Electron Beam Irradiation’s Effect on Polyaniline/LiClO4/CuO Nanocomposite: A Study of Dielectric, Conductivity and Electrochemical Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
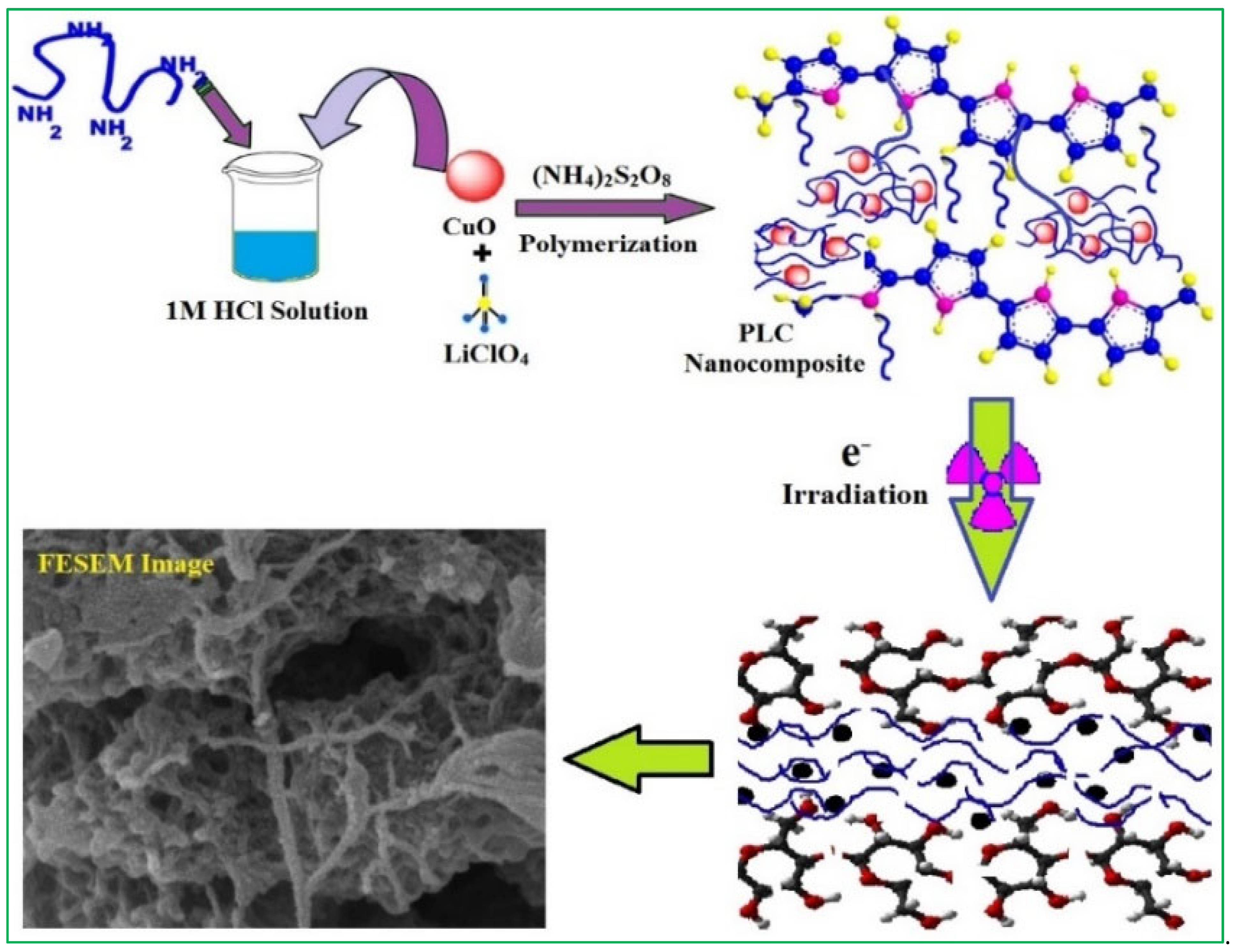
2.2. Synthesis of Polyaniline (PANI)/LiClO4/CuO Nanocomposite
2.3. Instrumentation
2.4. Electron Beam Irradiation
3. Results and Discussion
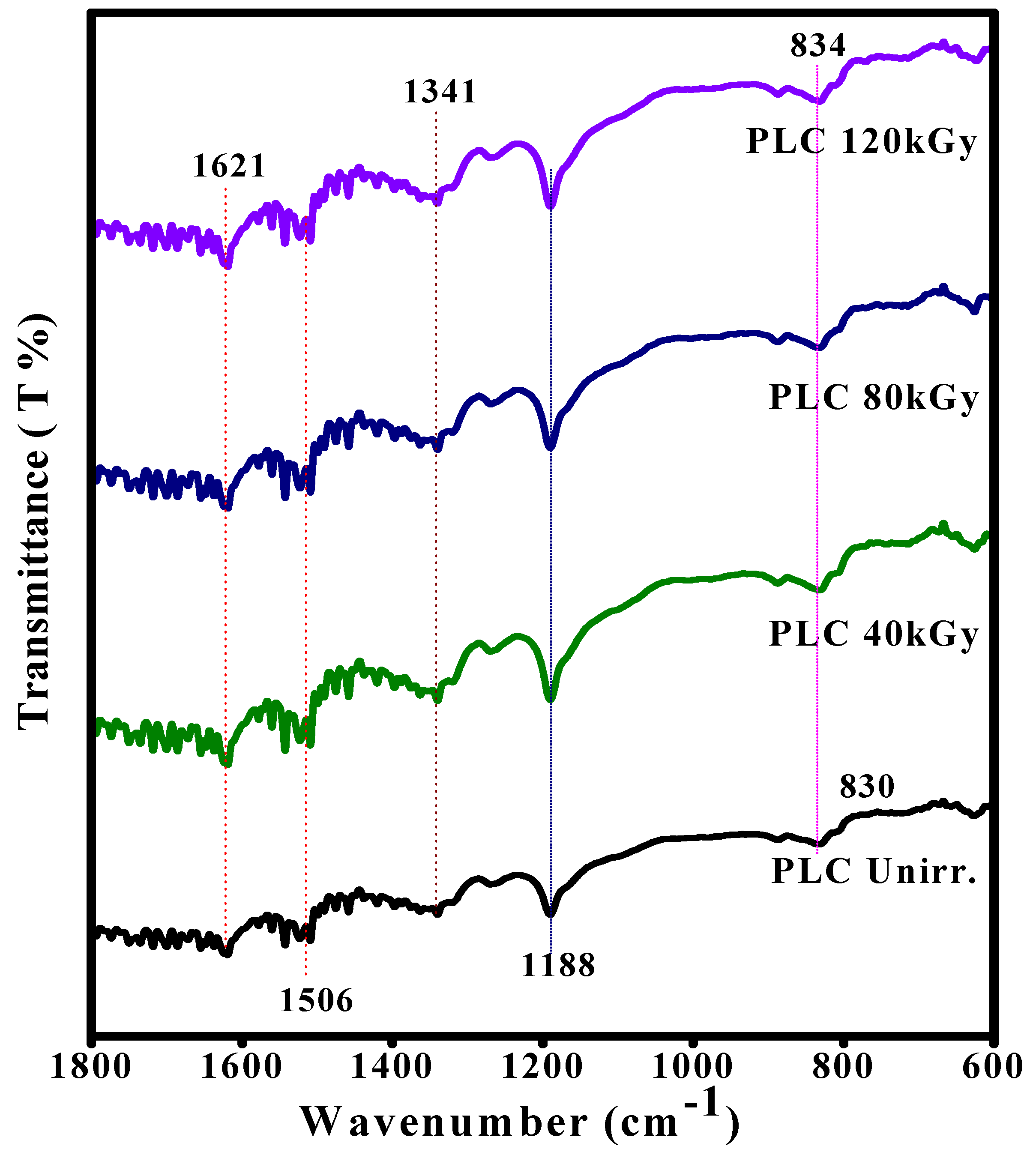
3.1. FTIR Analysis of EB-Irradiated PLC Nanocomposite
3.2. FESEM Studies of the PLC Nanocomposite
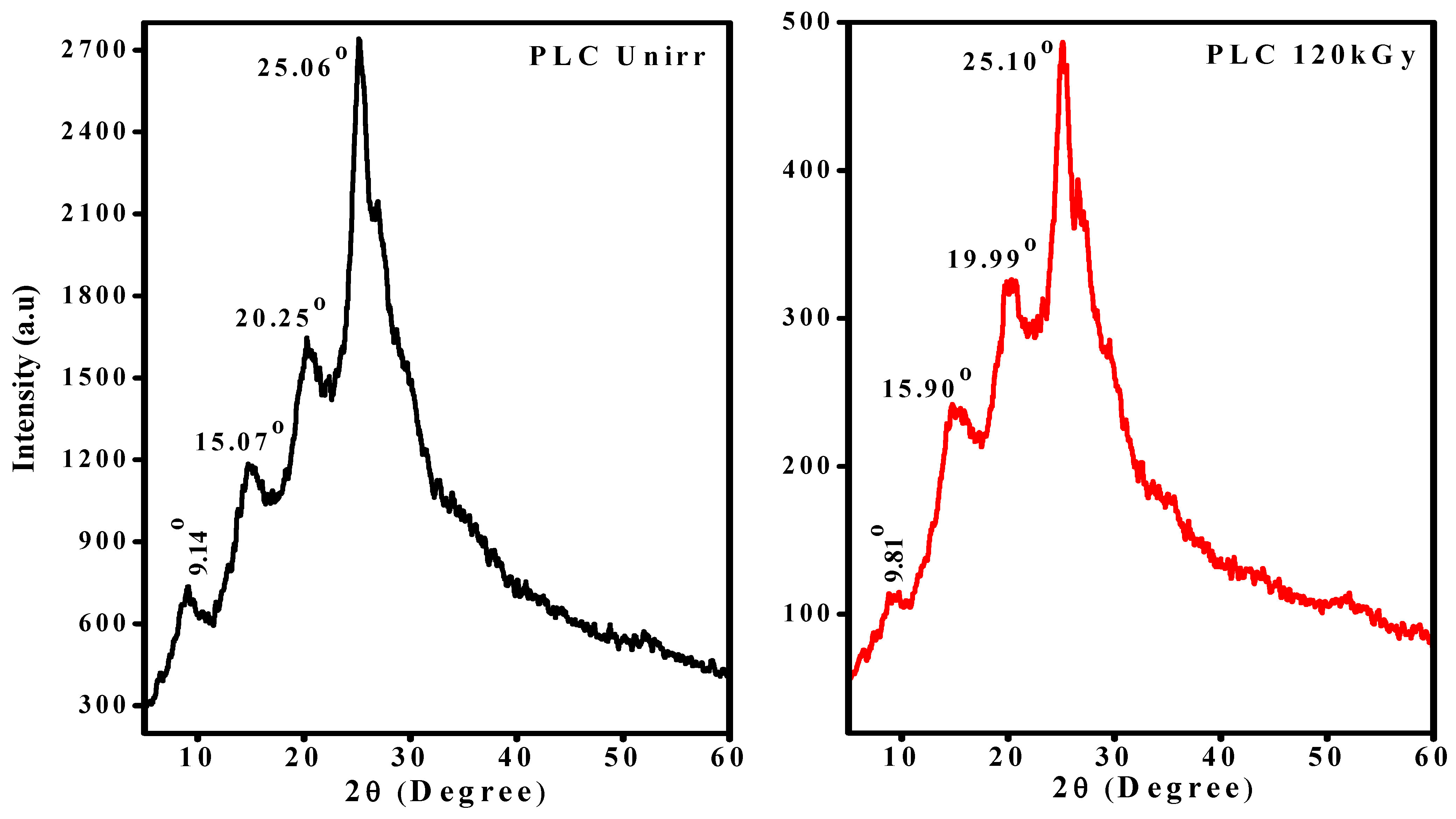
3.3. X-Ray Diffraction Study of EB-Irradiated PLC Nanocomposite
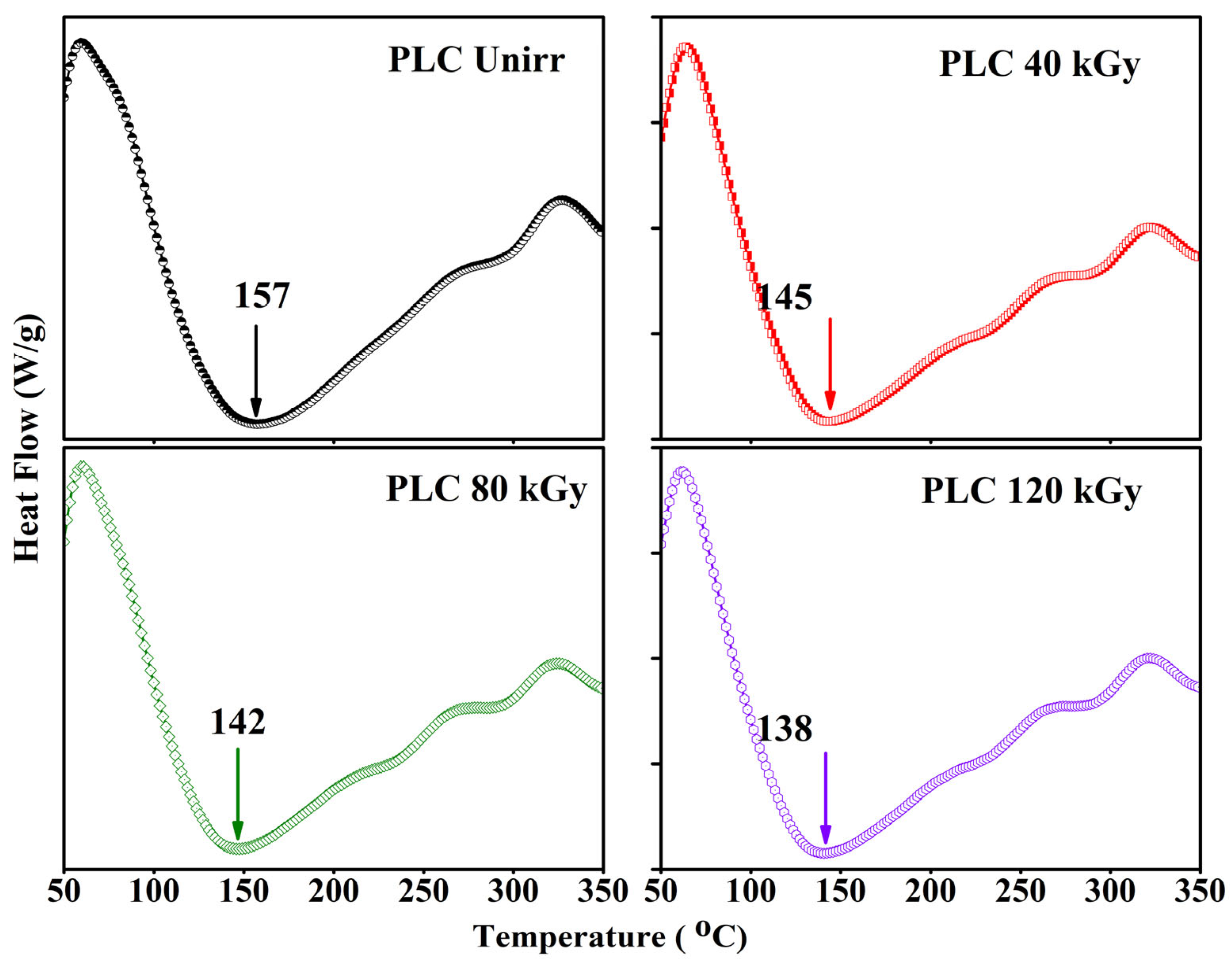
3.4. Thermal Analysis
3.5. UV-Visible Spectroscopy of the PLC Nanocomposite
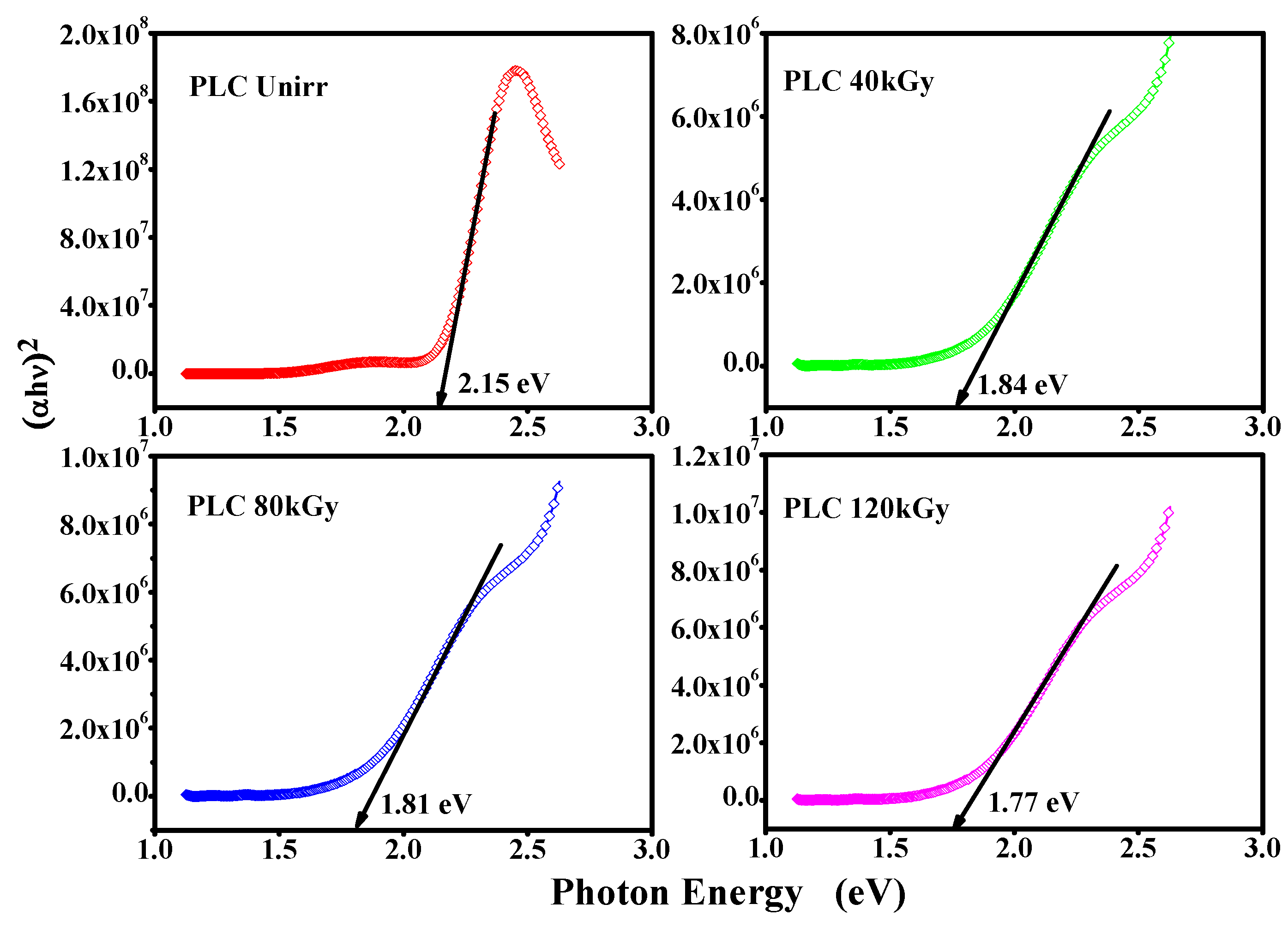
3.6. Optical Band Gap Study of the PLC Nanocomposite
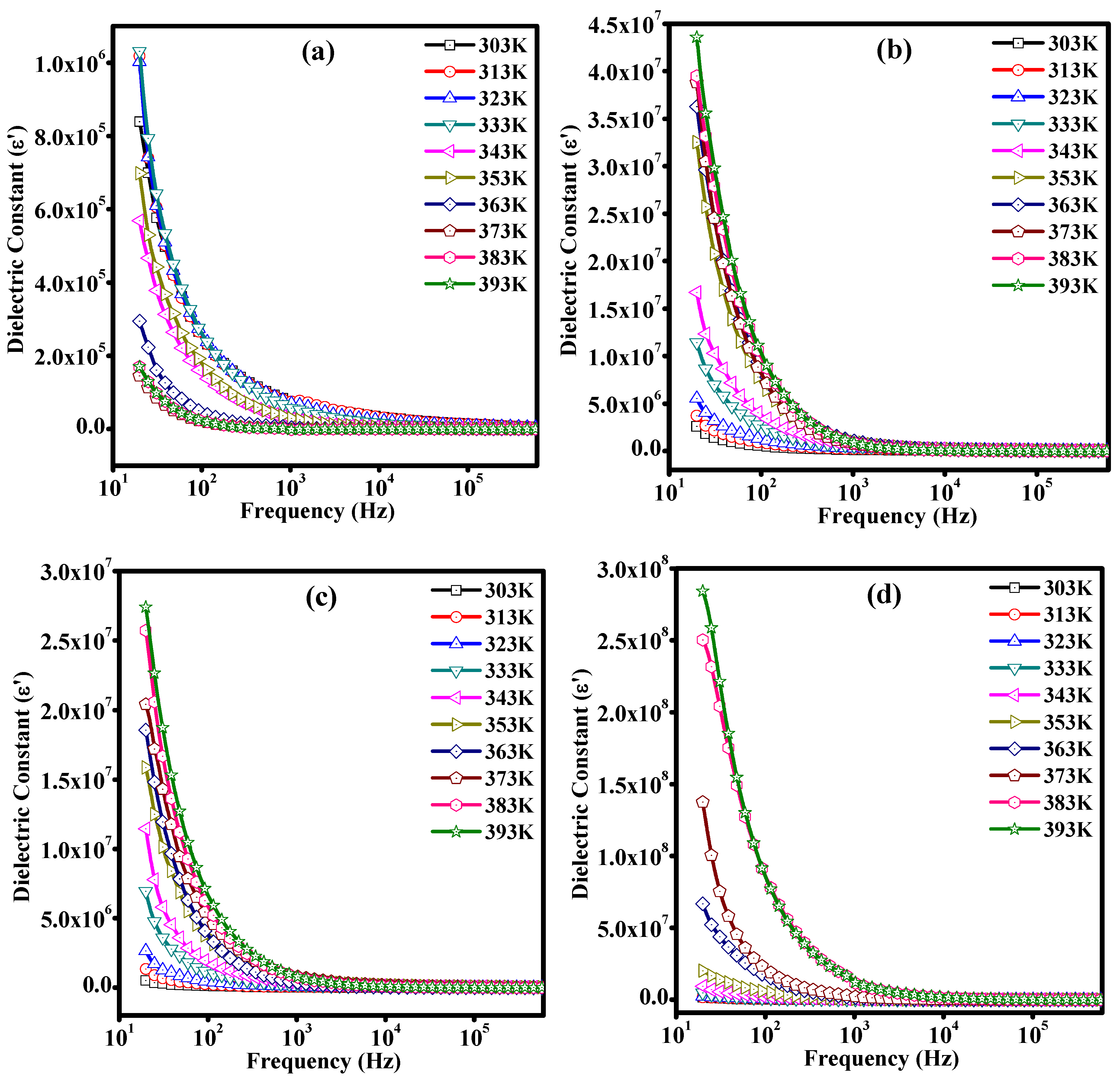
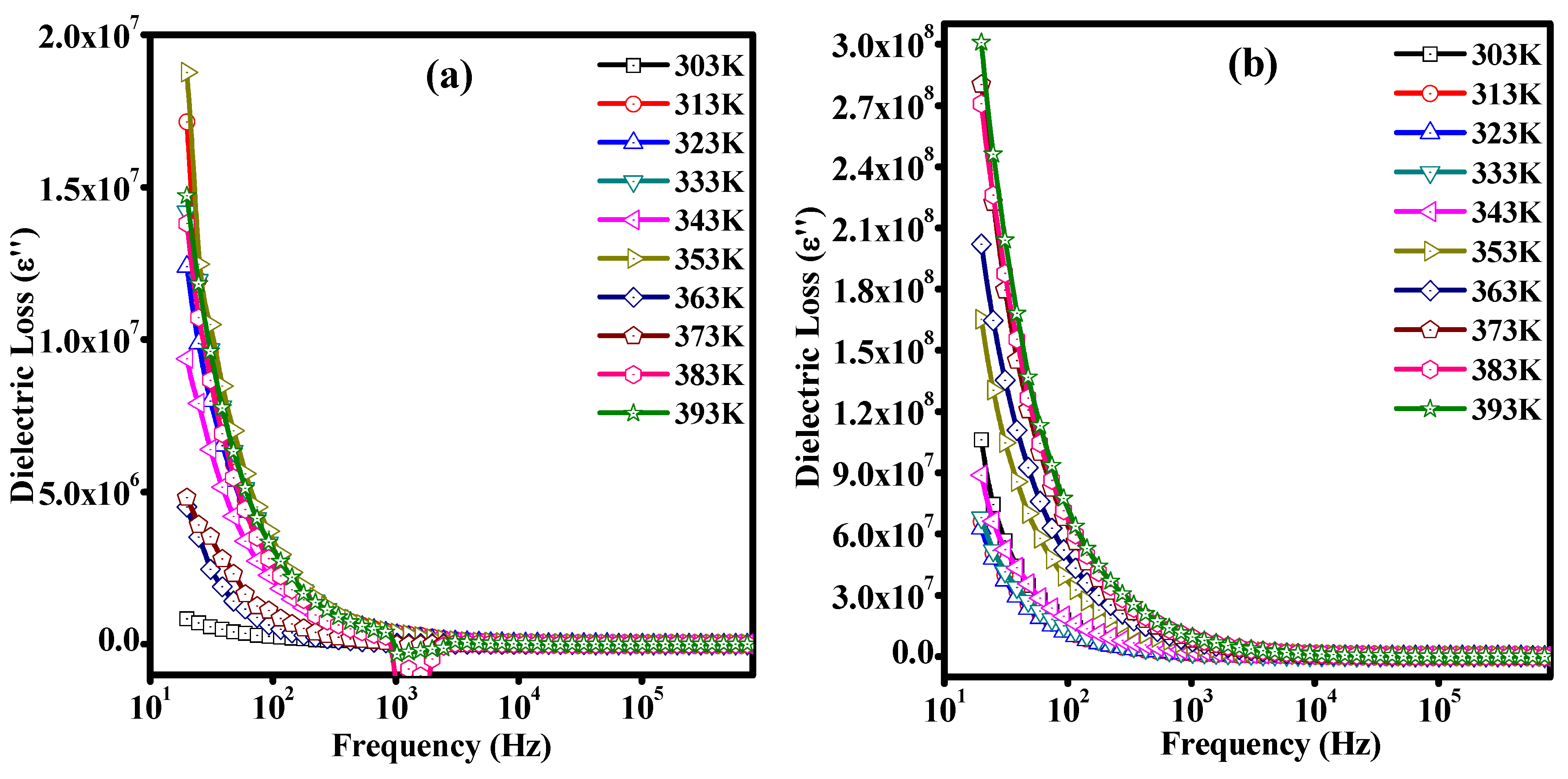
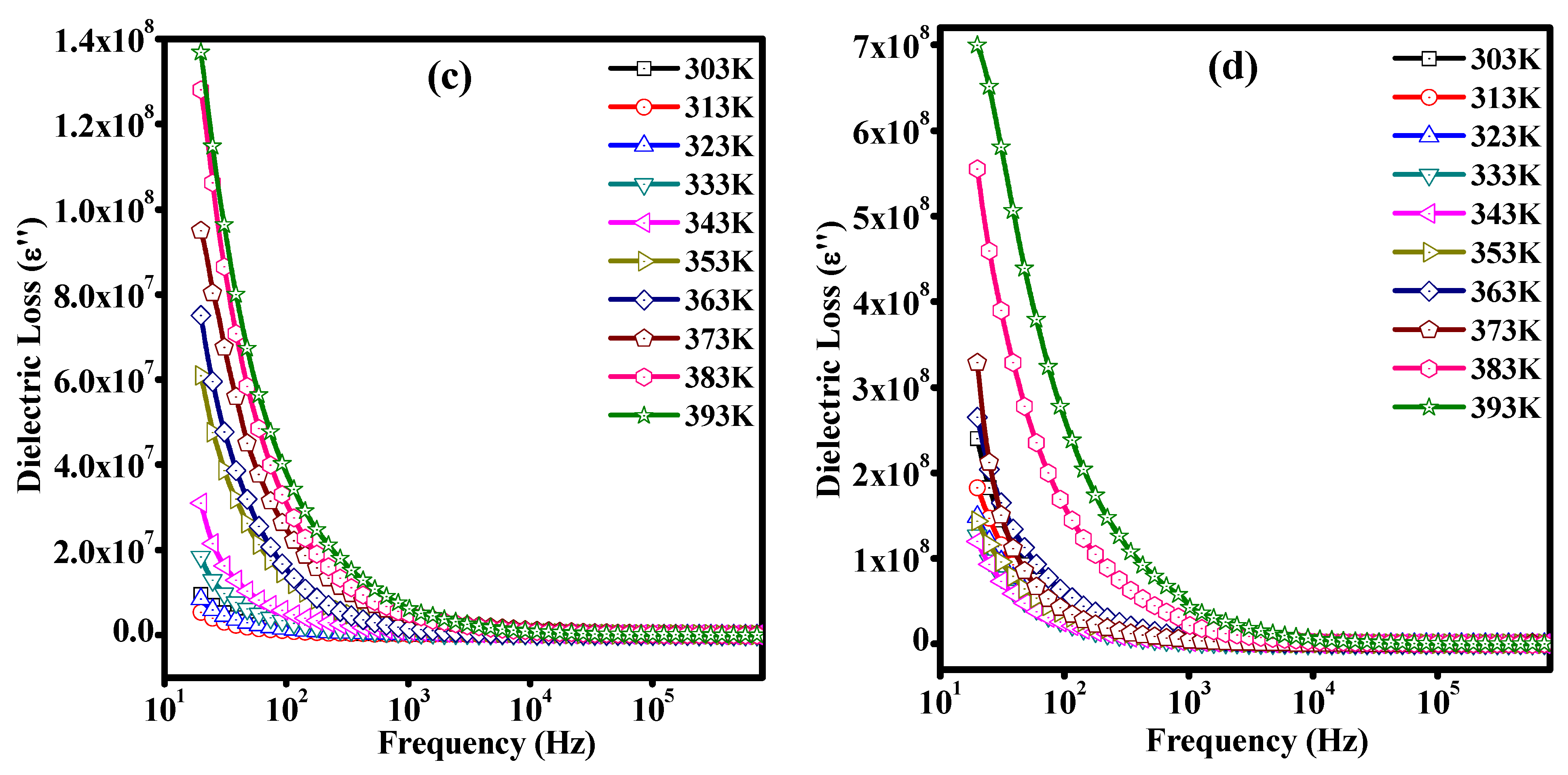
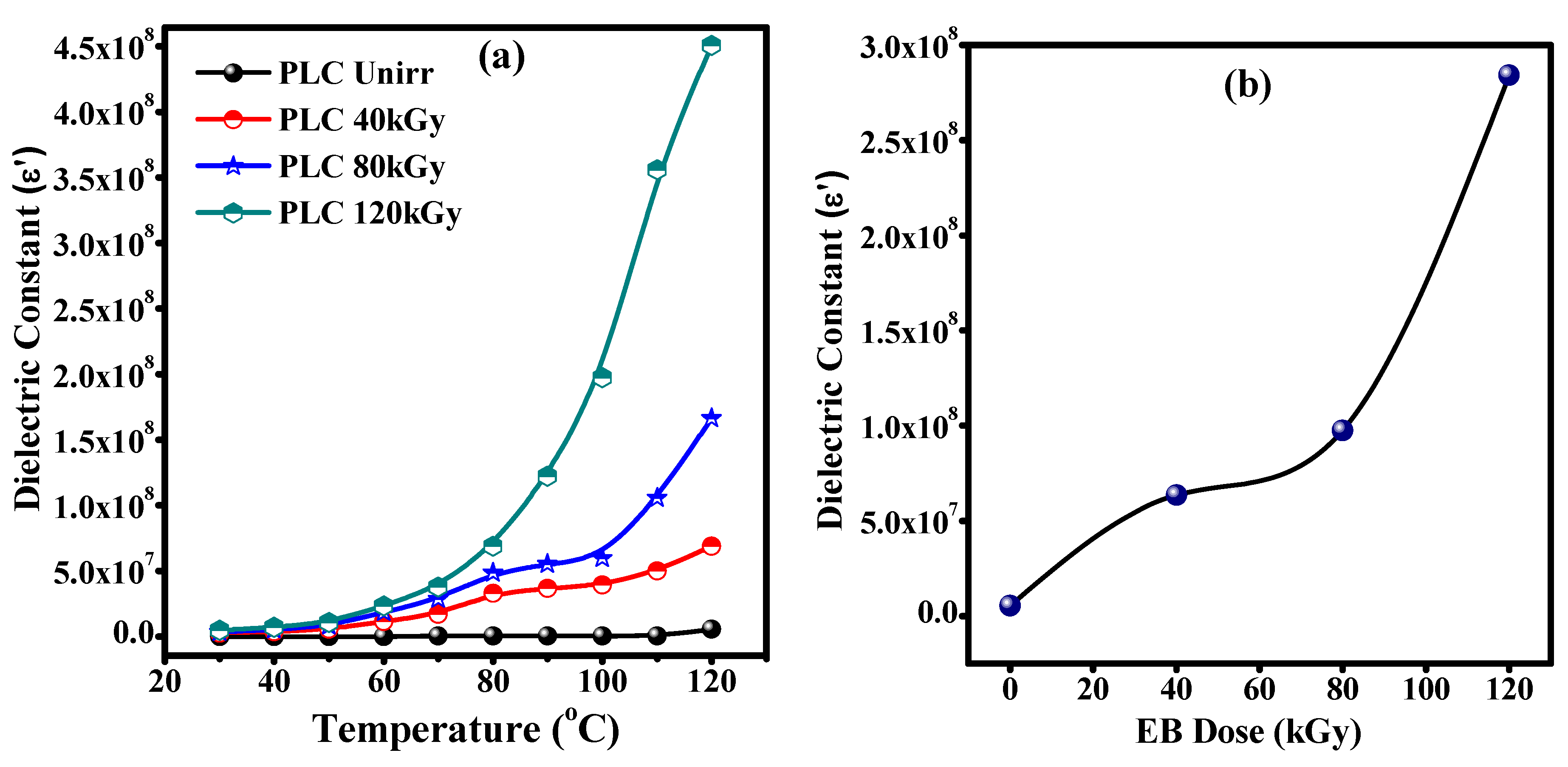
3.7. Dielectric Studies of Unirradiated and Irradiated PLC Nanocomposites
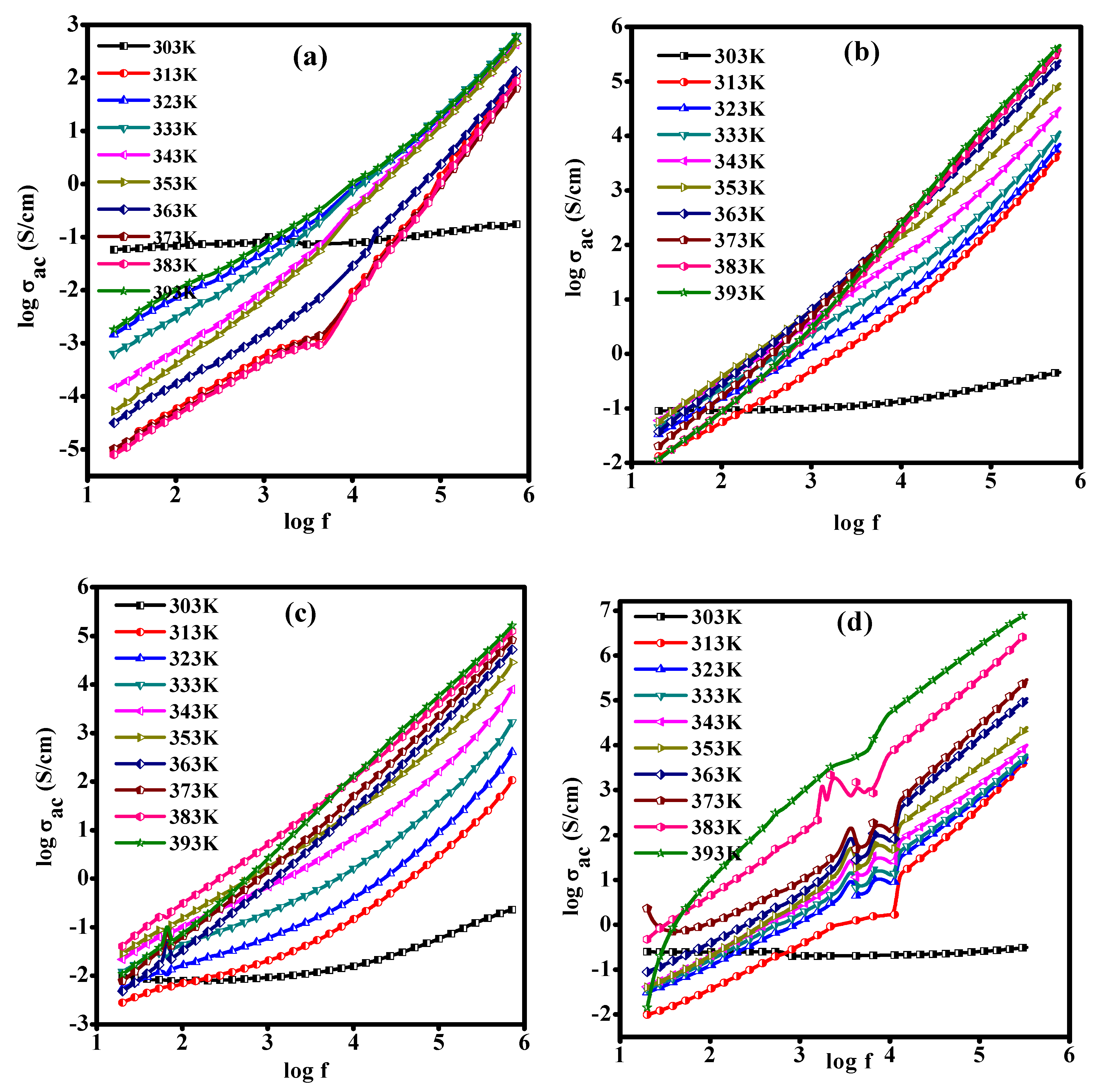
3.8. AC Conductivity of the PLC Nanocomposite
3.9. Temperature-Dependent Electrical Conductivity of the PLC Nanocomposite
3.10. Cyclic Voltammetry of EB-Irradiated PLC Nanocomposite
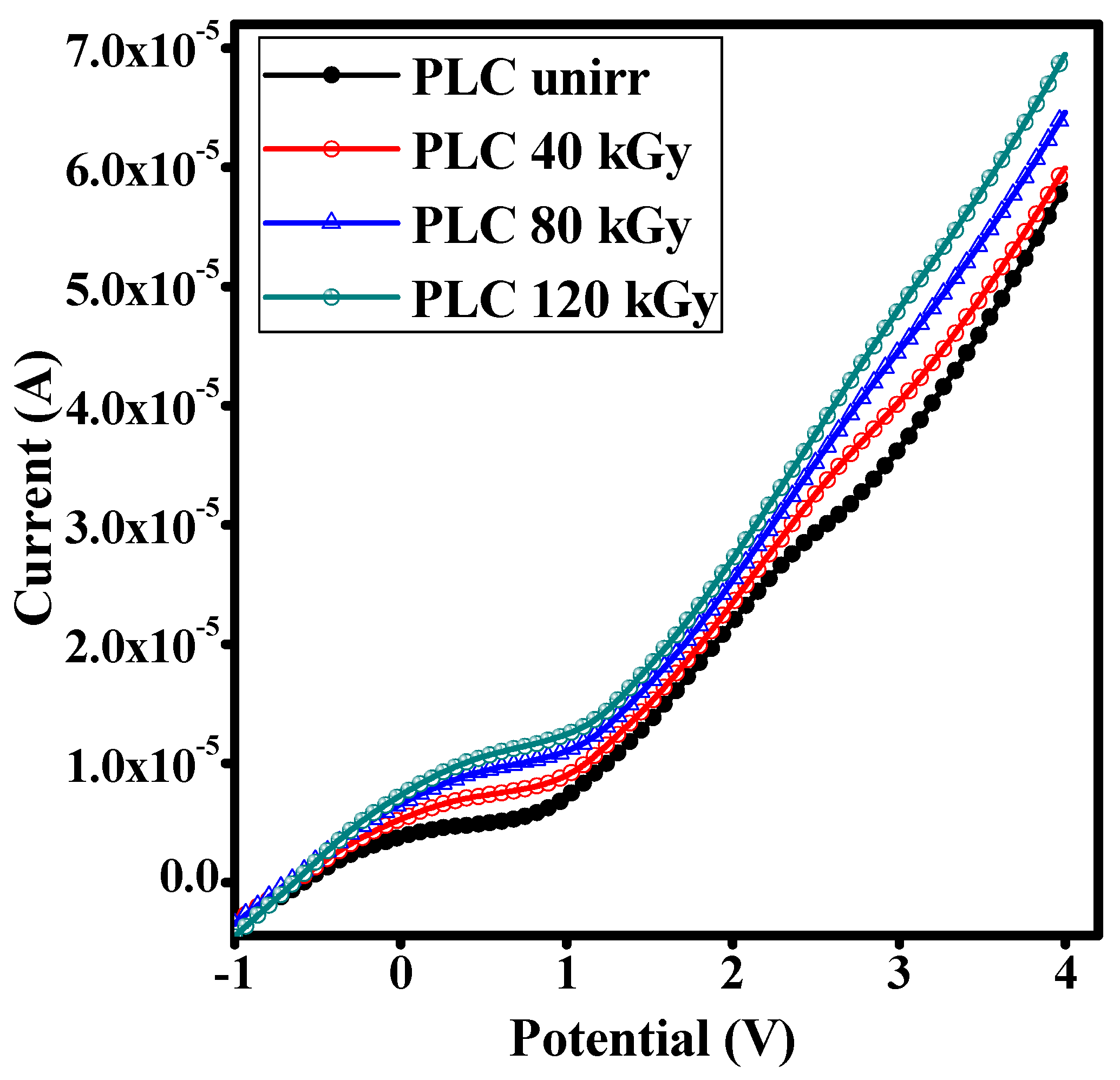
3.11. I-V Characteristic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drobny, J.G. Radiation Technology for Polymers; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Fifield, L.S.; Pharr, M.; Staack, D.; Pillai, S.D.; Nichols, L.; McCoy, J.; Faucette, T.; Bisel, T.T.; Huang, M.; Hasan, M.K. Direct comparison of gamma, electron beam and X-ray irradiation effects on single-use blood collection devices with plastic components. Radiat. Phys. Chem. 2021, 180, 109282. [Google Scholar] [CrossRef]
- Czvikovszky, T. Reactive recycling of multiphase polymer systems through electron beam. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1995, 105, 233–237. [Google Scholar] [CrossRef]
- Raghu, S.; Kilarkaje, S.; Sanjeev, G.; Nagaraja, G.; Devendrappa, H. Effect of electron beam irradiation on polymer electrolytes: Change in morphology, crystallinity, dielectric constant and AC conductivity with dose. Radiat. Phys. Chem. 2014, 98, 124–131. [Google Scholar] [CrossRef]
- Hong, Y.K.; Park, D.H.; Park, S.H.; Park, S.K.; Joo, J. Effects of electron-beam irradiation on conducting polypyrrole nanowires. Appl. Phys. Lett. 2009, 94, 053111. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials. J. Phys. Chem. B 2001, 105, 8475–8491. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. “Synthetic metals”: A novel role for organic polymers. Curr. Appl. Phys. 2001, 1, 269–279. [Google Scholar] [CrossRef]
- Rayar, A.; Chapi, S.; Murugendrappa, M.G.; Babaladimath, G.; Harish, K.; Kakarla, R.R.; Raghu, A.V. Organic conjugated polymers and their nanostructured composites: Synthesis methodologies and electrochemical applications. Nano-Struct. Nano-Objects 2024, 37, 101102. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liu, C.-J.; Mori, T. Polymer based thermoelectric nanocomposite materials and devices: Fabrication and characteristics. Nano Energy 2020, 78, 105186. [Google Scholar] [CrossRef]
- Kuznetsova, L.S.; Arlyapov, V.A.; Plekhanova, Y.V.; Tarasov, S.E.; Kharkova, A.S.; Saverina, E.A.; Reshetilov, A.N. Conductive polymers and their nanocomposites: Application features in biosensors and biofuel cells. Polymers 2023, 15, 3783. [Google Scholar] [CrossRef]
- Bhattacharyya, A. Conducting Polymers in Biosensing: A review. Chem. Phys. Impact 2024, 8, 100642. [Google Scholar] [CrossRef]
- Sood, Y.; Singh, K.; Mudila, H.; Lokhande, P.; Singh, L.; Kumar, D.; Kumar, A.; Mubarak, N.M.; Dehghani, M.H. Insights into properties, synthesis and emerging applications of polypyrrole-based composites, and future prospective: A review. Heliyon 2024, 10, e33643. [Google Scholar] [CrossRef] [PubMed]
- Clough, R. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 185, 8–33. [Google Scholar]
- Sabharwal, S. Electron beam irradiation applications. In Proceedings of the 25th North American Particle Accelerator Conference, Pasadena, CA, USA, 29 September–4 October 2013; pp. 745–748. [Google Scholar]
- Permana, A.A.; Chirasatitsin, S.; Putson, C. Electron-beam irradiation for boosting storage energy density of tuned poly (vinylidene fluoride-hexafluoropropylene)/graphene nanoplatelet polymer composites. Crystals 2020, 10, 633. [Google Scholar] [CrossRef]
- Yesappa, L.; Niranjana, M.; Ashokkumar, S.; Vijeth, H.; Raghu, S.; Devendrappa, H. Characterization, electrical conductivity and electrochemical performance of polyaniline-LiClO4-CuO nano composite for energy storage applications. Polym.-Plast. Technol. Mater. 2019, 58, 193–205. [Google Scholar]
- Yesappa, L.; Niranjana, M.; Ashokkumar, S.; Vijeth, H.; Sharanappa, C.; Raghu, S.; Devendrappa, H. Investigation of the structure, optical and electrical properties of lithium perchlorate doped polyaniline composite: Aloe vera used as a bio-plasticizer. J. Electron. Mater. 2017, 46, 6965–6976. [Google Scholar] [CrossRef]
- Dhanabalan, A.; Malmonge, J.A.; Riul Jr, A.; Faria, R.M.; Oliveira Jr, O. A study on X-ray irradiation of composite polyaniline LB films. Thin Solid Film. 1998, 327, 808–812. [Google Scholar] [CrossRef]
- Bodugoz, H.; Sevil, U.; Güven, O. Radiation-induced conductance in the blends of poly (aniline-base) with poly (vinyl chloride) and poly [(vinylidene chloride)-co-(vinyl acetate)]. Macromol. Symp. 2001, 169, 289–295. [Google Scholar]
- Sevil, U.; Güven, O.; Kovacs, A.; Slezsak, I. Gamma and electron dose response of the electrical conductivity of polyaniline based polymer composites. Radiat. Phys. Chem. 2003, 67, 575–580. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D. Degradation and stability of polyaniline on exposure to electron beam irradiation (structure–property relationship). Polym. Degrad. Stab. 2007, 92, 1824–1832. [Google Scholar]
- Güven, O. Radiation-induced conductivity control in polyaniline blends/composites. Radiat. Phys. Chem. 2007, 76, 1302–1307. [Google Scholar]
- Relekar, B.; Fulari, A.; Rath, M.; Fulari, V.; Lohar, G. Modification in porous MnO2/PANI composite using high-energy electron irradiation for electrochemical supercapacitor. J. Mater. Sci. Mater. Electron. 2020, 31, 11741–11747. [Google Scholar]
- Vallée, A.; Besner, S.; Prud’Homme, J. Comparative study of poly (ethylene oxide) electrolytes made with LiN(CF3SO2)2, LiCF3SO3 and LiClO4: Thermal properties and conductivity behaviour. Electrochim. Acta 1992, 37, 1579–1583. [Google Scholar]
- Atchaya, S.; Meena Devi, J. Experimental Investigation on Structural, Optical, Electrical and Magnetic Properties of Copper Oxide Nanoparticles. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2024, 94, 153–160. [Google Scholar]
- Ates, M.; Serin, M.A.; Ekmen, I.; Ertas, Y.N. Supercapacitor behaviors of polyaniline/CuO, polypyrrole/CuO and PEDOT/CuO nanocomposites. Polym. Bull. 2015, 72, 2573–2589. [Google Scholar]
- Patil, P.G.; Langade, K.J.; Vyawahare, S.K. Development of the PANI-CuO and PANI-CuO-GO Nanocomposite Architecture and Comparative Investigation of the Physicochemical, Optical and Electrochemical Properties Towards Supercapacitor Applications. J. Inorg. Organomet. Polym. Mater. 2024, 1–12. [Google Scholar] [CrossRef]
- Ramaprasad, A.; Rao, V.; Sanjeev, G. Synthesis of chitin-polyaniline nanocomposite by electron beam irradiation. J. Appl. Polym. Sci. 2011, 121, 623–633. [Google Scholar] [CrossRef]
- Zeghioud, H.; Lamouri, S.; Mahmoud, Y.; Hadj-Ali, T. Preparation and characterization of a new polyaniline salt with good conductivity and great solubility in dimethyl sulfoxyde. J. Serbian Chem. Soc. 2015, 80, 1435–1448. [Google Scholar]
- Chapi, S.; Murugendrappa, M.; Rayar, A.; Babaladimath, G.; Raghavendra, N.; Yazdi, M.K.; Ryl, J.; Saeb, M.R. Enhanced structural, electrical, and electrochemical performance of polyethylene oxides (PEO)-based polymer electrolytes for solid state K+ ion batteries. J. Appl. Polym. Sci. 2024, 141, e55390. [Google Scholar]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and structural analysis of polyaniline and poly (o-anisidine) layers generated in a DC glow discharge plasma by using an oblique angle electrode deposition configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef]
- Du, X.; Xu, Y.; Xiong, L.; Bai, Y.; Zhu, J.; Mao, S. Polyaniline with high crystallinity degree: Synthesis, structure, and electrochemical properties. J. Appl. Polym. Sci. 2014, 131, 40827. [Google Scholar]
- Gul, S.; Bilal, S. Synthesis and characterization of processable polyaniline salts. J. Phys. Conf. Ser. 2013, 439, 012002. [Google Scholar] [CrossRef]
- Wang, S.; Tan, Z.; Li, Y.; Sun, L.; Zhang, T. Synthesis, characterization and thermal analysis of polyaniline/ZrO2 composites. Thermochim. Acta 2006, 441, 191–194. [Google Scholar]
- Raghu, S.; Archana, K.; Sharanappa, C.; Ganesh, S.; Devendrappa, H. The physical and chemical properties of gamma ray irradiated polymer electrolyte films. J. Non-Cryst. Solids 2015, 426, 55–62. [Google Scholar]
- Singh, D.; Singh, N.; Qureshi, A.; Kulriya, P.; Tripathi, A.; Avasthi, D.; Gulluoglu, A.N. Radiation induced modification of dielectric and structural properties of Cu/PMMA polymer composites. J. Non-Cryst. Solids 2010, 356, 856–863. [Google Scholar] [CrossRef]
- Wang, B.; Gao, X.; Piao, G. Preparation of Polyaniline-Doped Fullerene Whiskers. Int. J. Polym. Sci. 2013, 2013, 867934. [Google Scholar] [CrossRef]
- Sonkawade, R.; Kumar, V.; Annapoorni, S.; Vaijapurkar, S.; Dhaliwal, A. Effects of gamma ray and neutron radiation on polyanilne conducting polymer. Indian J. Pure Appl. Phys. 2010, 48, 453–456. [Google Scholar]
- Mohd Meftah, A.; Gharibshahi, E.; Soltani, N.; Mat Yunus, W.M.; Saion, E. Structural, optical and electrical properties of PVA/PANI/Nickel nanocomposites synthesized by gamma radiolytic method. Polymers 2014, 6, 2435–2450. [Google Scholar] [CrossRef]
- Yesappa, L.; Niranjana, M.; Ashokkumar, S.; Vijeth, H.; Basappa, M.; Dwivedi, J.; Petwal, V.; Ganesh, S.; Devendrappa, H. Optical properties and ionic conductivity studies of an 8 MeV electron beam irradiated poly (vinylidene fluoride-co-hexafluoropropylene)/LiClO 4 electrolyte film for opto-electronic applications. RSC Adv. 2018, 8, 15297–15309. [Google Scholar]
- Jali, V.; Aparna, S.; Sanjeev, G.; Krupanidhi, S. ac conductivity studies on the electron irradiated BaZrO3 ceramic. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 257, 505–509. [Google Scholar]
- Hanafy, T.A. Dielectric relaxation and Schottky conduction of IR laser irradiated Makrofol-DE polycarbonate. J. Appl. Polym. Sci. 2012, 124, 1–8. [Google Scholar]
- Raghu, S.; Archana, K.; Sharanappa, C.; Ganesh, S.; Devendrappa, H. Electron beam and gamma ray irradiated polymer electrolyte films: Dielectric properties. J. Radiat. Res. Appl. Sci. 2016, 9, 117–124. [Google Scholar]
- Vishnuvardhan, T.; Kulkarni, V.; Basavaraja, C.; Raghavendra, S. Synthesis, characterization and ac conductivity of polypyrrole/Y2O3 composites. Bull. Mater. Sci. 2006, 29, 77–83. [Google Scholar] [CrossRef]
- Shah, S.; Singh, N.; Qureshi, A.; Singh, D.; Singh, K.; Shrinet, V.; Tripathi, A. Dielectric and structural modification of proton beam irradiated polymer composite. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2008, 266, 1768–1774. [Google Scholar]
- Nandihalli, N.; Gregory, D.H.; Mori, T. Energy-saving pathways for thermoelectric nanomaterial synthesis: Hydrothermal/solvothermal, microwave-assisted, solution-based, and powder processing. Adv. Sci. 2022, 9, 2106052. [Google Scholar]
- Tiptipakorn, S.; Suwanmala, P.; Hemvichian, K.; Pornputtanakul, Y. Effects of electron beam on irradiated polyimide/polyaniline composites. Adv. Mater. Res. 2012, 550, 861–864. [Google Scholar]
- Mahantesha, B.; Ravindrachary, V.; Padmakumari, R.; Sahanakumari, R.; Tegginamata, P.; Sanjeev, G.; Petwal, V.; Verma, V. Effect of electron irradiation on optical, thermal and electrical properties of polymer electrolyte. J. Radioanal. Nucl. Chem. 2019, 322, 19–27. [Google Scholar]
- Nanda, P.; Maity, S.; Pandey, N.; Ray, R.; Thakur, A.; Tarafdar, S. Conductivity enhancement in polymer electrolytes on gamma irradiation. Radiat. Phys. Chem. 2011, 80, 22–27. [Google Scholar]
- de Souza, V.S.; da Frota, H.O.; Sanches, E.A. Polyaniline-CuO hybrid nanocomposite with enhanced electrical conductivity. J. Mol. Struct. 2018, 1153, 20–27. [Google Scholar]
- Kim, J.; Ju, H.; Inamdar, A.I.; Jo, Y.; Han, J.; Kim, H.; Im, H. Synthesis and enhanced electrochemical supercapacitor properties of Ag–MnO2–polyaniline nanocomposite electrodes. Energy 2014, 70, 473–477. [Google Scholar]
- Bhowmik, K.L.; Deb, K.; Bera, A.; Nath, R.K.; Saha, B. Charge transport through polyaniline incorporated electrically conducting functional paper. J. Phys. Chem. C 2016, 120, 5855–5860. [Google Scholar]
- Vijeth, H.; Ashokkumar, S.; Yesappa, L.; Niranjana, M.; Vandana, M.; Devendrappa, H. Flexible and high energy density solid-state asymmetric supercapacitor based on polythiophene nanocomposites and charcoal. RSC Adv. 2018, 8, 31414–31426. [Google Scholar]
- Bhat, N.V.; Nate, M.M.; Gore, A.V.; Bhat, R.M. Interaction of gamma radiation with PVA/PANi composite films: Structural and morphological studies. J. Appl. Polym. Sci. 2008, 110, 2243–2252. [Google Scholar] [CrossRef]
- Benykhlef, S.; Bekhoukh, A.; Berenguer, R.; Benyoucef, A.; Morallon, E. PANI-derived polymer/Al2O3 nanocomposites: Synthesis, characterization, and electrochemical studies. Colloid Polym. Sci. 2016, 294, 1877–1885. [Google Scholar] [CrossRef]
- Raghu, S.; Devendrappa, H.; Ganesh, S.; Matteppanavar, S. Modification of PEO-based polymer electrolytes by electron beam irradiation for energy storage applications. Polym. Bull. 2023, 80, 381–394. [Google Scholar] [CrossRef]
- Chapi, S. Optical, electrical and electrochemical properties of PCL5/ITO transparent conductive films deposited by spin-coating—Materials for single-layer devices. J. Sci. Adv. Mater. Devices 2020, 5, 322–329. [Google Scholar] [CrossRef]
- Kolhar, P.; Sannakki, B.; Verma, M.; Suresha, S.; Alshehri, M.; Shah, N.A. Investigation of structural, dielectric and optical properties of polyaniline—Magnesium ferrite composites. Nanomaterials 2023, 13, 2234. [Google Scholar] [CrossRef]
- Devendrappa, H.; Yesappa, L.; Niranjana, M.; Ashokkumar, S.; Vijeth, H.; Ganesh, S. Morphology, optical and ionic conductivity studies of electron beam irradiated polymer electrolyte film. AIP Conf. Proc. 2018, 1942, 110003. [Google Scholar]

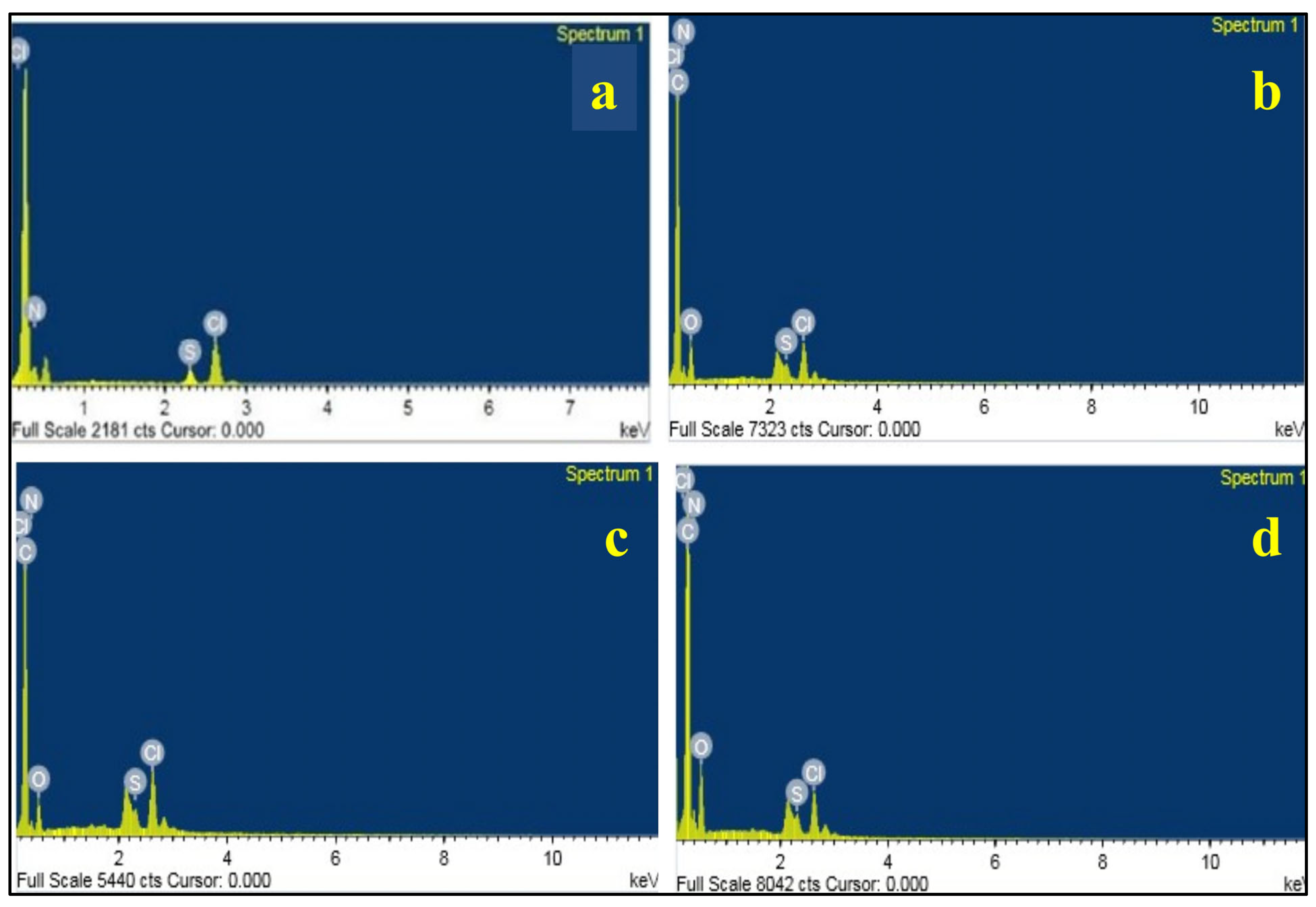




| Element | PLC Unirr | PLC 40 kGy | PLC 80 kGy | PLC 120 kGy | ||||
|---|---|---|---|---|---|---|---|---|
| Weight % | Atom % | Weight % | Atom % | Weight % | Atom % | Weight % | Atom % | |
| N | 0.05 | 23.89 | 0.57 | 24.52 | 0.41 | 23.23 | 1.01 | 25.35 |
| O | - | - | 1.00 | 37.55 | 0.67 | 32.98 | 1.83 | 40.26 |
| S | 0.02 | 4.58 | 0.11 | 2.07 | 0.10 | 2.46 | 0.18 | 1.99 |
| Cl | 0.11 | 22.00 | 0.81 | 13.68 | 0.89 | 19.88 | 0.99 | 9.81 |
| C | 0.03 | 17.98 | 0.44 | 22.19 | 0.33 | 21.46 | 0.77 | 22.58 |
| Sample | 2θ | d | Β | ɛ | R | D | % χ |
|---|---|---|---|---|---|---|---|
| PLC-Unirr | 25.06 | 3.550 | 4.8185 | 1.17 | 12.10 | 0.23 | 79.55 |
| 20.25 | 4.379 | 5.6662 | 1.34 | 7.35 | 0.24 | 76.49 | |
| 15.07 | 5.871 | 5.6662 | 1.40 | 5.48 | 0.25 | 73.76 | |
| 9.14 | 9.666 | 3.0989 | 0.77 | 4.44 | 0.44 | 59.19 | |
| PLC-120 kGy | 25.10 | 3.544 | 7.7873 | 1.90 | 11.27 | 0.18 | 59.23 |
| 19.99 | 4.436 | 6.9925 | 1.72 | 6.96 | 0.20 | 56.83 | |
| 15.90 | 5.567 | 6.9925 | 1.73 | 5.55 | 0.21 | 48.77 | |
| 9.81 | 9.006 | 5.8267 | 1.45 | 4.44 | 0.23 | 32.76 |
| Sl. No. | Name of the Sample | Dose Type (Irradiation) | Conductivity (S/cm) | Energy Band Gap (eV) | Ref. |
|---|---|---|---|---|---|
| 1. | PANI-derived polymer/Al2O3 nanocomposites | Unirr | 9.2 × 10−2 | - | [55] |
| 2. | PEO-based polymer electrolytes | Electron beam | 3.4 × 10−4 | - | [56] |
| 3 | IT5 (PCL5/ITO/glass) | Unirr. | 1.21 × 10−3 | 3.47 | [57] |
| 4 | MgFe2O4 Composites | Unirr. | - | 2.52 | [58] |
| 5 | PEO:Li2SO4 polymer electrolyte | Electron beam | 1.88 × 10−4 | - | [4] |
| 6 | PEO: CdCl2 (75:25%) | Electron beam | 1.74 × 10−4 | - | [43] |
| 7 | PVdF-co-HFP: LiClO4 = 90:10 | Electron beam | 8.28 × 10−4 | 2.65 | [59] |
| 8 | PEO-CdCl2 polymer electrolyte | Gamma ray | 1.15 × 10−4 | 3.80 | [35] |
| 9 | PANI/LiClO4/CuO nanocomposite | Unirr | 8.77 × 10–5 | 2.15 | Present work |
| 10 | PANI/LiClO4/CuO nanocomposite | Electron Beam | 1.05 × 10−4 | 1.77 | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laxmayyaguddi, Y.; Chapi, S.; Nandihalli, N. Electron Beam Irradiation’s Effect on Polyaniline/LiClO4/CuO Nanocomposite: A Study of Dielectric, Conductivity and Electrochemical Properties. Appl. Sci. 2025, 15, 4001. https://doi.org/10.3390/app15074001
Laxmayyaguddi Y, Chapi S, Nandihalli N. Electron Beam Irradiation’s Effect on Polyaniline/LiClO4/CuO Nanocomposite: A Study of Dielectric, Conductivity and Electrochemical Properties. Applied Sciences. 2025; 15(7):4001. https://doi.org/10.3390/app15074001
Chicago/Turabian StyleLaxmayyaguddi, Yesappa, Sharanappa Chapi, and Nagaraj Nandihalli. 2025. "Electron Beam Irradiation’s Effect on Polyaniline/LiClO4/CuO Nanocomposite: A Study of Dielectric, Conductivity and Electrochemical Properties" Applied Sciences 15, no. 7: 4001. https://doi.org/10.3390/app15074001
APA StyleLaxmayyaguddi, Y., Chapi, S., & Nandihalli, N. (2025). Electron Beam Irradiation’s Effect on Polyaniline/LiClO4/CuO Nanocomposite: A Study of Dielectric, Conductivity and Electrochemical Properties. Applied Sciences, 15(7), 4001. https://doi.org/10.3390/app15074001






