Usability and Acceptance Analysis of Wearable BCI Devices
Abstract
1. Introduction
2. Usability and Acceptability of Brain–Computer Interface Devices
3. Materials and Methods
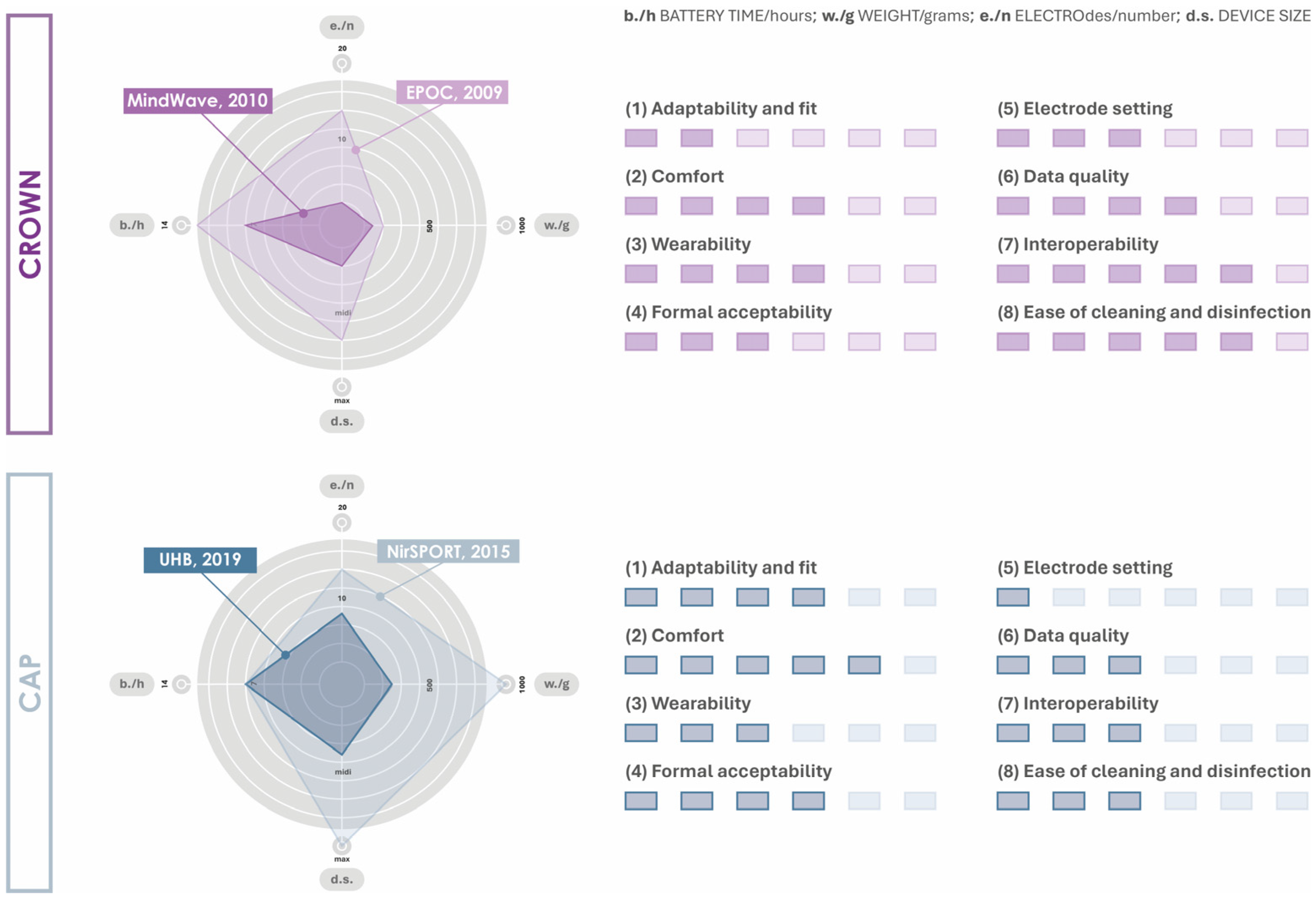
4. Wearable Neuroimaging Devices Usability Factor Identification
- (1)
- Adaptability and fit: the caps generally have a mechanism to adapt to the shape and size of the head, unlike crowns, which, being configured with rigid or semi-rigid structures, do not always manage to adapt to different head conformations;
- (2)
- Comfort: the caps, which are usually made of elastic and lightweight fabric, have a greater ability to adapt to the user, improving perceived comfort. Conversely, the rigid or semi-rigid configuration of the crowns may cause discomfort due to the lack of adaptability to the head, resulting in a feeling of instability and a more uneven weight distribution;
- (3)
- Wearability (ability of the device to be worn): crowns are usually quicker to put on than caps, which may require assistance, especially when supplemented with wet gel electrodes. In addition, crowns allow for one-handed use, which is essential for users with disabilities or operators in work contexts;
- (4)
- Formal acceptability: the configuration of crown devices is defined to have a small footprint and a desirable appearance in terms of aesthetics. In contrast, caps are often configured with a wiring system that is less practical and formally cumbersome;
- (5)
- Electrode setting: caps have guiding elements applied to the fabric, which allows correct positioning of the electrodes, according to the international standard 10–20. In contrast, crowns may cause electrode displacements in different sensing sessions and with respect to different configurations of the user’s head;
- (6)
- Data quality: caps, unlike crowns, can help maintain the correct positioning of the electrodes on the scalp, decreasing noise and disturbance due to small vibrations and/or movements. This ensures a better resolution of the data for their synthesis and interpretation;
- (7)
- Interoperability: the devices, regardless of their cap or crown configuration, can be integrated with other technologies. To ensure this, it is essential that the device can communicate with non-device software and allow direct access to data;
- (8)
- Ease of cleaning and disinfection: caps are configured to be disinfected by surface cleaning, whereas crowns require immersion in a disinfection solution for several minutes following removal of the electrodes. However, this also differs depending on the type of electrodes the device is equipped with (dry, semi-dry, and salt-based).
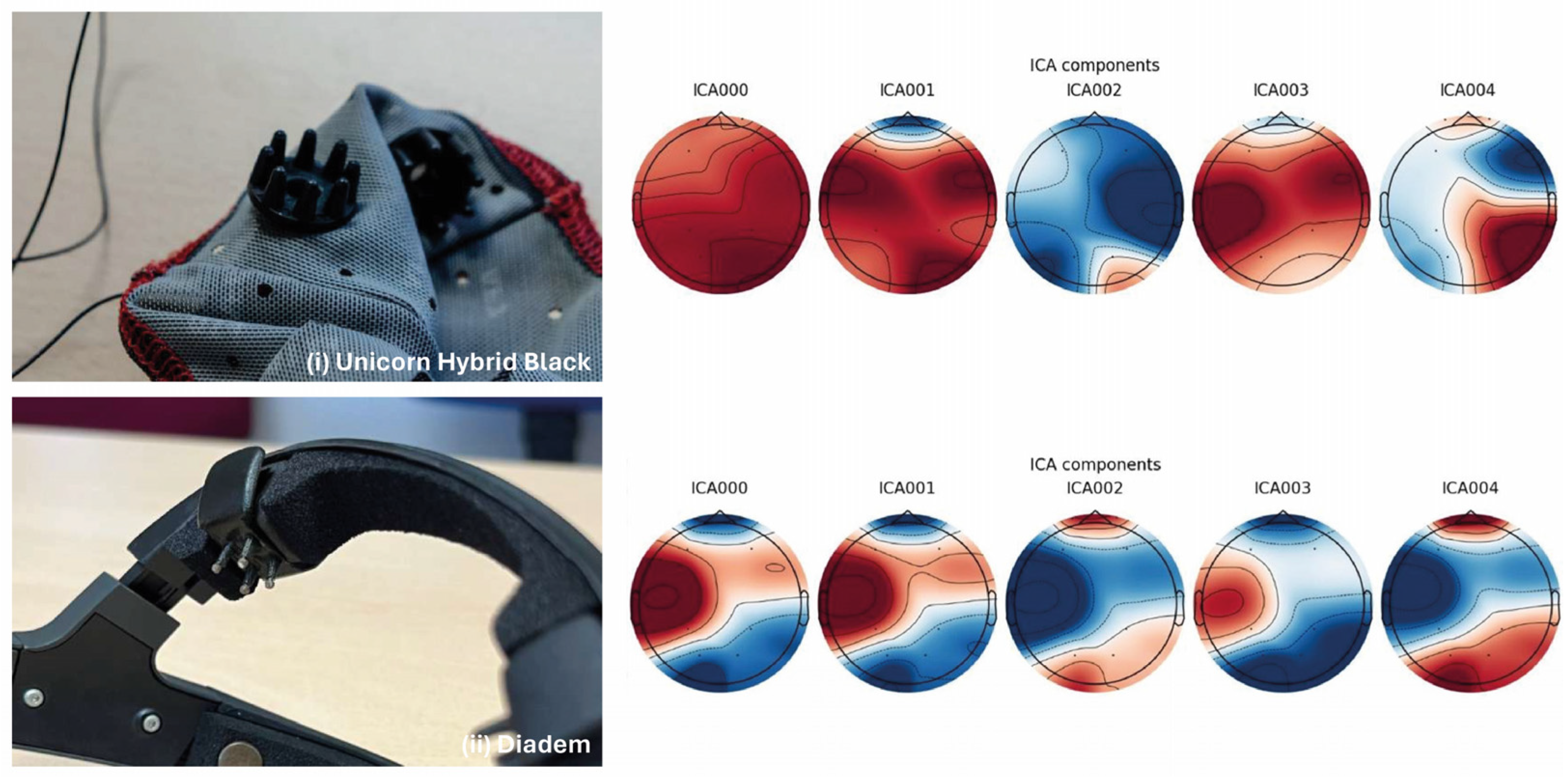
5. Results
5.1. State of the Art
5.2. Questionnaire Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parasuraman, R.; Rizzo, M. Neuroergonomics: The Brain at Work; Oxford University Press: Oxford, UK, 2007; Volume xii, p. 430. [Google Scholar]
- Dehais, F.; Lafont, A.; Roy, R.; Fairclough, S. A Neuroergonomics Approach to Mental Workload, Engagement and Human Performance. Front. Neurosci. 2020, 14, 268. [Google Scholar] [CrossRef]
- Moon, N.W.; Baker, P.M.; Goughnour, K. Designing Wearable Technologies for Users with Disabilities: Accessibility, Usability, and Connectivity Factors. J. Rehabil. Assist. Technol. Eng. 2019, 6, 2055668319862137. [Google Scholar] [CrossRef]
- Svertoka, E.; Saafi, S.; Rusu-Casandra, A.; Burget, R.; Marghescu, I.; Hosek, J.; Ometov, A. Wearables for Industrial Work Safety: A Survey. Sensors 2021, 21, 3844. [Google Scholar] [CrossRef]
- Khakurel, J.; Melkas, H.; Porras, J. Tapping into the Wearable Device Revolution in the Work Environment: A Systematic Review. Inf. Technol. People 2018, 31, 791–818. [Google Scholar] [CrossRef]
- Aljuaid, A.M. Theoretical Design of EEG-Based Neuroergonomics Integrated Portable System, Applying Direct Psychophysiological Indicators. In Proceedings of the 2019 Industrial & Systems Engineering Conference (ISEC), Jeddah, Saudi Arabia, 19–20 January 2019; pp. 1–6. [Google Scholar]
- Parasuraman, R.; Wilson, G.F. Putting the Brain to Work: Neuroergonomics Past, Present, and Future. Hum. Factors 2008, 50, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Xie, Z.; Wang, H.; Li, L.; Xu, X. Mental Stress and Safety Awareness during Human-Robot Collaboration—Review. Appl. Ergon. 2022, 105, 103832. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Ching, C.T.-S.; Wang, H.-M.D.; Liao, L.-D. Emerging Wearable Biosensor Technologies for Stress Monitoring and Their Real-World Applications. Biosensors 2022, 12, 1097. [Google Scholar] [CrossRef]
- Ferguson, S.A.; Allread, W.G.; Le, P.; Rose, J.; Marras, W.S. Shoulder Muscle Fatigue during Repetitive Tasks as Measured by Electromyography and Near-Infrared Spectroscopy. Hum. Factors 2013, 55, 1077–1087. [Google Scholar] [CrossRef]
- Matsumoto, M.; Sakurada, T.; Yamamoto, S.-I. Distinct Bilateral Prefrontal Activity Patterns Associated with the Qualitative Aspect of Working Memory Characterized by Individual Sensory Modality Dominance. PLoS ONE 2020, 15, e0238235. [Google Scholar] [CrossRef]
- Perrey, S.; Thedon, T.; Rupp, T. NIRS in Ergonomics: Its Application in Industry for Promotion of Health and Human Performance at Work. Int. J. Ind. Ergon. 2010, 40, 185–189. [Google Scholar] [CrossRef]
- Oyekan, J.; Chen, Y.; Turner, C.; Tiwari, A. Applying a Fusion of Wearable Sensors and a Cognitive Inspired Architecture to Real-Time Ergonomics Analysis of Manual Assembly Tasks. J. Manuf. Syst. 2021, 61, 391–405. [Google Scholar] [CrossRef]
- Savković, M.; Caiazzo, C.; Djapan, M.; Vukićević, A.M.; Pušica, M.; Mačužić, I. Development of Modular and Adaptive Laboratory Set-Up for Neuroergonomic and Human-Robot Interaction Research. Front. Neurorobot. 2022, 16, 863637. [Google Scholar] [CrossRef]
- Portillo-Lara, R.; Tahirbegi, B.; Chapman, C.A.R.; Goding, J.A.; Green, R.A. Mind the Gap: State-of-the-Art Technologies and Applications for EEG-Based Brain-Computer Interfaces. APL Bioeng. 2021, 5, 031507. [Google Scholar] [CrossRef]
- Toichoa Eyam, A.; Mohammed, W.M.; Martinez Lastra, J.L. Emotion-Driven Analysis and Control of Human-Robot Interactions in Collaborative Applications. Sensors 2021, 21, 4626. [Google Scholar] [CrossRef]
- Shahab, M.A.; Srinivasan, B.; Srinivasan, R. Analysis of Control Room Operators’ Competence Using Cognitive Engineering Approaches to Improve Process Safety. In Proceedings of the 2021 International Conference on Maintenance and Intelligent Asset Management (ICMIAM), Ballarat, Australia, 12–15 December 2021; pp. 1–6. [Google Scholar]
- He, C.; Chen, Y.-Y.; Phang, C.-R.; Stevenson, C.; Chen, I.-P.; Jung, T.-P.; Ko, L.-W. Diversity and Suitability of the State-of-the-Art Wearable and Wireless EEG Systems Review. IEEE J. Biomed. Health Inform. 2023, 27, 3830–3843. [Google Scholar] [CrossRef]
- Craik, A.; González-España, J.J.; Alamir, A.; Edquilang, D.; Wong, S.; Sánchez Rodríguez, L.; Feng, J.; Francisco, G.E.; Contreras-Vidal, J.L. Design and Validation of a Low-Cost Mobile EEG-Based Brain–Computer Interface. Sensors 2023, 23, 5930. [Google Scholar] [CrossRef] [PubMed]
- Žabčíková, M.; Koudelková, Z.; Jasek, R. Investigation of Emotiv Epoc+ Headset Usability by Measuring Various Stimuli. Wseas Trans. Appl. Theor. Mech. 2019, 14, 184–191. [Google Scholar]
- Lombardi, I.; Martínez, V.F.M.; Senese, V.P.; Capece, S. Design, Human Factors and Neuroergonomics for Safety in Manufacturing. In Human Factors in Accessibility and Assistive Technology; AHFE Open Access: Orlando, FL, USA, 2023; Volume 87. [Google Scholar]
- Pfeifer, M.D.; Scholkmann, F.; Labruyère, R. Signal Processing in Functional Near-Infrared Spectroscopy (fNIRS): Methodological Differences Lead to Different Statistical Results. Front. Hum. Neurosci. 2018, 11, 641. [Google Scholar] [CrossRef]
- Smulders, M.; van Dijk, L.N.M.; Song, Y.; Vink, P.; Huysmans, T. Dense 3D Pressure Discomfort Threshold (PDT) Map of the Human Head, Face and Neck: A New Method for Mapping Human Sensitivity. Appl. Ergon. 2023, 107, 103919. [Google Scholar] [CrossRef]
- Norman, D.A. The Design of Everyday Things; Basic Books: New York, NY, USA, 2014; ISBN 978-0-262-52567-1. [Google Scholar]
- Park, E. User Acceptance of Smart Wearable Devices: An Expectation-Confirmation Model Approach. Telemat. Inform. 2020, 47, 101318. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo-Mendez, G.; Dunwell, I.; Martínez-Mirón, E.A.; Vargas-Cerdán, M.D.; de Freitas, S.; Liarokapis, F.; García-Gaona, A.R. Assessing NeuroSky’s Usability to Detect Attention Levels in an Assessment Exercise. In Human-Computer Interaction. New Trends; Jacko, J.A., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 149–158. [Google Scholar]
- Ekandem, J.I.; Davis, T.A.; Alvarez, I.; James, M.T.; Gilbert, J.E. Evaluating the Ergonomics of BCI Devices for Research and Experimentation. Randomized Control. Trial. 2012, 55, 592–598. [Google Scholar] [CrossRef]
- Das, R.; Chatterjee, D.; Das, D.; Sinharay, A.; Sinha, A. Cognitive Load Measurement—A Methodology to Compare Low Cost Commercial EEG Devices. In Proceedings of the 2014 International Conference on Advances in Computing, Communications and Informatics (ICACCI), Delhi, India, 24–27 September 2014; pp. 1188–1194. [Google Scholar]
- Hairston, D.W.; Whitaker, K.W.; Ries, A.J.; Vettel, J.M.; Cortney Bradford, J.; Kerick, S.E.; McDowell, K. Usability of Four Commercially-Oriented EEG Systems. J. Neural. Eng. 2014, 11, 046018. [Google Scholar] [CrossRef]
- Maskeliunas, R.; Damasevicius, R.; Martisius, I.; Vasiljevas, M. Consumer-Grade EEG Devices: Are They Usable for Control Tasks? PeerJ 2016, 4, e1746. [Google Scholar] [CrossRef]
- Ratti, E.; Waninger, S.; Berka, C.; Ruffini, G.; Verma, A. Comparison of Medical and Consumer Wireless EEG Systems for Use in Clinical Trials. Front. Hum. Neurosci. 2017, 11, 398. [Google Scholar] [CrossRef]
- Noor, A.; Umer, A.; Noor-ul-Amin; Umar, A.I.; Ahmad, Z.; Khan, M.; Jan, S.A.; Iqbal, J. Usability Evaluation of Brain-Computer Interaction (BCI), Based Game for Normal Users. Int. J. Comput. Sci. Netw. Secur. 2018, 18, 168–175. [Google Scholar]
- Tavares, N.G.; Gad, R.S. Steady-State Visual Evoked Potential-Based Real-Time BCI for Smart Appliance Control. In Cognitive Informatics and Soft Computing; Mallick, P.K., Balas, V.E., Bhoi, A.K., Zobaa, A.F., Eds.; Springer: Singapore, 2019; pp. 795–805. [Google Scholar]
- Radüntz, T.; Meffert, B. User Experience of 7 Mobile Electroencephalography Devices: Comparative Study. JMIR Mhealth Uhealth 2019, 7, e14474. [Google Scholar] [CrossRef]
- Högman, L.; Dravniknes, H. Validation of a Consumer-Grade Functional Near-Infrared Spectroscopy Device for Measurement of Frontal Pole Brain Oxygenation—An Interim. 2020. Available online: https://mendi-webpage.s3.eu-north-1.amazonaws.com/Mendi_signal_validation_interim_report_final.pdf (accessed on 3 March 2025).
- Rupp, G.; Berka, C.; Meghdadi, A.; McConnell, M.; Storm, M.; Ramsøy, T.Z.; Verma, A. EEG Acquisition During the VR Administration of Resting State, Attention, and Image Recognition Tasks: A Feasibility Study; Springer: Cham, Switzerland, 2019; pp. 250–258. ISBN 978-3-030-23527-7. [Google Scholar]
- Gadea, M.; Herrero, N.; Picó, A.; Espert, R.; Salvador, A.; Sanjuán, J. Psychobiological Response to an Anger Induction Task in Schizophrenia: The Key Role of Anxiety. Psychiatry Res. 2019, 271, 541–547. [Google Scholar] [CrossRef]
- Zero, E.; Graffione, S.; Bersani, C.; Sacile, R. A BCI Driving System to Understand Brain Signals Related to Steering. In Proceedings of the 18th International Conference on Informatics in Control, Automation and Robotics (ICINCO 2021), Paris, France, 6–8 July 2021; p. 751. [Google Scholar]
- Erat, K.; Durdu, P.O. Düşük maliyetli EEG başlıklarının kullanıcı deneyimi değerlendirmesi. Pamukkale Üniversitesi Mühendislik Bilim. Derg. 2021, 27, 646–659. [Google Scholar]
- Zanetti, R.; Arza, A.; Aminifar, A.; Atienza, D. Real-Time EEG-Based Cognitive Workload Monitoring on Wearable Devices. IEEE Trans. Biomed. Eng. 2022, 69, 265–277. [Google Scholar] [CrossRef]
- Pontifex, M.B.; Coffman, C.A. Validation of the g.Tec Unicorn Hybrid Black Wireless EEG System. Psychophysiology 2023, 60, e14320. [Google Scholar] [CrossRef]
- Moontaha, S.; Schumann, F.; Arnrich, B. Online Learning for Wearable EEG-Based Emotion Classification. Sensors 2023, 23, 2387. [Google Scholar] [CrossRef] [PubMed]
- Sugden, R.J.; Pham-Kim-Nghiem-Phu, V.-L.L.; Campbell, I.; Leon, A.; Diamandis, P. Remote Collection of Electrophysiological Data with Brain Wearables: Opportunities and Challenges. Bioelectron. Med. 2023, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Mezzina, G.; Walchshofer, M.; Guger, C.; Venuto, D.D. Near-Brain Computation: Embedding P300-Based BCIs at EEG Headset Level. In Proceedings of the 2023 9th International Workshop on Advances in Sensors and Interfaces (IWASI), Bari, Italy, 8–9 June 2023; pp. 319–324. [Google Scholar]
- Gaspar-Figueiredo, D.; Abrahao, S.; Insfran, E.; Vanderdonckt, J. Measuring User Experience of Adaptive User Interfaces Using EEG: A Replication Study. In Proceedings of the 27th International Conference on Evaluation and Assessment in Software Engineering, Oulu, Finland, 14–16 June; Association for Computing Machinery: New York, NY, USA, 2023; pp. 52–61. [Google Scholar]
- Vidal-Rosas, E.E.; von Lühmann, A.; Pinti, P.; Cooper, R.J. Wearable, High-Density fNIRS and Diffuse Optical Tomography Technologies: A Perspective. NPh 2023, 10, 023513. [Google Scholar] [CrossRef] [PubMed]
- Kasyanova, Y.S.; Egorov, D.A.; Shevchenko, D.S. Biointerfaces: Types, Application and Perspectives. In Proceedings of the 2023 Seminar on Digital Medical and Environmental Systems and Tools (DMEST), Saint Petersburg, Russia, 24 November 2023; pp. 70–74. [Google Scholar]
- Beiramvand, M.; Lipping, T.; Karttunen, N.; Koivula, R. Mental Workload Assessment Using Low-Channel Prefrontal EEG Signals. In Proceedings of the 2023 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023; pp. 1–5. [Google Scholar]
- Boere, K.; Parsons, E.; Binsted, G.; Krigolson, O.E. How Low Can You Go? Measuring Human Event-Related Brain Potentials from a Two-Channel EEG System. Int. J. Psychophysiol. 2023, 187, 20–26. [Google Scholar] [CrossRef]
- Culau, L.; Pereira, R. Exploring the Usability and Acceptance of an EEG Headset in Game Playing; Department of Computer Science, Universidade Federal do Paraná: Paraná, Brasil, 2019. [Google Scholar]
- Warsito, I.F.; Komosar, M.; Bernhard, M.A.; Fiedler, P.; Haueisen, J. Flower Electrodes for Comfortable Dry Electroencephalography. Sci. Rep. 2023, 13, 16589. [Google Scholar] [CrossRef]
- Myles, K.; Kalb, J.T.; Lowery, J.; Kattel, B.P. The Effect of Hair Density on the Coupling between the Tactor and the Skin of the Human Head. Appl. Ergon. 2015, 48, 177–185. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardi, I.; Buono, M.; Giugliano, G.; Senese, V.P.; Capece, S. Usability and Acceptance Analysis of Wearable BCI Devices. Appl. Sci. 2025, 15, 3512. https://doi.org/10.3390/app15073512
Lombardi I, Buono M, Giugliano G, Senese VP, Capece S. Usability and Acceptance Analysis of Wearable BCI Devices. Applied Sciences. 2025; 15(7):3512. https://doi.org/10.3390/app15073512
Chicago/Turabian StyleLombardi, Ilaria, Mario Buono, Giovanna Giugliano, Vincenzo Paolo Senese, and Sonia Capece. 2025. "Usability and Acceptance Analysis of Wearable BCI Devices" Applied Sciences 15, no. 7: 3512. https://doi.org/10.3390/app15073512
APA StyleLombardi, I., Buono, M., Giugliano, G., Senese, V. P., & Capece, S. (2025). Usability and Acceptance Analysis of Wearable BCI Devices. Applied Sciences, 15(7), 3512. https://doi.org/10.3390/app15073512






