Plant Antimicrobial Compounds and Their Mechanisms of Action on Spoilage and Pathogenic Bacteria: A Bibliometric Study and Literature Review
Abstract
1. Introduction
2. Bibliometric Analysis
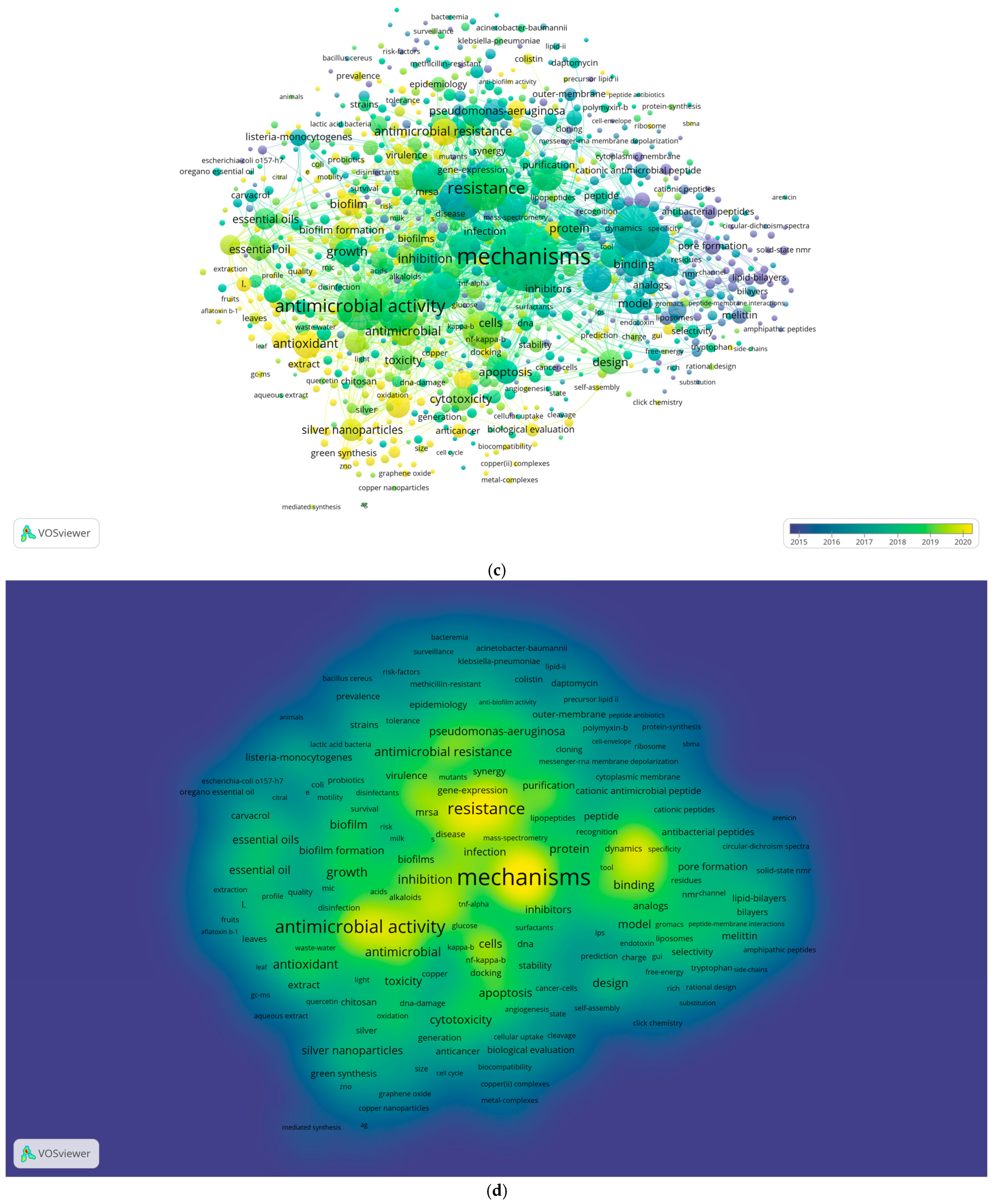
2.1. Bibliometric Mapping and Analysis
2.2. Authors’ Keywords Analysis
- The option “Create map based on bibliographic data” was selected to extract keyword co-occurrence relationships from the dataset;
- In the “Choose data source” section, the option “Read data from reference manager files” was selected;
- A RIS file (Research Information Systems file) containing the bibliometric dataset was uploaded in the “Select files” step;
- In the “Choose type of analysis and counting method” section, “Co-occurrence” was selected as the type of analysis, “Keywords” as the unit of analysis, and “Full counting” as the counting method. At this stage, a thesaurus file (CSV format) was included to standardize terms by grouping similar expressions. Specifically, the terms “mechanism”, “mechanisms”, “mechanism of action”, “mechanisms of action”, “molecular-mechanisms”, and “molecular-mechanism” were replaced with “mechanisms”. Similarly, “system” and “systems” were unified under “systems”, “enzyme” and “enzymes” were grouped as “enzymes”, and “gene” and “genes” were consolidated under “genes”;
- A threshold was applied by setting the “Minimum number of documents of an author” to five, filtering out less frequently occurring terms. Of the initial 29,361 keywords, 2794 met the threshold;
- To refine the visualization, the “Number of keywords to be selected” was set to 1000, focusing on the most relevant terms;
- The map was generated, and three visualizations—network, overlay, and density—were created to illustrate keyword relationships, temporal trends, and research intensity;
3. Mechanisms of Action of Antimicrobial Compounds
4. Concluding Remarks and Perspectives
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Feng, Y.; Han, X.; Cai, X.; Yang, L.; Liu, C.; Shen, L. Inhibition of virulence factors and biofilm formation by wogonin attenuates pathogenicity of Pseudomonas aeruginosa PAO1 via targeting PQS quorum-sensing system. Int. J. Mol. Sci. 2021, 22, 2699. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Smirnov, A.N.; Zavriev, S.K.; Grishin, E.V.; Vasilchenko, A.V.; Rogozhin, E.A. Novel thionins from black seed (Nigella sativa L.) demonstrate antimicrobial activity. Int. J. Pept. Res. Ther. 2017, 23, 171–180. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.; Zhao, Z.; Yu, S. The Antibiotic Activity and Mechanisms of Sugarcane (Saccharum Officinarum L.) Bagasse Extract against Food-Borne Pathogens. Food Chem. 2015, 185, 112–118. [Google Scholar] [CrossRef]
- He, N.; Wang, P.; Wang, P.; Ma, C.; Kang, W. Antibacterial mechanism of chelerythrine isolated from root of Toddalia asiatica (Linn) lam. BMC Complement. Altern. Med. 2018, 18, 261. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.D.; Hong, J.; Liu, C.; Zhang, X.L.; Zheng, J.P.; Xu, Y.J.; Ou, Z.Y.; Zheng, J.L.; Yu, D.J. Inhibitory effect of two traditional chinese medicine monomers, berberine and matrine, on the quorum sensing system of antimicrobial-resistant Escherichia coli. Front. Microbiol. 2019, 10, 2584. [Google Scholar] [CrossRef]
- Chen, D.H. In vitro antibacterial activity of alkaloids from Sophora flavescens. Chin. Anim. Health 2010, 12, 28–30. [Google Scholar]
- Zhang, Y.; Feng, R.; Li, L.; Zhou, X.; Li, Z.; Jia, R.; Song, X.; Zou, Y.; Yin, L.; He, C.; et al. The antibacterial mechanism of terpinen-4-ol against Streptococcus agalactiae. Curr. Microbiol. 2018, 75, 1214–1220. [Google Scholar] [CrossRef]
- Banerjee, M.; Parai, D.; Chattopadhyay, S.; Mukherjee, S.K. Andrographolide: Antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiol. 2017, 62, 237–244. [Google Scholar] [CrossRef]
- Gallegos-Flores, A.; Pozos-Guillén, A.; Martínez-Cruz, J. Evaluación de la actividad antibacteriana de compuestos terpenoides. Trop. Subtrop. Agroecosystems 2019, 22, 241–248. [Google Scholar]
- Reiter, J.; Hübbers, A.M.; Albrecht, F.; Leichert, L.I.O.; Slusarenko, A.J. Allicin, a natural antimicrobial defence substance from garlic, inhibits DNA gyrase activity in bacteria. Int. J. Med. Microbiol. 2020, 310, 151359. [Google Scholar] [CrossRef]
- Tang, Y.; Li, F.; Gu, D.; Wang, W.; Huang, J.; Jiao, X. Antimicrobial Effect and the Mechanism of Diallyl trisulfide against Campylobacter jejuni. Antibiotics 2021, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, T.H.; Warming, A.N.; Vejborg, R.M.; Moscoso, J.A.; Stegger, M.; Lorenzen, F.; Rybtke, M.; Andersen, J.B.; Petersen, R.; Andersen, P.S.; et al. A broad range quorum sensing inhibitor working through SRNA inhibition. Sci. Rep. 2017, 7, 9857. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, F.O.; Oyedeji, O.; Osundahunsi, M.T. Antimicrobial and antioxidant properties of Kaempferol-3-O-glucoside and 1-(4-hydroxyphenyl)-3-phenylpropan-1-one isolated from the leaves of Annona muricata (Linn.). J. Pharm. Res. Int. 2019, 26, 1–13. [Google Scholar] [CrossRef]
- Narmani, A.; Teponno, R.B.; Helaly, S.E.; Arzanlou, M.; Stadler, M. Cytotoxic, anti-biofilm and antimicrobial polyketides from the plant associated fungus Chaetosphaeronema achilleae. Fitoterapia 2019, 139, 104390. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Digra, S.; Nonzom, S. An insight into endophytic antimicrobial compounds: An updated analysis. Plant Biotechnol. Rep. 2023, 17, 427–457. [Google Scholar] [CrossRef]
- Teshome, E.; Forsido, S.F.; Rupasinghe, H.P.V.; Olika Keyata, E. Potentials of natural preservatives to enhance food safety and shelf life: A review. Sci. World J. 2022, 2022, 9901018. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Sridhar, S.; Forrest, S.; Pickard, D.; Cormie, C.; Lees, E.A.; Thomson, N.R.; Dougan, G.; Baker, S. Inhibitory concentrations of ciprofloxacin induce an adaptive response promoting the intracellular survival of Salmonella enterica serovar Typhimurium. mBio 2021, 12, e0109321. [Google Scholar] [CrossRef]
- Oliver, S.P.; Murinda, S.E.; Jayarao, B.M. Impact of antibiotic use in adult dairy cows on antimicrobial resistance of veterinary and human pathogens: A comprehensive review. Foodborne Pathog. Dis. 2011, 8, 337–355. [Google Scholar] [CrossRef]
- Abdallah, E.M.; Alhatlani, B.Y.; de Paula Menezes, R.; Martins, C.H.G. Back to nature: Medicinal plants as promising sources for antibacterial drugs in the post-antibiotic era. Plants 2023, 12, 3077. [Google Scholar] [CrossRef] [PubMed]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Zhou, W.; Deng, Z.; Liu, Y.; Shen, H.; Deng, H.; Xiao, H. Global research trends of artificial intelligence on histopathological images: A 20-year bibliometric analysis. Int. J. Environ. Res. Public Health 2022, 19, 1597. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rao, Y.; Yin, Y.; Li, Y.; Lin, Z.; Zhang, B. A bibliometric analysis of global trends in the research field of pharmaceutical care over the past 20 years. Front. Public. Health 2022, 10, 980866. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An r-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020; Available online: https://www.r-project.org/ (accessed on 20 December 2024).
- R Studio Team. R Studio: Integrated Development Environment for R; R Studio Team: Boston, MA, USA, 2022; Available online: http://www.rstudio.com/ (accessed on 10 December 2024).
- Yelamanchi, R.; Agrawal, H.; Gupta, N. Author level metrics and academic productivity. Int. J. Surg. 2021, 90, 105965. [Google Scholar] [CrossRef]
- Sinkovics, N. Enhancing the foundations for theorising through bibliometric mapping. Int. Mark. Rev. 2016, 33, 327–350. [Google Scholar] [CrossRef]
- García-Curiel, L.; Pérez-Flores, J.G.; González-Olivares, L.G.; Guerrero-Solano, J.A.; Contreras-López, E.; Pérez-Escalante, E.; Portillo-Torres, L.A.; Sebastián-Nicolás, J.L. Probiotics and metabolic syndrome: A bibliometric analysis and overview of dietary interventions. In Weight Loss—A Multidisciplinary Perspective; Himmerich, H., Ed.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Pérez-Flores, J.G.; García-Curiel, L.; Pérez-Escalante, E.; Contreras-López, E.; Olloqui, E.J. Arabinoxylans matrixes as a potential material for drug delivery systems development—A bibliometric analysis and literature review. Heliyon 2024, 10, e25445. [Google Scholar] [CrossRef]
- de Sousa, F. A simplified bibliometric mapping and analysis about sustainable polymers. Mater. Today Proc. 2021, 49, 2025–2033. [Google Scholar] [CrossRef]
- Dias, C.; Aires, A.; Saavedra, M.J. Antimicrobial activity of isothiocyanates from cruciferous plants against Methicillin-Resistant Staphylococcus aureus (MRSA). Int. J. Mol. Sci. 2014, 15, 19552–19561. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Nasário, F.; Silva-Gonçalves, L.; Tiera, V.; Arcisio-Miranda, M.; Tiera, M.; Cabrera, M. Chitosan derivatives targeting lipid bilayers: Synthesis, biological activity and interaction with model membranes. Carbohyd Polym. 2018, 181, 1213–1223. [Google Scholar] [CrossRef]
- Zhu, Z.; Min, T.; Zhang, X.; Wen, Y. Microencapsulation of thymol in poly(lactide-co-glycolide) (PLGA): Physical and antibacterial properties. Materials 2019, 12, 1133. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.; Barriga, A.; Alberício, F.; Romero, M.; Guzmán, F. Identification of peptides in flowers of Sambucus nigra with antimicrobial activity against aquaculture pathogens. Molecules 2018, 23, 1033. [Google Scholar] [CrossRef]
- Gao, F.; Ahmed, A.; Cong, H.; Yu, B.; Shen, Y. Effective strategies for developing potent, broad-spectrum antibacterial and wound healing promotion from short-chain antimicrobial peptides. ACS Appl. Mater. Interfaces 2023, 15, 32136–32147. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Li, H.; Tao, Y.; JiaYong, L.; Yuan, W.; Chen, Y.; Liu, S. In vitro and in vivo evaluation of mem-brane-active flavone amphiphiles: Semisynthetic kaempferol-derived antimicrobials against drug-resistant gram-positive bacteria. J. Med. Chem. 2020, 63, 5797–5815. [Google Scholar] [CrossRef]
- Nagabushan, C.; Govindaraju, S.; Shivamallu, C. Molecular interaction studies for inhibition of the Streptococcus pneumoniae competence stimulating peptide (CSP1) by potent plant-derived compounds. Int. J. Pharm. Bio Sci. 2020, 10, 154–166. [Google Scholar] [CrossRef]
- Moummou, H.; Meftah, I. Natural medicine: In-depth exploration of Moringa oleifera’s bioactive compounds and antimicrobial effects. In The Global Burden of Disease and Risk Factors—Understanding and Management; Mukadder, M., Murat, C., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Nourbakhsh, F.; Lotfalizadeh, M.; Badpeyma, M.; Shakeri, A.; Soheili, V. From plants to antimicrobials: Natural products against bacterial membranes. Phytother. Res. 2021, 36, 33–52. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards Advances in Medicinal Plant Antimicrobial Activity: A Review Study on Challenges and Future Perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Barreto-Santamaría, A.; Rivera-Monroy, Z.; García-Castañeda, J.; Curtidor, H.; Patarroyo, M.; Patarroyo, M.; Arévalo-Pinzón, G. Shorter antibacterial peptide having high selectivity for E. coli membranes and low potential for inducing resistance. Microorganisms 2020, 8, 867. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, and therapeutic strategies. Signal Transduct. Target Ther. 2022, 7, 199. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; Miguel, T. Antimicrobial activity of polyphenols and natural polyphenolic extracts on clinical isolates. Antibiotics 2021, 11, 46. [Google Scholar] [CrossRef]
- Ellward, G.; Binda, M.; Dzurny, D.; Bucher, M.; Dees, W.; Czyż, D. A screen of traditional chinese medicinal plant extracts reveals 17 species with antimicrobial properties. Antibiotics 2024, 13, 1220. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Silva, A.; Magalhães, L.; Magalhães, M.; Magalhães, T.; Franco, E.; Viana, D. Synthesis of silver nanoparticles and evaluation of antimicrobial activity using the aqueous extract of Pterodon emarginatus seeds. Cureus 2024, 16, e76382. [Google Scholar] [CrossRef]
- Sakata, N.; Haraguchi, T.; Masuo, S.; Ishiga, T.; Ishiga, Y. Pseudomonas cannabina pv. alisalensis virulence factors are involved in resistance to plant-derived antimicrobials during infection. Plants 2022, 11, 1742. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, Y.; Nishimura, T.; Harada, M.; Kashiwagi, R.; Yamamoto, M.; Noutoshi, Y.; Matsui, H. Role of two sets of RND-type multidrug efflux pump transporter genes, mexAB-oprM and mexEF-oprN, in virulence of Pseudomonas syringae pv. tabaci 6605. Plant Pathol. J. 2020, 36, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Costa, P.A.D.; Ribon, A.; Purgato, G.; Diaz-Muñoz, G.; Diaz, M. Plant extracts display synergism with different classes of antibiotics. An Aca Bras Ciênc 2019, 91, e20180117. [Google Scholar] [CrossRef]
- Zhai, X.; Wu, G.; Tao, X.; Yang, S.; Lv, L.; Zhu, Y.; Xiang, H. Success stories of natural product-derived compounds from plants as multidrug resistance modulators in microorganisms. RSC Adv. 2023, 13, 7798–7817. [Google Scholar] [CrossRef]
- Nguyen, T.; Thi, N.; Diep, X.; Nguyen, T.; Bui, L. Antimicrobial resistance tendency and collateral sensitivity of Staphylococcus aureus adapted to antibiotics or extracts of medicinal plants grown in Viet Nam. Lett. Appl. Microbiol. 2022, 75, 616–622. [Google Scholar] [CrossRef]
- Jubair, N.; Rajagopal, M.; Chinnappan, S.; Abdullah, N.; Fatima, A. Review on the antibacterial mechanism of plant-derived compounds against multidrug-resistant bacteria (MDR). Evid. Based Complement. Alternat Med. 2021, 16, 1–30. [Google Scholar] [CrossRef]
- Gangwar, B.; Kumar, S.; Darokar, M. Antioxidant phytochemicals as novel therapeutic strategies against drug-resistant bacteria. In Importance of Oxidative Stress and Antioxidant System in Health and Disease; Suna Sabuncuoğlu, S., Yalcinkaya, A., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Khan, H. Exploring the combined efficacy of carvacrol and friedelin against multi-drug resistant bacteria in upper and lower respiratory tract infections. Ann. Experiment Mol. Biol. 2024, 6, 1–7. [Google Scholar] [CrossRef]
- Rahman, H.; Khan, I.; Hussain, A.; Shahat, A.; Tawab, A.; Qasim, M.; Khan, S. Glycyrrhiza glabra HPLC fractions: Identification of aldehydo isoophiopogonone and liquirtigenin having activity against multidrug resistant bacteria. BMC Complement Altern. Med. 2018, 18, 140. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, H.; Kurushima, J.; Hashimoto, Y.; Tomita, H. Overview of bacterial two-component regulatory systems as potential targets for antimicrobial chemotherapy. Antibiotics 2020, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Multari, R.; Cremers, D.; Bostian, M.; Dupre, J.; Gustafson, J. Proof of principle for a real-time pathogen isolation media diagnostic: The use of laser-induced breakdown spectroscopy to discriminate bacterial pathogens and antimicrobial-resistant Staphylococcus aureus strains grown on blood agar. J. Pathog. 2013, 2013, 898106. [Google Scholar] [CrossRef]
- Reiber, C.; Bodendoerfer, E.; Brugger, S.; Eberhard, N.; Hitz, E.; Hofmaenner, D.; Hasse, B. Rapid antimicrobial susceptibility testing in patients with bacteraemia due to enterobacterales: An implementation study. Swiss Med. Wkly. 2023, 153, 40066. [Google Scholar] [CrossRef] [PubMed]
- Orelle, C.; Szal, T.; Klepacki, D.; Shaw, K.; Vázquez-Laslop, N.; Mankin, A. Identifying the targets of aminoacyl-tRNA synthetase inhibitors by primer extension inhibition. Nucleic Acids Res. 2013, 41, e144. [Google Scholar] [CrossRef]
- Vasala, A.; Hytönen, V.; Laitinen, O. Modern tools for rapid diagnostics of antimicrobial resistance. Front. Cell Infect. Microbiol. 2020, 10, 308. [Google Scholar] [CrossRef]
- Mesli, F.; Bouchentouf, S.; Ghomri, A.; Noureddine, M.; Ghalem, S. In silico comparison of synthetic and natural molecules bindings with acetylcholinesterase enzyme using molecular docking. J. Adv. Mol. Biol. 2018, 2, 17–26. [Google Scholar] [CrossRef]
- Luo, L.; Cai, Y.; Su, Y.; Li, C.; Tian, G.; Wang, X.; Zhang, Z. Novel tree shrew-derived antimicrobial peptide with broad-spectrum antibacterial activity. Acs Omega 2024, 9, 45279–45288. [Google Scholar] [CrossRef]
- Banerjee, R.; Teng, C.; Cunningham, S.; Ihde, S.; Steckelberg, J.; Moriarty, J.; Patel, R. Randomized trial of rapid multiplex Polymerase Chain Reaction–based blood culture identification and susceptibility testing. Clin. Infect. Dis. 2015, 61, 1071–1080. [Google Scholar] [CrossRef]
- Sharma, M.; Gangakhedkar, R.; Bhattacharya, S.; Walia, K. Understanding complexities in the uptake of in-digenously developed rapid point-of-care diagnostics for containment of antimicrobial resistance in India. BMJ Glob. Health 2021, 6, e006628. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The potential health benefits of gallic acid: Therapeutic and food applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Gao, Y.; Hao, L. Synergistic effects and mechanisms of combined treatment with harmine hydrochloride and azoles for resistant Candida albicans. Front. Microbiol. 2019, 10, 2295. [Google Scholar] [CrossRef]
- Rassu, G.; Sorrenti, M.; Catenacci, L.; Pavan, B.; Ferraro, L.; Gavini, E.; Dalpiaz, A. Conjugation, prodrug, and co-administration strategies in support of nanotechnologies to improve the therapeutic efficacy of phytochemicals in the central nervous system. Pharmaceutics 2023, 15, 1578. [Google Scholar] [CrossRef]
- Vora, R.; Joshi, A.; Joshi, N. Green synthesis and characterization of gold nanoparticles using Mucuna monosperma. J. Nanosci. aTechnol. 2020, 6, 901–904. [Google Scholar] [CrossRef]
- Ghobadi, N.; Asoodeh, A. Co-administration of curcumin with other phytochemicals improves anticancer activity by regulating multiple molecular targets. Phytother. Res. 2023, 37, 1688–1702. [Google Scholar] [CrossRef]
- Kumar, G.; Virmani, T.; Sharma, A.; Pathak, K. Codelivery of phytochemicals with conventional anticancer drugs in form of nanocarriers. Pharmaceutics 2023, 15, 889. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Kumar, A. Polychemotherapy with curcumin and doxorubicin via biological nanoplatforms: Enhancing antitumor activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef]
- Pandey, P.; Verma, M.; Lakhanpal, S.; Pandey, S.; Kumar, M.; Bhat, M.; Khan, F. An updated review summarizing the anticancer potential of poly (lactic-co-glycolic acid) (PLGA) based curcumin, epigallocatechin gallate, and resveratrol nanocarriers. Biopolymers 2024, 116. [Google Scholar] [CrossRef]
- Jahangir, M. Phytonutrients and technological development in formulations. J. Pharm. Res. Sci. Technol. 2022, 6, 38–66. [Google Scholar] [CrossRef]
- Sibuyi, N.; Thipe, V.; Panjtan-Amiri, K.; Meyer, M.; Katti, K. Green synthesis of gold nanoparticles using acai berry and elderberry extracts and investigation of their effect on prostate and pancreatic cancer cells. Nanobiomedicine 2021, 8. [Google Scholar] [CrossRef]
- Thatyana, M.; Dube, N.; Kemboi, D.; Manicum, A.; Mokgalaka, N.; Tembu, J. Advances in phytonanotechnology: A plant-mediated green synthesis of metal nanoparticles using phyllanthus plant extracts and their antimicrobial and anticancer applications. Nanomaterials 2023, 13, 2616. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Hakeem, K.R.; Rehman, R.U. Synergistic effect of plant extract coupled silver nanoparticles in various therapeutic applications-present insights and bottlenecks. Chemosphere 2022, 288, 132527. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Patra, J.; Debnath, T.; Ansari, A.; Shin, H. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus. PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S.; Shim, M. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 2016, 6, 235. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, J.; Manikandan, D.; Riyaz, S.; Gopal, M.; Khan, M.; Simal-Gándara, J.; Cid, A. Green synthesis of silver nanoparticles using Allium cepa var. aggregatum natural extract: Antibacterial and cytotoxic properties. Nanomaterials 2022, 12, 1725. [Google Scholar] [CrossRef]
- Elchaghaby, M.; Rashad, S.; Yousry, Y. Inhibitory effect of silver nanoparticles synthesized using the chamomile extract against Streptococcus mutans cariogenic pathogen. Dent. Med. Probl. 2023, 60, 483–488. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef]
- Chandran, N.; Ramesh, S.; Shanmugam, R.; Jayalakshmi, S. A comparative evaluation of antimicrobial and cytotoxic efficacy of biosynthesized silver nanoparticles and chemically synthesized silver nanoparticles against Enterococcus faecalis: An in vitro study. Cureus 2024, 16, e58428. [Google Scholar] [CrossRef]
- Ali, E.; Abdallah, B. Effective inhibition of candidiasis using an eco-friendly leaf extract of Calotropis-gigantean-mediated silver nanoparticles. Nanomaterials 2020, 10, 422. [Google Scholar] [CrossRef]
- Borase, H.; Patil, C.; Salunkhe, R.; Suryawanshi, R.; Salunke, B.; Patil, S. Catalytic and synergistic antibacterial potential of green synthesized silver nanoparticles: Their ecotoxicological evaluation on Poecillia reticulata. Biotechnol. Appl. Biochem. 2014, 61, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Shathviha, P.; Ezhilarasan, D.; Rajeshkumar, S.; Jayaraman, S. Β-sitosterol mediated silver nanoparticles induce cytotoxicity in human colon cancer HT-29 cells. Avicenna J. Med. Biotechnol. 2020, 13, 42–46. [Google Scholar] [CrossRef]
- Camaioni, L.; Ustyanowski, B.; Buisine, M.; Lambert, D.; Sendid, B.; Billamboz, M.; Jawhara, S. Natural compounds with antifungal properties against Candida albicans and identification of hinokitiol as a promising antifungal drug. Antibiotics 2023, 12, 1603. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.; Kedia, A.; Singh, A.; Yadav, S.; Singh, A.; Yadav, A.; Dubey, N. Antifungal, antiaflatoxin and antioxidant activity of plant essential oils and their in vivo efficacy in protection of chickpea seeds. J. Food Qual. 2015, 39, 36–44. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernández, J.; López-Ibáñez, S.; Miguélez, E.; Villar, C.; Lombó, F. Plant phytochemicals in food preservation: Antifungal bioactivity: A review. J. Food Protect 2020, 83, 163–171. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Abdolmaleki, K.; Javanmardi, F.; Hadidi, M.; Khaneghah, A. Recent advances in plant-based compounds for mitigation of mycotoxin contamination in food products: Current status, challenges and perspectives. Int. J. Food Sci. Technol. 2022, 57, 2159–2170. [Google Scholar] [CrossRef]
- Cacaci, M.; Squitieri, D.; Palmieri, V.; Torelli, R.; Perini, G.; Campolo, M.; Bugli, F. Curcumin-functionalized graphene oxide strongly prevents Candida parapsilosis adhesion and biofilm formation. Pharmaceuticals 2023, 16, 275. [Google Scholar] [CrossRef]
- Khwaza, V.; Aderibigbe, B. Antifungal activities of natural products and their hybrid molecules. Pharmaceutics 2023, 15, 2673. [Google Scholar] [CrossRef]
- Sonderegger, C.; Váradi, G.; Galgóczy, L.; Kocsubé, S.; Posch, W.; Borics, A.; Marx, F. The evolutionary conserved γ-core motif influences the anti-candida activity of the Penicillium chrysogenum antifungal protein PAF. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef]
- Tóth, L.; Boros, É.; Poór, P.; Ördög, A.; Kele, Z.; Váradi, G.; Galgóczy, L. The potential use of the Penicillium chrysogenum antifungal protein PAF, the designed variant pafopt and its γ-core peptide pγopt in plant protection. Microb. Biotechnol. 2020, 13, 1403–1414. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, S.; Fan, C.; Du, Q.; Jin, P. Enhanced antifungal activities of eugenol-entrapped casein nanoparticles against anthracnose in postharvest fruits. Nanomaterials 2019, 9, 1777. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, M.; Tayel, A.; Zidan, N.; Rabey, H. Bioactive coatings from nano-biopolymers/plant extract composites for complete protection from mycotoxigenic fungi in dates. J. Sci. Food Agric. 2019, 99, 4338–4343. [Google Scholar] [CrossRef]
- Myint, K.; Yu, Q.; Xia, Y.; Qing, J.; Zhu, S.; Fang, Y.; Shen, J. Bioavailability and antioxidant activity of nanotechnology-based botanic antioxidants. J. Food Sci. 2021, 86, 284–292. [Google Scholar] [CrossRef]
- Jiménez-Reyes, M.; Carrasco, H.; Olea, A.; Silva-Moreno, E. Natural compounds: A sustainable alternative to the phytopathogens control. J. Chil. Chem. Soc. 2019, 64, 4459–4465. [Google Scholar] [CrossRef]
- Munguia, J.; Nizet, V. Pharmacological targeting of the host-pathogen interaction: Alternatives to classical antibiotics to combat drug-resistant superbugs. Trends Pharmacol. Sci. 2018, 39, 1–12. [Google Scholar] [CrossRef]
- Narrowe, A.; Lemons, J.; Mahalak, K.; Firrman, J.; Abbeele, P.; Baudot, A.; Liu, L. Targeted remodeling of the human gut microbiome using Juemingzi (Senna seed extracts). Front. Cell Infect. Microbiol. 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.; Kornfeld, H. Interactions between naïve and infected macrophages reduce Mycobacterium tuberculosis viability. PLoS ONE 2011, 6, e27972. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Otal, J.; Torrent, D.; Porcar, M.; Vilanova, C.; Cuadras, F. A mouthwash formulated with O-cymen-5-ol and zinc chloride specifically targets potential pathogens without impairing the native oral microbiome in healthy individuals. J. Oral. Microbiol. 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Busetti, A.; Maggs, C.; Gilmore, B. Marine macroalgae and their associated microbiomes as a source of antimicrobial chemical diversity. Eur. J. Phycol. 2017, 52, 452–465. [Google Scholar] [CrossRef]
- Ellis, M.; Tsai, C.; Johnson, J.; French, S.; Elhenawy, W.; Porwollik, S.; Brown, E. A macrophage-based screen identifies antibacterial compounds selective for intracellular Salmonella typhimurium. Nat. Commun. 2019, 10, 197. [Google Scholar] [CrossRef]
- Gaire, T.; Scott, H.; Noyes, N.; Ericsson, A.; Tokach, M.; Menegat, M.; Volkova, V. Age influences the temporal dynamics of microbiome and antimicrobial resistance genes among fecal bacteria in a cohort of production pigs. Anim. Microbiome 2023, 5. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, D.; Alagumuthu, M.; Subramaniam, P.; Bakthavachalam, D.; Arumugam, S.; Chellam, S. Antimicrobial effects of vanillin-based pyridyl-benzylidene-5-fluoroindolins. J. HeterocyclChem 2021, 58, 1515–1524. [Google Scholar] [CrossRef]
- Moussa, A.Y. Streptomyces endophytes in edible plants: New insights into their chemistry and health benefits. Chem. Biodivers. 2024, 21, e202400888. [Google Scholar] [CrossRef]
- Teles, R.H.G.; Moralles, H.F.; Cominetti, M.R. Global trends in nanomedicine research on triple negative breast cancer: A bibliometric analysis. Int. J. Nanomedicine 2018, 13, 2321–2336. [Google Scholar] [CrossRef]
- Yang, Y.; Lv, K.; Xue, J.; Huang, X. A bibliometric analysis and visualization of fractional order research in China over two decades (2001–2020). J. Math. 2021, 2021, 7996776. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Qian, M.; Ismail, B.B.; He, Q.; Zhang, X.; Yang, Z.; Ding, T.; Ye, X.; Liu, D.; Guo, M. Inhibitory mechanisms of promising antimicrobials from plant byproducts: A review. Comp. Rev. Food Sci. Food Saf. 2023, 22, 2523–2590. [Google Scholar] [CrossRef]
- Ammendolia, D.A.; Boulanger, W.M.; Boulanger, J.H. Plasma membrane integrity: Implications for health and disease. BMC Biology 2021, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Berditsch, M.; Hawecker, J.; Ardakani, M.F.; Gerthsen, D.; Ulrich, A.S. Damage of the bacterial cell envelope by antimicrobial peptides gramicidinS and PGLA as revealed by transmission and scanning electron microscopy. Antimicrob. Agents Chemother. 2010, 54, 3132–3142. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.; Liu, Z.; Kumar, V. Exploring phytochemicals for combating antibiotic resistance in microbial pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Wu, Y.; Bai, J.; Zhong, K.; Huang, Y.; Qi, H.; Jiang, Y.; Gao, H. Antibacterial activity and membrane-disruptive mechanism of 3-P-trans-coumaroyl-2-hydroxyquinic acid, a novel phenolic compound from pine needles of Cedrus deodara, against Staphylococcus aureus. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Benfield, A.H.; Henriques, S.T. Mode-of-action of antimicrobial peptides: Membrane disruption vs. intracellular mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Tian, L.; Wang, X.; Liu, R.; Zhang, D.; Wang, X.; Sun, R.; Guo, W.; Yang, S.; Li, H.; Gong, G. Antibacterial mechanism of thymol against Enterobacter sakazakii. Food Control 2020, 123, 107716. [Google Scholar] [CrossRef]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of bactericidal action of eugenol against Escherichia coli. J. Herb. Med. 2020, 26, 100406. [Google Scholar] [CrossRef]
- Wang, L.H.; Wang, M.S.; Zeng, X.A.; Xu, X.M.; Brennan, C.S. Membrane and genomic DNA dual-targeting of citrus flavonoid naringenin against Staphylococcus aureus. Integr. Biol. 2017, 16, 820–829. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; Islam, K.T.; Hobson, E.; Shah, D.M. Modes of action of a bi-domain plant defensin MtDef5 against a Bacterial pathogen Xanthomonas campestris. Front. Microbiol. 2018, 9, 934. [Google Scholar] [CrossRef]
- Shinde, S.; Lee, L.H.; Chu, T. Inhibition of biofilm formation by the synergistic action of EGCG-S and antibiotics. Antibiotics 2021, 10, 102. [Google Scholar] [CrossRef]
- Nohynek, L.J.; Alakomi, H.L.; Kähkönen, M.P.; Heinonen, M.; Helander, I.M.; Oksman-Caldentey, K.M.; Puupponen-Pimiä, R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer 2006, 54, 18–32. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Li, G.; Chunhong, Y.; Yunfeng, X.; Yuqing, F.; Qian, W.; Xiaoying, L.; Baowei, Y.; Xin, W.; Xiaodong, X. Punicalagin inhibits salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Luís, Â.; Silva, F.; Sousa, S.; Duarte, A.P.; Domingues, F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling 2014, 30, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.N.; Wang, F.; Yuan, Y.T.; Liu, J.; Liu, Y.Z.; Yi, X. Antibacterial activity and mode of action of dihydromyricetin from Ampelopsis grossedentata leaves against food-borne bacteria. Molecules 2019, 24. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Hillaert, U.; Van Calenbergh, S.; Nelis, H.J.; Coenye, T. Use of quorum sensing inhibitors to interfere with biofilm formation and development in Burkholderia multivorans and Burkholderia cenocepacia. Res. Microbiol. 2009, 160, 144–151. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, Y.G.; Cho, H.S.; Ryu, S.Y.; Cho, M.H.; Lee, J. Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 2014, 21, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Portillo-Torres, L.A.; Bernardino-Nicanor, A.; Gómez-Aldapa, C.A.; González-Montiel, S.; Rangel-Vargas, E.; Villagó-mez-Ibarra, J.R.; González-Cruz, L.; Cortés-López, H.; Castro-Rosas, J. Hibiscus acid and chromatographic fractions from Hi-biscus sabdariffa calyces: Antimicrobial activity against Multidrug-Resistant pathogenic bacteria. Antibiotics 2019, 8, 218. [Google Scholar] [CrossRef]
- Gu, Y.; Dong, J.; Li, J.; Luo, Q.; Dong, X.; Tang, G.; Zhang, J.; Du, X.; Pu, Q.; He, L.; et al. Antibacterial activity and mechanism of sanguinarine against staphylococcus aureus by interfering with the permeability of the cell wall and membrane and inducing bacterial ROS production. Front. Vet. Sci. 2023, 10, 1121082. [Google Scholar] [CrossRef]
- Du, G.F.; Le, Y.J.; Sun, X.; Yang, X.Y.; He, Q.Y. Proteomic investigation into the action mechanism of berberine against Strep-tococcus pyogenes. J. Proteomics 2020, 215, 103666. [Google Scholar] [CrossRef]
- Yang, S.K.; Yusoff, K.; Ajat, M.; Yap, W.S.; Lim, S.H.E.; Lai, K.S. Antimicrobial activity and mode of action of linaly anthranilate against carbapenemase-producing Klebsiella pneumoniae. J. Pharm. Anal. 2021, 11, 210–219. [Google Scholar] [CrossRef]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Silva, L.N.; Zimmer, K.R.; Macedo, A.J.; Trentin, D.S. Plant natural products targeting bacterial virulence factors. Chem. Rev. 2016, 116, 9162–9236. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Siddiqui, M.W.; Ayub, Q. Characterization of Salmonella enterica biofilms and antibiofilm effect of carvacrol and 2-aminobenzimidazole. Foodborne Pathog. Dis. 2024, 21, 52–60. [Google Scholar] [CrossRef]
- Gambino, E.; Maione, A.; Guida, M.; Albarano, L.; Carraturo, F.; Galdiero, E. Evaluation of the pathogenic-mixed biofilm formation of Pseudomonas aeruginosa/Staphylococcus aureus and treatment with limonene on three different materials by a dy-namic model. Int. J. Environ. Res. Public. Health 2022, 19, 3741. [Google Scholar] [CrossRef] [PubMed]
- Salim, A.; Deiana, P.; Fancello, F.; Molinu, M.G.; Santona, M.; Zara, S. Antimicrobial and antibiofilm activities of pomegranate peel phenolic compounds: Varietal screening through a multivariate approach. J. Bioresour. Bioprod. 2023, 8, 146–161. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Pacyga, K.; Kupczyński, R.; Maćzka, W.; Grabarczyk, M.; Pacyga, P.; Topola, E.; Ostrówka, M.; Bania, J. Antibiofilm and antimicrobial potentials of novel synthesized sulfur camphor derivatives. Int. J. Mol. Sci. 2024, 25, 10895. [Google Scholar] [CrossRef]
- Cho, H.; Uehara, T.; Bernhardt, T.G. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell 2014, 159, 1300–1311. [Google Scholar] [CrossRef]
- Apostolos, A.J.; Pires, M.M. Impact of crossbridge structure on peptidoglycan crosslinking: A synthetic stem peptide approach. Methods Enzymol. 2022, 665, 259–279. [Google Scholar] [CrossRef]
- Al Alsheikh, H.M.; Sultan, I.; Kumar, V.; Rather, I.A.; Al-sheikh, H.; Jan, A.T.; Haq, Q.M.R. Plant-based phytochemicals as possible alternative to antibiotics in combating bacterial drug resistance. Antibiotics 2020, 9, 1–23. [Google Scholar] [CrossRef]
- Chabán, M.F.; Hrast, M.; Frlan, R.; Graikioti, D.G.; Athanassopoulos, C.M.; Carpinella, M.C. Inhibition of MURA enzyme from Escherichia coli and Staphylococcus aureus by diterpenes from Lepechinia meyenii and their synthetic analogs. Antibiotics 2021, 10, 1535. [Google Scholar] [CrossRef]
- Hu, Y.; Keniry, M.; Palmer, S.O.; Bullard, J.M. Discovery and analysis of natural-product compounds inhibiting protein syn-thesis in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2016, 60, 4820–4829. [Google Scholar] [CrossRef]
- Calvo, J.; Martínez-Martínez, L. Mecanismos de acción de los antimicrobianos. Enferm. Infecc. Microbiol. Clin. 2009, 27, 44–52. [Google Scholar] [CrossRef] [PubMed]
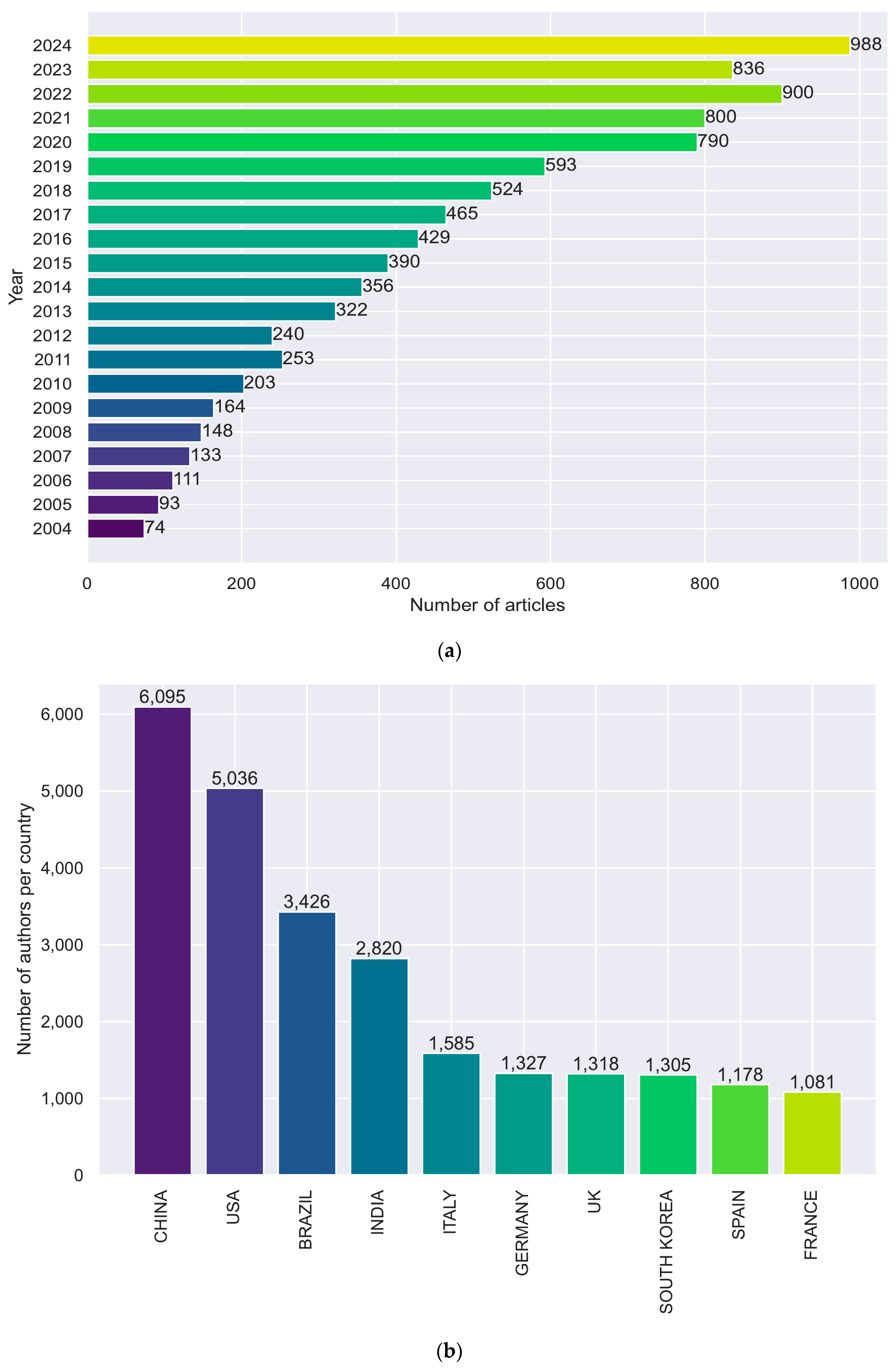
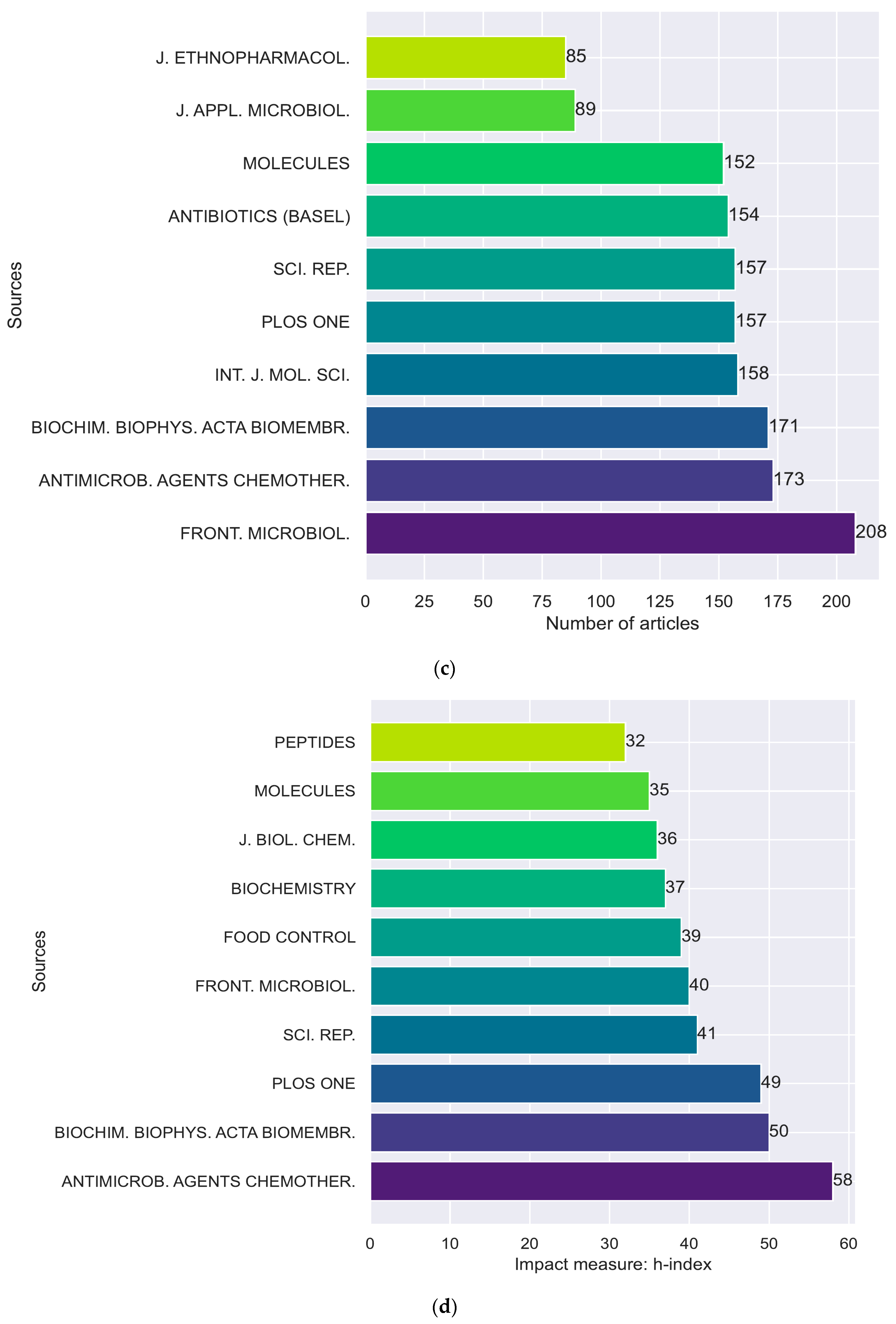
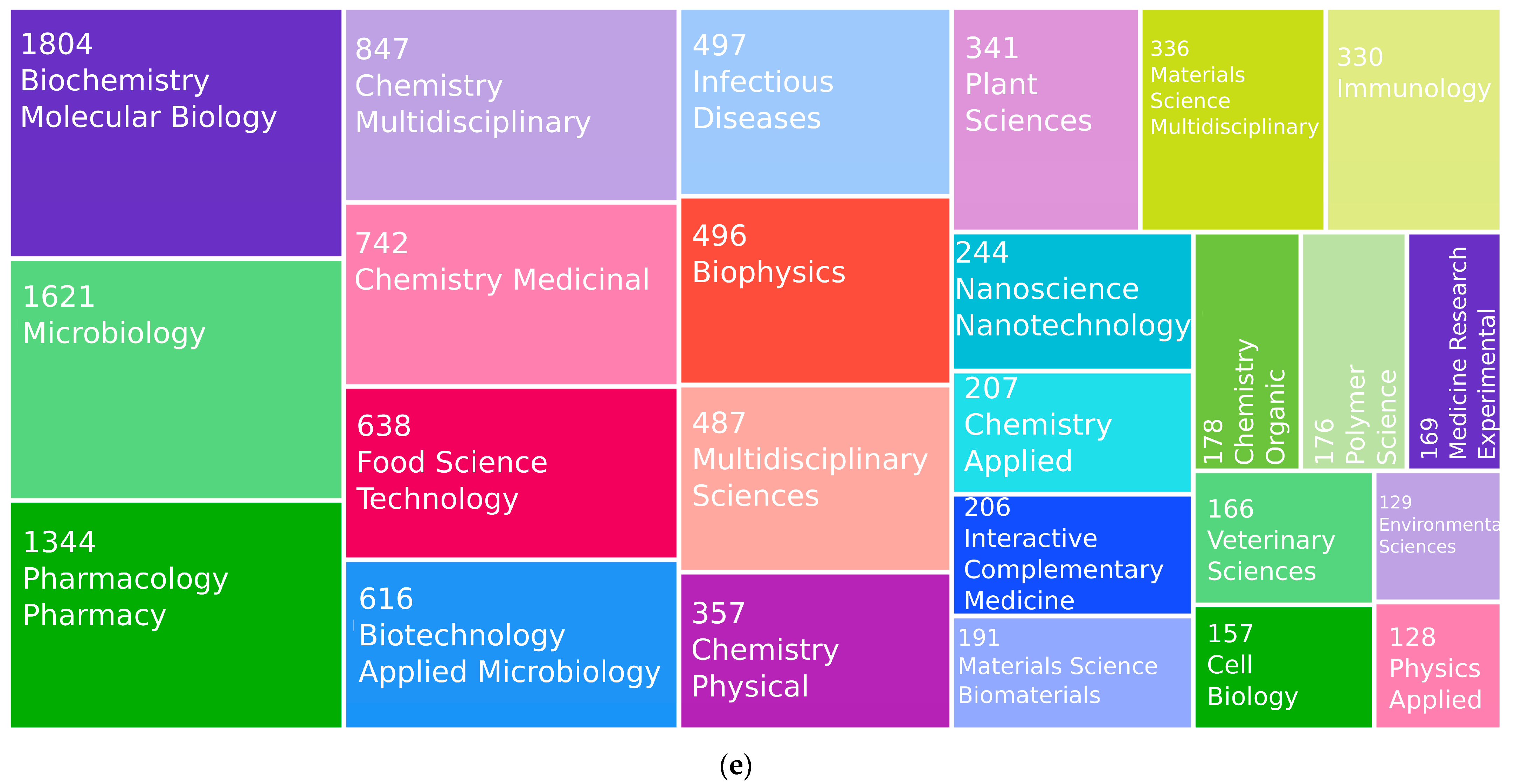
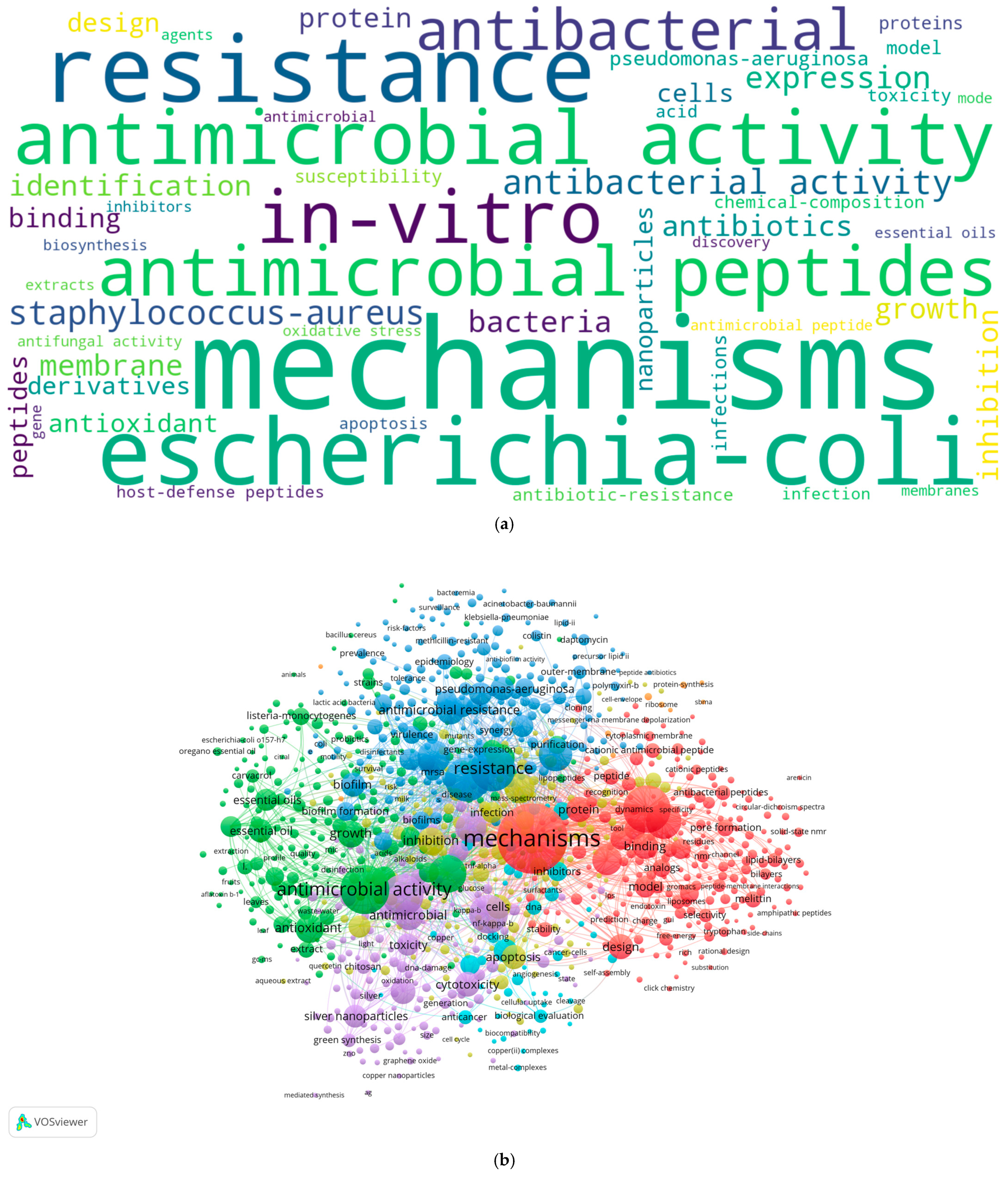

| Description | Results |
|---|---|
| Timespan | 2004–2024 |
| Sources (Journals, Books, etc.) | 1698 |
| Documents | 8812 |
| Annual Growth Rate % | 13.84 |
| Document Average Age | 7.01 |
| Average citations per doc | 32.03 |
| DOCUMENT CONTENTS | |
| Keywords Plus (ID) | 15,722 |
| Author’s Keywords (DE) | 17,417 |
| AUTHORS | |
| Authors | 42,819 |
| Authors of single-authored docs | 134 |
| AUTHORS COLLABORATION | |
| Single-authored docs | 142 |
| Co-Authors per doc | 7.23 |
| International co-authorships % | 29.35 |
| DOCUMENT TYPES | |
| Article | 8716 |
| Plant Source | Compound | Mechanism of Action | Susceptible Microorganism | Concentration (µg/mL) | Reference |
|---|---|---|---|---|---|
| Antimicrobial peptides | |||||
| Nigella sativa | Thionins NsW1 and NsW2 | Disruption of cell membrane integrity | Bacillus subtilis and Staphylococcus aureus | 3.25 6.25 | [3] |
| Medicago truncatula | Defensin MtDef5 | Disruption of cell membrane integrity/Inhibition of protein biosynthesis/Interfering with nucleic acid synthesis or expression | Xanthomonas campestris | 12 | [121] |
| Phenolic compounds | |||||
| Camellia sinensis | Epigallocatechin-3-gallate-stearate (EGCG-S) | Inhibition of bacterial biofilm formation | Escherichia coli | 100 | [122] |
| Agrimonia pilosa Ledeb. | Wogonin | Inhibition of bacterial biofilm formation | Pseudomonas aeruginosa | 30 | [2] |
| Saccharum officinarum | Mixture of gallic acid coumaric acid, and chlorogenic acid | Disruption of cell membrane integrity | S. aureus | 0.625 | [4] |
| Vaccinium myrtillus, V. vitis-idaea, V. oxycoccos, Rubus idaeus var. Ottawa, and others | Ellagic acid | Disruption of cell membrane integrity | Salmonella enterica serovar Typhimurium | 40 | [123] |
| Vaccinium myrtillus, V. vitis-idaea, V. oxycoccos, Rubus idaeus var. Ottawa, and others | Gallic acid | Disruption of cell membrane integrity | S. Typhimurium | 600 | [123] |
| Punica granatum | Punicalagin | Disruption of cell membrane integrity /Inhibition against quorum sensing | S. aureus S. Typhimurium SL1344 | 250 500 | [124] [125] |
| Bambusa vulgaris, Oryza sativa, and others | Ferulic acid | Disruption of cell membrane integrity | E. coli and P. aeruginosa S. aureus Listeria monocytogenes | 100 1100 1250 | [126] |
| Coffea arabica, Olea europaea, and others | Caffeic acid | Inhibition of energy metabolism/Disruption of cell membrane integrity | S. aureus ATCC 25923 | 62.5 | [127] |
| Ampelopsis grossedentata | Dihydromyricetin | Inhibition of energy metabolism/Disruption of cell membrane integrity/Inhibition of cell wall biosynthesis | S. aureus E. coli | 625 312.5 | [128] [128] |
| Sonchus grandifolius, Aesculus turbinata, and others | Esculetin | Inhibition against quorum sensing | Burkholderia cepacia | 500 | [129] |
| Cinnamomum cassia | Coumarin | Inhibition against quorum sensing | E. coli O157:H7 | 50 | [130] |
| Hibiscus sabdariffa | Hibiscus acid | Disruption of cell membrane integrity | S. Typhimurium Enterohemorrhagic E. coli | 7000 7000 | [131] |
| Alkaloids | |||||
| Toddalia asiatica (Linn) Lam | Chelerythrine | Disruption of cell membrane integrity/Inhibition of protein biosynthesis | S. aureus Methicillin-resistant S. aureus | 156 156 | [5] |
| Macleaya cordata | Sanguinarine | Disruption of cell membrane integrity | S. aureus | 128 | [132] |
| Rhizoma coptidis and, Cortex phellodendri | Berberine | Inhibition against quorum sensing Inhibition of energy metabolism/ Inhibition of protein biosynthesis/ Interfering with nucleic acid synthesis or expression | E. coli Streptococcus pyogenes | 2560 80 | [6] [133] |
| Sophora flavescens | Matrine | Inhibition of protein biosynthesis Inhibition against quorum sensing | E. coli S. aureus E. coli | 2500 10000 5120 | [7] [6] |
| Organosulfur compound | |||||
| Allium sativum | Allicin | Interfering with nucleic acid synthesis or expression | E. coli | 130 | [11] |
| Allium sativum | Diallyl trisulfide | Disruption of cell membrane integrity | Campylobacter jejuni | 32 | [12] |
| Allium sativum | Ajoene | Inhibition against quorum sensing | S. aureus | 20 | [13] |
| Terpenes | |||||
| Andrographis paniculata | Andrographolide | Interfering with nucleic acid synthesis or expression/ Inhibition of cell wall biosynthesis | S. aureus MTCC 96 | 100 | [9] |
| Cinnamomum camphora L. | Terpinen-4-ol | Interfering with nucleic acid synthesis or expression/inhibition of protein biosynthesis/Disruption of cell membrane integrity | Streptococcus agalactiae | 98 | [8] |
| Origanum majorana, Agastache mexicana, Lavandula angustifolia, and Thymus vulgaris L. | Linalyl anthranilate | Disruption of cell membrane integrity | Klebsiella pneumoniae | 2.5 | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Flores, J.G.; García-Curiel, L.; Pérez-Escalante, E.; Contreras-López, E.; Aguilar-Lira, G.Y.; Ángel-Jijón, C.; González-Olivares, L.G.; Baena-Santillán, E.S.; Ocampo-Salinas, I.O.; Guerrero-Solano, J.A.; et al. Plant Antimicrobial Compounds and Their Mechanisms of Action on Spoilage and Pathogenic Bacteria: A Bibliometric Study and Literature Review. Appl. Sci. 2025, 15, 3516. https://doi.org/10.3390/app15073516
Pérez-Flores JG, García-Curiel L, Pérez-Escalante E, Contreras-López E, Aguilar-Lira GY, Ángel-Jijón C, González-Olivares LG, Baena-Santillán ES, Ocampo-Salinas IO, Guerrero-Solano JA, et al. Plant Antimicrobial Compounds and Their Mechanisms of Action on Spoilage and Pathogenic Bacteria: A Bibliometric Study and Literature Review. Applied Sciences. 2025; 15(7):3516. https://doi.org/10.3390/app15073516
Chicago/Turabian StylePérez-Flores, Jesús Guadalupe, Laura García-Curiel, Emmanuel Pérez-Escalante, Elizabeth Contreras-López, Guadalupe Yoselín Aguilar-Lira, Carlos Ángel-Jijón, Luis Guillermo González-Olivares, Elena Saraí Baena-Santillán, Israel Oswaldo Ocampo-Salinas, José Antonio Guerrero-Solano, and et al. 2025. "Plant Antimicrobial Compounds and Their Mechanisms of Action on Spoilage and Pathogenic Bacteria: A Bibliometric Study and Literature Review" Applied Sciences 15, no. 7: 3516. https://doi.org/10.3390/app15073516
APA StylePérez-Flores, J. G., García-Curiel, L., Pérez-Escalante, E., Contreras-López, E., Aguilar-Lira, G. Y., Ángel-Jijón, C., González-Olivares, L. G., Baena-Santillán, E. S., Ocampo-Salinas, I. O., Guerrero-Solano, J. A., & Portillo-Torres, L. A. (2025). Plant Antimicrobial Compounds and Their Mechanisms of Action on Spoilage and Pathogenic Bacteria: A Bibliometric Study and Literature Review. Applied Sciences, 15(7), 3516. https://doi.org/10.3390/app15073516










