Featured Application
This work presents important data about the impact of the coagulation and flocculation process on the industrial-scale treatment of brewery wastewater, providing technical operators with essential information about the efficacy of various reagents under varied conditions.
Abstract
Brewery wastewater (BWW) often contains a high concentration of organic matter and nutrients, requiring pre-treatment before it can be effectively treated in conventional wastewater treatment plants. This study focuses on the use of coagulation–flocculation techniques to treat real industrial wastewater. Firstly, lab-scale tests have been carried out to determine the most effective coagulant and flocculant type and concentration. The levels of pollutants, including chemical oxygen demand (COD), biological oxygen demand in five days (BOD5), total nitrogen (Ntot), total phosphorus (Ptot), and orthophosphate (P-PO43−) have been measured to assess the efficiency of the procedure. Industrial scale tests were performed in optimal conditions in order to evaluate the effectiveness of the treatment on a larger scale and estimate the generation of chemical sludge. The most effective substances for coagulation and flocculation were polyaluminium chloride (PAC) and anion-active flocculant (AAF) ZETAG 4139 0.1%, respectively, at concentrations of 675 mg·L−1 and 40 mg·L−1. During industrial-scale tests, the process allowed the effective removal of TSS (86.8%), Ntot (51.8%), Ptot (95.5%), and P-PO43− (99.6%), while the limited removal of organic substances has been highlighted (BOD5: 34.3%; COD: 26.5%). The dry matter (DM) content of the separated sludge was found to be 4.5–5%, and a yield of 1.01 kgDM per kg of COD removed was obtained after flocculation treatment of the BWW. These findings can be beneficial for both the scientific community and technical operators, offering insights into the effectiveness of various coagulants and flocculants on industrial-scale wastewater treatment.
1. Introduction
Beer is the predominant alcoholic beverage globally, and it plays a crucial role in the economies of numerous countries [1,2]. China is the world’s leading producer of beer, manufacturing 360 million kiloliters annually in 2022 [2]. Beer manufacture involves the exploitation of a liquid called wort, which contains carbohydrates. This liquid undergoes fermentation, a biological process helped by yeast, in which the carbohydrates in the wort are transformed into ethanol and carbon dioxide. During production, yeast functions under anaerobic conditions to catalyze the conversion of sugar in the wort into ethanol and carbon dioxide. The average fermenting process lasts for almost one week [3,4].
The beer production process involves several stages, including malting, milling, mashing, wort boiling and filtering, hopping, fermentation and maturation, stabilization, clarifying, and packaging. Boiling and clarifying of wort resulted in the generation of around 3 to 10 L of brewery wastewater (BWW) for every liter of beer produced [2]. This wastewater (WW) is characterized in terms of quality by a high concentration of organic matter (2000–10,000 mg·L−1). The range concentration of biological oxygen demand (BOD) in BWW is between 1200 mg·L−1 and 3600 mg·L−1. The pH of this WW varies between 3 and 12. Clean-in-Place (CIP) sanitization process has an impact on this parameter. Breweries employ standardized CIP cycles. The initial phase of sterilizing fermentation tanks commences with an acid cycle and concludes with an alkaline cycle. These CIP cycles are also distinguished by elevated temperatures ranging from 18 to 40 °C.
BWW also contains significant levels of Ntot, ranging from 25 mg·L−1 to 80 mg·L−1, and Ptot, ranging from 10 mg·L−1 to 50 mg·L−1, because of utilizing processed raw materials [5,6,7]. Due to the presence of these pollutants, untreated wastewater can have a detrimental impact on the ecosystem. When breweries are connected to the sewer system, the most appropriate approach for reducing chemical oxygen demand (COD), BOD, and total phosphorus (Ptot) is to first implement pre-treatment methods [7,8,9], and then use biological processes [10,11,12].
Preliminary treatments are essential to enhance the quality of the WW, hence enhancing the potential of the organic matter to be broken down by microorganisms in conventional activated sludge plants. Moreover, it is essential to modify the pH in order to facilitate an effective subsequent biological process in wastewater treatment plants (WWTPs). The ideal pH range for BWW is from six to nine, which enables it to be discharged into the sewage system without causing harm, and it can then undergo additional treatment at WWTPs. Pre-treatment methods including sedimentation, flow equalization, and filtering are commonly employed in dealing with suspended particles in BWW [4,9]. However, their effectiveness is limited to addressing these specific particles. In contrast, chemical methods such as coagulation and flocculation have been proven to be extremely efficient in removing pollutants in both liquid and solid phases [6,7]. Coagulation is a commonly used physicochemical technique to remove colloidal particles and color from wastewater. In the context of BWW treatment, coagulation pertains to the destabilization of particles through the introduction of a coagulant. Perikinetic coagulation, also known as the creation of tiny aggregates by the Brownian motion of particles, may occur during this phase [9,13].
For instance, aluminum chlorohydrate and polyamine were tested as coagulants for the removal of particulate COD and turbidity in BWW [9]. The results highlighted that the effectiveness of using the combined coagulation dosage of polyamine–aluminum chlorohydrate (PAC) treatment was 90% and 59% for turbidity and COD, respectively, comparable to the use of polyamine alone (75% and 50%, respectively). In the case of the use of aluminum chlorohydrate alone, 37% and 54% of chemical oxygen demand and turbidity were removed, respectively [9]. In another study, chitosan was used as a biopolymer coagulant, obtaining, with 2 g L−1 of coagulant dose, pH equal to 8, and 43 min of contact time, the removal of 91% turbidity, 89% total organic carbon (TOC), and 65% of orthophosphate [7].
However, most of the investigations were carried out under controlled laboratory conditions and tested small volumes of (mainly synthetic) BWWs. The aim of this study was to overcome this current gap in the literature by evaluating the effectiveness of the coagulation process on real BWW on a larger scale in order to better estimate the actual effectiveness of the process and the production of chemical sludge. The work was divided into two distinct phases: (i) a lab-scale phase for the identification of the optimal conditions for coagulation and flocculation, and (ii) a subsequent industrial-scale phase where the effectiveness of the previously identified optimal conditions (type of reagent and concentrations) was evaluated on a larger scale for almost one month. Moreover, this stage also allowed us to estimate the production of chemical sludge that has to be managed after the flocculation process. The findings can be valuable for the scientific community, offering insights into the impact of chemical coagulation on industrial-scale wastewater treatment and providing technical operators with crucial data on the efficacy of various reagents under different conditions.
2. Materials and Methods
The tests were performed on BWW produced by a brewery located in southern Europe, in a city with a population of around 950,000 inhabitants and a developed industrial sector. The urban wastewater treatment plant (WWTP) processes wastewater (WW) from people as well as brewery wastewater (BWW).
The main problem in this WWTP is the absence of technology to effectively treat wastewater containing high levels of organic matter and nutrients, namely phosphorus. Furthermore, the WWTP is currently running at its maximum capacity [14,15]. This work aims to evaluate the effectiveness of the chemical pre-treatment method in order to decrease the levels of organic compounds and nutrients in BWW prior to its release into the sewer system.
2.1. Characteristics of the Brewery Wastewater
BWW was sampled from a brewery in southern Europe before discharge into the sewer system. The samples have been stored in plastic accumulation tanks and homogenized using submersible mixers.
For the lab-scale phase, a total of 25 L of BWW has been collected in each plastic tank. Samples were then stored in the incubation unit at a temperature of 5 °C to prevent organic degradation. For the industrial-scale phase, 350 m3 were sampled and used in the tests. Table 1 reports the main characteristics of the untreated BWW. Compared to urban wastewater, BWW was characterized by a higher concentration of organic matter (with a lower biodegradability index) and nutrients. The low amount of total suspended solids (TSS) suggests that the organic pollutants are primarily present in a dissolved form rather than in particulate. Furthermore, a high variability in pH in the case of BWW was detected. The characteristics of raw BWW agree with those measured in previous studies [2,3,4,9,13,16,17,18,19].

Table 1.
Chemical and biochemical properties of the BWW compared to a typical urban WW. u.m.: unit of measure. Ibiod: biodegradability index. a: data was taken from Henze and Comeau [20], b: expressed as total BOD.
2.2. Design of the Experimental Activities
2.2.1. Lab-Scale Phase
The effectiveness of two coagulants, FeCl3 (40% solution, density: 1.45 g·L−1; Fetot: 13%) and polyaluminium chloride (PAC—density: 1.35 g·L−1; active Al: 9%) have been evaluated. In the lab-scale phase, five diverse doses were studied to determine the optimal dosage. COD has been selected as a target parameter to evaluate the effectiveness of the process.
In order to define the proper flocculant, an anion-active reagent (ZETAG 4139—AAF) and a cation-active reagent (ZETAG 9246 FS—CAF), both at a 0.1% concentration in solution, were selected in this study. In this case, turbidity has been selected as the target parameter.
A multi-chamber mixer with five reactor vessels has been used. A total of 1 L of BWW was fed into each reactor vessel, keeping it at a temperature of 25 °C. A paddle mixer (170 RPM) was used to stir all the vessels to achieve homogeneity, and H2SO4 solution (20%) was used for neutralization. Within 2 min, a steady pH value was achieved.
Five different amounts of coagulant agent were tested in the study. The concentrations of FeCl3 were 290, 435, 580, 725, and 1088 mg·L−1, and the concentrations of PAC were 270, 405, 540, 675, and 1013 mg·L−1.
After the coagulation (3–5 min), the stirrer speed was decreased to 30 RPM and samples were treated with two different (40 mg·L−1 and 90 mg·L−1) flocculating solutions (20–25 min). The sludge formed and precipitated rapidly. The settling time was 30 min. Tests have been carried out to test different doses and types of flocculating solutions for every possible combination of samples.
2.2.2. Industrial-Scale Phase
The main aim of this phase was to validate, on a larger scale, the efficacy of the chemical pre-treatment and chemical dosage, and to estimate sludge production. Moreover, this phase was designed to evaluate the system’s steady operation over an extended period (1 month), while also observing and addressing any variations in BWW quality. Figure 1 provides details about the layout of the plant used for BWW treatment.
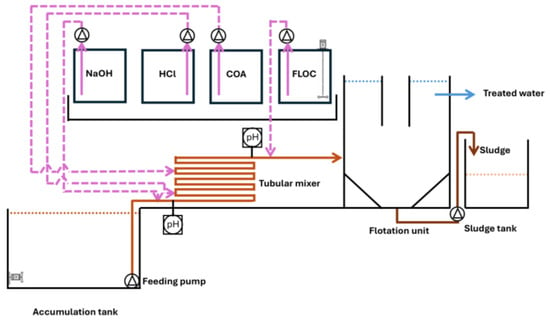
Figure 1.
Scheme of the industrial scale plant used for the BWW treatment. COA: coagulant; FLOC: flocculant. Pink and orange arrows indicate the dosage of chemicals and the untreated BWW, respectively. Blue and brown arrows refer to treated water and chemical sludge produced in the process, respectively.
The BWW was stored in the accumulation tank, which had a capacity of 350 m3. The tank was operated manually for the purpose of the test. The untreated BWW was initially pumped into a tubular mixer, a device designed to accurately dispense chemicals and ensure the necessary amount of time for appropriate mixing of the wastewater with the reagents.
Tests were performed on a portable chemical pre-treatment device equipped with a pumping station capable of handling 1 m3·h−1; diaphragm pumps for dispensing alkali, acid, and coagulant; a tubular mixer; a flotation unit with a hydraulic capacity of 1 m3·h−1; and a storage tank for collecting separated sludge with a volume of 0.5 m3 (Figures S1 and S2). NaOH (30%) and HCl (31%) were used for adjusting the pH levels to alkaline and acidic, respectively. The sludge separation process occurred in the flotation unit, which had a volume of 1.2 m3, and involved the separation of sludge from the treated water. The processed BWW descended with gravity into the municipal sewer system. Temperature, pH, and conductivity were monitored continuously while chemical parameters were evaluated on a daily basis. The temperature in the flotation unit stayed consistently between 35 and 40 °C without the need for a heat exchanger, thanks to the elevated temperature of the BWW.
2.3. Analytical Methods
Total suspended solids (TSSs) have been measured using Method 8006 [21]. The biochemical oxygen consumption was determined after a period of 5 days (BOD5) using a membrane electrode to estimate the initial and post-incubation dissolved oxygen concentration and estimate the consumed oxygen according to the Standard Methods for the Examination of Water and Wastewater [22]. The COD was analyzed with Hach kits (150–1000 mg L−1 O2), which provided all reagents after proper dilutions of the initial sample were made (Hach Company, Loveland, CO, USA) [23]. To reduce the analytical error, after filtration (pore size, 0.45 µm), interference from chlorides was ruled out by verifying that the chloride concentration was lower than the maximum accepted by the method (1500 mg L−1). A total of 2 mL of sample was injected into the cuvette and heated in a HACH HT 200 S (Hach Company, Loveland, CO, USA) for 15 min, then a HACH DR-5000 (Hach Company, Loveland, CO, USA) was used for the spectrophotometric measurements, respectively.
A Hach-Lange IL 550 TOC-TN device (Hach Company, Loveland, CO, USA), which operates on the principle of oxidation pyrolysis (800 °C), was used to determine total nitrogen (Ntot). In this case, samples were digested using thermocatalytic high-temperature oxidation (800 °C and platinum as catalyst), and nitrogen was evaluated using a CLD chemiluminescence detector.
Total phosphorous (Ptot) was analyzed with Hach kits (Hach Company, Loveland, CO, USA), which provided all reagents after proper dilutions of the initial sample were made. A total of 5 mL of sample was injected into the cuvette and one potassium persulfate pillow was added to the cuvette. Then, the cuvette was heated in a COD Reactor 115/230 VAC (Hach Company, Loveland, CO, USA) for 30 min, and 2 mL of 1.54 N Sodium Hydroxide was added. Then, one PhosVer 3 pillow was added, and the cuvette was analyzed by a Molybdovanadate DR 6000 (Hach Company, Loveland, CO, USA) [24]. P-PO43− was measured using Hach kits (Hach Company, Loveland, CO, USA), which provided all reagents after proper dilutions of the initial sample were made. A total of 5 mL of sample was injected into the cuvette, then analyzed by a Molybdovanadate DR 6000 (Hach Company, Loveland, CO, USA) [25].
Temperature and pH were monitored using a portable multiparameter instrument (WTW 3410 SET4, Xylem Inc., Washington, DC, USA) and the probe WTW-IDS Model SenTix® 940 (Xylem Inc., Washington, DC, USA), while conductivity was measured using the probe TetraCon® 925. A HACH 2100Q turbidimeter (Hach Company, Loveland, CO, USA) was used to quantify turbidity.
For each sampling activity, one sample was taken, and all the measurements and analyses were performed immediately. Possible sources of analytical error in the use of the kits could be the inoculation of an incorrect volume, possible turbidity that alters the spectrophotometric measure, the presence of interfering substances, and failure to respect the reaction times and temperatures [26]. For COD and Ptot, to be sure that the results were robust, each analysis of the same sample was conducted in triplicate to verify the difference is less than 10%. These values are presented in the results as the mean, along with their 95% confidence interval.
3. Results and Discussion
3.1. Lab-Scale Phase
The dosages of each coagulation agent (FeCl3 and PAC) ranged from 270 mg·L−1 to 1088 mg·L−1. Prior to each test, the pH level was adjusted to a range close to 7 in order to establish the most favorable conditions for the coagulation process. After evaluating the test’s design, it can be determined that the most effective coagulant was PAC with a dosage of 675 mg·L−1, leading to a 30% reduction in COD (Figure 2a). Increasing the dosage of coagulant did not contribute to enhancing the performance of the process. The use of PAC resulted in an observed rise in the content of chlorides in the treated BWW that should be further investigated in future studies.
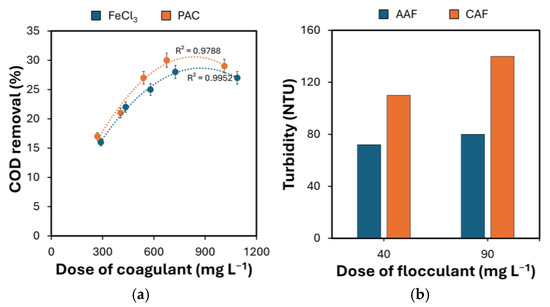
Figure 2.
(a) COD removal as a function of the dosage of different coagulants, and (b) final turbidity of the BWW treated with PAC as a function of the dosage of different flocculants. The bars represent the 95% confidence interval of the results from diverse analyses (n = 3) made on the same sample.
This same approach was also used in the subsequent laboratory tests for optimizing the dosage of the flocculation agent. Based on the findings, it can be concluded that CAF is not suitable for treating this particular type of wastewater since it does not form solid flocs and there is an undissolved substance present in the liquid above the sediment (Figure 2b).
In the case of optimal conditions (PAC: 675 mg·L−1, AAF: 40 mg·L−1), the lab-scale tests showed that the treatment was highly effective in removing nutrients (Ntot: 89%, Ptot: 89.9%, P-PO43−: 99.7%) and TSS (53.2%). In this study, the high removal of nitrogenous forms and phosphates reaches the values obtained in previous research, where BWWs were treated with coagulants assisted by the use of electric current (electrocoagulation), probably due to the optimal conditions of pH and reagent dosage [27]. In particular, the nearly total removal of P-PO43− obtained in the present study can be attributed to a series of adsorption (incorporation of phosphates to the solid particles) and chemical precipitation, which involves reversible and irreversible precipitation processes [28]. The insoluble precipitate will then be removed from BWW by the final setting to end up in the sludge. This value is also confirmed in previous studies where different coagulants were used. For instance, the use of aluminum sulfate in the coagulation process removed 90% of P-PO43− under optimal pH and dosage conditions [28], while polyacrylamide and PAC removed more than 94% of phosphorous from real phosphate mine WW [29]. However, the removal efficiency for organic substances was limited, with COD removal at 29.5% and BOD5 removal at 31.9% when PAC was used.
Figure S3 illustrates the visible difference between the BWW prior to and following coagulation and flocculation, using the most favorable conditions. The findings have also been examined on a broader scope in industrial-scale facilities (Section 3.2).
3.2. Industrial-Scale Phase
Optimal conditions for coagulation (PAC, 675 mg·L−1) and flocculation (AAF 0.1%, 40 mg·L−1) were tested at an industrial scale for almost 1 month.
3.2.1. Physico-Chemical Parameters and Consumption of Chemicals
Parameters such as pH, conductivity, and temperature were constantly monitored to ensure constant operation of the flotation unit. BWW had elevated temperatures compared to urban WW, and its quality demonstrated significant variations, particularly in terms of influent pH, with values ranging from 5.6 to 10.2 (Table S1). These variations may be related to the production process that necessitates the implementation of CIP cycles to facilitate the preparation of tanks for the start-up of new products [30]. The brewery from which BWW was sampled is engaged in the production of multiple varieties of beer, resulting in regular variations in the quality of the effluent and the pH levels of the incoming inputs. It was feasible to limit the substantial fluctuations and establish a stable equilibrium of this value within the pH range of 6.5 to 7.5 providing the ideal conditions for the process of chemical precipitation using a coagulant [3]. In fact, to contrast the fluctuation of pH in the influent load, the industrial-scale plant was properly equipped with two tanks able to dose NaOH and HCl to adjust the pH levels to alkaline and acidic, respectively, depending on the necessity.
The conductivity was constant during the testing process, both during the input and output phases, with no significant modifications detected. In terms of chemical consumption for pH adjustment, the mean dosage for NaOH was found to be 1004 mg·h−1, while HCl was dosed at 658 mg·h−1 on average. Additionally, the PAC showed an average dosage of 930 mg·h−1, whereas the 0.1% AAF solution was dosed at 52 mg·h−1 on average.
The industrial scale tests also showed high temperatures due to the industrial production process, with an average temperature of approximately 35–40 °C, as confirmed in other studies [6,7,9]. It should be taken into account that the high temperature of the BWW could limit the adequate dissolution of oxygen in the water, reducing the effectiveness of clarification [31,32]. Consequently, this might have adverse effects on the creation of microbubbles and subsequently decrease the efficiency of the separating process. This aspect should be further investigated in future studies given the possible influence on the process performance. However, preliminary results showed that the performances obtained with temperatures between 35 and 40 °C (industrial scale) are completely comparable to those obtained at 25 °C (laboratory scale), demonstrating that the impact of temperature on the process efficiency seems to be limited.
3.2.2. Chemical Parameters
The WW generated by breweries contains both organic and inorganic contaminants, including nitrogen and phosphorus compounds.
These forms exist because processed natural components are used in beer production. BOD5, COD, TSS, Ntot, Ptot, and P-PO43− were monitored to confirm the results obtained in lab-scale tests. The influent concentrations of organic contaminants are comparable to those reported in previous studies. During tests, the average removal for BOD5, COD, and TSS was found to be 34.3%, 26.5%, and 86.8%, respectively (Figure 3a). The outcomes can be interpreted as positive and confirm the results obtained at the scale in similar previous studies [33,34,35].
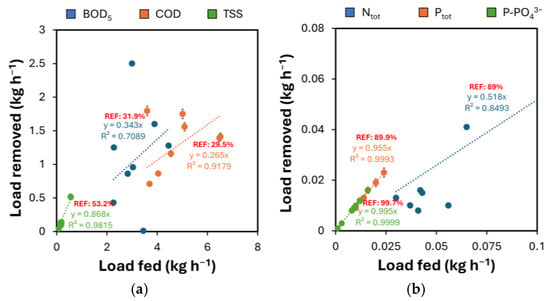
Figure 3.
(a) Removal of BOD5, COD, and TSS, and (b) removal of Ntot, Ptot, and P-PO43− as a function of the load fed. Dotted lines refer to the linear fitting of the data. REF indicates the average percentage of removal obtained in lab-scale tests. The bars represent the 95% confidence interval of the results from diverse analyses (n = 3) made on the same sample.
Comparing the results with those obtained in a 14-day experiment, where three different coagulation agents were used to achieve the highest level of effectiveness in eliminating organic pollutants, the use of PAC allowed the removal of 50% of COD [9]. However, it is important to note that the concentration of COD at the intake in that study was 219 mg L−1 compared to 3.544 mg L−1 in the present work, and this could have influenced the more limited removal of organic matter. In other studies, Fosso-Kankeu et al. [36] estimated the effectiveness of the use of FeCl3 with a non-ionic flocculant for BWW pre-treatment highlighting that almost 4–26 mg of COD were removed per mg of coagulant used, a value higher than the 1–3 mg of COD removed per mg of coagulant obtained in this work. This difference could be attributed to the different types of coagulants but also to the diverse composition of the BWW since a complete characterization of the water was not carried out. For this reason, in general, due to different conditions of testing and initial characteristics of the BWW, a quantitative comparison between different case studies remains difficult.
Swain et al. [35] tested sequential electrocoagulation and chemical coagulation, using aluminum sulfate, in order to increase the performance of the organic matter degradation process in BWW. They obtained a COD removal of 26%, in line with that obtained in the present study, where electrochemical treatment was not applied.
In any case, the current effectiveness of the coagulation–flocculation process therefore makes it necessary to post-treat the BWW at a municipal WWTP before it can be discharged into a surface water body. The effectiveness of an alum-based coagulation agent for the removal of organic contaminants from water was also confirmed by Aremanda et al. [13], who successfully treated a high-strength BWW in lab-scale tests.
During the monitoring, the average decrease efficiencies for the parameters Ntot, Ptot, and P-PO43− were 51.8%, 95.5%, and 99.55%, respectively (Figure 3b). Results about phosphorous removal confirm preliminary evaluation in lab-scale tests, while nitrogen removal at the industrial scale was shown to be less effective. Future studies will further explore this aspect, which remains unclarified. However, the results obtained in the present study are higher than those reported in previous works in the literature [37,38,39,40,41,42]. For instance, Tonhato Junior et al. [37] used AlCl3 as a coagulant, obtaining a removal of Ntot and Ptot of 19.9% and 82.3%, respectively. The difference could be attributed to the diverse flocculant used: an organic one made from vegetable tannin was dosed in the study of Tonhato Junior et al. [37], different from the AAF tested in this work.
In fact, recently research interest has also shifted to the use of natural-based coagulants and flocculants, such as tannin and vegetable and fruit peels, in order to reduce the impact on the ecosystem related to the use of conventional chemical coagulants and flocculants [43,44]. A future development of this study could therefore be to evaluate the technical and economic feasibility of replacing PAC and AAF in the treatment of BWW with alternative natural-based coagulants and flocculants.
3.2.3. Production of Chemical Sludge
The production of chemical sludge underwent evaluation during the industrial-scale phase. The significance of this data comes from its relevance to the future design of the dewatering process for the sludge. Samples of chemical sludge were collected the quantity of dry matter (DM) has been evaluated as 4.5–5%.
On average, 25.5 L of sludge was made per m3 of BWW treated. Since 1.33 kg of COD was taken out of every m3 of BWW that was fed, when the BWW was treated chemically and flocculated, 1.01 kgDM of chemical sludge was released per kilogram of chemical oxygen demand removed (Figure 4).
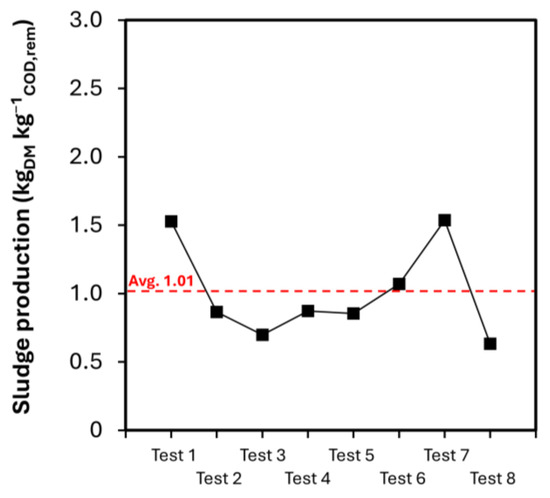
Figure 4.
Amount of chemical sludge produced in the industrial-scale tests. Avg. indicates the average value of sludge production.
The possible forms of reuse of this sludge are dependent on the chemical and chemical–physical characteristics of the sludge itself. A complete characterization of the sludge and additional tests will be carried out in the future to determine (i) the effectiveness of the dewatering process to reduce the volume and therefore the disposal costs, and (ii) the possible forms of management appropriate to its characteristics. For example, the Czech Republic, as well as other EU countries, have very stringent rules on the quality of sludge that is disposed of in agriculture, and for this reason, a complete analysis of its characteristics is necessary to be able to choose the most appropriate option [45]. Furthermore, research on the possibility of recovering coagulants from the chemical sludge to minimize its production is giving promising results, providing a possible alternative to the more traditional forms of disposal [46].
A quantitative comparison of the present results with previous studies remains difficult considering that the previous literature is mainly based on tests carried out on a laboratory scale, and for this reason, sludge production has not been estimated.
4. Conclusions
The primary aim of this study was to investigate the application of coagulation–flocculation as a treatment method for real BWW containing a high amount of organic substances and nutrients. In order to assess the effectiveness of coagulation and flocculation as a pre-treatment, the concentration of COD, BOD5, Ntot, Ptot, and P-PO43− have been monitored. The results indicated that the most effective coagulant and flocculant were PAC (dosage: 675 mg L−1) and AAF (dosage: 40 mg L−1), respectively. The mean removal efficiencies for BOD5, COD, TSS, Ntot, Ptot, and P-PO43− were 34.3%, 26.5%, 86.8%, 51.8%, 95.5%, and 95.5%, respectively. The elevated temperature of the BWW may have hindered the assimilation of sufficient oxygen into the water, reducing the sedimentation efficiency. The separated sludge had a DM content ranging from 4.5% to 5%. Following the chemical/flocculation treatment of the BWW, approximately 1.01 kgDM of chemical sludge was released per kilogram of chemical oxygen demand removed. This data will be helpful for both the scientific community and technical operators, providing information about (i) the effectiveness of the pre-treatment of BWW before discharge into the sewer system, and (ii) the amount of chemical sludge produced during the treatment of this type of industrial WW. These results can be useful for evaluating the applicability of this treatment to other specific contexts, sizing the plant, and considering the most advantageous solution from an economic and environmental point of view for the disposal or valorization of chemical sludge.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app15062999/s1, Figure S1: The treatment plant used in industrial-scale tests; Figure S2: Details of the flocculation unit in the industrial-scale plant; Figure S3: A sample of raw WW before chemical pre-treatment (left) and a sample after coagulation/flocculation process (right); Table S1: Temperature, pH, and conductivity in the inlet and outlet and consumption of chemicals of the industrial-scale plant.
Author Contributions
Conceptualization, M.R., P.H. and J.T.; methodology, M.R. and P.H.; validation, M.R. and P.H.; formal analysis, M.R., P.H. and J.T.; investigation, J.T.; resources, M.R. and P.H.; data curation, M.C.M. and J.T.; writing—original draft preparation, M.R., P.H. and J.T.; writing—review and editing, M.C.M. and V.T.; visualization, M.C.M. and V.T.; supervision, M.R. and P.H.; project administration, M.R. and P.H.; funding acquisition, M.R. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAF | Anion-active flocculants |
| BOD | Biological oxygen demand |
| BOD5 | Biological oxygen demand in five days |
| BWW | Brewery wastewater |
| CAF | Cation-active reagent |
| COD | Chemical oxygen demand |
| CIP | Clean-in-Place |
| DM | Dry matter |
| P-PO43− | Orthophosphate |
| PAC | Polyaluminium chloride |
| WW | Wastewater |
| Ntot | Total nitrogen |
| Ptot | Total phosphorus |
| TSS | Total suspended solids |
References
- Pascari, X.; Ramos, A.J.; Marín, S.; Sanchís, V. Mycotoxins and Beer. Impact of Beer Production Process on Mycotoxin Contamination. A Review. Food Res. Int. 2018, 103, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Ramamurthy, R.; Rene, E.R. Wastewater Treatment and Resource Recovery Technologies in the Brewery Industry: Current Trends and Emerging Practices. Sustain. Energy Technol. Assess. 2021, 47, 101432. [Google Scholar] [CrossRef]
- de Oliveira Gomes, F.; Guimarães, B.P.; Ceola, D.; Ghesti, G.F. Advances in Dry Hopping for Industrial Brewing: A Review. Food Sci. Technol. 2022, 42, e60620. [Google Scholar] [CrossRef]
- Costa, A.M.; Zanoelo, E.F.; Benincá, C.; Freire, F.B. A Kinetic Model for Electrocoagulation and Its Application for the Electrochemical Removal of Phosphate Ions from Brewery Wastewater. Chem. Eng. Sci. 2021, 243, 116755. [Google Scholar] [CrossRef]
- Dabrowski, W.; Karolinczak, B. Application of Trickling Filter and Vertical Flow Constructed Wetland Bed to Treat Sewage from Craft Brewery. J. Ecol. Eng. 2019, 20, 211–217. [Google Scholar] [CrossRef]
- Khumalo, S.M.; Bakare, B.F.; Rathilal, S.; Tetteh, E.K. Characterization of South African Brewery Wastewater: Oxidation-Reduction Potential Variation. Water 2022, 14, 1604. [Google Scholar] [CrossRef]
- Khumalo, S.M.; Bakare, B.F.; Tetteh, E.K.; Rathilal, S. Application of Response Surface Methodology on Brewery Wastewater Treatment Using Chitosan as a Coagulant. Water 2023, 15, 1176. [Google Scholar] [CrossRef]
- Mwewa, B.; Stopić, S.; Ndlovu, S.; Simate, G.S.; Xakalashe, B.; Friedrich, B. Synthesis of Poly-Alumino-Ferric Sulphate Coagulant from Acid Mine Drainage by Precipitation. Metals 2019, 9, 1166. [Google Scholar] [CrossRef]
- Shabangu, K.P.; Bakare, B.F.; Bwapwa, J.K. The Treatment Effect of Chemical Coagulation Process in South African Brewery Wastewater: Comparison of Polyamine and Aluminum-Chlorohydrate Coagulants. Water 2022, 14, 2495. [Google Scholar] [CrossRef]
- Egamberdiev, N.B.; Sharipjonova, Z.; Nasibov, B.; Khomidov, A.O.; Alimova, M.I.; Abdumalikov, A.A. Biological Treatment of Industrial and Domestic Wastewater of a Brewery in Uzbekistan. E3S Web Conf. 2021, 264, 01055. [Google Scholar] [CrossRef]
- Strelkov, A.K.; Ponomarenko, O.S.; Avdeenkov, P.P.; Zontova, E.R.; Tuktasheva, E.Y. Results of Studies on Biological Wastewater Treatment of a Brewery. Urban Constr. Archit. 2022, 12, 34–39. [Google Scholar] [CrossRef]
- Tuktasheva, E.Y.; Stepanov, A.S.; Kumpeisov, M.S. Biological Treatment of Brewery Wastewater in a Sequencing Batch Reactor. IOP Conf. Ser. Earth Environ. Sci. 2021, 867, 012053. [Google Scholar] [CrossRef]
- Aremanda, R.B.; Berhane, F.; Daniel, H.; Mehari, A.; Tekle, A. Brewery Effluent Treatment with Conventional and Natural Coagulants. Equilib. J. Chem. Eng. 2022, 6, 105–116. [Google Scholar] [CrossRef]
- Wagner, T.V.; Rempe, F.; Hoek, M.; Schuman, E.; Langenhoff, A. Key Constructed Wetland Design Features for Maximized Micropollutant Removal from Treated Municipal Wastewater: A Literature Study Based on 16 Indicator Micropollutants. Water Res. 2023, 244, 120534. [Google Scholar] [CrossRef]
- Reif, D.; Weisz, L.; Kobsik, K.; Schaar, H.; Saracevic, E.; Krampe, J.; Kreuzinger, N. Adsorption/Precipitation Prototype Agent for Simultaneous Removal of Phosphorus and Organic Micropollutants from Wastewater. J. Environ. Chem. Eng. 2023, 11, 110117. [Google Scholar] [CrossRef]
- Aziz, N.; Effendy, N.; Basuki, K.T. Comparison of Poly Aluminium Chloride (PAC) and Aluminium Sulphate Coagulants Efficiency in Waste Water Treatment Plant. J. Inov. Tek. Kim. 2017, 2, 24–31. [Google Scholar]
- Wang, Y.; Chen, M.; Xu, J.; Qi, N.; Dong, L.; Cao, G.; Zhao, X. Potential and Characteristics of Bio-H2 Production from Brewery Wastewater by a Maltose-Preferring Butyrate-Type Producer: Investigation in Batch and Semi-Continuous Cultures. Environ. Res. 2022, 205, 112457. [Google Scholar] [CrossRef]
- Gorfie, B.N.; Tuhar, A.W.; Keraga, A.S.; Woldeyohannes, A.B. Effect of Brewery Wastewater Irrigation on Soil Characteristics and Lettuce (Lactuca Sativa) Crop in Ethiopia. Agric. Water Manag. 2022, 269, 107633. [Google Scholar] [CrossRef]
- Arambarri, J.; Abbassi, B.; Zytner, P. Enhanced Removal of Phosphorus from Wastewater Using Sequential Electrocoagulation and Chemical Coagulation. Water Air Soil Pollut. 2019, 230, 312. [Google Scholar] [CrossRef]
- Henze, M.; Comeau, Y. Wastewater Characterisation. In Biological Wastewater Treatment: Principles, Modelling and Design; Henze, M., van Loosdrecht, M.C.M., Ekama, G.A., Brdjanovic, D., Eds.; IWA Publishing: London, UK, 2008; Volume 7. [Google Scholar]
- Hach Company. Suspended Solids Photometric Method 1 Method 8006 5 to 750 Mg/L TSS Scope and Application: For Water and Wastewater. Test Preparation Instrument-Specific Information Before Starting; Hach Company: Loveland, CO, USA, 2014. [Google Scholar]
- American Public Health Association. APHA Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2022. [Google Scholar]
- Hach Company. HACH LCK 114—Chemical Oxygen Demand (COD); Hach Company: Loveland, CO, USA, 2019. [Google Scholar]
- Hach Company. HACH Phosphorus, Total|Method 8190; Hach Company: Loveland, CO, USA, 2017. [Google Scholar]
- Hach Company. HACH Phosphorus, Reactive (Orthophosphate)|Method 10214; Hach Company: Loveland, CO, USA, 2014. [Google Scholar]
- Hach Company. HACH Error Sources During Analysis—DOC062.52.00304; Hach Company: Loveland, CO, USA, 2017. [Google Scholar]
- Garomsa, F.S.; Mehari, Y.; Desta, W.M.; Bidira, F.; Asaithambi, P. Removal of NO3-, PO3-, and Color from Brewery Wastewater by the Use of Indigenous Bio-Coagulant-Assisted Electrocoagulation. Prog. Eng. Sci. 2024, 1, 100032. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S.; Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S. A Review on Revolutionary Technique for Phosphate Removal in Wastewater Using Green Coagulant. J. Water Process Eng. 2023, 52, 103573. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Y.; Ke, Y.; Li, B. Efficiency and Mechanism of Advanced Treatment for Phosphate Wastewater by High Efficiency and Low Consumption Coagulation and Phosphorus Removal System. IOP Conf. Ser. Earth Environ. Sci. 2021, 631, 012002. [Google Scholar] [CrossRef]
- Avila-Sierra, A.; Zhang, Z.J.; Fryer, P.J. Effect of Surface Characteristics on Cleaning Performance for CIP System in Food Processing. Energy Procedia 2019, 161, 115–122. [Google Scholar] [CrossRef]
- Rajapakse, N.; Zargar, M.; Sen, T.; Khiadani, M. Effects of Influent Physicochemical Characteristics on Air Dissolution, Bubble Size and Rise Velocity in Dissolved Air Flotation: A Review. Sep. Purif. Technol. 2022, 289, 120772. [Google Scholar] [CrossRef]
- Dayarathne, H.N.P.; Angove, M.J.; Jeong, S.; Aryal, R.; Paudel, S.R.; Mainali, B. Effect of Temperature on Turbidity Removal by Coagulation: Sludge Recirculation for Rapid Settling. J. Water Process Eng. 2022, 46, 102559. [Google Scholar] [CrossRef]
- Bakare, B.F.; Shabangu, K.; Chetty, M. Brewery Wastewater Treatment Using Laboratory Scale Aerobic Sequencing Batch Reactor. S. Afr. J. Chem. Eng. 2017, 24, 128–134. [Google Scholar] [CrossRef]
- Tuktasheva, E.Y. Study of efficiency of pre-reagent treatment of waste water from brewery. Urban Constr. Archit. 2021, 11, 56–61. [Google Scholar] [CrossRef]
- Swain, K.; Abbassi, B.; Kinsley, C. Combined Electrocoagulation and Chemical Coagulation in Treating Brewery Wastewater. Water 2020, 12, 726. [Google Scholar] [CrossRef]
- Fosso-Kankeu, E.; Lunga, O.T.G.; Moyakhe, D.; Waanders, F.B.; de Klerk, C. Effects of Pretreatment on the Removal of COD from Brewery Wastewater. In Proceedings of the SETWM-19, ACBES-19, EEHSS-19, Johannesburg, South Africa, 18–19 November 2019. Eminent Association of Pioneers. [Google Scholar]
- Tonhato Junior, A.; Hasan, S.D.M.; Sebastien, N.Y. Optimization of Coagulation/Flocculation Treatment of Brewery Wastewater Employing Organic Flocculant Based of Vegetable Tannin. Water Air Soil Pollut. 2019, 230, 202. [Google Scholar] [CrossRef]
- Alavijeh, H.N.; Sadeghi, M.; Kashani, M.R.K.; Moheb, A. Efficient Chemical Coagulation-Electrocoagulation-Membrane Filtration Integrated Systems for Baker’s Yeast Wastewater Treatment: Experimental and Economic Evaluation. Clean. Chem. Eng. 2022, 3, 100032. [Google Scholar] [CrossRef]
- Hultberg, M.; Bodin, H. Fungi-Based Treatment of Brewery Wastewater—Biomass Production and Nutrient Reduction. Appl. Microbiol. Biotechnol. 2017, 101, 4791–4798. [Google Scholar] [CrossRef]
- Duan, J.; Wang, J.; Guo, T.; Gregory, J. Zeta Potentials and Sizes of Aluminum Salt Precipitates—Effect of Anions and Organics and Implications for Coagulation Mechanisms. J. Water Process Eng. 2014, 4, 224–232. [Google Scholar] [CrossRef]
- Aigboje, E.O. Effect of the Production Processed Effluence on the Environment: A Case Study of a Typical Brewery Industry in Nigeria. J. Adv. Sci. Eng. 2022, 7, 9–17. [Google Scholar] [CrossRef]
- Jaiyeola, A.T.; Bwapwa, J.K. Treatment Technology for Brewery Wastewater in a Water-Scarce Country: A Review. S. Afr. J. Sci. 2016, 112, 8. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Agunbiade, M.; Oladipo, B.; Ademakinwa, A.N.; Awolusi, O.; Adesiyan, I.M.; Oyekola, O.; Ololade, O.; Ojo, A. Bioflocculant Produced by Bacillus Velezensis and Its Potential Application in Brewery Wastewater Treatment. Sci. Rep. 2022, 12, 10945. [Google Scholar] [CrossRef]
- Badawi, A.K.; Salama, R.S.; Mostafa, M.M.M. Natural-Based Coagulants/Flocculants as Sustainable Market-Valued Products for Industrial Wastewater Treatment: A Review of Recent Developments. RSC Adv. 2023, 13, 19335–19355. [Google Scholar] [CrossRef]
- Collivignarelli, M.; Abbà, A.; Frattarola, A.; Carnevale Miino, M.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the Reuse of Biosolids on Agricultural Land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef]
- Nayeri, D.; Mousavi, S.A. A Comprehensive Review on the Coagulant Recovery and Reuse from Drinking Water Treatment Sludge. J. Environ. Manag. 2022, 319, 115649. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).