Evaluating the Degradation of WE43 for Implant Applications: Optical and Mechanical Insights
Abstract
Featured Application
Abstract
1. Introduction
2. State of the Art
2.1. Classification of Magnesium and Its Alloys

2.2. Degradation Experiments on Magnesium Alloys
2.3. Magnesium Alloy WE43
3. Materials and Methods
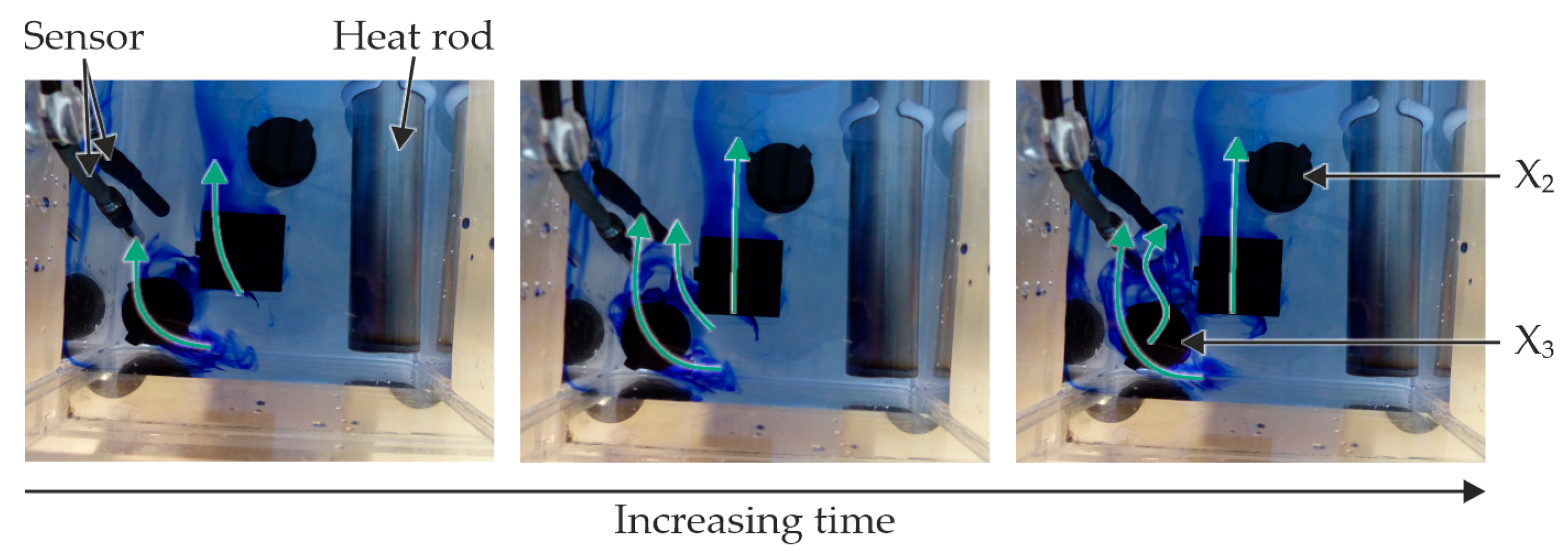
3.1. Experimental Setup and Preparation of the Experiments
3.2. Preparing and Conducting the Experimental Tests
4. Results and Discussion
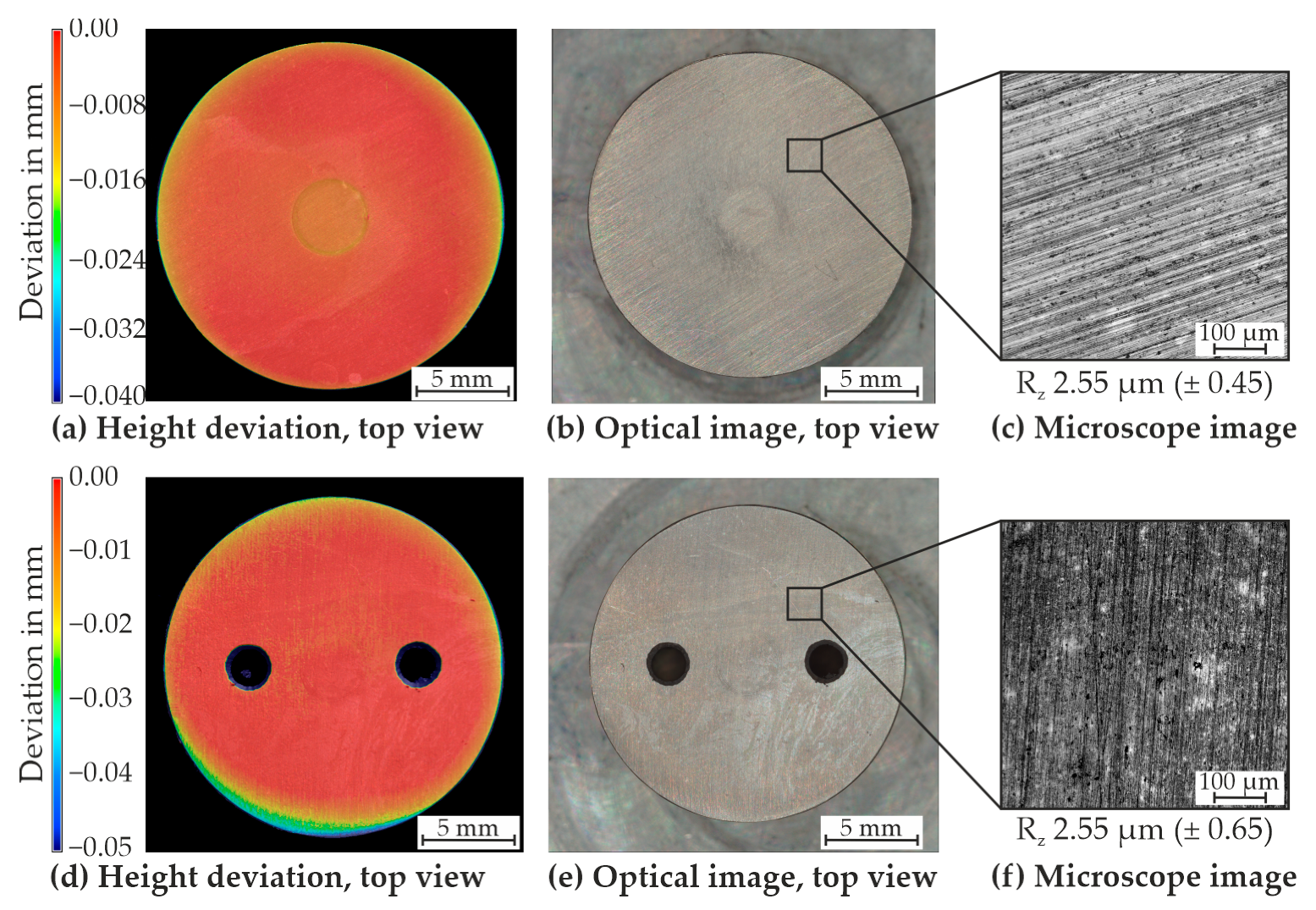
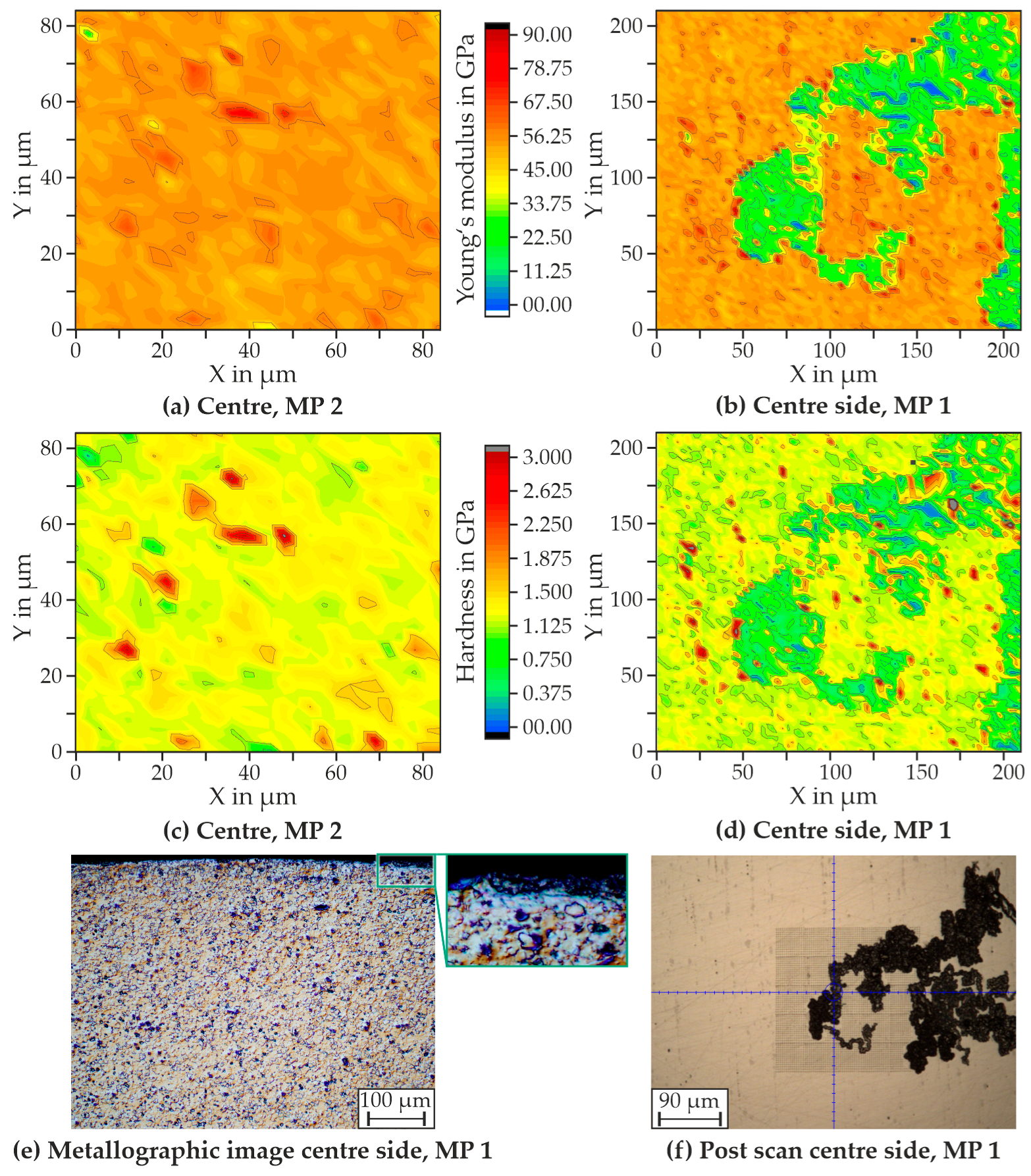
4.1. Initial State
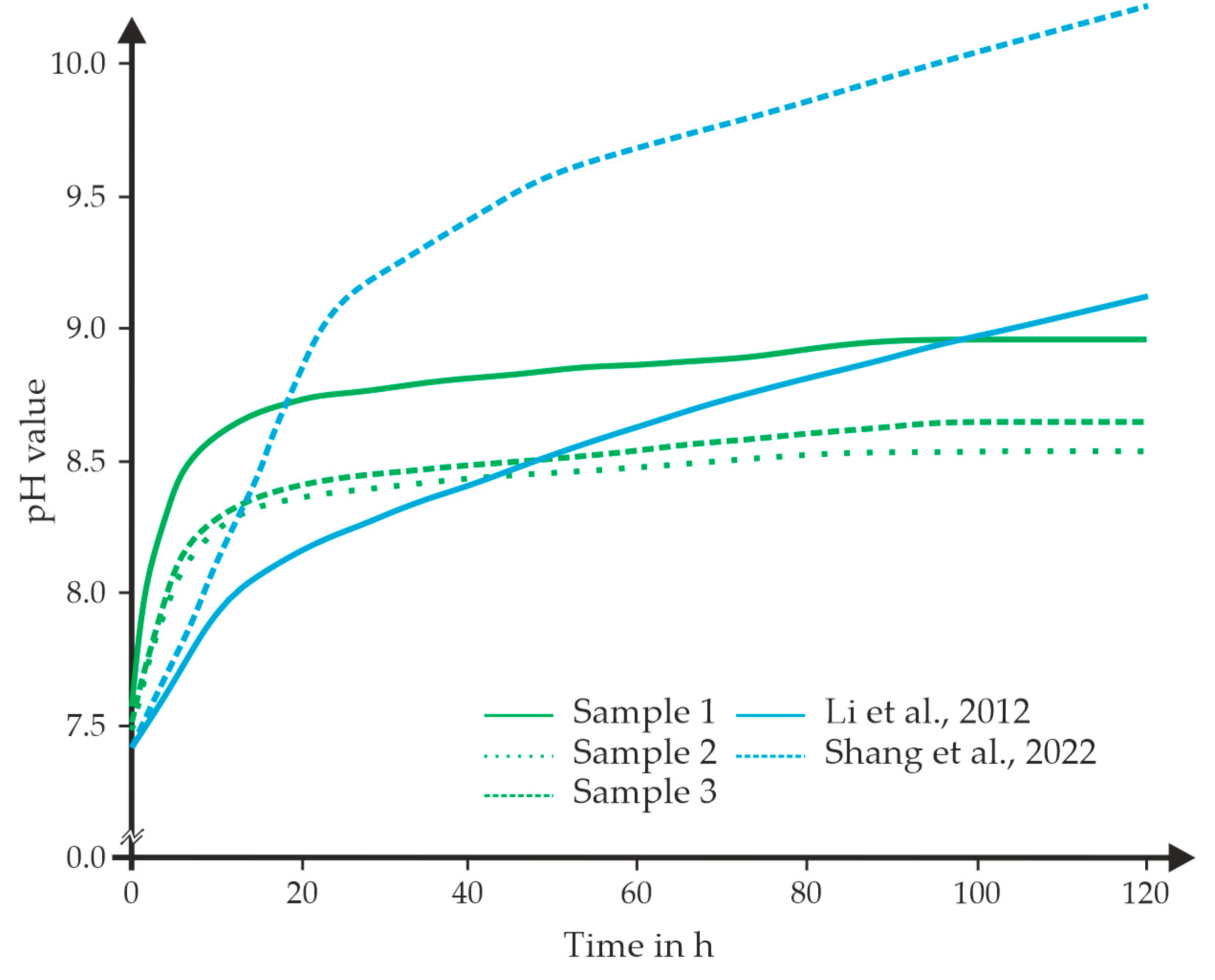
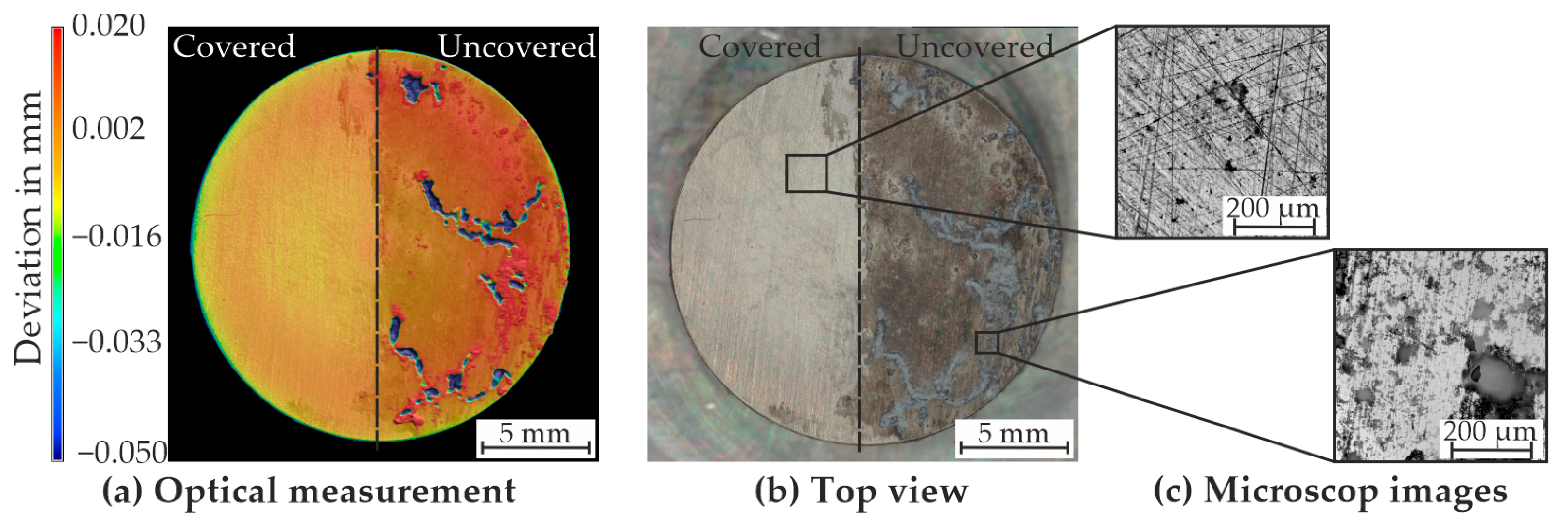
4.2. Results from the Degradation Experiments
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gesundheitsberichterstattung des Bundes: Diagnosedaten der Krankenhäuser ab 2000. Available online: https://www.gbe-bund.de/gbe/pkg_isgbe5.prc_menu_olap?p_uid=gast&p_aid=57722364&p_sprache=D&p_help=0&p_indnr=550&p_indsp=&p_ityp=H&p_fid= (accessed on 25 March 2023).
- Wintermantel, E.; Ha, S.-W. Medizintechnik: Life Science Engineering, 5th ed.; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Rahim, M.I.; Tavares, A.; Evertz, F.; Kieke, M.; Seitz, J.-M.; Eifler, R.; Weizbauer, A.; Willbold, E.; Jürgen Maier, H.; Glasmacher, B.; et al. Phosphate conversion coating reduces the degradation rate and suppresses side effects of metallic magnesium implants in an animal model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1622–1635. [Google Scholar] [CrossRef] [PubMed]
- Grün, N.G.; Donohue, N.; Holweg, P.; Weinberg, A.-M. Resorbierbare Implantate in der Unfallchirurgie. J. Miner. Muskuloskelettale Erkrank. 2018, 25, 82–89. [Google Scholar] [CrossRef]
- Seitz, J.-M. Entwicklung von Magnesiumlegierungen Mit Seltenen Erden Für Medizintechnische Anwendungen. Ph.D. Thesis, Leibniz University Hannover, Hannover, Germany, 2013. [Google Scholar]
- Liu, L.; Gebresellasie, K.; Collins, B.; Zhang, H.; Xu, Z.; Sankar, J.; Lee, Y.-C.; Yun, Y. Degradation Rates of Pure Zinc, Magnesium, and Magnesium Alloys Measured by Volume Loss, Mass Loss, and Hydrogen Evolution. Appl. Sci. 2018, 8, 1459. [Google Scholar] [CrossRef]
- Durisin, M.; Reifenrath, J.; Weber, C.M.; Eifler, R.; Maier, H.J.; Lenarz, T.; Seitz, J.-M. Biodegradable nasal stents (MgF2 -coated Mg-2 wt %Nd alloy)-A long-term in vivo study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 350–365. [Google Scholar] [CrossRef]
- Han, P.; Cheng, P.; Zhang, S.; Zhao, C.; Ni, J.; Zhang, Y.; Zhong, W.; Hou, P.; Zhang, X.; Zheng, Y.; et al. In vitro and in vivo studies on the degradation of high-purity Mg (99.99wt.%) screw with femoral intracondylar fractured rabbit model. Biomaterials 2015, 64, 57–69. [Google Scholar] [CrossRef]
- Manescu, V.; Antoniac, I.; Antoniac, A.; Laptoiu, D.; Paltanea, G.; Ciocoiu, R.; Nemoianu, I.V.; Gruionu, L.G.; Dura, H. Bone Regeneration Induced by Patient-Adapted Mg Alloy-Based Scaffolds for Bone Defects: Present and Future Perspectives. Biomimetics 2023, 8, 618. [Google Scholar] [CrossRef]
- Tan, J.; Ramakrishna, S. Applications of Magnesium and Its Alloys: A Review. Appl. Sci. 2021, 11, 6861. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, H.; Guo, X.; Bu, C. Design and Characterization of Mg Alloy Pedicle Screws for Atlantoaxial Fixation. Metals 2023, 13, 352. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloys 2021, 6, 1884–1905. [Google Scholar] [CrossRef]
- Md Saad, A.P.; Prakoso, A.T.; Sulong, M.A.; Basri, H.; Wahjuningrum, D.A.; Syahrom, A. Impacts of dynamic degradation on the morphological and mechanical characterisation of porous magnesium scaffold. Biomech. Model. Mechanobiol. 2019, 18, 797–811. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Seitz, J.-M.; Eifler, R.; Bach, F.-W.; Maier, H.J. Magnesium degradation products: Effects on tissue and human metabolism. J. Biomed. Mater. Res. Part A 2014, 102, 3744–3753. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.-C.; Yu, B.-Y.; Hao, J.-F.; Zhu, H.-W.; Wu, H.-S.; Chen, B.; Li, W.-R.; Li, Y.-F.; Zheng, L.; Li, R.-X. Improvement of microstructure, mechanical properties, and corrosion resistance of WE43 alloy by squeeze casting. China Foundry 2022, 19, 419–426. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Römer, R.; Feyerabend, F. Magnesium degradation under physiological conditions—Best practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Wang, L.; He, J.; Yu, J.; Arthanari, S.; Lee, H.; Zhang, H.; Lu, L.; Huang, G.; Xing, B.; Wang, H.; et al. A Review: Degradable Magnesium Corrosion Control for Implant Applications. Materials 2022, 15, 6197. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef]
- Shuai, C.; Li, S.; Peng, S.; Feng, P.; Lai, Y.; Gao, C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloys 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Lachmayer, R.; Lippert, R.B. Additive Manufacturing Quantifiziert: Visionäre Anwendungen und Stand der Technik, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Shang, T.; Wang, K.; Tang, S.; Shen, Y.; Zhou, L.; Zhang, L.; Zhao, Y.; Li, X.; Cai, L.; Wang, J. The Flow-Induced Degradation and Vascular Cellular Response Study of Magnesium-Based Materials. Front. Bioeng. Biotechnol. 2022, 10, 940172. [Google Scholar] [CrossRef]
- Kim, W.-C.; Han, K.-H.; Kim, J.-G.; Yang, S.-J.; Seok, H.-K.; Han, H.-S.; Kim, Y.-Y. Effect of surface area on corrosion properties of magnesium for biomaterials. Met. Mater. Int. 2013, 19, 1131–1137. [Google Scholar] [CrossRef]
- van Gaalen, K.; Gremse, F.; Benn, F.; McHugh, P.E.; Kopp, A.; Vaughan, T.J. Automated ex-situ detection of pitting corrosion and its effect on the mechanical integrity of rare earth magnesium alloy—WE43. Bioact. Mater. 2022, 8, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Guo, C.; Wu, Y.H.; Zheng, Y.F.; Ruan, L.Q. Comparative study on corrosion behaviour of pure Mg and WE43 alloy in static, stirring and flowing Hank’s solution. Corros. Eng. Sci. Technol. 2012, 47, 346–351. [Google Scholar] [CrossRef]
- Song, G.; Atrens, A.; StJohn, D. An Hydrogen Evolution Method for the Estimation of the Corrosion Rate of Magnesium Alloys. In Magnesium Technology 2001; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 254–262. [Google Scholar]
- Willumeit, R.; Feyerabend, F.; Huber, N. Magnesium degradation as determined by artificial neural networks. Acta Biomater. 2013, 9, 8722–8729. [Google Scholar] [CrossRef]
- Klauser, H. Internal fixation of three-dimensional distal metatarsal I osteotomies in the treatment of hallux valgus deformities using biodegradable magnesium screws in comparison to titanium screws. Foot Ankle Surg. 2019, 25, 398–405. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Z.; Zhu, Y.; Zhao, R.; Zhang, S.; Shi, X.; Li, G.; Chen, Z.; Zhao, Y. Degradation Resistance and In Vitro Cytocompatibility of Iron-Containing Coatings Developed on WE43 Magnesium Alloy by Micro-Arc Oxidation. Coatings 2020, 10, 1138. [Google Scholar] [CrossRef]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable magnesium-based screw clinically equivalent to titanium screw in hallux valgus surgery: Short term results of the first prospective, randomized, controlled clinical pilot study. Biomed. Eng. Online 2013, 12, 62. [Google Scholar] [CrossRef]
- Sahin, A.; Gulabi, D.; Buyukdogan, H.; Agar, A.; Kilic, B.; Mutlu, I.; Erturk, C. Is the magnesium screw as stable as the titanium screw in the fixation of first metatarsal distal chevron osteotomy? A comparative biomechanical study on sawbones models. J. Orthop. Surg. 2021, 29, 23094990211056439. [Google Scholar] [CrossRef]
- Denkena, B.; Köhler, J.; Stieghorst, J.; Turger, A.; Seitz, J.; Fau, D.R.; Wolters, L.; Angrisani, N.; Reifenrath, J.; Helmecke, P. Influence of Stress on the Degradation Behavior of Mg LAE442 Implant Systems. Procedia CIRP 2013, 5, 189–195. [Google Scholar] [CrossRef]
- Kirkland, N.T.; Birbilis, N.; Staiger, M.P. Assessing the corrosion of biodegradable magnesium implants: A critical review of current methodologies and their limitations. Acta Biomater. 2012, 8, 925–936. [Google Scholar] [CrossRef]
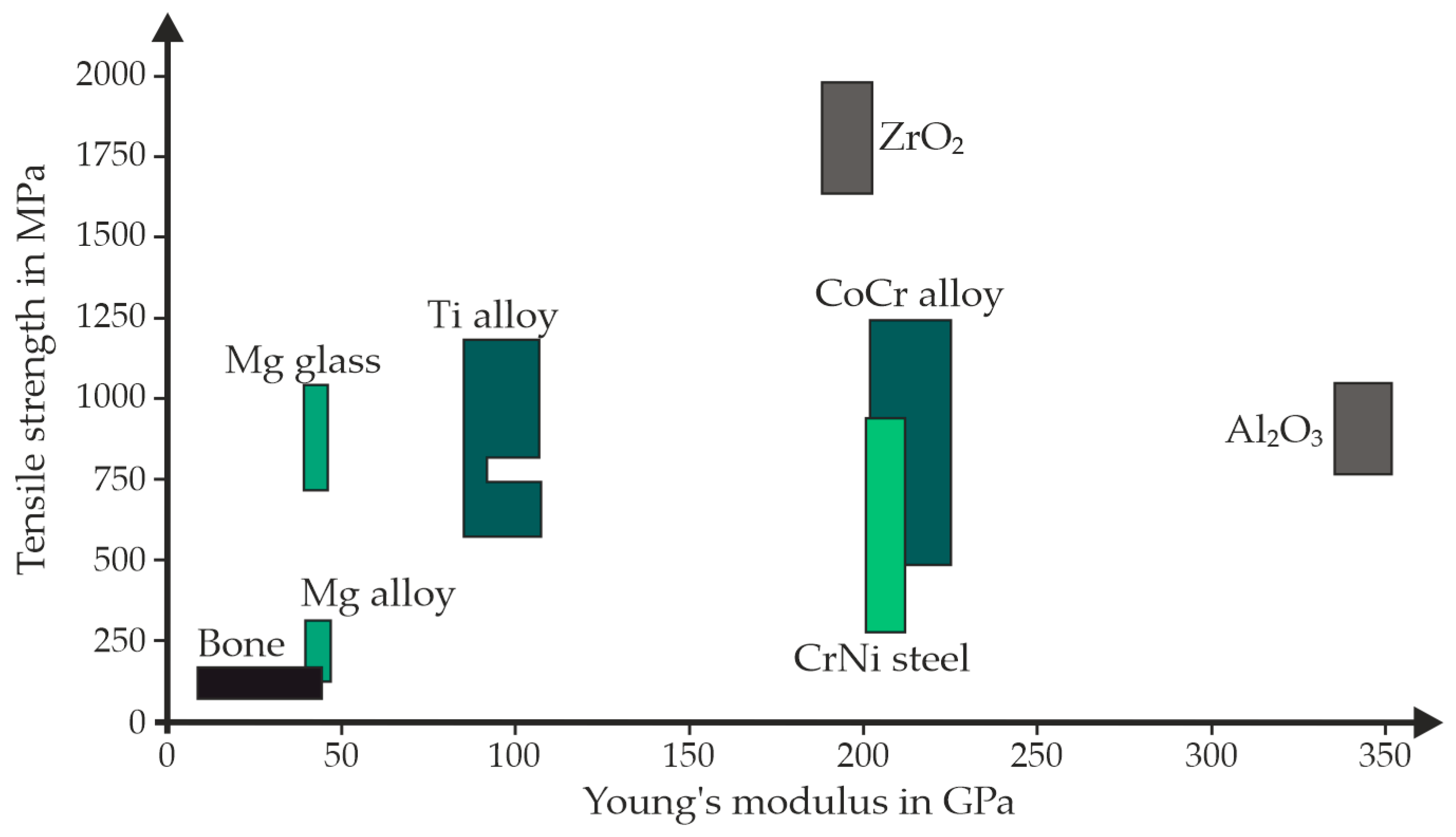
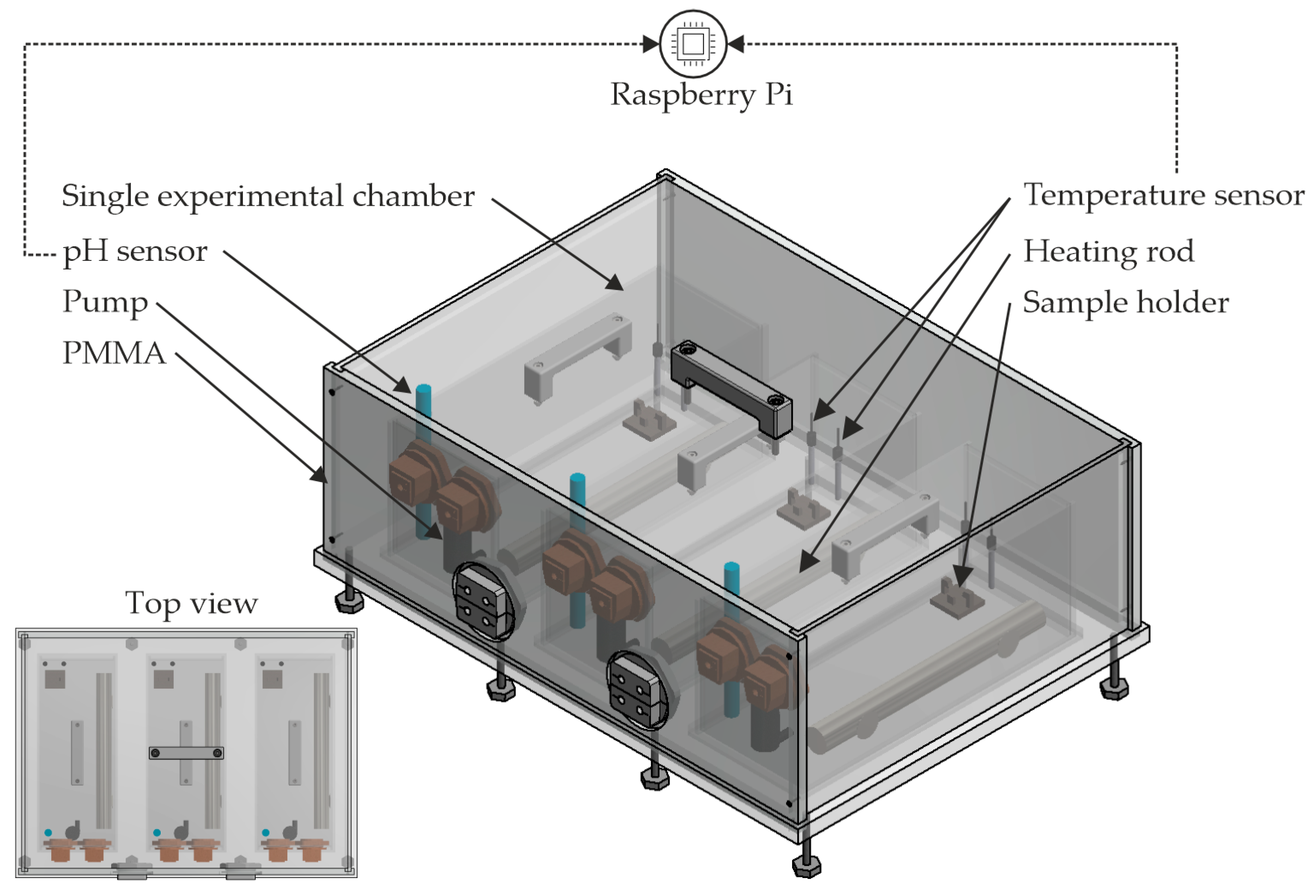
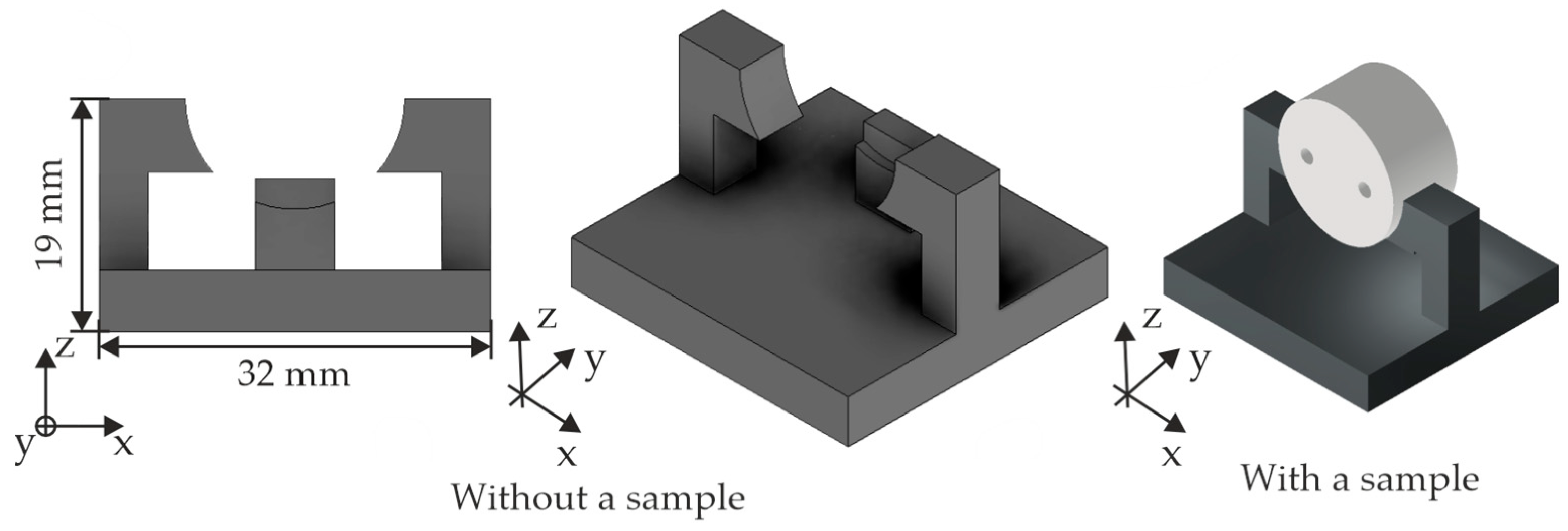
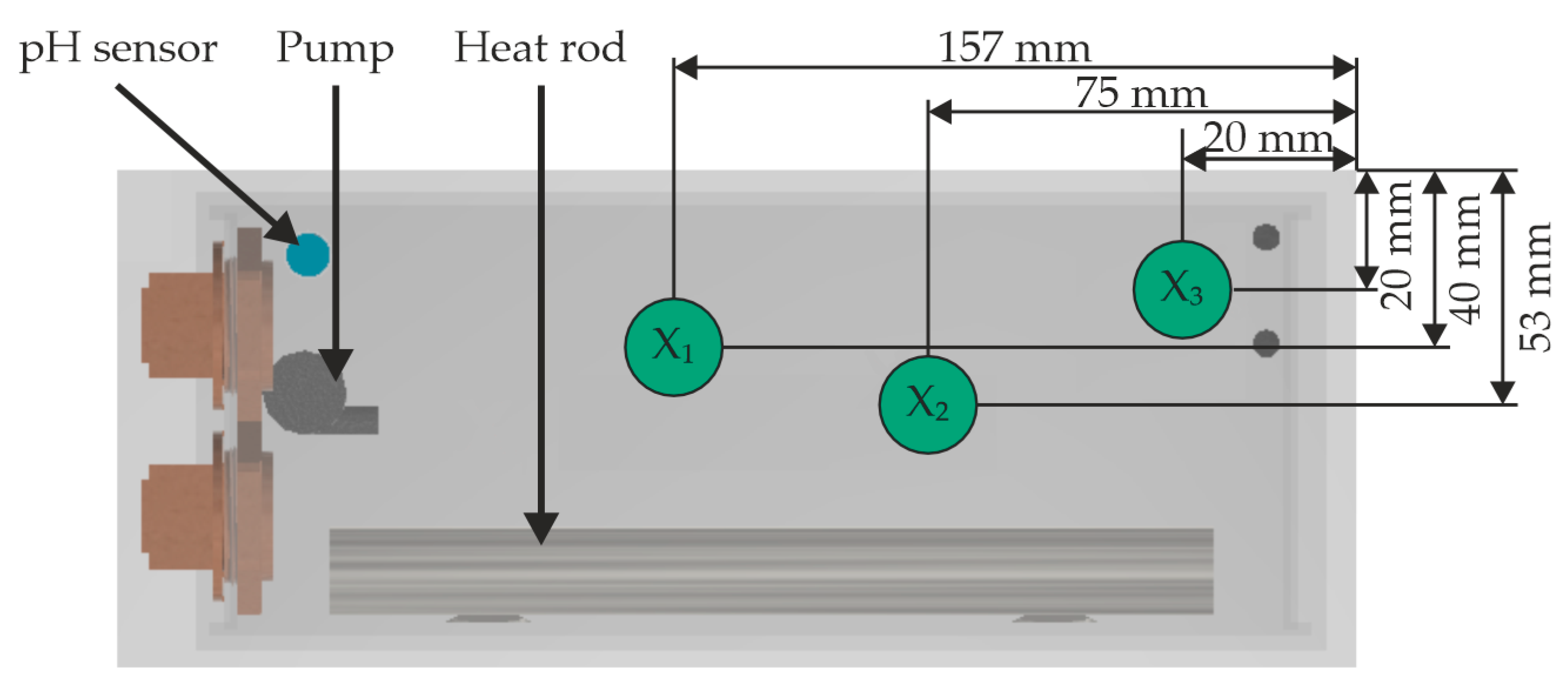
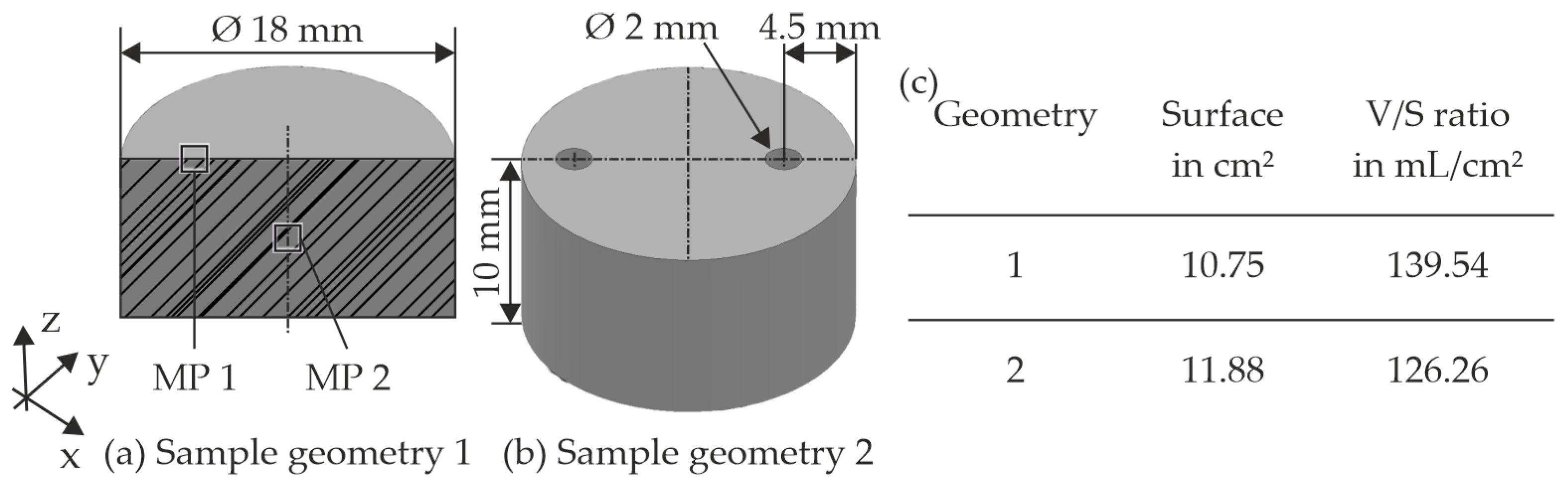




| Material | Density in g/cm3 | Tensile Strength in MPa | Young’s Modulus in GPa |
|---|---|---|---|
| Average human bone | 1.80–2.10 | 110–130 | 10–30 |
| Pure magnesium | 1.74–2.00 | 90–190 | 41–45 |
| WE43 | 1.83–1.84 | ~220 | ~44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siring, J.; Cökelek, A.; Mohnfeld, N.; Wester, H.; Behrens, B.-A. Evaluating the Degradation of WE43 for Implant Applications: Optical and Mechanical Insights. Appl. Sci. 2025, 15, 3300. https://doi.org/10.3390/app15063300
Siring J, Cökelek A, Mohnfeld N, Wester H, Behrens B-A. Evaluating the Degradation of WE43 for Implant Applications: Optical and Mechanical Insights. Applied Sciences. 2025; 15(6):3300. https://doi.org/10.3390/app15063300
Chicago/Turabian StyleSiring, Janina, Anil Cökelek, Norman Mohnfeld, Hendrik Wester, and Bernd-Arno Behrens. 2025. "Evaluating the Degradation of WE43 for Implant Applications: Optical and Mechanical Insights" Applied Sciences 15, no. 6: 3300. https://doi.org/10.3390/app15063300
APA StyleSiring, J., Cökelek, A., Mohnfeld, N., Wester, H., & Behrens, B.-A. (2025). Evaluating the Degradation of WE43 for Implant Applications: Optical and Mechanical Insights. Applied Sciences, 15(6), 3300. https://doi.org/10.3390/app15063300







