et al. Anti-Acanthamoeba activity of Tunisian Thymus capitatus essential oil and organic extracts. Exp. Parasitol. 2017, 183, 231–235. [Google Scholar] [CrossRef]
- Randag, A.C.; van Rooij, J.; van Goor, A.T.; Verkerk, S.; Wisse, R.P.L.; Saelens, I.E.Y.; Stoutenbeek, R.; van Dooren, B.T.H.; Cheng, Y.Y.Y.; Eggink, C.A. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE 2019, 14, e0222092. [Google Scholar] [CrossRef]
- Garg, D.; Daigavane, S. A Comprehensive review on Acanthamoeba keratitis: An overview of epidemiology, risk factors, and therapeutic strategies. Cureus 2024, 16, e67803. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.N.; Ronquillo, Y.; Moshirfar, M. Acanthamoeba keratitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Carnt, N.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis risk factors for daily wear contact lens users: A case-control study. Ophthalmology 2023, 130, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Verani, J.R.; Lorick, S.A.; Yoder, J.S.; Beach, M.J.; Braden, C.R.; Roberts, J.M.; Conover, C.S.; Chen, S.; McConnell, K.A.; Chang, D.C.; et al. Acanthamoeba keratitis investigation team. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg. Infect. Dis. 2009, 15, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Kalra, P.; Joseph, J. Non-contact lens related Acanthamoeba keratitis. Indian J. Ophthalmol. 2017, 65, 1079–1086. [Google Scholar] [CrossRef]
- Brown, A.C.; Ross, J.; Jones, D.B.; Collier, S.A.; Ayers, T.L.; Hoekstra, R.M.; Backensen, B.; Roy, S.L.; Beach, M.J.; Yoder, J.S. Acanthamoeba keratitis investigation team. Risk factors for Acanthamoeba keratitis-a multistate case-control study, 2008–2011. Eye Contact Lens 2018, 44 Suppl. S1, S173–S178. [Google Scholar] [CrossRef]
- Sauter, I.P.; dos Santos, J.C.; Apel, M.A.; Cibulski, S.P.; Roehe, P.M.; von Poser, G.L.; Rott, M.B. Amoebicidal activity and chemical composition of Pterocaulon polystachyum (Asteraceae) essential oil. Parasitol. Res. 2011, 109, 1367–1371. [Google Scholar] [CrossRef]
- Polat, Z.A.; Vural, A.; Ozan, F.; Tepe, B.; Ozcelik, S.; Cetin, A. In vitro evaluation of the amoebicidal activity of garlic (Allium sativum) extract on Acanthamoeba castellanii and its cytotoxic potential on corneal cells. J. Ocul. Pharmacol. Ther. 2008, 24, 8–14. [Google Scholar] [CrossRef]
- Vural, A.; Polat, Z.A.; Topalkara, A.; Toker, M.I.; Erdogan, H.; Arici, M.K.; Cetin, A. The effect of propolis in experimental Acanthamoeba keratitis. Clin. Exp. Ophthalmol. 2007, 35, 749–754. [Google Scholar] [CrossRef]
- Benitez, L.B.; Caumo, K.; Brandelli, A.; Rott, M.B. Bacteriocin-like substance from Bacillus amyloliquefaciens shows remarkable inhibition of Acanthamoeba polyphaga. Parasitol. Res. 2011, 108, 687–691. [Google Scholar] [CrossRef]
- Hadas, E.; Ozarowski, M.; Derda, M.; Thiem, B.; Cholewinski, M.; Skrzypczak, L.; Gryszczynska, A.; Piasecka, A. The Use of Extracts from Passiflora spp. in Helping the Treatment of Acanthamoebiasis. Acta Pol. Pharm. 2017, 74, 921–928. [Google Scholar] [PubMed]
- Kikowska, M.; Kruszka, D.; Derda, M.; Hadaś, E.; Thiem, B. Phytochemical Screening and Acanthamoebic activity of shoots from in vitro cultures and in vivo plants of Eryngium alpinum L.-The endangered and protected species. Molecules 2020, 25, 1416. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Ting, E.L.S.; Anwar, A.; Ain, N.U.; Faizi, S.; Shah, M.R.; Khan, N.A.; Siddiqui, R. Antiamoebic activity of plant-based natural products and their conjugated silver nanoparticles against Acanthamoeba castellanii (ATCC 50492). AMB Express 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Leigh-de Rapper, S.; Viljoen, A.; van Vuuren, S. Essential oil blends: The potential of combined use for respiratory tract infections. Antibiotics 2021, 10, 1517. [Google Scholar] [CrossRef] [PubMed]
- Simbu, S.; Orchard, A.; van de Venter, M.; van Vuuren, S. Ibuprofen as an adjuvant to conventional antimicrobials and essential oil compounds against skin pathogens. J. Appl. Microbiol. 2024, 135, lxae186. [Google Scholar] [CrossRef]
- Elkadery, A.A.S.; Elsherif, E.A.; Ezz Eldin, H.M.; Fahmy, I.A.F.; Mohammad, O.S. Efficient therapeutic effect of Nigella sativa aqueous extract and chitosan nanoparticles against experimentally induced Acanthamoeba keratitis. Parasitol. Res. 2019, 118, 2443–2454. [Google Scholar] [CrossRef]
- Sharma, G.; Kalra, S.K.; Tejan, N.; Ghoshal, U. Nanoparticles based therapeutic efficacy against Acanthamoeba: Updates and future prospect. Exp. Parasitol. 2020, 218, 108008. [Google Scholar] [CrossRef]
- Anacarso, I.; Guerrieri, E.; Bondi, M.; de Niederhäusern, S.; Iseppi, R.; Sabia, C.; Contri, M.; Borella, P.; Messi, P. Influence of Legionella pneumophila and other water bacteria on the survival and growth of Acanthamoeba polyphaga. Arch. Microbiol. 2010, 192, 877–882. [Google Scholar] [CrossRef]
- Anacarso, I.; de Niederhäusern, S.; Messi, P.; Guerrieri, E.; Iseppi, R.; Sabia, C.; Bondi, M. Acanthamoeba polyphaga, a potential environmental vector for the transmission of food-borne and opportunistic pathogens. J. Basic Microbiol. 2012, 52, 261–268. [Google Scholar] [CrossRef]
- Messi, P.; Bargellini, A.; Anacarso, I.; Marchesi, I.; de Niederhäusern, S.; Bondi, M. Protozoa and human macrophages infection by Legionella pneumophila environmental strains belonging to different serogroups. Arch. Microbiol. 2013, 195, 89–96. [Google Scholar] [CrossRef]
- King, C.H.; Shotts, E.B., Jr.; Wooley, R.E.; Porter, K.G. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 1988, 54, 3023–3033. [Google Scholar] [CrossRef]
- Snelling, W.J.; McKenna, J.P.; Lecky, D.M.; Dooley, J.S. Survival of Campylobacter jejuni in waterborne protozoa. Appl. Environ. Microbiol. 2005, 71, 5560–5571. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.D.; Falkow, S.; Tompkins, L.S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 1994, 62, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Scaife, H.; Brown, M.R.W. Intraphagocytic growth induces an antibiotic resistant phenotype of Legionella pneumophila. Antimicrob. Agents Chemother. 1995, 39, 2684–2688. [Google Scholar] [CrossRef] [PubMed]
- Hilbi, H.; Weber, S.S.; Ragaz, C.; Nyfeler, Y.; Urwyler, S. Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 2007, 9, 563–575. [Google Scholar] [CrossRef]
- Barker, J.; Brown, M.R.W.; Collier, P.J.; Farrell, I.; Gilbert, P. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: Physiological status and susceptibility to chemical inactivation. Appl. Environ. Microbiol. 1992, 58, 2420–2425. [Google Scholar] [CrossRef]
- Miltner, E.C.; Bermudez, L.E. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 2000, 44, 1990–1994. [Google Scholar] [CrossRef]
- Souhaiel, N.; Sifaoui, I.; Ben Hassine, D.; Bleton, J.; Bonose, M.; Moussa, F.; Piñero, J.E.; Lorenzo-Morales, J.; Abderrabba, M. Ammoides pusilla (Apiaceae) essential oil: Activity against Acanthamoeba castellanii Neff. Exp. Parasitol. 2017, 183, 99–103. [Google Scholar] [CrossRef]
- Santos, I.G.; Scher, R.; Rott, M.B.; Menezes, L.R.; Costa, E.V.; Cavalcanti, S.C.; Blank, A.F.; Aguiar Jdos, S.; da Silva, T.G.; Dolabella, S.S. Amebicidal activity of the essential oils of Lippia spp. (Verbenaceae) against Acanthamoeba polyphaga trophozoites. Parasitol. Res. 2016, 115, 535–540. [Google Scholar] [CrossRef]
- Hadaś, E.; Derda, M.; Cholewiński, M. Evaluation of the effectiveness of tea tree oil in treatment of Acanthamoeba infection. Parasitol. Res. 2017, 116, 997–1001. [Google Scholar] [CrossRef][Green Version]
- Martín-Pérez, T.; Heredero-Bermejo, I.; Verdú-Expósito, C.; Pérez-Serrano, J. In vitro evaluation of the combination of Melaleuca alternifolia (tea tree) oil and dimethyl sulfoxide (DMSO) against trophozoites and cysts of Acanthamoeba strains. Oxygen Consumption Rate (OCR) Assay as a Method for Drug Screening. Pathogens 2021, 10, 491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).