Abstract
Biofilms pose a major challenge to the food industry, as they develop on both biotic and abiotic surfaces and contribute to the persistence of antibiotic-resistant pathogens. This study evaluated the antimicrobial and antibiofilm potential of Satureja thymbra, Thymus capitatus, and Origanum hirtum essential oils (EOs), their main components (thymol, carvacrol, p-cymene, and γ-terpinene), and ethanolic and ethyl acetate extracts of the water-steam distillation residue of T. capitatus (WSTRTc). Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values of EOs and WSTRTc extracts ranged from 0.6 to 56.8 mg/mL. The corresponding MIC values of the main components ranged in lower values (0.5–2.4 mg/mL). Minimum Biofilm Inhibition Concentration (MBIC) and Minimum Biofilm Eradication Concentration (MBEC) were also determined. MBIC values for the EOs ranged from 0.6 to 4.7 mg/mL against biofilms of Escherichia coli, Listeria monocytogenes, Proteus mirabilis, and Salmonella Enteritidis. Significant antibiofilm activity at concentrations > 61 mg/mL and > 20 mg/mL was recorded by the ethanolic and ethyl acetate WSTRTc extracts, respectively, but no activity against L. monocytogenes and P. mirabilis biofilms was documented. These findings highlighted the efficacy of EOs and extracts derived from Greek herbs, suggesting their application in the food and pharmaceutical industry as natural antimicrobials and biofilm inhibitors.
1. Introduction
Pathogenic biofilms pose a serious threat to public health, as many food poisoning outbreaks have been associated with their formation. The development of biofilms by bacteria is a self-protection growth pattern and a complex process, including the following stages: initial attachment, irreversible attachment, biofilm development, biofilm maturation, and biofilm dispersion [1,2]. Specifically, biofilm formation develops when free planktonic cells adhere to a substrate, begin to replicate, and establish a cell-to-cell communication called quorum sensing (QS) and response, which allows them to be more resistant/tolerant to all kinds of stress conditions, including antibiotics and disinfectants. QS is also involved in virulence gene expression [1,2]. Biofilms can be mono-species but usually are multi-species, some of which are beneficial for the food industry, e.g., in fermented food production, but, most often, biofilms cause food contamination and spoilage [3] that can occur at any stage of the food processing chain, from food production to consumption, and can also occur due to cross-contamination from the processing environment. These disturbances not only constitute a major financial loss to the food industry, but may also affect the health of consumers by compromising food safety.
At the same time, the emergence of inherited antimicrobial resistance remains one of the greatest public health challenges and the formation of biofilm has been postulated as a mechanism by which microbes resist antimicrobial agents, rendering already used disinfectants ineffective. As a result, bacteria survive, grow, and cause serious infections [4]. Antibiotic resistance is on the rise, as is the tolerance of biofilm-embedded cells (even in the planktonic state), prompting researchers to look for new and more effective methods of eliminating pathogenic biofilms. Chemicals may be detrimental to the environment; therefore, the discovery of new non-harmful substances with antimicrobial activity is really important [4].
The medicinal properties of plants have been known since ancient times. The essential oils (EOs), plant extracts, and by-products of the distillation of certain aromatic plants have significant antimicrobial properties [5,6]. Therefore, in recent years, natural antimicrobials have emerged as alternatives, among which are plant-derived EOs, in addition to the positive consumers’ perception, in contrast to chemical preservatives, especially in food and pharmaceutical industry applications. Plant-based EOs and extracts are primarily composed of a variety of bioactive and volatile compounds at distinct concentrations. EOs and plant extract compositions vary among plant species, the plant parts used for the extraction of the bioactive compound, the extraction method, the climate, the time of harvesting, and the cultivation conditions [7].
However, it is important to note that the EO represents only a minor fraction of the plant. A significant portion of the plant biomass, termed “solid waste”, remains after the recovery of EO during the distillation process. This residual material is rich in polyphenolic compounds, which have potential applications as antimicrobial or antioxidant agents. For example, Skoula and Grayer [8] detected flavonoids in the aerial parts of the herb Satureja spp. These polyphenols could be valuable for further investigation, as they may provide additional antimicrobial properties or enhance the antimicrobial activity of plant extracts, contributing to sustainable and comprehensive use of aromatic plants in both the food and pharmaceutical industries. Exploring the bioactivity of such compounds could lead to the development of new natural antimicrobial agents, thus maximizing the use of plant resources beyond EO extraction. Flavonoids, flavonoid glycosides, and phenolic acids may be fractionated and recovered from aromatic plants by successive extractions with organic solvents.
Several studies are focusing on the research of the antimicrobial activity of EOs and plant by-products against biofilms [3,4,9] or in combination with nanocapsules [10,11], nanomaterials [12], and antibiotics [13,14]. In this vein, the aim of the present study was to evaluate the antibiofilm effect of Satureja thymbra, Thymus capitatus, and Origanum hirtum EOs, cultivated in Greece, and their main components (thymol, carvacrol, p-cymene, and γ-terpinene), along with the ethanolic and ethyl acetate extracts of the water-steam distillation residue of T. capitatus against biofilm formation of the common pathogens Escherichia coli, Listeria monocytogenes, Proteus mirabilis, and Salmonella Enteritidis.
2. Materials and Methods
2.1. EOs and Extracts of the Water-Steam Distillation Residue of Thymus Capitatus
Satureja thymbra, Thymus capitatus, and Origanum hirtum EOs, as well as the ethanolic (Et) and ethyl acetate (EtAc) extracts of the water-steam distillation residue of T. capitatus (WSTRTc), were kindly provided by Prof. V. Oreopoulou (School of Chemical Engineering, NTUA).
The main components of the EOs (thymol, carvacrol, p-cymene, and γ-terpinene) were commercially purchased in powder form from Sigma-Aldrich (St. Louis, MO, USA).
S. thymbra and O. hirtum EOs were extracted as described by Tsimogiannis et al. [15] and Mitropoulou et al. [16], respectively. Briefly, S. thymbra and T. capitatus leaves were harvested during the flowering season in May 2014 and O. hirtum in 2016, during the maximum blooming period. S. thymbra and T. capitatus EOs were obtained by water-steam distillation of 500 g of dry leaves in a pilot-scale distiller of 10 L useful capacity with an inner perforated grid to hold the plant material above the boiling water. The initial volume of boiling water was 3.5 L, and the distillation processes lasted for 180 and 250 min, respectively. Likewise, 9.6 kg of O. hirtum dry leaves were submerged in 150 L of water, and the water-steam distillation lasted for 16 h.
For the preparation of the extracts of the water-steam distillation residue of T. capitatus, the procedure described below was followed: After the completion of water-steam distillation, the wet herbal residue was dried in a ventilated oven at 39 °C over a period of 24 h. Part of the distilled and dried herb was powdered with the use of a domestic blender. The powder was then subjected to two successive Soxhlet extractions with ethyl acetate and ethanol [15].
2.2. Microbial Strains
Escherichia coli ATCC 25922, Listeria monocytogenes NCTC 10527 serotype 4b, Salmonella enterica subsp. Enterica ser. Enteritidis FMCC B56 PT4, and the clinically isolated Proteus mirabilis (opportunistic foodborne pathogen) were grown in Brain Heart Infusion (BHI) broth (LABM, Hertfordshire, UK) at 37 °C for 24 h.
2.3. Analytical Procedures
2.3.1. Phytochemical Analyses
The two extracts of T. capitatus were analyzed by HPLC-DAD as described by Kouri et al. [17]. Briefly, the analyses were conducted with a system that consisted of an autosampler (Agilent Infinity 1260, Agilent, Santa Clara, CA, USA), a gradient quaternary pump (HP 1100, Hewlett Packard, Waldbronn, Germany), and a diode array detector (HP 1100), which was connected to a ZORBAX Eclipse XDB-C18 column (5 μm, 250 × 4.6 mm). The solvent system comprised water (A), methanol (B), and acetonitrile (C), each containing 0.2% trifluoroacetic acid. The initial composition of the mobile phase was 90% A, 6% B, and 4% C, and changed with linear gradients to 85% A, 9% B, and 6% C within 5 min, 71% A, 17.4% B, and 11.6% C within 30 min, and 0% A, 85% B, and 15% C within 60 min. The solvent flow rate was 1 mL/min, and the injection volume was 20 μL. Detection was performed at 280 nm, and the data were processed by the ChemStation for LC 3D software (Agilent, Rev. B.04.03). Standard rosmarinic acid, carvacrol, and quercetin were used as external standards for the quantifications of phenolics in extracts. Specifically, quercetin was used for the determination of total flavonoids.
The GC/MS analyses of EOs were carried out according to Choulitoudi et al. [18]. An Agilent 6890 GC system (plus+) coupled to an Agilent 5973 mass selective detector (Agilent, Santa Clara, CA, USA), equipped with an HP-5 MS column (30 m × 20 μm × 0.25 μm), was used for the analyses. The oven temperature was started at 50 °C, increased to 100 °C at a 10 °C min−1 rate, then to 220 °C at a 15 °C min−1 rate, and held at 220 °C for 7 min. Helium was used as a carrier gas at 1 mL min−1 column flow, inlet temperature 220 °C, and split 20:1. The mass range was 40–400, and compounds were identified by comparison of their mass spectra with the data of NIST and Wiley mass spectral libraries.
2.3.2. Determination of Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC and MBC values, expressed as mg/mL, were determined using the microdilution method, as previously described [6,19]. All compounds were tested in one microplate per microorganism and concentrations ranged from 0.02 to 10 mg/mL for EOs and 3.1–100 mg/mL for WSTRTc extracts. Standard polystyrene microplates with lids were used to avoid the transition of the vapors between the wells [20,21,22].
2.3.3. Adherent Biofilm Inhibition Formation Assays
Pathogenic biofilm inhibition formation activity expressed as Minimum Biofilm Inhibition Concentration (MBIC) of EOs was assessed using a microtiter plate-based crystal violet staining assay [4,11,23]. Specifically, 200 μL of 107 CFU/mL bacterial suspensions in ¼ Ringer’s solution (VWR International S.A.S, Darmstadt, Germany) were transferred into a well of a 96-well polystyrene flat-bottomed microtiter plate and placed at 30 °C for 2 h under static conditions, allowing bacteria to adhere to the well surface. After the incubation, the suspensions were carefully removed, and the wells were washed once with ¼ Ringer’s solution (VWR International S.A.S) to remove any remaining planktonic bacteria. The adherent biofilm was treated with EOs, as previously described by Vetas et al. [4], for 48 h. Two hundred μL of Brain Heart Infusion broth (LABM) supplemented with EOs or their main components or WSTRTc extracts at concentrations tested for MIC and MBC determination (Section 2.3.2), followed by incubation at 30 °C for 48 h. After 48 h of incubation, the wells were washed once with ¼ Ringer’s solution (VWR International S.A.S.) to remove all planktonic bacteria, and the growth medium was renewed with fresh medium without the addition of EOs or main compounds or WSTRTc extracts. After medium renewal, biofilms were incubated again for 48 h in order to reach their maximum formation [24,25,26,27].
Afterward, the supernatant was carefully removed, and the wells were washed once with ¼ Ringer’s solution (VWR International S.A.S). The biofilms were then fixed with methanol (Chem-Lab NV, Zedelgem, Belgium), stained with 1% w/v Crystal Violet (Sigma-Aldrich) for 30 min, washed once with distilled water to remove any excess Crystal Violet (Sigma-Aldrich) and quantified by eluting Crystal Violet with an ethanol–acetone mix (80/20 v/v). The optical absorbance of the eluted dye was determined at 620 nm using a microplate reader (Tecan, Sunrise, Program XFLUOR4, Männedorf, Switzerland). Wells containing only Brain Heart Infusion broth (LABM) and wells containing Brain Heart Infusion broth (LABM) and pathogens with no treatment served as negative and positive controls, respectively.
MBEC is defined as the lowest concentration of an antimicrobial substance that eradicates 99.9% of biofilm-embedded bacteria. The Minimum Biofilm Eradication Concentration (MBEC) was determined as described by Castaneda et al. [28] with some modifications. MBEC determinations were performed on biofilms grown in triplicate for 2 h under static conditions and rinsed once with ¼ Ringer’s solution to remove planktonic cells keeping the biofilm on the bottom of the wells intact. The growth media was replaced with 200 μL of Brain Heart Infusion broth containing the EOs or the main compounds, or the WSTRTc extracts (antimicrobials), at various concentrations. After 48 h of incubation, the wells were washed once with ¼ Ringer’s solution to remove all planktonic cells and the growth medium was renewed with fresh medium without the addition of the antimicrobials. Following medium renewal, biofilms were incubated for another 48 h to allow maximum formation. Then, the biofilms were rinsed four times with 200 μL Brain Heart Infusion broth to minimize residual antimicrobial agents. Biofilms were then removed from each well by scraping with sterile transfer pipettes (Thermo Fisher Scientific, Waltham, MA, USA). The collected biofilm fragments, after scraping, were placed in 3 mL of Brain Heart Infusion broth for subculturing. The subcultures were then incubated at 37 °C for 21 days with aliquots plated onto Brain Heart Agar every 48 h for colony enumeration. The MBEC was recorded as the lowest concentration, and at least two of the three replicates were culture-negative after subculture incubation. The resulting data were noncontinuous, ordinal in nature, and characterized by variable intervals between concentrations [28].
2.4. Statistical Analysis
All experiments were performed in at least four independent replicates and the mean values are presented. The results were analyzed with analysis of variance (ANOVA) using Tukey’s multiple range test to determine significant differences (p < 0.05) among results (coefficients, ANOVA tables, and significance (p < 0.05) were computed using Statistica v.10.0). Specifically, MIC, MBC, MBIC, and MBEC values were treated as dependent variables, while bacterial strains and the antimicrobials were considered as independent factors.
3. Results
3.1. Chemical Composition of EOs
Chemical analyses of EOs [18] were performed with GC-MS and revealed that above 80% of the EOs composition consisted of the same four ingredients, namely thymol, carvacrol, p-cymene, and γ-terpinene, but in different concentrations. The main components of S. thymbra EO were γ-terpinene (40.1%) and carvacrol (30.8%). In the case of O. hirtum EO, the dominant compounds were thymol (48.1%) and carvacrol (27.2%), while, in T. capitatus, carvacrol was, by far, the main volatile (81.8%) (Table 1).

Table 1.
Percentage (%) of the main components of S. thymbra, O. hirtum, and T. capitatus EOs, as derived by GC-MS analyses.
3.2. Chemical Composition of T. capitatus Extracts
The successive extractions of T. capitatus were performed with two solvents of increasing polarity and different properties as far as the hydrogen bonding is concerned. Ethyl acetate is a solvent of low polarity and non-protic (hydrogen bond acceptor), while ethanol presents higher polarity, and the extraction of compounds is based on the donation of hydrogen bonds by the solvent (protic, hydrogen bond donor). Ethyl acetate was expected to extract compounds of low polarity, such as residual EO components, lipids, etc., and, to a lesser extent, phenolics.
Ethyl acetate extract yielded 4% of the total solids of distilled T. capitatus, while the main component was residual carvacrol. Since a residual quantity of carvacrol was detected in the ethyl acetate extract, the compound was quantified according to a calibration curve performed with the respective standard using HPLC analysis. Carvacrol was quantified at 176 mg/g dried extract. Terpenic monophenols, such as thymol and carvacrol, presented quite a high signal each, at 280 nm, while terpenic hydrocarbons, like p-cymene and γ-terpinene, did not respond in the HPLC-DAD analysis. The extract was also injected into the GC-MS system in order to verify the composition of the residual EO. The analysis revealed that only carvacrol remained as residual volatile in the herbal material after distillation, a fact that is reasonable since carvacrol is a “heavy” volatile with a boiling point of 238 °C, distilling slowly, while γ-terpinene and p-cymene are “lighter” volatiles, (boiling points at 183 and 177 °C, respectively), and their distillation rates are much higher than those of carvacrol. The distillation process quantitatively recovered the terpenic hydrocarbons, and a residue of carvacol remained. Thymol is an isomer of carvacrol with an equally high boiling point (232 °C), but it was barely detectable in the ethyl acetate extract, according to the GC-MS analysis, attributed to the very low content of the compound in the herb, i.e., 0.4%, as presented in Table 1.
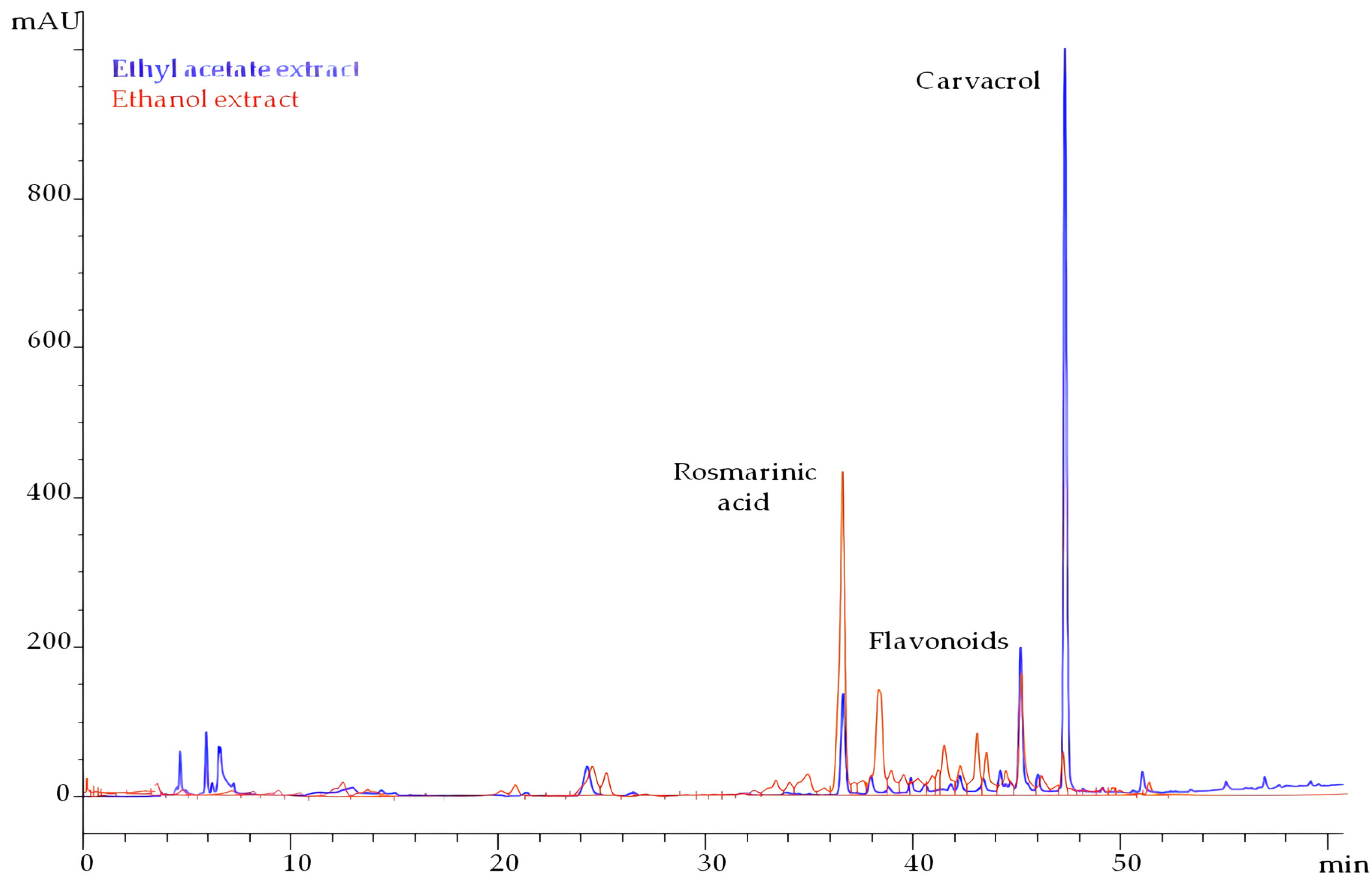
The extract also contained rosmarinic acid, but at a lower concentration of 13 mg/g dried extract. Both chromatograms of ethyl acetate and ethanol extracts are presented in Figure 1. The peaks eluted between rosmarinic acid and carvacrol possessed characteristic UV spectra of flavonoids. The majority belongs to the subclasses of flavonols and flavones. Ethyl acetate extract contained 60 mg total flavonoids/g dried extract.
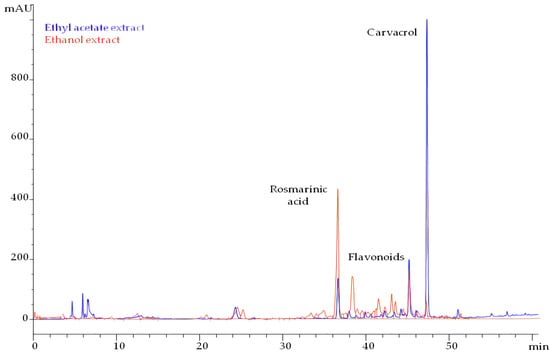
Figure 1.
HPLC chromatograms of ethyl acetate and ethanol extract from pre-distilled T. capitatus, monitored at 280 nm.
Ethanol yielded 6.1% of total solids, and carvacrol was detected in traces (0.2%). The main group of compounds was flavonoids that amounted to 117 mg/g dried extract. Rosmarinic acid was determined at 31 mg/g dried extract.
3.3. Antimicrobial Activity
The antimicrobial activity of EOs, WSTRTc extracts, and their main components were determined, and the results are presented in Table 2 and Table 3. The MIC values of EOs and WSTRTc extracts ranged from 0.6 to 3.1 mg/mL and 20.1 to 40.7 mg/mL, respectively, while the MIC values of the main components varied from 0.5 to 2.4 mg/mL. The MBC values of EOs and WSTRTc extracts were 2.7 and 56.8 mg/mL, respectively, while those of the main components ranged from 1.9 to 8.6 mg/mL.

Table 2.
Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) (mg/mL) of EOs and extracts of the water-steam distillation residue of T. capitatus against pathogenic bacteria.

Table 3.
Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) (mg/mL) of thymol, carvacrol, p-cymene, and γ-terpinene against pathogenic bacteria.
3.4. Anti-Biofilm Activity
The inhibition of biofilm formation activity of EOs, their main components, and WSTRTc extracts was evaluated against four common pathogens. MBIC and MBEC values are presented in Table 4 and Table 5. MBIC values of S. thymbra, T. capitatus, and O. hirtum EOs ranged from 0.6 to 1.1, 1.1 to 2.3, and 1.2 to 4.7 mg/mL, respectively, while the corresponding values for thymol and carvacrol were 0.3–0.6 and 0.6–2.4 mg/mL. Likewise, MBIC values of p-cymene and γ-terpinene ranged from 0.1 to 0.3 mg/mL and 0.5 to 2.1 mg/mL, respectively. Similarly, inhibition of biofilm formation was documented by the EtAc WSTRTc extract against all strains at concentrations of 20–40 mg/mL. On the other hand, the ethanolic WSTRTc extract was effective against biofilm formation by E. coli and S. Enteritidis (MBIC 61 mg/mL), but had no effect on L. monocytogenes or P. mirabilis at the concentrations tested, and, thus, MBIC was not determined. Notably, the MBEC values of the EOs ranged 2.3–9.4 mg/mL, while no biofilm eradication effect of WSTRTc extracts was observed at the concentrations tested.

Table 4.
Minimum Biofilm Inhibition Concentration (MBIC) and Minimum Biofilm Eradication Concentration (MBEC) (mg/mL) of EOs and extracts of the water-steam distillation residue of T. capitatus against pathogenic bacteria.

Table 5.
Minimum Biofilm Inhibition Concentration (MBIC) and Minimum Biofilm Eradication Concentration (MBEC) (mg/mL) of the main components of the EOs (thymol, carvacrol, p-cymene, and γ-terpinene) against pathogenic bacteria.
Both the bacterial strains and the antimicrobial agents (EOs, main compounds, or WSTRTc extracts) significantly affected the MIC, MBC, MBIC, and MBEC values (p < 0.05), and a strong interaction between the two factors was observed (p < 0.05). In general, the strongest antibiofilm activity between the EOs tested was recorded by S. thymbra followed by T. capitatus, while O. hirtum had the least effect on biofilm inhibition (p < 0.05) in all bacteria tested except E. coli (p > 0.05 to S. thymbra) and L, monocytogenes (p > 0.05 to T. capitatus). The EOs and their main compounds showed significantly (p < 0.05) stronger antibiofilm activity against all bacteria tested than the WSTRTc extracts. O. hirtum EO inhibited biofilm formation by L. monocytogenes, S. Enteritidis, and P. mirabilis at higher concentrations compared to E. coli (p < 0.05). Thymol demonstrated significantly higher (p < 0.05) anti-biofilm activity than the EOs in all bacteria tested, except for S. thymbra against L. monocytogenes where no significant differences (p > 0.05) were noted. Carvacrol’s biofilm inhibition activity was significantly higher (p < 0.05) than that of O. hirtum EO against E. coli and S. Enteritidis, significantly lower (p < 0.05) than S. thymbra EO against L. monocytogenes, and significantly higher (p < 0.05) against P. mirabilis than T. capitatus and O. hirtum EOs. Likewise, γ-terpinene demonstrated stronger (p < 0.05) antibiofilm activity against E. coli and S. Enteritidis, as well as stronger (p < 0.05) inhibition of biofilm formation than T. capitatus EO against S. Enteritidis and E. coli, than O. hirtum EO against E. coli, S. Enteritidis, and P. mirabilis, but showed weaker (p < 0.05) antibiofilm activity than S. thymbra EO against L. monocytogenes and P. mirabilis. Additionally, significantly lower (p < 0.05) antibiofilm activity of γ-terpinene was noted against L. monocytogenes compared to p-cymene and thymol and against P. mirabilis compared to all compounds tested.
Or note, the MBEC values of the EOs were significantly (p < 0.05) higher than the corresponding MBIC values in all cases.
4. Discussion
S. thymbra, T. capitatus, and O. hirtum EOs have been previously tested as potential antimicrobial and antibiofilm agents [3,15,29,30,31,32]. In the present study, determining the Minimum Inhibitory Concentration (MIC) first allowed for a primary understanding of the antimicrobial efficacy of the EOs, WSTRTc extracts, and their main components. This step served as a precursor to the subsequent evaluation of their MBIC, enabling a comprehensive characterization of their antimicrobial activity. The MIC values indicated that the tested EOs, WSTRTc extracts, and their main components were effective antimicrobial agents against selected pathogenic bacteria. Notably, all main compounds tested (thymol, carvacrol, p-cymene, and γ-terpinene) exhibited low MIC values, underscoring their antimicrobial activity. These findings are consistent with previous studies demonstrating the efficacy of these compounds as natural antimicrobial agents [33,34,35,36].
The present study showed that all EOs and their four main components had antibiofilm activity against common pathogens. A variation between MIC and MBIC values was observed, highlighting the differences between inhibiting planktonic bacterial growth and preventing biofilm formation. MIC values represent the minimum concentration needed to inhibit the growth of planktonic bacteria, whereas MBIC values reflect the higher concentrations often required to interfere with biofilm formation, due to the enhanced resistance of biofilms. The observed differences underscored the resilience of biofilms, which derive protection from mechanisms, such as the production of an extracellular polymeric substance (EPS) matrix and the activation of quorum-sensing systems. Interestingly, in some cases, MBIC values were lower than MIC values, potentially due to stress-induced suppression of bacterial adhesion genes during the initial biofilm formation phase. This phenomenon suggested that the antimicrobial compounds tested may effectively disrupt early biofilm formation steps by interfering with adhesion mechanisms, as previously reported in the literature [37,38,39,40]. Moreover, the observed variation between MIC and MBIC values highlighted the importance of strain-dependent and environmental factors in influencing bacterial adherence and biofilm formation. These factors include the structural properties of bacterial surface appendages, the composition of the EPS matrix, and the specific interactions between bacterial cells and their environment [41].
Many studies indicated the antimicrobial effect of S. thymbra EO [42,43], but the antibiofilm activity was less examined. Chorianopoulos et al. [3] tested the ability of S. thymbra ΕO and its hydrosol to eliminate preformed monoculture and mixed culture biofilms of L. monocytogenes compared to hydrochloric acid, lactic acid, and sodium hydroxide. The results suggested a substantial bactericidal effect of the oil and its hydrosol, but the only concentration examined was 1% (v/v). In the present study, the MBIC of S. thymbra EO against L. monocytogenes was estimated at 0.6 (mg/mL).
With the exception of S. thymbra against L. monocytogenes, the EOs tested showed antibiofilm potential, but MBIC ranged at similar or higher values than those of their main compounds (p < 0.05). The antagonistic effect between these compounds could possibly explain the difference in MBIC values against E. coli, S. Enteritidis, and P. mirabilis species. Although S. thymbra EO consists mainly of γ-terpinene and carvacrol, the MBIC of S. thymbra EO against L. monocytogenes biofilm formation was almost 4-fold lower than the MBIC of the two main compounds when tested alone (p < 0.05), suggesting a possible synergistic effect with the other components of the oil against this strain. Al-Shuneigat et al. [44] tested the antibiofilm activity of Thymus vulgaris EO against seven clinically biofilm-forming bacteria. The MBIC determined for E. coli and P. mirabilis at 0.125% (v/v) and 0.25% (v/v), respectively, was in accordance with the results of the present study. T. vulgaris EO inhibited the growth of E. coli up to almost 70% at 0.75 mg/mL [45]. Kerekes et al. [46] found a strong inhibitory effect of T. vulgaris EO against the biofilm formation of E. coli and L. monocytogenes at 0.5 mg/mL and 2 mg/mL, respectively, in line with the results of this study, as E. coli was more sensitive to T. capitatus EO than L. monocytogenes. Noumi et al. [47] showed that the methanolic extract of Thymus musilii Velen. can inhibit the biofilm formation of S. Typhimurium, E. coli, and L. monocytogenes by 36.59 ± 2.84% at 5 mg/mL, 53.96 ± 4.21% at 10 mg/mL and 41.96 ± 3.42% at 5 mg/mL, respectively.
The MBIC of O. hirtum EO against E. coli in this study was 1.2 (mg/mL), which was in agreement with Bīlge Oral et al. [48], as they reported inhibition of biofilm formation and removal of established E. coli biofilm at 0.1% (v/v). Lee et al. [49] screened 79 EOs for antibiofilm activity against uropathogenic E. coli and noticed that oregano oil, thyme red oil, carvacrol, and thymol significantly inhibited biofilm formation at subinhibitory concentrations of less than 0.01% (v/v).
Apparently, the antimicrobial and antibiofilm potential of EOs is closely linked to the composition of their main compounds. Variations in the abundances of carvacrol, thymol, p-cymene, and γ-terpinene can influence the antimicrobial efficacy of EOs against certain pathogens, suggesting either a possible antagonistic or synergistic effect depending on each case. Between the four main compounds tested, the strongest biofilm inhibition formation activity was noted by p-cymene followed by thymol, while carvacrol and γ-terpinene had the least effect on biofilm inhibition. The MBIC values of p-cymene against all bacterial biofilms tested were significantly (p < 0.05) lower than those of the EOs, suggesting that other EOs components may influence the biofilm inhibition activity, potentially through antagonistic interactions. In accordance with the presented results, Maniki et al. [50] also demonstrated the strong antibiofilm activity of T. capitatus EO, consisting mainly of p-cymene and thymol, against S. Enteritidis and L. monocytogenes [MBIC values ranged between 0.03 and 0.13% (v/v)]. Recently, Yuan et al. [32] suggested that p-cymene could directly affect some of the virulence genes of pathogenic bacteria, such as Streptococcus mutans. In addition, the combination of p-cymene with other compounds like carvacrol and linalool has shown increased synergistic effects against biofilms [51]. In their study, Sousa et al. [51] observed a reduction of approximately 30% in the total biomass of Gardnerella species biofilms due to p-cymene. Besides diminishing established biofilms, p-cymene was effective in inhibiting the initial attachment of microbial cells to surfaces, thus preventing biofilm formation. While the specific mechanism by which p-cymene exhibits its anti-biofilm activity is still being investigated, it has been suggested that its ability to disrupt quorum sensing and interfere with the production of extracellular polymeric substances is a potential mechanism of action [52].
Yuan et al. [32] suggested that thymol can alter cell membrane permeability, disturbing both protein synthesis and binary fission and thus inhibiting bacterial growth. In their study, they tested thymol against Staphylococcus aureus ATCC BAA-1717 (MRSA) biofilm formation at concentrations ranging between 8 and 64 mg/mL, and they observed a reduction in biofilm formation between 27% and 63.9%. In accordance with the results of the present study, Khan et al. [53] showed that thymol possessed stronger antimicrobial activity and a stronger inhibitory effect against S. mutans biofilm formation than carvacrol and clove oil was recorded. Chatrath et al. [54] demonstrated that thymol exhibited stronger antibiofilm activity against Candida tropicalis than citrol. Moreover, thymol can have a synergistic effect with other naturally derived or chemical compounds (i.e., citral, antibiotics, etc.). Valliammai et al. [55] unveiled the synergistic effect of citral and thymol in biofilm inhibition formation by MRSA ATCC 33591, compared to the antibiofilm activity when the compounds were used separately. According to Tokam Kuaté et al. [56], the combination of thymol with three different antibiotics (amikacin, kanamycin, and streptomycin) against four Salmonella enterica serovars decreased the MBICs of antibiotics against biofilm formation. Additionally, a combination of amikacin with thymol resulted in a 32-fold decrease in the MBIC against S. Enteritidis biofilm formation, while a combination of kanamycin with thymol in a 1-fold decrease and streptomycin with thymol in an 8-fold reduction.
Carvacrol, as a natural compound found in various plants, including oregano, thyme, and marjoram, is well-known for its antibacterial properties and is effective in the treatment of biofilm formation [57]. In a recent study by Fernández-Babiano et al. [58], carvacrol was evaluated for its antibiofilm activity against S. mutans and Streptococcus sanguinis, and the MBIC values were determined at 93.4 μg/mL for both strains. Furthermore, carvacrol has been found to possess anti-virulence potential and disrupt the extracellular matrix of biofilms, inhibiting their formation and promoting their dispersal [59]. The mechanism of action is probably attributed to the ability of carvacrol to disrupt the extracellular matrix of biofilms. A synergistic effect of carvacrol with other antimicrobial agents and compounds has also been observed in L. monocytogenes, in accordance with the above results, suggesting that carvacrol may be used in combination therapies to enhance its antibiofilm activity and overcome antibiotic resistance [51].
γ-Τerpinene was lesseffective against pathogenic bacterial biofilms compared to p-cymene and thymol. It is a naturally occurring secondary metabolite (monoterpene hydrocarbon) present in many medicinal plants [60]. Miladi et al. [60] showed that γ-terpinene alone or in combination with tetracycline was generally less effective against pathogenic biofilms, in accordance with the results of the present study, as we observed higher MBIC values for γ-terpinene in comparison to thymol, carvacrol, and p-cymene in most bacteria tested.
The results of this study emphasized the potential of EOs, WSTRTc extracts, and their primary components as alternative or complementary treatments for addressing biofilm-related infections. Further exploration into the specific mechanisms through which these components demonstrate their antibiofilm effects will be crucial for optimizing their therapeutic effectiveness and devising successful combination therapies to improve their efficacy. Additionally, the findings underscore the significance of evaluating the composition of EOs and WSTRTc extracts when assessing their antimicrobial and antibiofilm capabilities. Different compounds found in EOs and WSTRTc extracts may exhibit varying levels of effectiveness against biofilms, highlighting the need for additional investigation into their activity and the potential ability of pathogenic bacteria to adapt to EO and plant extracts constituents.
5. Conclusions
The results revealed that EOs of S. thymbra, T. capitatus, and O. hirtum, as well as their main components (thymol, carvacrol, p-cymene, and γ-terpinene), exhibited significant antibiofilm activity against the common pathogens. Additionally, a substantial antibiofilm effect of the ethanolic and ethyl acetate WSTRTc extracts was observed. Studying the antimicrobial and antibiofilm activities of water-steam distillation residues is an emerging approach that has attracted considerable interest due to its potential in providing novel bioactive compounds. While the use of EOs is well-documented, the investigation of their distillation residues as bioactive compounds is less explored. The above findings contributed to this growing field, suggesting that WSTRTc extracts, along with EOs derived from Greek herbs, may serve as effective and inexpensive sources of potent natural antimicrobial agents with applications in the food and pharmaceutical industry. However, further research is required to elucidate their mode of action and determine the safety of long-term use, particularly at high doses.
Author Contributions
Conceptualization, V.L. and Y.K.; data curation, G.M., D.T., V.O., V.L. and Y.K.; formal analysis, G.M., D.T., V.O., V.L. and Y.K.; funding acquisition, Y.K.; investigation, G.M., I.K. and D.T.; methodology, G.M., I.K., D.T., V.O., V.L. and Y.K.; project administration, Y.K.; resources, V.O., V.L. and Y.K.; supervision, V.L. and Y.K.; validation, G.M., D.T., V.O., V.L. and Y.K.; visualization, G.M., V.O. and Y.K.; writing—original draft, I.K., and D.T.; writing—review and editing, G.M., V.O., V.L. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Salmonella enterica subsp. Enterica ser. Enteritidis FMCC B56 PT4 was kindly provided by Nychas G. J. E., Agricultural University of Athens, Athens, Greece, and the clinically isolated Proteus mirabilis was kindly provided by the Laboratory of Clinical Microbiology, Sismanoglio General Hospital, Greece.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, X.; Yao, H.; Zhao, X.; Ge, C. Biofilm Formation and Control of Foodborne Pathogenic Bacteria. Molecules 2023, 28, 2432. [Google Scholar] [CrossRef] [PubMed]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Chorianopoulos, N.G.; Giaouris, E.D.; Skandamis, P.N.; Haroutounian, S.A.; Nychas, G.-J.E. Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: Bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid–base sanitizers. J. Appl. Microbiol. 2008, 104, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Vetas, D.; Dimitropoulou, E.; Mitropoulou, G.; Kourkoutas, Y.; Giaouris, E. Disinfection efficiencies of sage and spearmint essential oils against planktonic and biofilm Staphylococcus aureus cells in comparison with sodium hypochlorite. Int. J. Food Microbiol. 2017, 257, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Fitsiou, E.; Stavropoulou, E.; Papavassilopoulou, E.; Vamvakias, M.; Pappa, A.; Oreopoulou, A.; Kourkoutas, Y. Composition, antimicrobial, antioxidant, and antiproliferative activity of Origanum Dictamnus (dittany) essential oil. Microb. Ecol. Health Dis. 2015, 26, 26543. [Google Scholar]
- Awad, A.M.; Kumar, P.; Ismail-Fitry, M.R.; Jusoh, S.; Ab Aziz, M.F.; Sazili, A.Q. Green extraction of bioactive compounds from plant biomass and their application in meat as natural antioxidant. Antioxidants 2021, 10, 1465. [Google Scholar] [CrossRef]
- Skoula, M.; Grayer, R. Volatile oils of Coridothymus capitatus, Satureja thymbra, Satureja spinosa and Thymbra calostachya (Lamiaceae) from Crete. Flavour Fragr. J. 2005, 20, 573–576. [Google Scholar] [CrossRef]
- Rubini, D.; Banu, S.F.; Nisha, P.; Murugan, R.; Thamotharan, S.; Percino, M.J.; Subramani, P.; Nithyanand, P. Essential oils from unexplored aromatic plants quench biofilm formation and virulence of methicillin resistant Staphylococcus aureus. Microb. Pathog. 2018, 122, 162–173. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.; Cafiso, V.; Stefani, S.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef]
- Liakos, I.; Grumezescu, A.; Holban, A.; Florin, I.; D’Autilia, F.; Carzino, R.; Bianchini, P.; Athanassiou, A. Polylactic acid—Lemongrass essential oil nanocapsules with antimicrobial properties. Pharmaceuticals 2016, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.; Holban, A.; Carzino, R.; Lauciello, S.; Grumezescu, A. Electrospun fiber pads of cellulose acetate and essential oils with antimicrobial activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Kissels, W.; Wu, X.; Santos, R.R. Short communication: Interaction of the isomers carvacrol and thymol with the antibiotics doxycycline and tilmicosin: In vitro effects against pathogenic bacteria commonly found in the respiratory tract of calves. J. Dairy Sci. 2017, 100, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Shemchuk, O.; d’Agostino, S.; Fiore, C.; Sambri, V.; Zannoli, S.; Grepioni, F.; Braga, D. Natural antimicrobials meet a synthetic antibiotic: Carvacrol/thymol and ciprofloxacin cocrystals as a promising solid-state route to activity enhancement. Cryst. Growth Des. 2020, 20, 6796–6803. [Google Scholar] [CrossRef]
- Tsimogiannis, D.; Choulitoudi, E.; Bimpilas, A.; Mitropoulou, G.; Kourkoutas, Y.; Oreopoulou, V. Exploitation of the biological potential of Satureja thymbra essential oil and distillation by-products. J. Appl. Res. Med. Aromat. Plants 2017, 4, 12–20. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Oreopoulou, A.; Papavassilopoulou, E.; Vamvakias, M.; Panas, P.; Fragias, S.; Kourkoutas, Y. Origanum vulgare ssp. hirtum essential oil as a natural intrinsic hurdle against common spoilage and pathogenic microbes of concern in tomato juice. Appl. Microbiol. 2021, 1, 1–10. [Google Scholar]
- Kouri, G.; Tsimogiannis, D.; Bardouki, H.; Oreopoulou, V. Extraction and analysis of antioxidant components from Origanum Dictamnus. Innov. Food Sci. Emerg. Technol. 2007, 8, 155–162. [Google Scholar] [CrossRef]
- Choulitoudi, E.; Bravou, K.; Bimpilas, A.; Tsironi, T.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Antimicrobial and antioxidant activity of Satureja thymbra in gilthead seabream fillets edible coating. Food Bioprod. Process. 2016, 100, 570–577. [Google Scholar] [CrossRef]
- Hulankova, R. Methods for Determination of Antimicrobial Activity of Essential Oils In Vitro—A Review. Plants 2024, 13, 2784. [Google Scholar] [CrossRef]
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; de Carvalho, M.G.; Catunda, F.E.A., Jr. Antibacterial and Antibiofilm Activities of Cinnamomum Sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018, 2018, 7405736. [Google Scholar] [CrossRef]
- Sulistyani, H.; Sulastri, S.; Risnawati, D.; Agustina, D. Can Moringa Oleifera Leaf Ethyl Acetate Extract Inhibit Candida Albicans Planktonic Cell Growth and Biofilm Formation in Vitro? J. Drug Deliv. Ther. 2023, 13, 34–37. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter Dish Biofilm Formation Assay. J. Vis. Exp. 2011, 47, e2437. [Google Scholar] [CrossRef]
- Farjami, A.; Hatami, M.S.; Siahi-Shadbad, M.R.; Lotfipour, F. Peracetic acid activity on biofilm formed by Escherichia coli isolated from an industrial water system. Lett. Appl. Microbiol. 2022, 74, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Manville, E.; Kaya, E.C.; Yucel, U.; Boyle, D.; Trinetta, V. Evaluation of Listeria monocytogenes biofilms attachment and formation on different surfaces using a CDC biofilm reactor. Int. J. Food Microbiol. 2023, 399, 110251. [Google Scholar] [CrossRef]
- Ivers, C.; Kaya, E.C.; Yucel, U.; Boyle, D.; Trinetta, V. Evaluation of Salmonella biofilm attachment and hydrophobicity characteristics on food contact surfaces. BMC Microbiol. 2024, 24, 387. [Google Scholar] [CrossRef]
- Wasfi, R.; Hamed, S.M.; Amer, M.A.; Fahmy, L.I. Proteus mirabilis Biofilm: Development and Therapeutic Strategies. Front. Cell. Infect. Microbiol. 2020, 10, 414. [Google Scholar] [CrossRef]
- Castaneda, P.; McLaren, A.; Tavaziva, G.; Overstreet, D. Biofilm Antimicrobial Susceptibility Increases with Antimicrobial Exposure Time. Clin. Orthop. Relat. Res. 2016, 474, 1659–1664. [Google Scholar] [CrossRef]
- Giweli, A.; Džamić, A.M.; Soković, M.; Ristić, M.S.; Marin, P.D. Antimicrobial and antioxidant activities of essential oils of Satureja thymbra growing wild in Libya. Molecules 2012, 17, 4836–4850. [Google Scholar] [CrossRef]
- Jemaa, M.B.; Falleh, H.; Serairi, R.; Neves, M.A.; Snoussi, M.; Isoda, H.; Nakajima, M.; Ksouri, R. Nanoencapsulated thymus capitatus essential oil as natural preservative. Innov. Food Sci. Emerg. Technol. 2018, 45, 92–97. [Google Scholar] [CrossRef]
- Kulaksız, B.; Er, S.; Üstündağ Okur, N.; Saltan İşcan, G. Investigation of antimicrobial activities of some herbs containing essential oils and their mouthwash formulations. Turk. J. Pharm. Sci. 2018, 15, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Dai, Y.; Ouyang, P.; Rehman, T.; Hussain, S.; Zhang, T.; Yin, Z.; Fu, H.; Lin, J.; He, C.; et al. Thymol inhibits biofilm formation, eliminates pre-existing biofilms, and enhances clearance of methicillin-resistant Staphylococcus aureus (MRSA) in a mouse peritoneal implant infection model. Microorganisms 2020, 8, 99. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Raei, P.; Alizadeh, N.; Akbari Aghdam, M.; Kafil, H.S. Carvacrol and Thymol: Strong antimicrobial agents against resistant isolates. Rev. Med. Microbiol. 2017, 28, 63–68. [Google Scholar] [CrossRef]
- Hajibonabi, A.; Yekani, M.; Sharifi, S.; Nahad, J.S.; Dizaj, S.M.; Memar, M.Y. Antimicrobial activity of nanoformulations of Carvacrol and Thymol: New Trend and Applications. OpenNano 2023, 13, 100170. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and Thymol. Crit. Rev. Food Sci. Nutr. 2019, 60, 3042–3053. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- da Silva, B.D.; do Rosário, D.K.A.; Neto, L.T.; Lelis, C.A.; Conte-Junior, C.A. Antioxidant, Antibacterial and Antibiofilm Activity of Nanoemulsion-Based Natural Compound Delivery Systems Compared with Non-Nanoemulsified Versions. Foods 2023, 12, 1901. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of Microorganisms to Cold Temperatures, Weak Acid Preservatives, Low PH, and Osmotic Stress: A Review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Gloag, E.S.; Fabbri, S.; Wozniak, D.J.; Stoodley, P. Biofilm Mechanics: Implications in Infection and Survival. Biofilm 2020, 2, 100017. [Google Scholar] [CrossRef]
- Lou, Z.X.; Letsididi, K.S.; Yu, F.H.; Pei, Z.J.; Wang, H.X.; Letsididi, R. Inhibitive Effect of Eugenol and Its Nanoemulsion on Quorum Sensing-Mediated Virulence Factors and Biofilm Formation by Pseudomonas aeruginosa. J. Food Prot. 2019, 82, 379–389. [Google Scholar] [CrossRef]
- Yu, L.; Shi, H. Recent advances in anti-adhesion mechanism of natural antimicrobial agents on fresh produce. Curr. Opin. Food Sci. 2021, 42, 8–14. [Google Scholar] [CrossRef]
- Al Hafi, M.; El Beyrouthy, M.; Ouaini, N.; Stien, D.; Rutledge, D.; Chaillou, S. Chemical composition and antimicrobial activity of satureja, thymus, and thymbra species grown in Lebanon. Chem. Biodivers. 2017, 14, e1600236. [Google Scholar] [CrossRef] [PubMed]
- Vanti, G.; Tomou, E.-M.; Stojković, D.; Ćirić, A.; Bilia, A.R.; Skaltsa, H. Nanovesicles loaded with Origanum Onites and Satureja thymbra essential oils and their activity against food-borne pathogens and spoilage microorganisms. Molecules 2021, 26, 2124. [Google Scholar] [CrossRef]
- Al-Shuneigat, J.; Al-Sarayreh, S.; Al-Saraireh, Y.; Al-Qudah, M.; Al-Tarawneh, I.; Albataineh, E. Effects of wild thymus vulgaris essential oil on clinical isolates biofilm-forming bacteria. J. Dent. Med. Sci. 2014, 13, 62–66. [Google Scholar] [CrossRef]
- Martínez, A.; Manrique-Moreno, M.; Klaiss-Luna, M.C.; Stashenko, E.; Zafra, G.; Ortiz, C. Effect of essential oils on growth inhibition, biofilm formation and membrane integrity of escherichia coli and Staphylococcus aureus. Antibiotics 2021, 10, 1474. [Google Scholar] [CrossRef]
- Kerekes, E.B.; Vidács, A.; Takó, M.; Petkovits, T.; Vágvölgyi, C.; Horváth, G.; Balázs, V.L.; Krisch, J. Anti-Biofilm effect of selected essential oils and main components on mono- and polymicrobic bacterial cultures. Microorganisms 2019, 7, 345. [Google Scholar] [CrossRef]
- Noumi, E.; Ahmad, I.; Bouali, N.; Patel, H.; Ghannay, S.; ALrashidi, A.A.; Abdulhakeem, M.A.; Patel, M.; Ceylan, O.; Badraoui, R.; et al. Thymus musilii velen. Methanolic extract: In vitro and in silico screening of its antimicrobial, antioxidant, anti-quorum sensing, Antibiofilm, and anticancer activities. Life 2022, 13, 62. [Google Scholar] [CrossRef]
- Bilge Oral, N.; Sezer, Ç.; Başer, K.H.C.; Vatansever, L.; Duman Aydin, B.; Güven, A.; Gülmez, M.; Kürkçüoğlu, M. Effect of Oregano Essential Oil on Biofilms Formed By Staphylococci and Escherichia coli. Kafkas Univ. Vet. Fak. Derg. 2010, 16, 23–29. [Google Scholar]
- Lee, J.-H.; Kim, Y.-G.; Lee, J. Carvacrol-Rich oregano oil and thymol-rich thyme red oil inhibit biofilm formation and the virulence of uropathogenic Escherichia coli. J. Appl. Microbiol. 2017, 123, 1420–1428. [Google Scholar] [CrossRef]
- Maniki, E.; Kostoglou, D.; Paterakis, N.; Nikolaou, A.; Kourkoutas, Y.; Papachristoforou, A.; Giaouris, E. Chemical composition, antioxidant, and antibiofilm properties of essential oil from Thymus capitatus plants organically cultured on the greek island of Lemnos. Molecules 2023, 28, 1154. [Google Scholar] [CrossRef]
- Sousa, L.G.V.; Castro, J.; Cavaleiro, C.; Salgueiro, L.; Tomás, M.; Palmeira-Oliveira, R.; Martinez-Oliveira, J.; Cerca, N. Synergistic effects of carvacrol, α-terpinene, γ-terpinene, ρ-cymene and linalool against Gardnerella species. Nat. Portf. 2022, 12, 4417. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Sánchez, D.; Galvão, J.A.; Mazine, M.R.; Gloria, E.M.; Oetterer, M. Control of Staphylococcus aureus biofilms by the application of single and combined treatments based in plant essential oils. Int. J. Food Microbiol. 2018, 286, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Chatrath, A.; Gangwar, R.; Kumari, P.; Prasad, R. In vitro Anti-Biofilm activities of citral and Thymol against Candida tropicalis. J. Fungi 2019, 5, 13. [Google Scholar] [CrossRef]
- Valliammai, A.; Selvaraj, A.; Mathumitha, P.; Aravindraja, C.; Pandian, S.K. Polymeric antibiofilm coating comprising synergistic combination of citral and thymol prevents methicillin-resistant Staphylococcus aureus biofilm formation on titanium. Mater. Sci. Eng. C 2021, 121, 111863. [Google Scholar] [CrossRef]
- Tokam Kuaté, C.R.; Bisso Ndezo, B.; Dzoyem, J.P. Synergistic antibiofilm effect of thymol and piperine in combination with aminoglycosides antibiotics against four Salmonella enterica serovars. Evid. Based Complement. Altern. Med. 2021, 2021, 1567017. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.A.; Ortega-Ramírez, L.A.; Leyva JF, G.; Siddiqui, M.W.; Cruz-Valenzuela, M.R.; González-Aguilar, G.A.; Ayala-Zavala, J.F. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef]
- Fernández-Babiano, I.; Navarro-Pérez, M.L.; Pérez-Giraldo, C.; Fernández-Calderón, M.C. Antibacterial and Antibiofilm Activity of Carvacrol against Oral Pathogenic Bacteria. Metabolites 2022, 12, 1255. [Google Scholar] [CrossRef]
- Wang, J.; Qin, T.; Chen, K.; Pan, L.; Xie, J.; Xi, B. Antimicrobial and Antivirulence Activities of Carvacrol against Pathogenic Aeromonas hydrophila. Microorganisms 2022, 10, 2170. [Google Scholar] [CrossRef]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).