Melaleuca alternifolia Cheel (Tea Tree) and Eucalyptus globulus Labill. Essential Oils’ Effectiveness Against an Acanthamoeba polyphaga Strain Responsible for Human Keratitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Amoeba Strain
2.2. Genotypic Characterization of Amoeba Strain
2.3. Essential Oils
2.4. EOs Minimum Inhibitory Concentration (MIC)
2.5. Determination of the Fractional Inhibitory Concentration (FIC) Index
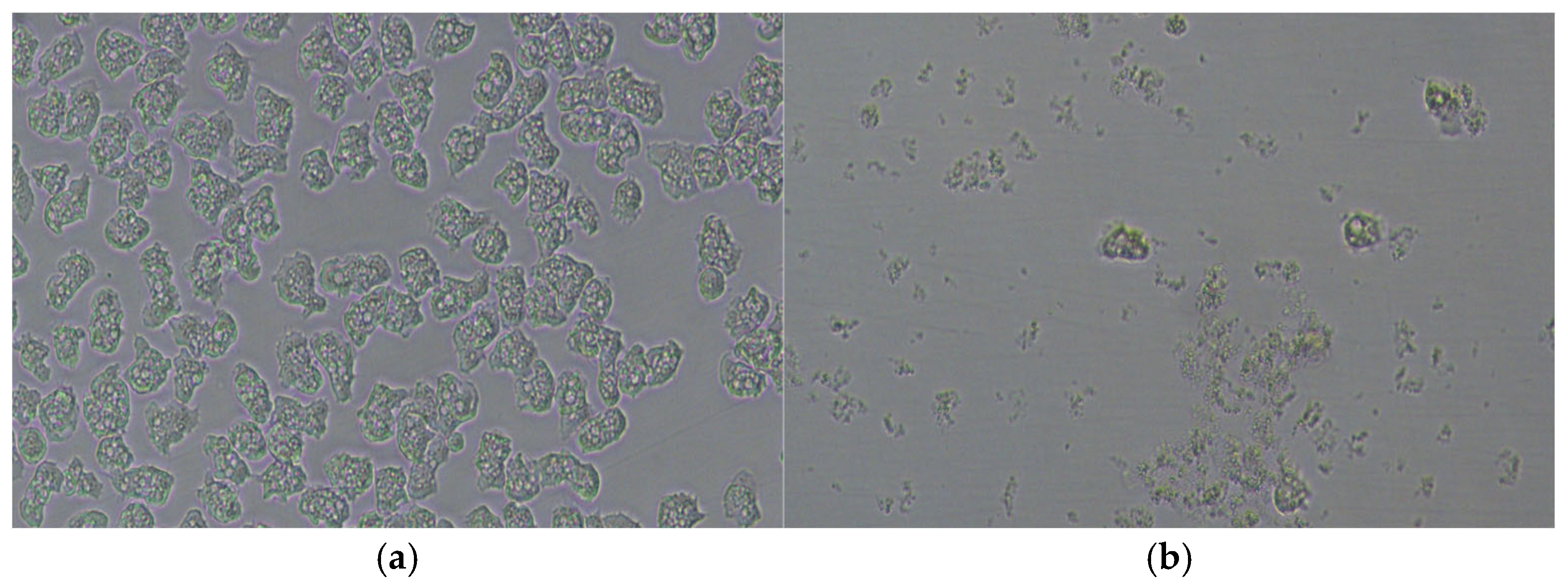
2.6. Amoebicidal Activity of EOs
2.7. Statistical Analysis
3. Results
3.1. Genotypic Characterization of Amoeba Strain
3.2. Minimum Inhibitory Concentration (MIC) and Fractional Inhibitory Concentration (FIC) Index of TTO and EEO
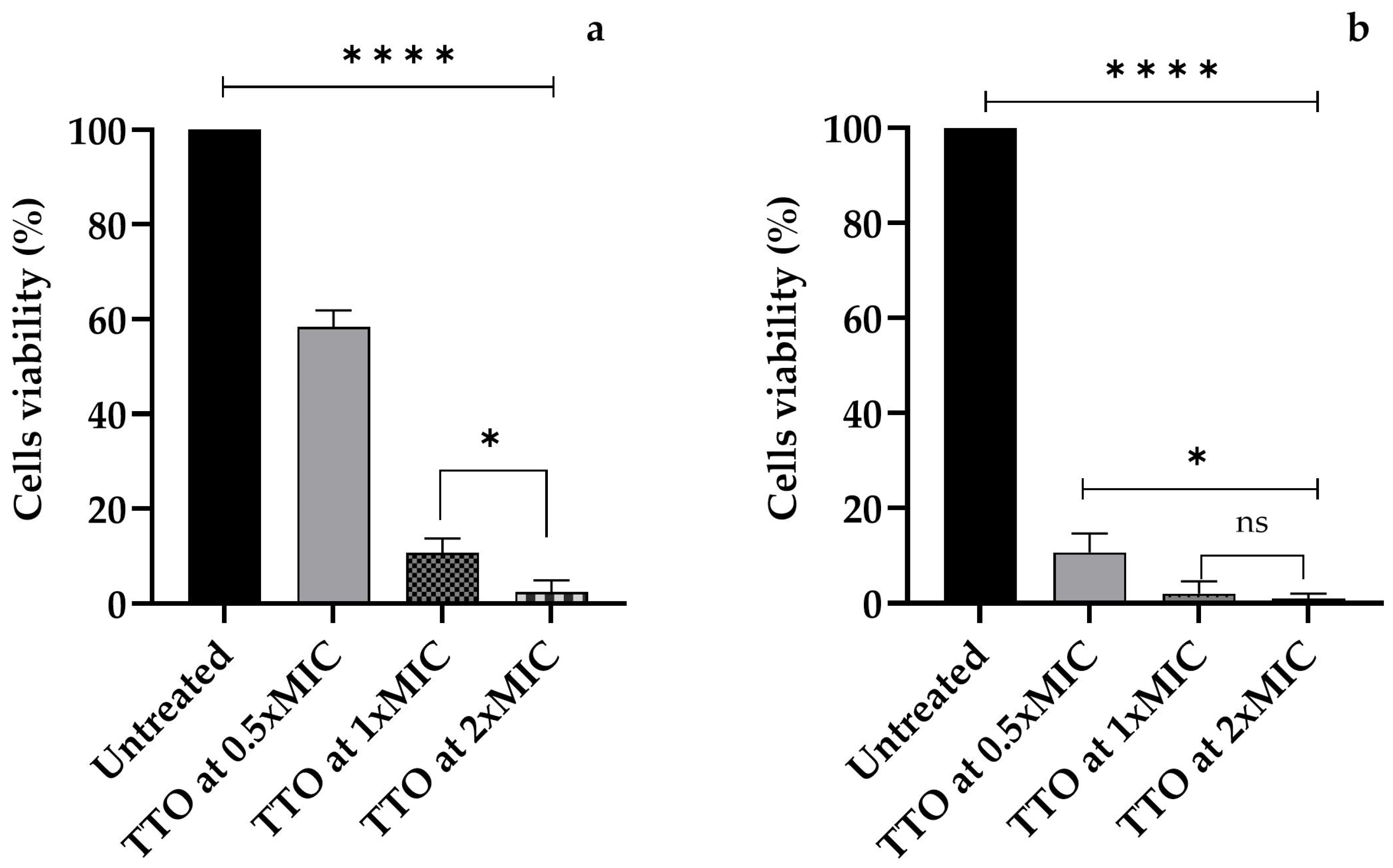
3.3. Amoebicidal Activity of M. alternifolia (Tea Tree) (TTO)
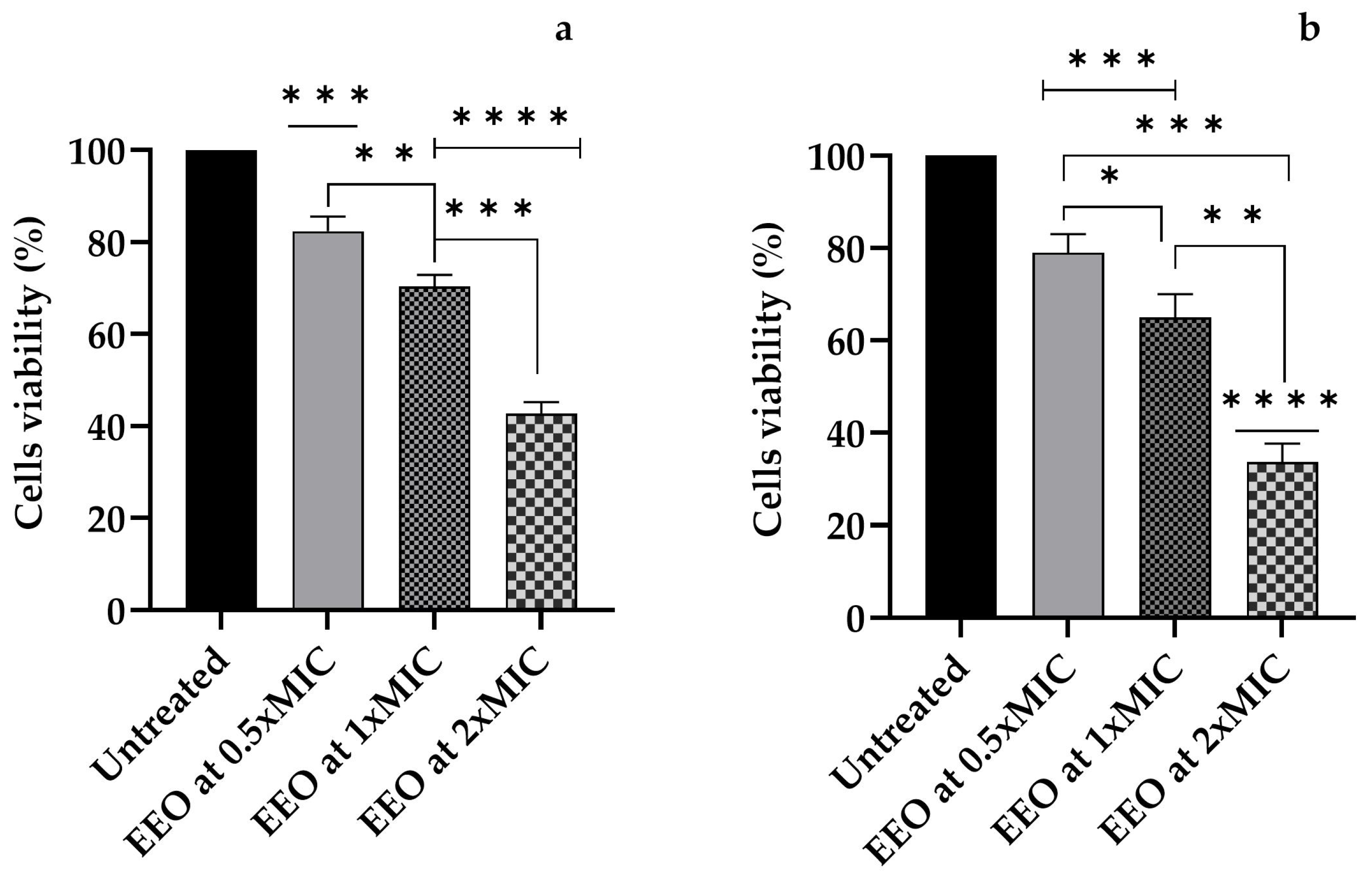
3.4. Amoebicidal Activity of E. globulus Labill. (EEO)
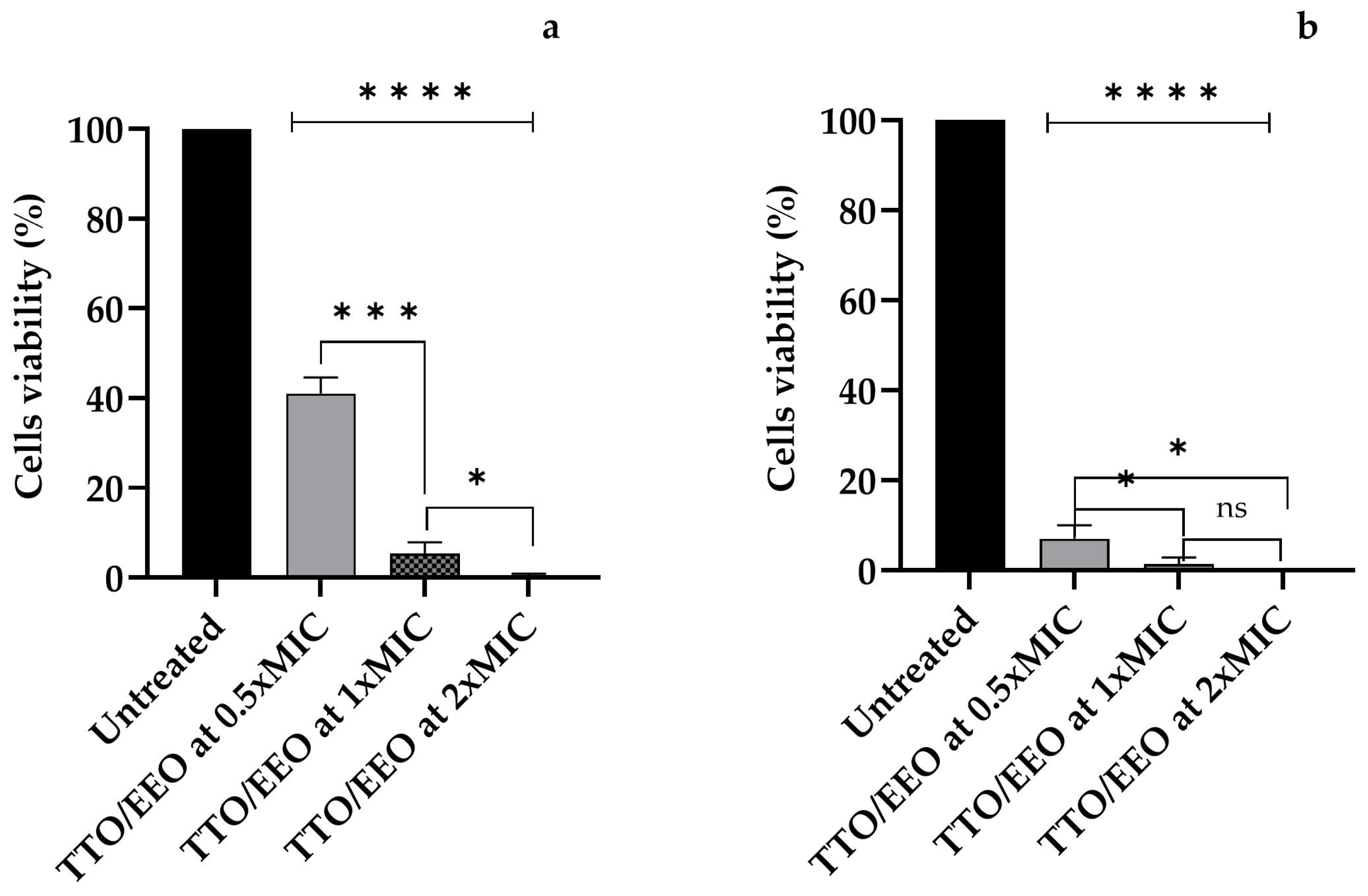
3.5. Amoebicidal Activity of Associated TTO/EEO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corsaro, D. Update on Acanthamoeba phylogeny. Parasitol. Res. 2020, 119, 3327–3338, Erratum in Parasitol. Res. 2021, 120, 1927–1928. [Google Scholar] [CrossRef] [PubMed]
- Putaporntip, C.; Kuamsab, N.; Nuprasert, W.; Rojrung, R.; Pattanawong, U.; Tia, T.; Yanmanee, S.; Jongwutiwes, S. Analysis of Acanthamoeba genotypes from public freshwater sources in Thailand reveals a new genotype, T23 Acanthamoeba bangkokensis sp. nov. Sci. Rep. 2021, 11, 17290. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.C.B.; Chaúque, B.J.M.; Benitez, G.B.; Rott, M.B. Global prevalence of potentially pathogenic free-living amoebae in sewage and sewage-related environments-systematic review with meta-analysis. Parasitol. Res. 2024, 123, 148. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhee, B.; Williams, N.L.R.; Siboni, N.; Rodgers, K.; Willcox, M.; Henriquez, F.L.; Seymour, J.R.; Potts, J.; Johnson, C.; Scanes, P.; et al. Identification and quantification of Acanthamoeba spp. within seawater at four coastal lagoons on the east coast of Australia. Sci. Total Environ. 2023, 901, 165862. [Google Scholar] [CrossRef]
- Fabros, M.R.L.; Diesta, X.R.S.; Oronan, J.A.; Verdejo, K.S.; Garcia, J.S.M.; Sophia Romey, M.; Milanez, G.J. Current report on the prevalence of free-living amoebae (FLA) in natural hot springs: A systematic review. J. Water Health 2021, 19, 563–574. [Google Scholar] [CrossRef]
- Hoffmann, R.; Michel, R. Distribution of free-living amoebae (FLA) during preparation and supply of drinking water. Int. J. Hyg. Environ. Health 2001, 203, 215–219. [Google Scholar] [CrossRef]
- Wopereis, D.B.; Bazzo, M.L.; de Macedo, J.P.; Casara, F.; Golfeto, L.; Venancio, E.; de Oliveira, J.G.; Rott, M.B.; Caumo, K.S. Free-living amoebae and their relationship to air quality in hospital environments: Characterization of Acanthamoeba spp. obtained from air-conditioning systems. Parasitology 2020, 147, 782–790. [Google Scholar] [CrossRef]
- Chaúque, B.J.M.; Dos Santos, D.L.; Anvari, D.; Rott, M.B. Prevalence of free-living amoebae in swimming pools and recreational waters, a systematic review and meta-analysis. Parasitol. Res. 2022, 121, 3033–3050. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological characteristics and pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 1147077. [Google Scholar] [CrossRef]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An update on Acanthamoeba keratitis: Diagnosis, pathogenesis and treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef]
- Haston, J.C.; O’Laughlin, K.; Matteson, K.; Roy, S.; Qvarnstrom, Y.; Ali, I.K.M.; Cope, J.R. The Epidemiology and Clinical Features of Non-Keratitis Acanthamoeba Infections in the United States, 1956–2020. Open Forum Infect. Dis. 2023, 10, ofac682. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and its pathogenic role in granulomatous amebic encephalitis. Exp. Parasitol. 2020, 208, 107788. [Google Scholar] [CrossRef] [PubMed]
- Torno, M.S., Jr.; Babapour, R.; Gurevitch, A.; Witt, M.D. Cutaneous acanthamoebiasis in AIDS. J. Am. Acad. Dermatol. 2000, 42 Pt 2, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Kot, K.; Łanocha-Arendarczyk, N.; Kosik-Bogacka, D. Immunopathogenicity of Acanthamoeba spp. in the brain and lungs. Int. J. Mol. Sci. 2021, 22, 1261. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, X.; Wei, Z.; Cao, K.; Zhang, Z.; Liang, Q. The global epidemiology and clinical diagnosis of Acanthamoeba keratitis. J. Infect. Public Health 2023, 16, 841–852. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Siddiqui, R.; Khan, N.A. Drug Discovery against Acanthamoeba Infections: Present Knowledge and Unmet Needs. Pathogens 2020, 9, 405. [Google Scholar] [CrossRef]
- Dickson, A.; Cooper, E.; Fakae, L.B.; Wang, B.; Chan, K.L.A.; Elsheikha, H.M. In Vitro Growth- and Encystation-Inhibitory Efficacies of Matcha Green Tea and Epigallocatechin Gallate Against Acanthameoba Castellanii. Pathogens 2020, 9, 763. [Google Scholar] [CrossRef]
- Fakae, L.B.; Stevenson, C.W.; Zhu, X.Q.; Elsheikha, H.M. In vitro activity of Camellia sinensis (green tea) against trophozoites and cysts of Acanthamoeba castellanii. Int. J. Parasitol. Drugs Drug Resist. 2020, 13, 59–72. [Google Scholar] [CrossRef]
- Deans, S.G.; Ritchie, G. Antibacterial properties of plant essential oils. Int. J. Food Microbiol. 1987, 5, 165–180. [Google Scholar] [CrossRef]
- Oussalah, M.; Caillet, S.; Lacroix, M. Mechanism of action of Spanish oregano, Chinese cinnamon, and savory essential oils against cell membranes and walls of Escherichia coli O157: H7 and Listeria monocytogenes. J. Food Prot. 2006, 69, 1046–1055. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans: Microscopic observations and chemical characterization of Cymbopogon citratus. BMC Complement. Altern. Med. 2010, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Garozzo, A.; Timpanaro, R.; Stivala, A.; Bisignano, G.; Castro, A. Activity of Melaleuca alternifolia (tea tree) oil on influenza virus A/PR/8: Study on the mechanism of action. Antivir. Res. 2011, 89, 83–88. [Google Scholar] [CrossRef] [PubMed]
- George, D.R.; Smith, T.J.; Shiel, R.S.; Sparagano, O.A.E.; Guy, J.H. Mode of action and variability in efficacy of plant essential oils showing toxicity against the poultry red mite, Dermanyssus gallinae. Vet. Parasitol. 2009, 161, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Mariani, M.; Benvenuti, S.; Truzzi, E.; Messi, P. Effects of Melaleuca alternifolia Chell (Tea Tree) and Eucalyptus globulus Labill. essential oils on antibiotic-resistant bacterial biofilms. Molecules 2023, 28, 1671. [Google Scholar] [CrossRef]
- Casero, R.D.; Mongi, F.; Laconte, L.; Rivero, F.; Sastre, D.; Teherán, A.; Herrera, G.; Ramírez, J.D. Molecular and morphological characterization of Acanthamoeba isolated from corneal scrapes and contact lens wearers in Argentina. Infect. Genet. Evol. 2017, 54, 170–175. [Google Scholar] [CrossRef]
- Megha, K.; Sharma, M.; Gupta, A.; Sehgal, R.; Khurana, S. Protein profiling of Acanthamoeba species using MALDI-TOF MS for specific identification of Acanthamoeba genotype. Parasitol. Res. 2018, 117, 729–736. [Google Scholar] [CrossRef]
- Sama-Ae, I.; Sangkanu, S.; Siyadatpanah, A.; Norouzi, R.; Chuprom, J.; Mitsuwan, W.; Surinkaew, S.; Boonhok, R.; Paul, A.K.; Mahboob, T.; et al. Targeting Acanthamoeba proteins interaction with flavonoids of propolis extract by in vitro and in silico studies for promising therapeutic effects. F1000Research 2022, 11, 1274. [Google Scholar] [CrossRef]
- Hemaiswaryaa, S.; Kruthiventib, A.K.; Doblea, M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine 2008, 15, 639–652. [Google Scholar] [CrossRef]
- Saoudi, S.; Sifaoui, I.; Chammem, N.; Reyes-Batlle, M.; López-Arencibia, A.; Pacheco-Fernández, I.; Pino, V.; Hamdi, M.; Jiménez, I.A.; Bazzocchi, I.L.; et al. Anti-Acanthamoeba activity of Tunisian Thymus capitatus essential oil and organic extracts. Exp. Parasitol. 2017, 183, 231–235. [Google Scholar] [CrossRef]
- Randag, A.C.; van Rooij, J.; van Goor, A.T.; Verkerk, S.; Wisse, R.P.L.; Saelens, I.E.Y.; Stoutenbeek, R.; van Dooren, B.T.H.; Cheng, Y.Y.Y.; Eggink, C.A. The rising incidence of Acanthamoeba keratitis: A 7-year nationwide survey and clinical assessment of risk factors and functional outcomes. PLoS ONE 2019, 14, e0222092. [Google Scholar] [CrossRef]
- Garg, D.; Daigavane, S. A Comprehensive review on Acanthamoeba keratitis: An overview of epidemiology, risk factors, and therapeutic strategies. Cureus 2024, 16, e67803. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.N.; Ronquillo, Y.; Moshirfar, M. Acanthamoeba keratitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Carnt, N.; Minassian, D.C.; Dart, J.K.G. Acanthamoeba keratitis risk factors for daily wear contact lens users: A case-control study. Ophthalmology 2023, 130, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Verani, J.R.; Lorick, S.A.; Yoder, J.S.; Beach, M.J.; Braden, C.R.; Roberts, J.M.; Conover, C.S.; Chen, S.; McConnell, K.A.; Chang, D.C.; et al. Acanthamoeba keratitis investigation team. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg. Infect. Dis. 2009, 15, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Kalra, P.; Joseph, J. Non-contact lens related Acanthamoeba keratitis. Indian J. Ophthalmol. 2017, 65, 1079–1086. [Google Scholar] [CrossRef]
- Brown, A.C.; Ross, J.; Jones, D.B.; Collier, S.A.; Ayers, T.L.; Hoekstra, R.M.; Backensen, B.; Roy, S.L.; Beach, M.J.; Yoder, J.S. Acanthamoeba keratitis investigation team. Risk factors for Acanthamoeba keratitis-a multistate case-control study, 2008–2011. Eye Contact Lens 2018, 44 Suppl. S1, S173–S178. [Google Scholar] [CrossRef]
- Sauter, I.P.; dos Santos, J.C.; Apel, M.A.; Cibulski, S.P.; Roehe, P.M.; von Poser, G.L.; Rott, M.B. Amoebicidal activity and chemical composition of Pterocaulon polystachyum (Asteraceae) essential oil. Parasitol. Res. 2011, 109, 1367–1371. [Google Scholar] [CrossRef]
- Polat, Z.A.; Vural, A.; Ozan, F.; Tepe, B.; Ozcelik, S.; Cetin, A. In vitro evaluation of the amoebicidal activity of garlic (Allium sativum) extract on Acanthamoeba castellanii and its cytotoxic potential on corneal cells. J. Ocul. Pharmacol. Ther. 2008, 24, 8–14. [Google Scholar] [CrossRef]
- Vural, A.; Polat, Z.A.; Topalkara, A.; Toker, M.I.; Erdogan, H.; Arici, M.K.; Cetin, A. The effect of propolis in experimental Acanthamoeba keratitis. Clin. Exp. Ophthalmol. 2007, 35, 749–754. [Google Scholar] [CrossRef]
- Benitez, L.B.; Caumo, K.; Brandelli, A.; Rott, M.B. Bacteriocin-like substance from Bacillus amyloliquefaciens shows remarkable inhibition of Acanthamoeba polyphaga. Parasitol. Res. 2011, 108, 687–691. [Google Scholar] [CrossRef]
- Hadas, E.; Ozarowski, M.; Derda, M.; Thiem, B.; Cholewinski, M.; Skrzypczak, L.; Gryszczynska, A.; Piasecka, A. The Use of Extracts from Passiflora spp. in Helping the Treatment of Acanthamoebiasis. Acta Pol. Pharm. 2017, 74, 921–928. [Google Scholar] [PubMed]
- Kikowska, M.; Kruszka, D.; Derda, M.; Hadaś, E.; Thiem, B. Phytochemical Screening and Acanthamoebic activity of shoots from in vitro cultures and in vivo plants of Eryngium alpinum L.-The endangered and protected species. Molecules 2020, 25, 1416. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Ting, E.L.S.; Anwar, A.; Ain, N.U.; Faizi, S.; Shah, M.R.; Khan, N.A.; Siddiqui, R. Antiamoebic activity of plant-based natural products and their conjugated silver nanoparticles against Acanthamoeba castellanii (ATCC 50492). AMB Express 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Leigh-de Rapper, S.; Viljoen, A.; van Vuuren, S. Essential oil blends: The potential of combined use for respiratory tract infections. Antibiotics 2021, 10, 1517. [Google Scholar] [CrossRef] [PubMed]
- Simbu, S.; Orchard, A.; van de Venter, M.; van Vuuren, S. Ibuprofen as an adjuvant to conventional antimicrobials and essential oil compounds against skin pathogens. J. Appl. Microbiol. 2024, 135, lxae186. [Google Scholar] [CrossRef]
- Elkadery, A.A.S.; Elsherif, E.A.; Ezz Eldin, H.M.; Fahmy, I.A.F.; Mohammad, O.S. Efficient therapeutic effect of Nigella sativa aqueous extract and chitosan nanoparticles against experimentally induced Acanthamoeba keratitis. Parasitol. Res. 2019, 118, 2443–2454. [Google Scholar] [CrossRef]
- Sharma, G.; Kalra, S.K.; Tejan, N.; Ghoshal, U. Nanoparticles based therapeutic efficacy against Acanthamoeba: Updates and future prospect. Exp. Parasitol. 2020, 218, 108008. [Google Scholar] [CrossRef]
- Anacarso, I.; Guerrieri, E.; Bondi, M.; de Niederhäusern, S.; Iseppi, R.; Sabia, C.; Contri, M.; Borella, P.; Messi, P. Influence of Legionella pneumophila and other water bacteria on the survival and growth of Acanthamoeba polyphaga. Arch. Microbiol. 2010, 192, 877–882. [Google Scholar] [CrossRef]
- Anacarso, I.; de Niederhäusern, S.; Messi, P.; Guerrieri, E.; Iseppi, R.; Sabia, C.; Bondi, M. Acanthamoeba polyphaga, a potential environmental vector for the transmission of food-borne and opportunistic pathogens. J. Basic Microbiol. 2012, 52, 261–268. [Google Scholar] [CrossRef]
- Messi, P.; Bargellini, A.; Anacarso, I.; Marchesi, I.; de Niederhäusern, S.; Bondi, M. Protozoa and human macrophages infection by Legionella pneumophila environmental strains belonging to different serogroups. Arch. Microbiol. 2013, 195, 89–96. [Google Scholar] [CrossRef]
- King, C.H.; Shotts, E.B., Jr.; Wooley, R.E.; Porter, K.G. Survival of coliforms and bacterial pathogens within protozoa during chlorination. Appl. Environ. Microbiol. 1988, 54, 3023–3033. [Google Scholar] [CrossRef]
- Snelling, W.J.; McKenna, J.P.; Lecky, D.M.; Dooley, J.S. Survival of Campylobacter jejuni in waterborne protozoa. Appl. Environ. Microbiol. 2005, 71, 5560–5571. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, J.D.; Falkow, S.; Tompkins, L.S. Growth of Legionella pneumophila in Acanthamoeba castellanii enhances invasion. Infect. Immun. 1994, 62, 3254–3261. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Scaife, H.; Brown, M.R.W. Intraphagocytic growth induces an antibiotic resistant phenotype of Legionella pneumophila. Antimicrob. Agents Chemother. 1995, 39, 2684–2688. [Google Scholar] [CrossRef] [PubMed]
- Hilbi, H.; Weber, S.S.; Ragaz, C.; Nyfeler, Y.; Urwyler, S. Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 2007, 9, 563–575. [Google Scholar] [CrossRef]
- Barker, J.; Brown, M.R.W.; Collier, P.J.; Farrell, I.; Gilbert, P. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: Physiological status and susceptibility to chemical inactivation. Appl. Environ. Microbiol. 1992, 58, 2420–2425. [Google Scholar] [CrossRef]
- Miltner, E.C.; Bermudez, L.E. Mycobacterium avium grown in Acanthamoeba castellanii is protected from the effects of antimicrobials. Antimicrob. Agents Chemother. 2000, 44, 1990–1994. [Google Scholar] [CrossRef]
- Souhaiel, N.; Sifaoui, I.; Ben Hassine, D.; Bleton, J.; Bonose, M.; Moussa, F.; Piñero, J.E.; Lorenzo-Morales, J.; Abderrabba, M. Ammoides pusilla (Apiaceae) essential oil: Activity against Acanthamoeba castellanii Neff. Exp. Parasitol. 2017, 183, 99–103. [Google Scholar] [CrossRef]
- Santos, I.G.; Scher, R.; Rott, M.B.; Menezes, L.R.; Costa, E.V.; Cavalcanti, S.C.; Blank, A.F.; Aguiar Jdos, S.; da Silva, T.G.; Dolabella, S.S. Amebicidal activity of the essential oils of Lippia spp. (Verbenaceae) against Acanthamoeba polyphaga trophozoites. Parasitol. Res. 2016, 115, 535–540. [Google Scholar] [CrossRef]
- Hadaś, E.; Derda, M.; Cholewiński, M. Evaluation of the effectiveness of tea tree oil in treatment of Acanthamoeba infection. Parasitol. Res. 2017, 116, 997–1001. [Google Scholar] [CrossRef][Green Version]
- Martín-Pérez, T.; Heredero-Bermejo, I.; Verdú-Expósito, C.; Pérez-Serrano, J. In vitro evaluation of the combination of Melaleuca alternifolia (tea tree) oil and dimethyl sulfoxide (DMSO) against trophozoites and cysts of Acanthamoeba strains. Oxygen Consumption Rate (OCR) Assay as a Method for Drug Screening. Pathogens 2021, 10, 491. [Google Scholar] [CrossRef]
| EO | MIC EOs (μg/mL) | MIC EO/EO (μg/mL) | FIC Index |
|---|---|---|---|
| TTO | 16 | 4 | 0.5 |
| EEO | 32 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iseppi, R.; Mariani, M.; Sabia, C.; Messi, P. Melaleuca alternifolia Cheel (Tea Tree) and Eucalyptus globulus Labill. Essential Oils’ Effectiveness Against an Acanthamoeba polyphaga Strain Responsible for Human Keratitis. Appl. Sci. 2025, 15, 4198. https://doi.org/10.3390/app15084198
Iseppi R, Mariani M, Sabia C, Messi P. Melaleuca alternifolia Cheel (Tea Tree) and Eucalyptus globulus Labill. Essential Oils’ Effectiveness Against an Acanthamoeba polyphaga Strain Responsible for Human Keratitis. Applied Sciences. 2025; 15(8):4198. https://doi.org/10.3390/app15084198
Chicago/Turabian StyleIseppi, Ramona, Martina Mariani, Carla Sabia, and Patrizia Messi. 2025. "Melaleuca alternifolia Cheel (Tea Tree) and Eucalyptus globulus Labill. Essential Oils’ Effectiveness Against an Acanthamoeba polyphaga Strain Responsible for Human Keratitis" Applied Sciences 15, no. 8: 4198. https://doi.org/10.3390/app15084198
APA StyleIseppi, R., Mariani, M., Sabia, C., & Messi, P. (2025). Melaleuca alternifolia Cheel (Tea Tree) and Eucalyptus globulus Labill. Essential Oils’ Effectiveness Against an Acanthamoeba polyphaga Strain Responsible for Human Keratitis. Applied Sciences, 15(8), 4198. https://doi.org/10.3390/app15084198









