Subcritical Extraction of Coal Tar Slag and Analysis of Extracts and Raffinates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
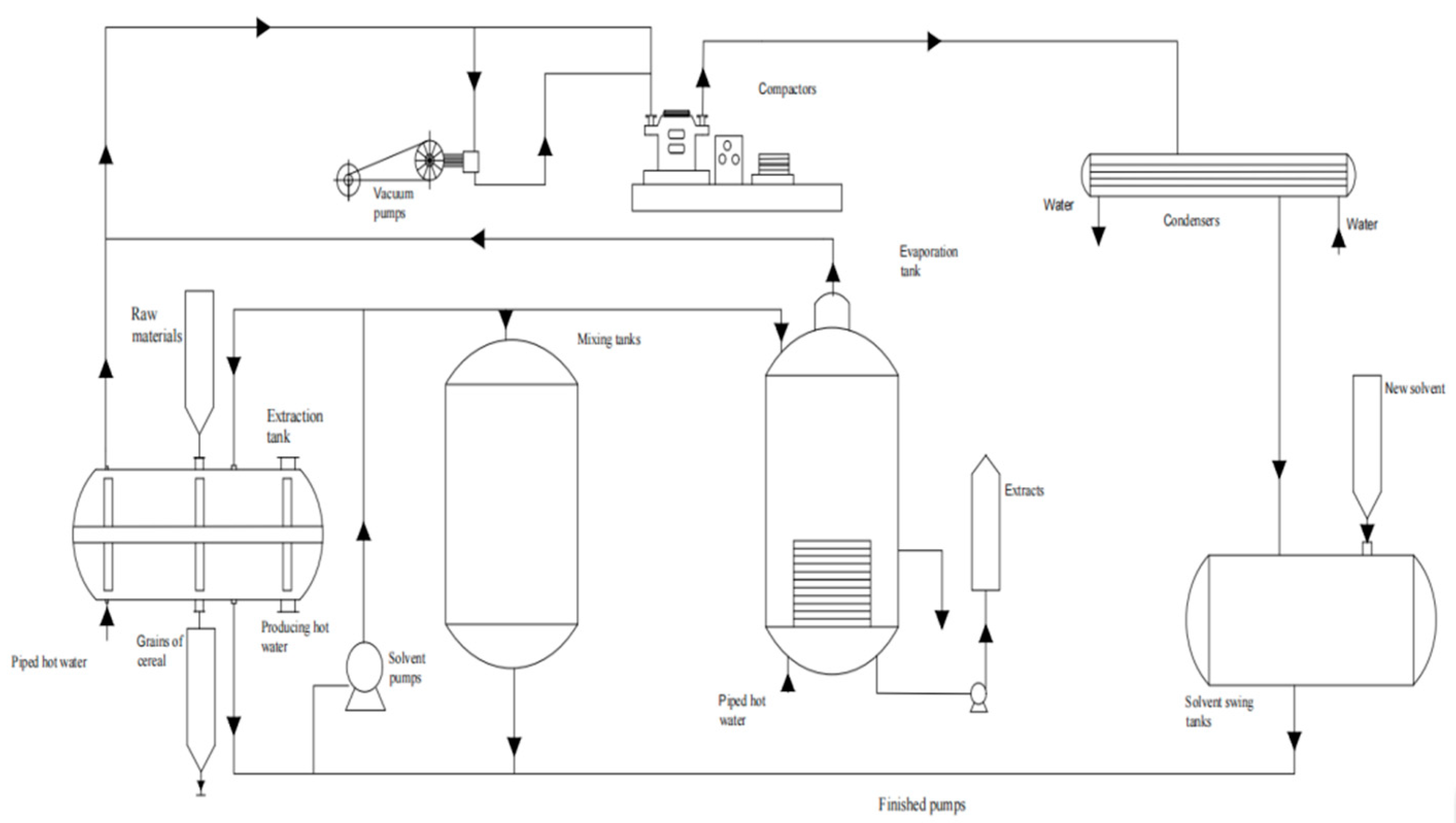
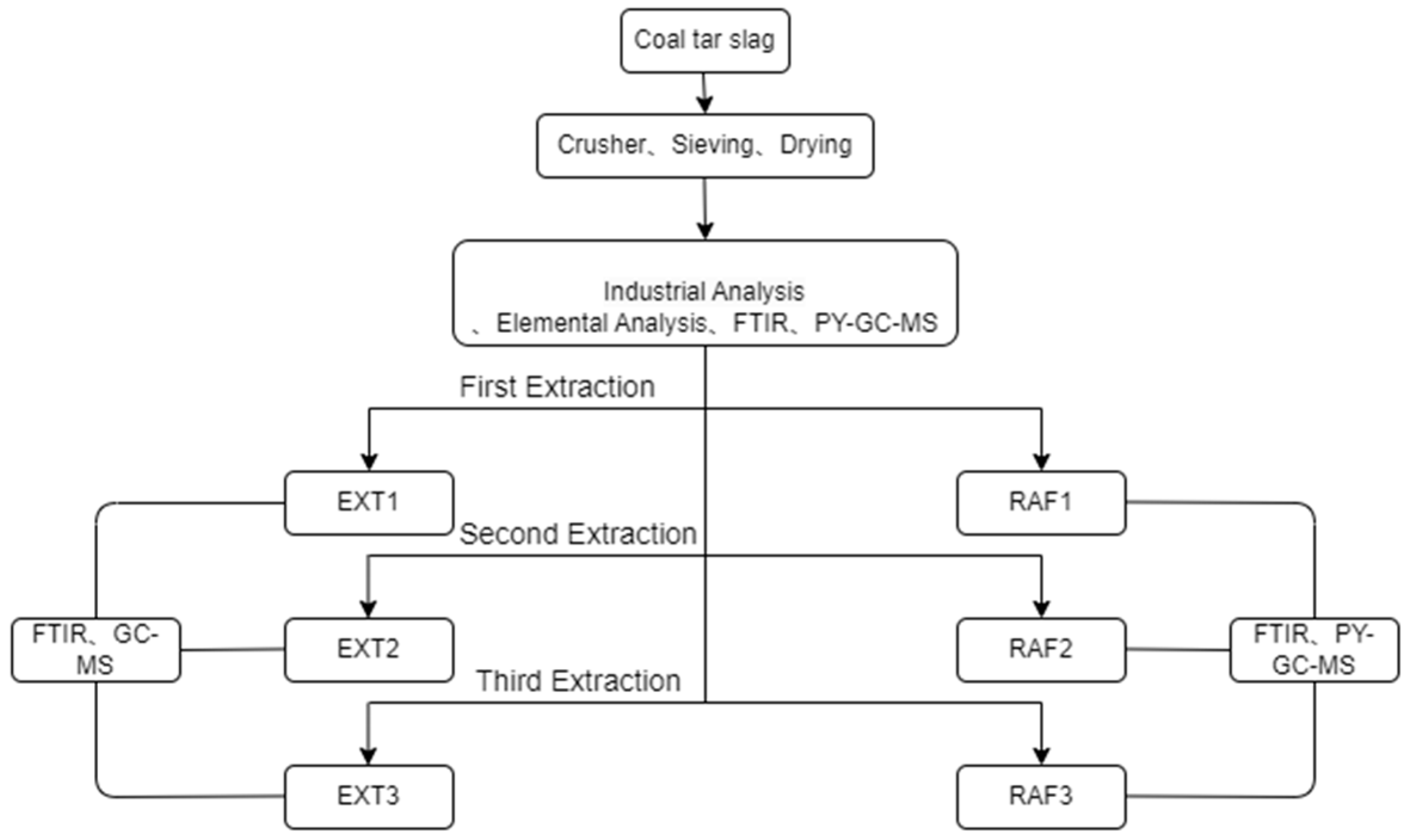
2.2. Subcritical Extraction Experiments
2.3. Test Analysis
3. Results and Discussion
3.1. Coal Tar Slag Basic Property Test
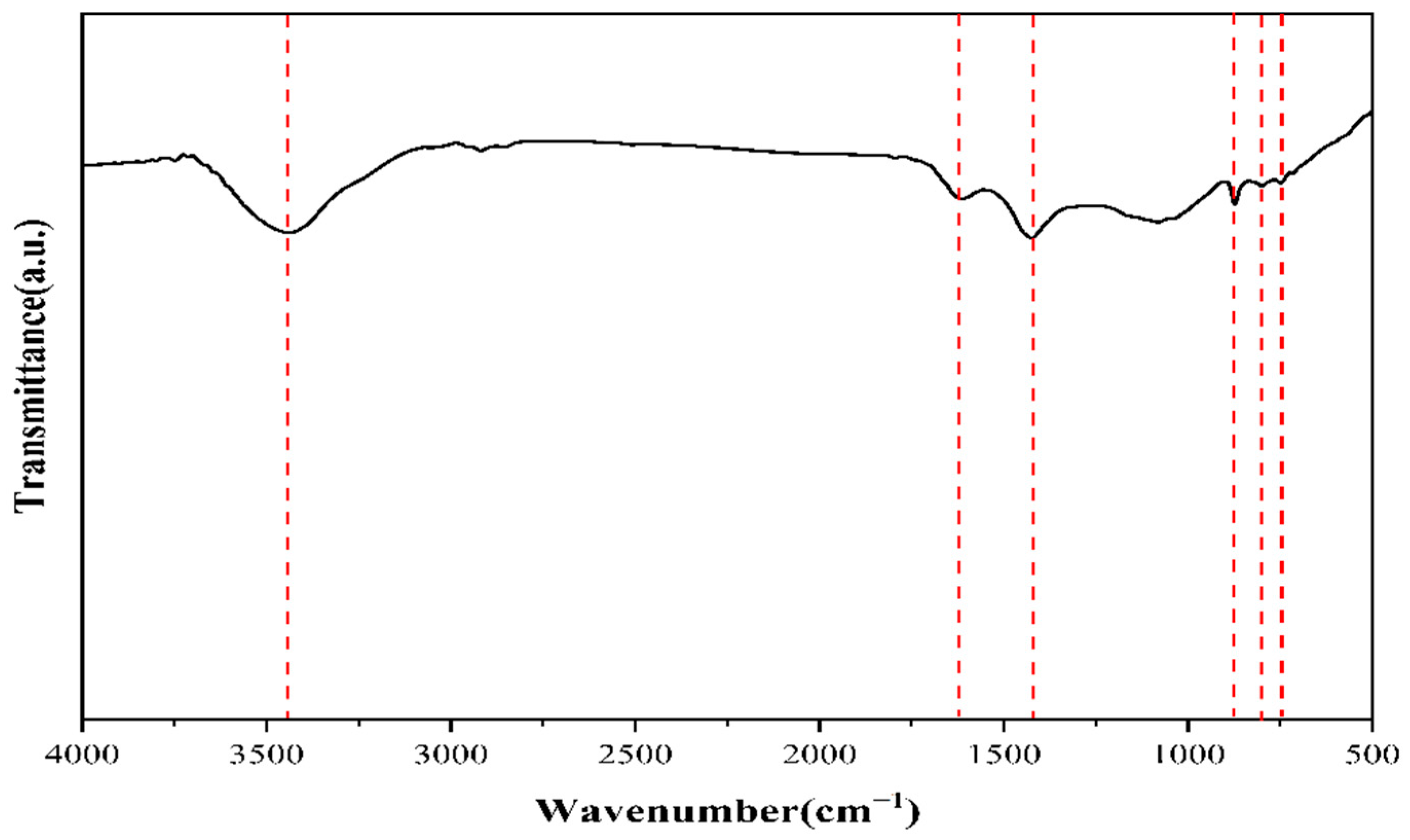
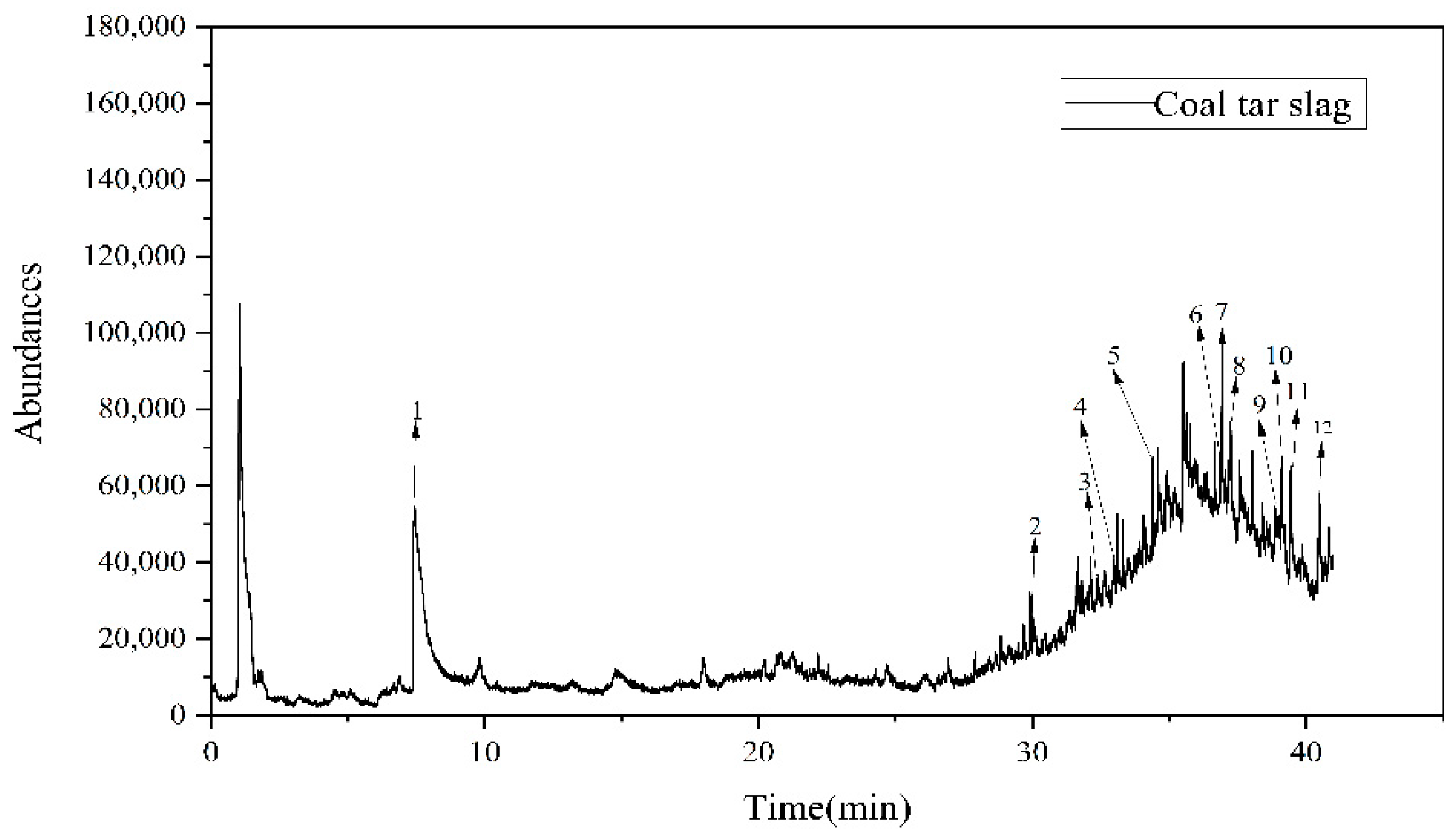
3.2. Infrared Spectroscopy and PY-GC-MS Tests on Raw Coal Tar Slag Samples
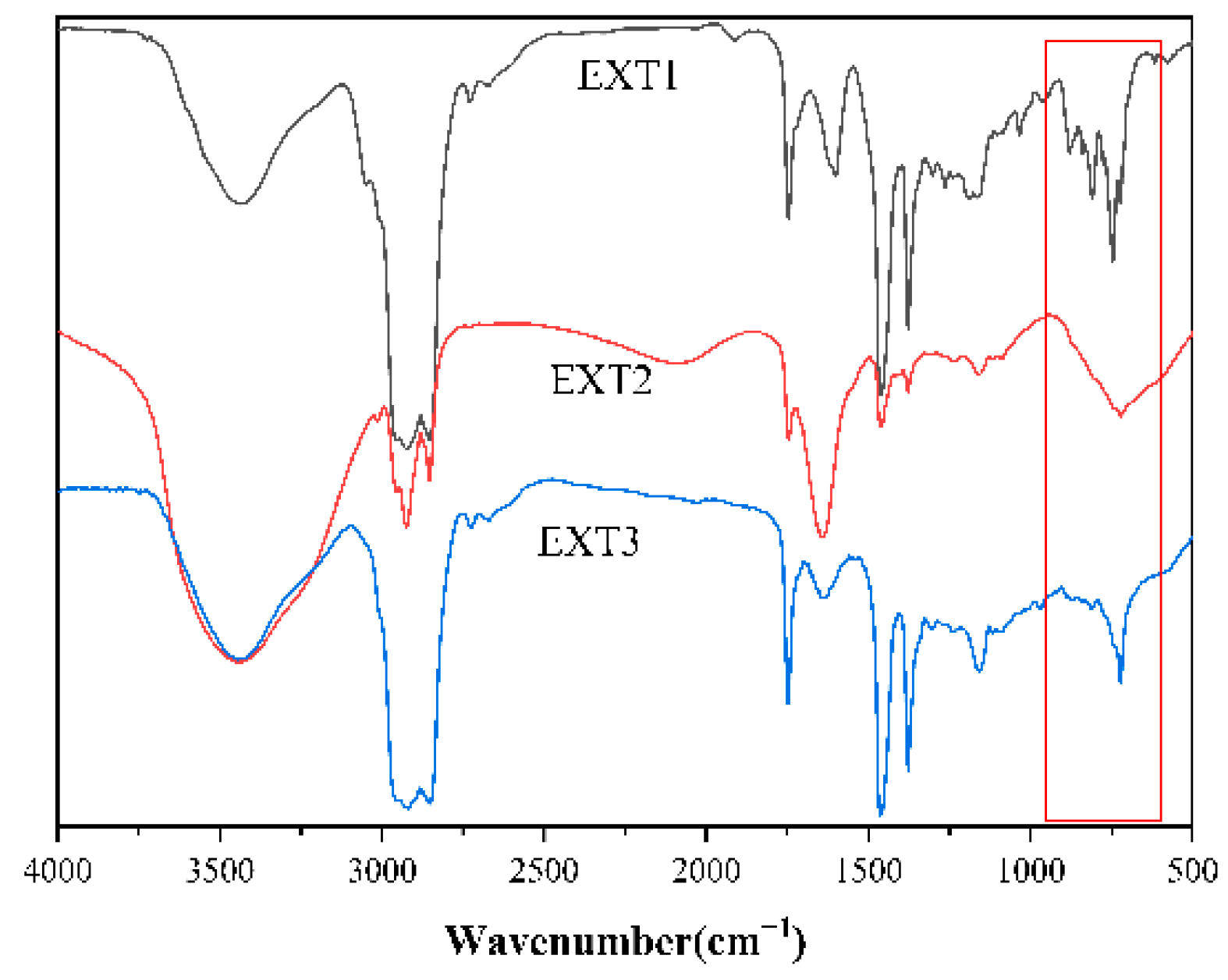
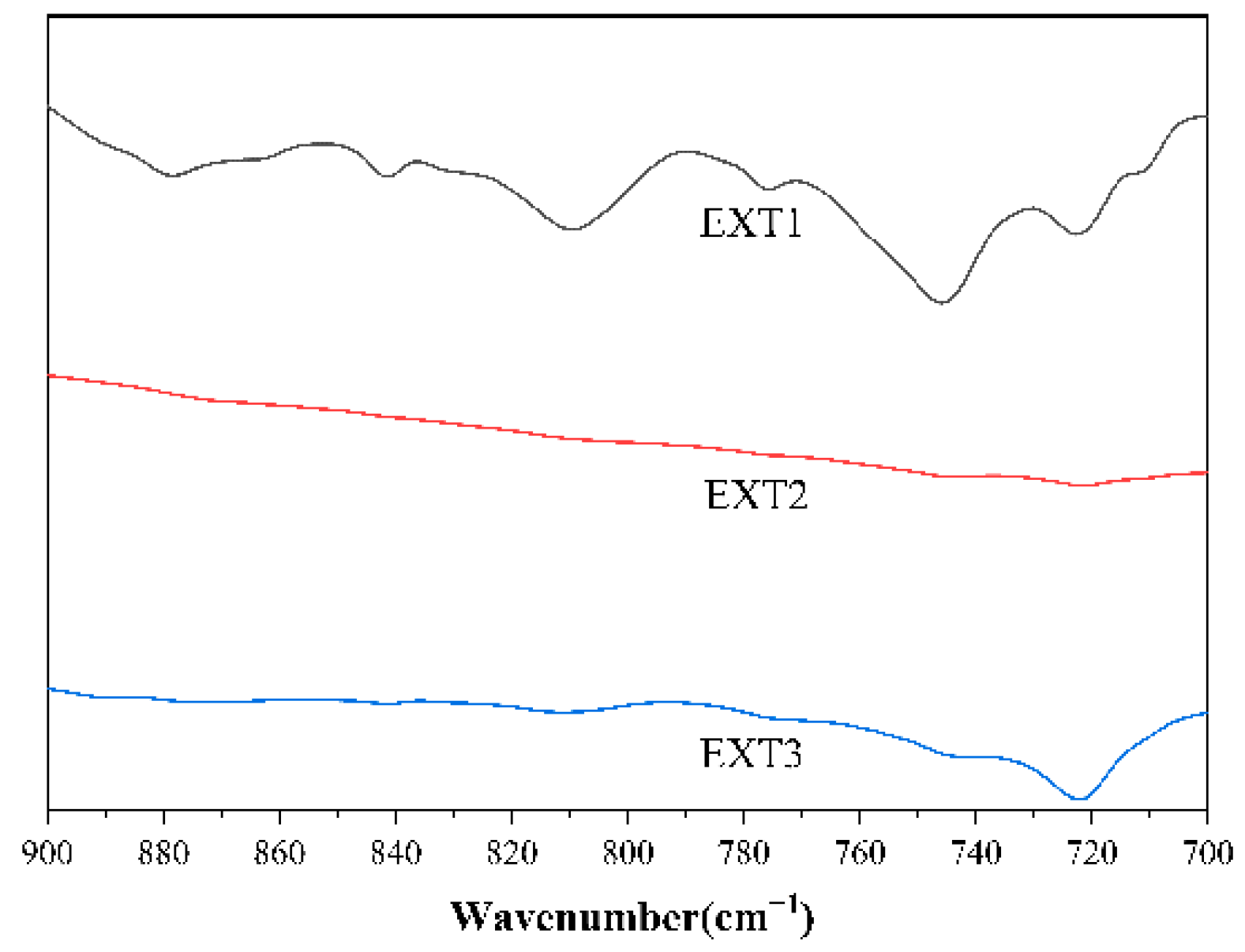
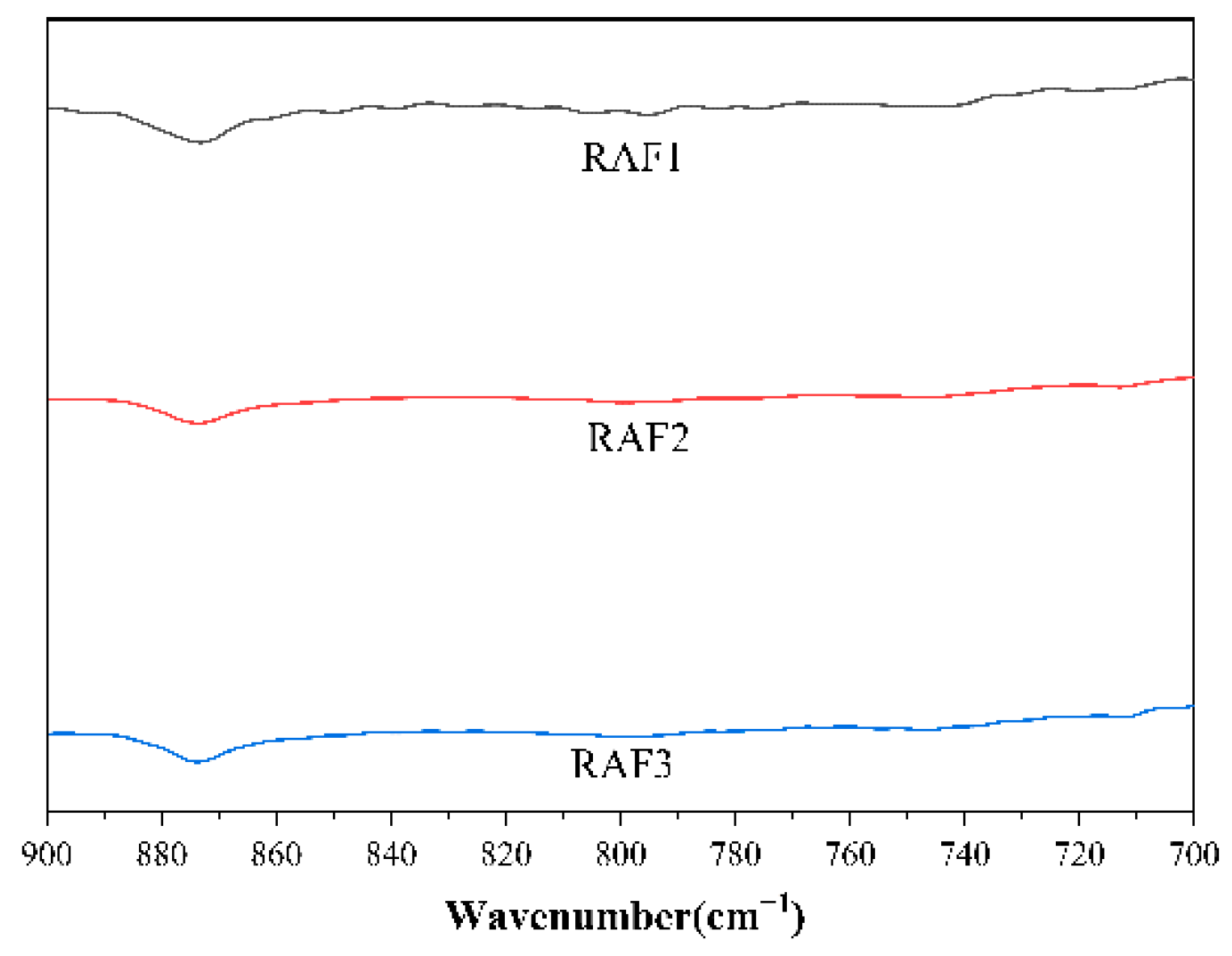
3.3. Infrared Spectroscopic Testing of Coal Tar Slag Raffinates and Extracts
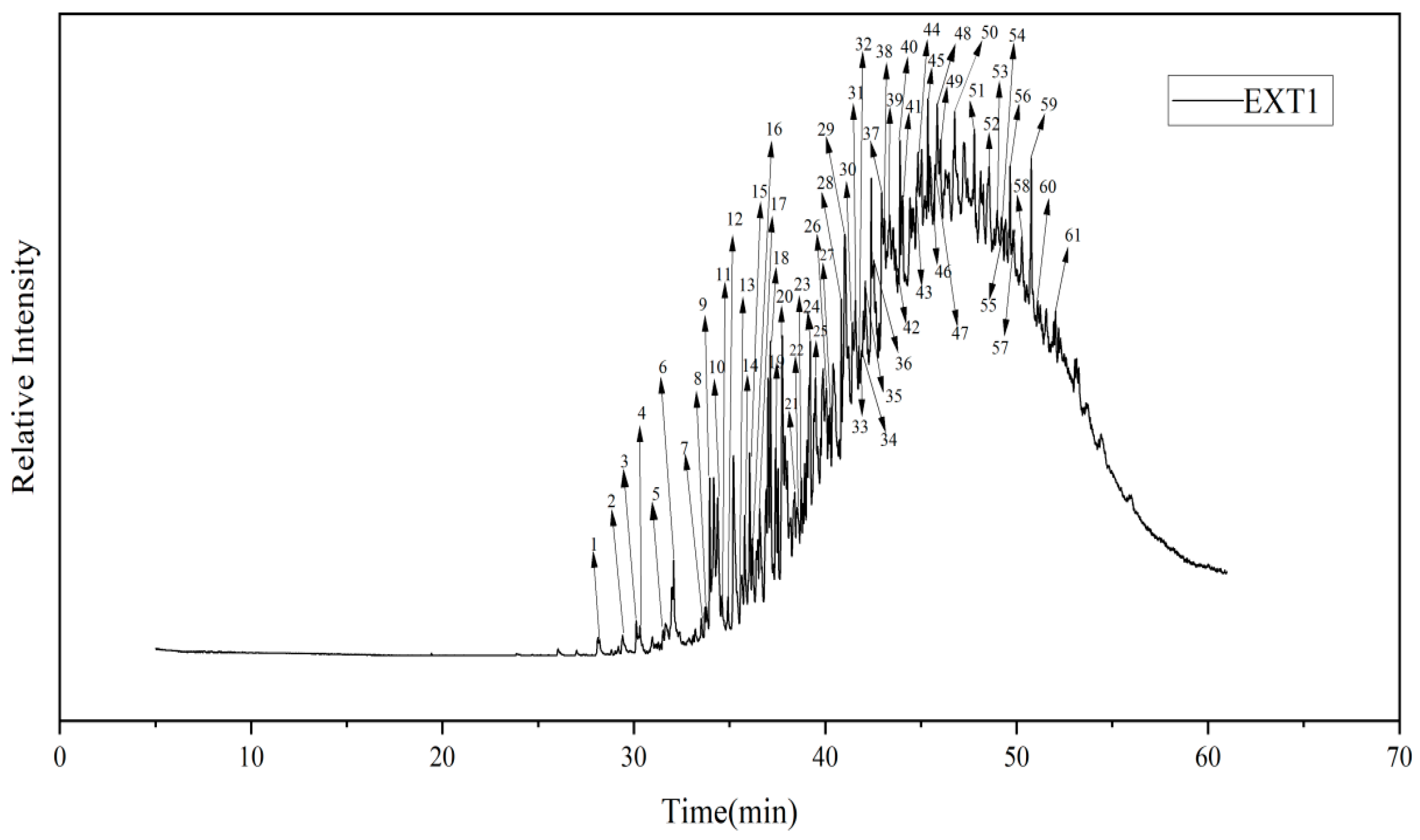
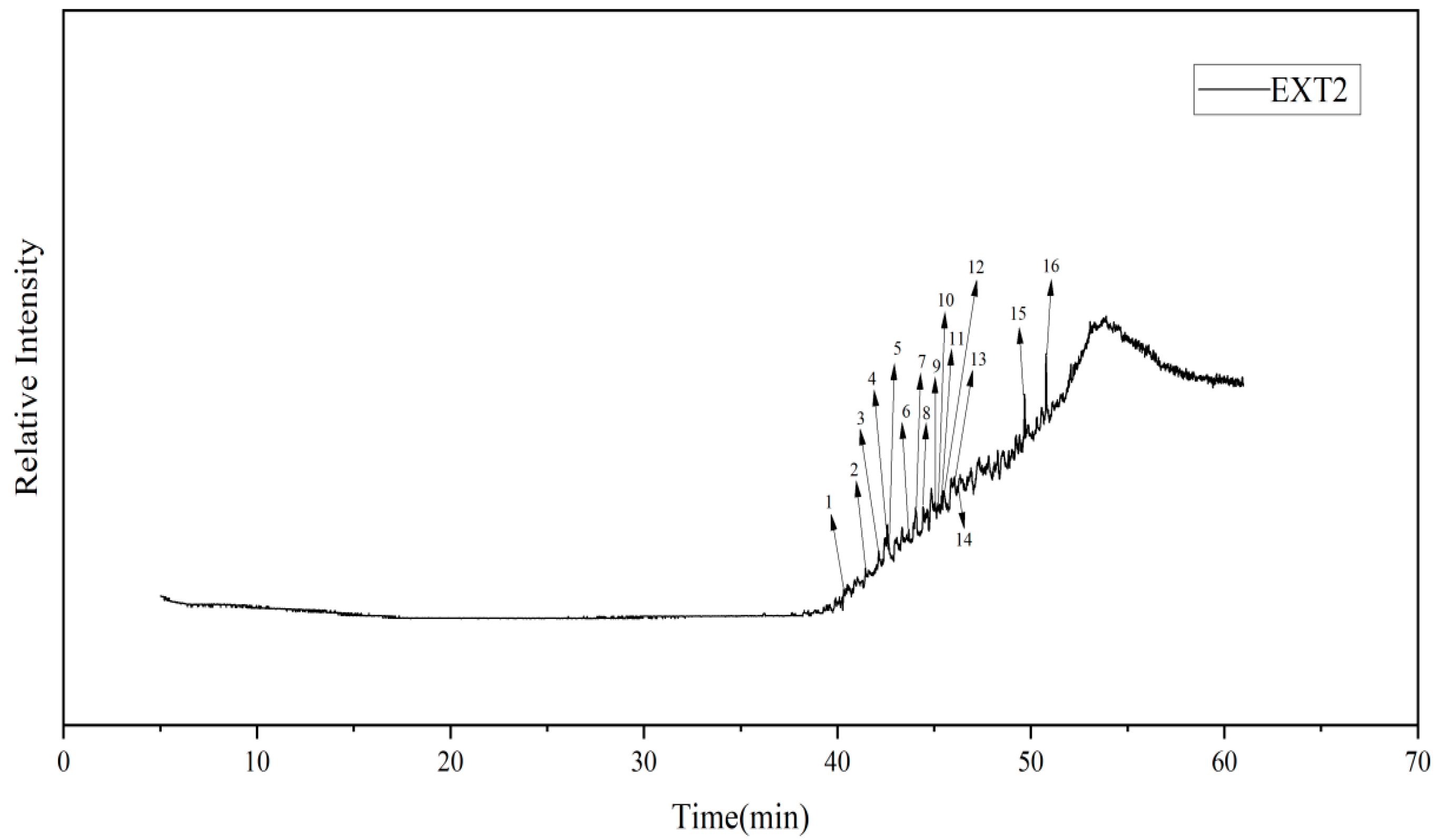
3.4. GC-MS Testing of Coal Tar Slag Extracts
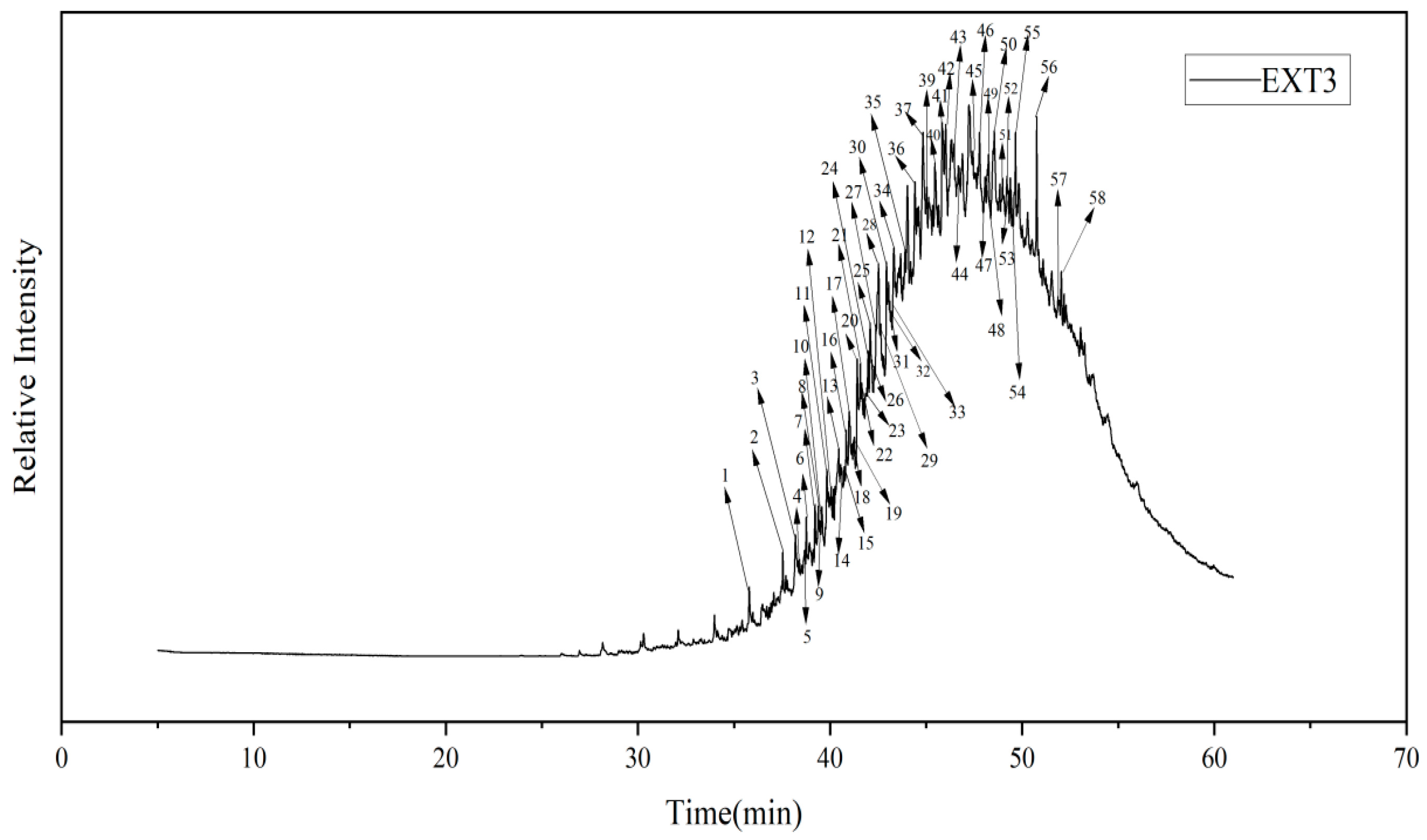
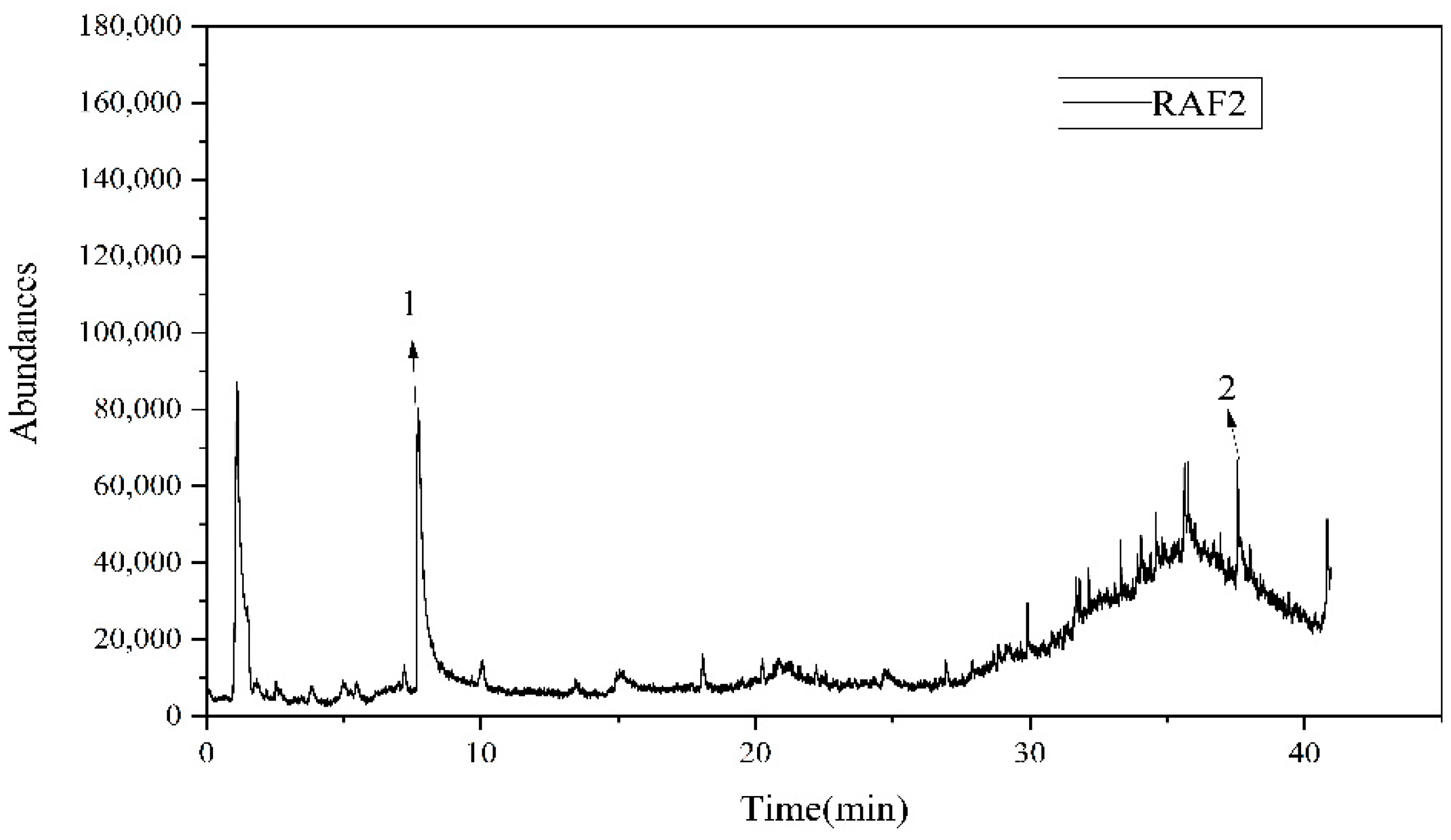
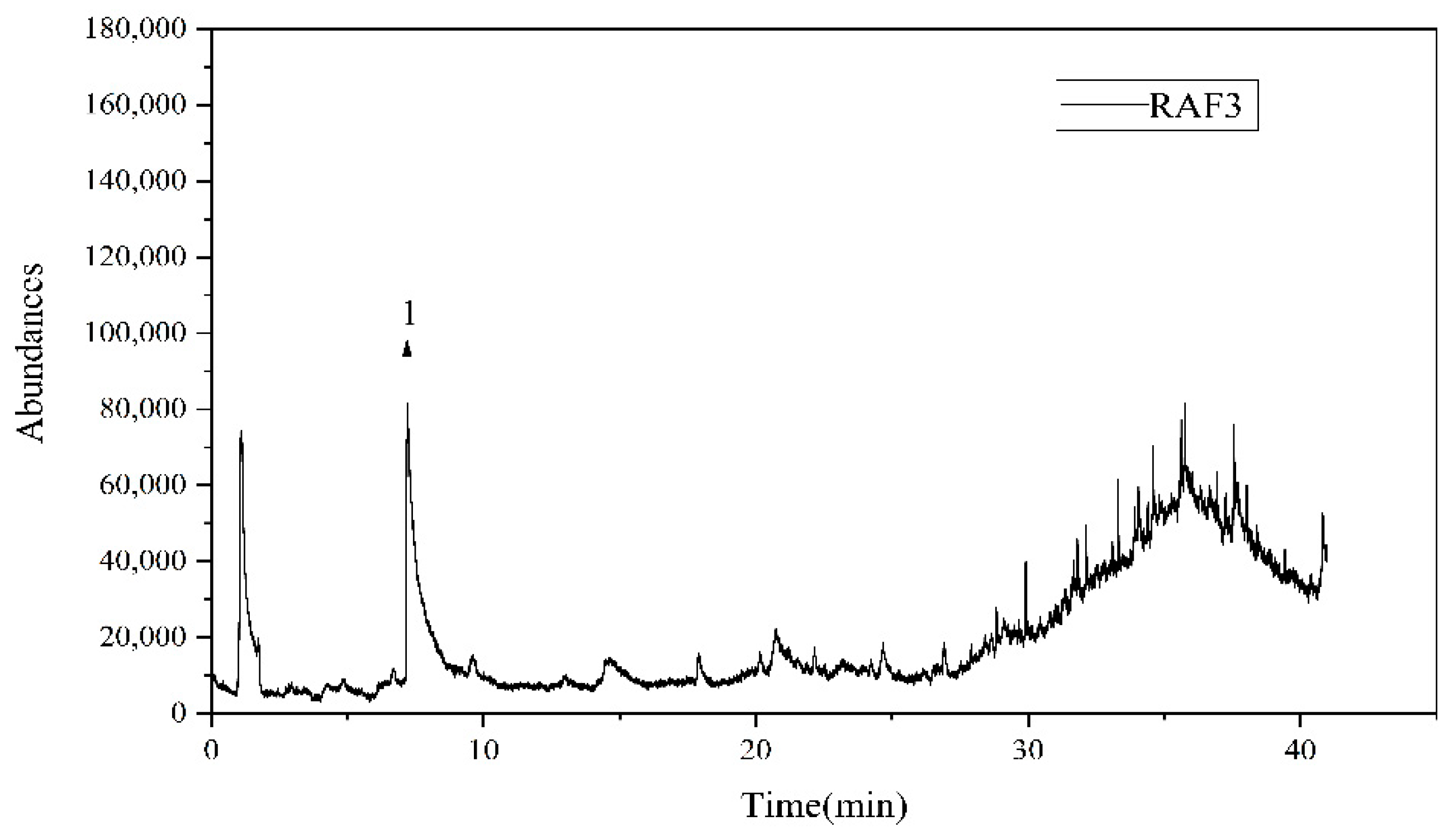
3.5. Coal Tar Slag Raffinate PY-GC/MS Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, X.B.; Liu, X.C.; Du, Z.M.; Hou, C.R.; Li, M.J.; Cao, F.B.; Chen, M.F.; Zhang, T. Advances in development of safe and efficient mining of coexisting coal and uranium resources. Processes 2024, 12, 1340. [Google Scholar] [CrossRef]
- Yue, G.X.; Lyu, J.F.; Li, S.Q. Clean and highly-efficient utilization of coal. Front. Energy 2021, 15, 1–3. [Google Scholar] [CrossRef]
- Wang, X.L.; He, X.; Wang, X.H. FTIR analysis of the functional group composition of coal tar residue extracts and extractive residues. Appl. Sci. 2023, 13, 5162. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.L.; Niu, Y.X.; Liu, G.; Sheng, Q.T.; Wang, Y.G. Combustion properties and toxicity analysis of coal gasification tar residue. J. Clean. Prod. 2016, 139, 567–575. [Google Scholar] [CrossRef]
- Gao, F.Y.; Zhou, C.C.; Wang, Z.H.; Zhu, W.W.; Wang, X.; Liu, G.J. Solid-oil separation of coal tar residue to reduce polycyclic aromatic hydrocarbons via microwave-assisted extraction. J. Environ. Manag. 2023, 337, 117679. [Google Scholar] [CrossRef]
- Apicella, B.; Russo, C.; Senneca, O. Analytics for recovery and reuse of solid wastes from refineries. Energies 2022, 15, 4026. [Google Scholar] [CrossRef]
- Ma, Z.H.; Wei, X.Y.; Liu, G.H.; Liu, F.J.; Zong, Z.M. Value-added utilization of high-temperature coal tar: A review. Fuel 2021, 292, 119954. [Google Scholar] [CrossRef]
- Yang, B.; Fan, X.Y.; Li, D.; Cui, L.W.; Chang, C.R.; Yan, L.; Lu, B.W.; Li, J. Simulation and experimental study of solid–liquid extraction of coal tar residue based on different extractants. ACS Omega 2023, 8, 47835–47845. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wu, Y.Q.; Huang, S.; Wu, S.Y.; Gao, J.S. Coking behavior and mechanism of direct coal liquefaction residue in coking of coal blending. Fuel 2020, 280, 118488. [Google Scholar] [CrossRef]
- Xu, Z.W.; Shao, H.S.; Wang, Y.G.; Zhao, Q.X.; Liang, Z.Y. Characteristics of coal tar residue treated with microwave-assisted hydrothermal treatment. Fuel Process. Technol. 2021, 211, 106580. [Google Scholar] [CrossRef]
- Liang, L.P.; Gao, F.; Mao, L.T.; Wang, Y.K.; Zhu, B.S.; Zhang, R.; Li, G.M. Resource utilization of coal hydrogasification residue to Ni/carbon-based composites for efficient microwave absorption. J. Mater. Sci. Mater. Electron. 2022, 33, 12857–12870. [Google Scholar] [CrossRef]
- Yao, Q.X.; Liu, Y.Q.; Zhang, D.; Sun, M.; Ma, X.X. Catalytic conversion of a≥ 200° C fraction separated from low-temperature coal tar into light aromatic hydrocarbons. ACS Omega 2021, 6, 4062–4073. [Google Scholar] [CrossRef] [PubMed]
- Yabalak, E.; Aminzai, M.T.; Gizir, A.M.; Yang, Y. A review: Subcritical water extraction of organic pollutants from environmental matrices. Molecules 2024, 29, 258. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xue, F.M.; Yu, S.; Du, S.C.; Yang, Y. Subcritical water extraction of natural products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Zhang, J.X.; Wen, C.T.; Zhang, H.H.; Duan, Y.Q.; Ma, H.L. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Techol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Fărcaș, A.C.; Socaci, S.A.; Nemeș, S.A.; Salanță, L.C.; Chiș, M.S.; Pop, C.R.; Borșa, A.; Diaconeasa, Z.; Vodnar, D.C. Cereal waste valorization through conventional and current extraction techniques—An up-to-date overview. Foods 2022, 11, 2454. [Google Scholar] [CrossRef]
- Ding, Y.H.; Chen, H.; Wang, D.F.; Ma, W.G.; Wang, J.F.; Xu, D.P.; Wang, Y.G. Supercritical fluid extraction and fractionation of high-temperature coal tar. J. Fuel Chem. Technol. 2010, 38, 140–143. [Google Scholar] [CrossRef]
- Meziani, A.; Verloy, S.; Ferroukhi, O.; Roca, S.; Curat, A.; Tisse, S.; Peulon-Agasse, V.; Gardeniers, H.; Desmet, G.; Cardinael, P. Evaluation of gas chromatography columns with radially elongated pillars as second-dimension columns in comprehensive two-dimensional gas chromatography. Anal. Chem. 2022, 94, 14126–14134. [Google Scholar] [CrossRef]
- Yu, Q.; Xu, S.Y.; Shi, W.Y.; Tian, Y.; Wang, X.H. Mass spectrometry coupled with vacuum thermal desorption for enhanced volatile organic sample analysis. Anal. Methods 2020, 12, 1852–1857. [Google Scholar] [CrossRef]
- Qi, Y.; Hann, W.; Subagyono, D.J.N.; Fei, Y.; Marshall, M.; Jackson, W.R.; Patti, A.F.; Chaffee, A.L. Characterisation of the products of low temperature pyrolysis of Victorian brown coal in a semi-continuous/flow through system. Fuel 2018, 234, 1422–1430. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, D.; Yao, Q.X.; Liu, Y.Q.; Su, X.P.; Jia, C.Q.; Hao, Q.Q.; Ma, X.X. Separation and composition analysis of GC/MS analyzable and unanalyzable parts from coal tar. Energ. Fuels 2018, 32, 7404–7411. [Google Scholar] [CrossRef]
- GBT212-2022; Industrial Analysis Method of Coal. State Administration for Market Regulation: Beijing, China, 2022.


| Equipment Name | Equipment Model | Address | Manufacturer |
|---|---|---|---|
| Muffle furnace | KSL-1100X | Hefei, China | Hefei Kejing Material Technology Co., Ltd. |
| Electrically heated blast drying oven | 101-OAB | Tianjin, China | Tianjin Taist Instrument Co., Ltd. |
| Elemental Analyser | Elementar Vario EL II | Hebi, China | Hebi Yingtai Electronic and Electrical Appliance Co., Ltd. |
| Sulfur meter | 5E-S3200 | Yuncheng, China | Nanfeng Chemical Group Co., Ltd. |
| Subcritical extraction equipment | CBE-T-1L | Henan, China | Henan Subcritical Extraction Research Institute |
| Infrared spectrometer | Nicolet iN10&iZ10 | Waltham, MA, USA | Thermo Fisher Sciedtific, USA |
| GC-MS | 7890A-5975C | Nishinokyo Kuwabara-cho, Japan | Shimadzu Manufacturing, Japan |
| PY-GC-MS | 7890A-5975C | Santa Clara, CA, USA | Agilent Corporation of the United States |
| Sample | Industrial Analysis/W% | Elemental Analysis/W%, daf | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Aad | Vdaf | FCad | C | H | N | S | O* | |
| Coal tar slag | 5.33 | 13.43 | 17.58 | 67.55 | 74.64 | 2.46 | 1.83 | 0.56 | 20.51 |
| No. | Compound Names | Proposed Formula | Molecular Weight | Relative Content, % |
|---|---|---|---|---|
| Aliphatic Hydrocarbons | ||||
| 5 | Tetradecane | C14H30 | 198.39 | 1.23 |
| Dodecane | C12H26 | 170.33 | ||
| Hentriacontane | C31H64 | 436.80 | ||
| Aromatic Hydrocarbons | ||||
| Bicyclic Aromatic Hydrocarbons | ||||
| 2 | 2,7-dimethyl-Naphthalene | C12H12 | 156.22 | 0.15 |
| 2,3-dimethyl-Naphthalene | ||||
| 1,8-dimethyl-Naphthalene | ||||
| 3 | 2,3,6-trimethyl-Naphthalene | C13H14 | 170.25 | 0.55 |
| 4,6,8-Trimethylazulene | ||||
| 1,6,7-trimethyl-Naphthalene | ||||
| 4 | 4,6,8-Trimethylazulene | C13H14 | 170.25 | 1.34 |
| 1,4,5-trimethyl-Naphthalene | ||||
| 1,6,7-trimethyl-Naphthalene | ||||
| Tricyclic Aromatic Hydrocarbons | ||||
| 6 | 1-methyl-Phenanthrene | C15H12 | 192.25 | 4.22 |
| 4-methyl-Phenanthrene | ||||
| l-methyl-Anthracene | ||||
| 7 | 4-methyl-Phenanthrene | C15H12 | 192.25 | 3.86 |
| 4-methyl-Phenanthrene | ||||
| 2-methyl-Anthracene | ||||
| 8 | 1-methyl-Anthracene | C15H12 | 192.25 | 1.80 |
| 1-methyl-Phenanthrene | ||||
| 4-methyl-Phenanthrene | ||||
| 9 | 3,4-Dimethylphenanthrene | C16H14 | 206.28 | 2.57 |
| di-p-Tolylacetylene | ||||
| 2,7-dimethyl-Phenanthrene | ||||
| 10 | 3,6-dimethyl-Phenanthrene | C16H14 | 206.28 | 1.36 |
| 2,7-dimethyl-Phenanthrene | ||||
| l,2-dihydro-1-phenyl-Naphthalene | ||||
| Tetracyclic Aromatic Hydrocarbons | ||||
| 11 | Fluoranthene | C16H10 | 202.25 | 2.16 |
| Fluoranthene | ||||
| Pyrene | ||||
| 12 | Pyrene | C16H10 | 202.25 | 1.18 |
| Pyrene | ||||
| Fluoranthene | ||||
| Aldehyde | ||||
| 1 | Benzaldehyde | C7H6O | 106.12 | 9.47 |
| Benzaldehyde | ||||
| Benzaldehyde |
| No. | Compound Names | Proposed Formula | Molecular Weight | Relative Content, % |
|---|---|---|---|---|
| Aliphatic hydrocarbons | ||||
| 3 | Heptadecane, 2,6,10,15-tetramethyl- | C21H44 | 296.60 | 0.51 |
| Heptadecane, 2,6,10,15-tetramethyl- | C21H44 | 296.60 | ||
| 2-Bromo dodecan | C32H40BrN5O5 | 654.60 | ||
| 4 | Nonane, 3,7-dimethyl- | C11H24 | 156.31 | 0.30 |
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| Carbonic acid, tridecyl vinyl ester | C16H30O3 | 270.41 | ||
| 6 | Octacosane, 2-methyl- | C29H60 | 408.80 | 0.74 |
| 2-Methyltetracosane | C25H52 | 352.70 | ||
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| 14 | Heneicosane | C21H44 | 296.60 | 2.10 |
| 2-Methylhexacosane | C27H56 | 380.70 | ||
| 2-Methyltetracosan | C25H52 | 352.70 | ||
| 22 | Cyclohexane, (1-butylhexadecyl)- | C26H52 | 364.70 | 0.22 |
| Fumaric acid, dodecyl 2,3,4,6-tetrachlorophenyl ester | C22H28Cl4O4 | 498.30 | ||
| Fumaric acid, heptadecyl octyl ester | C29H54O4 | 466.70 | ||
| 24 | Heneicosane | C21H44 | 296.60 | 4.45 |
| 2-Methylhexacosane | C27H56 | 380.70 | ||
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 29 | Tetrapentacontane | C54H110 | 759.40 | 0.99 |
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| Heptacosyl trifluoroacetate | C29H55F3O2 | 492.70 | ||
| 32 | 2-Methylhexacosane | C27H56 | 380.70 | 0.53 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 5-Methyl-Z-5-docosene | C23H46 | 322.60 | ||
| 34 | Cyclohexane, octadecyl- | C24H48 | 336.60 | 0.34 |
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| 35 | Dotriacontane | C32H66 | 450.90 | 2.07 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| 39 | Squalane | C30H62 | 422.80 | 1.24 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 1-Dodecanol, 2-octyl- | C20H42O | 298.50 | ||
| 40 | Tetracontane | C40H82 | 563.10 | 2.11 |
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| 1-Decanol, 2-octyl- | C18H38O | 270.50 | ||
| 41 | Dotriacontane | C32H66 | 450.90 | 5.65 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| 48 | 11-Methyltricosane | C24H50 | 338.70 | 1.50 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| 49 | 2-Methylhexacosane | C27H56 | 380.70 | 0.68 |
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| 51 | 17.alpha.(H),21.beta.(H)-Homohopane | C31H54 | 426.80 | 0.31 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 2-Dodecen-1-yl(-)succinic anhydride | C16H26O3 | 266.38 | ||
| 52 | Pentatriacontane | C35H72 | 492.90 | 1.13 |
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| 53 | Cholestane, (5.alpha.,14.beta.)- | C27H48 | 372.70 | 0.40 |
| E-10,13,13-Trimethyl-11-tetradecen-1-ol acetate | C19H36O2 | 296.50 | ||
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| 55 | 2-Methylhexacosane | C27H56 | 380.70 | 0.39 |
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| 56 | 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | 0.64 |
| 2-Dodecen-1-yl(-)succinic anhydride | C16H26O3 | 266.38 | ||
| 7-Hexadecenal, (Z)- | C16H30O | 238.41 | ||
| 57 | Tetrapentacontane | C54H110 | 759.40 | 0.66 |
| E-10,13,13-Trimethyl-11-tetradecen-1-ol acetate | C19H36O2 | 296.50 | ||
| Heptacosyl trifluoroacetate | C29H55F3O2 | 492.70 | ||
| 59 | Tetracontane | C40H82 | 563.10 | 0.39 |
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| Heptacosyl trifluoroacetate | C29H55F3O2 | 492.70 | ||
| 60 | 17.alpha.(H),21.beta.(H)-Hopane | C30H52 | 412.70 | 0.93 |
| E-10,13,13-Trimethyl-11-tetradecen-1-ol acetate | C19H36O2 | 296.50 | ||
| Undec-10-ynoic acid, tetradecyl ester | C25H46O2 | 378.60 | ||
| 61 | 17.alpha.(H),21.beta.(H)-Homohopane | C31H54 | 426.80 | 0.40 |
| E-10,13,13-Trimethyl-11-tetradecen-1-ol acetate | C19H36O2 | 296.50 | ||
| cis-1-Chloro-9-octadecene | C18H35Cl | 286.90 | ||
| Aromatic hydrocarbons | ||||
| Tricyclic aromatic hydrocarbons | ||||
| 2 | 9H-Fluorene, 9-methylene- | C14H10 | 178.23 | 0.89 |
| 9,10-Ethanoanthracene-11,12-dicarbonitrile, 9,10-dihydro-, cis- | C18H12N2 | 256.30 | ||
| Phenanthrene | C14H10 | 178.23 | ||
| 5 | Phenanthrene, 2-methyl- | C15H12 | 192.25 | 2.70 |
| Phenanthrene, 2-methyl- | ||||
| Phenanthrene, 4-methyl- | ||||
| 7 | Phenanthrene, 9-ethyl- | C16H14 | 206.28 | 0.42 |
| Anthracene, 9-dodecyltetradecahydro- | C26H48 | 360.70 | ||
| Phenanthrene, 9-dodecyltetradecahydro- | C26H48 | 360.70 | ||
| 8 | Phenanthrene, 2,5-dimethyl- | C16H14 | 206.28 | 2.46 |
| Phenanthrene, 2,5-dimethyl- | ||||
| Phenanthrene, 4,5-dimethyl- | ||||
| 11 | Anthracene, 9-(1-methylethyl)- | C17H16 | 220.31 | 2.16 |
| Phenanthrene, 2,3,5-trimethyl- | ||||
| Anthracene, 9-(1-methylethyl)- | ||||
| 12 | [14]Annulene, 1,6:7,12-bis(methano)-, anti- | C16H14 | 206.28 | 0.29 |
| 18.alpha.-Olean-3.beta.-ol, acetate | C32H54O2 | 470.80 | ||
| 5-Fluoro-2-trifluoromethylbenzoic acid, eicosyl ester | C28H44F4O2 | 488.60 | ||
| 15 | 2,3,5-trimethyl-Phenanthrene, | C17H16 | 220.31 | 0.63 |
| 2,3,5-trimethyl-Phenanthrene, | ||||
| 2,3,5-trimethyl-Phenanthrene, | ||||
| 17 | Retene | C18H18 | 234.30 | 2.23 |
| Retene | ||||
| Retene | ||||
| 21 | 1-benzyl-3-methylnaphthalene | C18H16 | 232.30 | 0.50 |
| Fumaric acid, dodecyl 2,3,4,6-tetrachlorophenyl ester | C22H28Cl4O4 | 498.30 | ||
| 5-Methyl-Z-5-docosene | C23H46 | 322.60 | ||
| Tetracyclic aromatic hydrocarbons | ||||
| 10 | Pyrene | C16H10 | 202.25 | 9.43 |
| Fluoranthene | ||||
| Pyrene | ||||
| 16 | 11H-Benzo[b]fluorene | C17H12 | 216.28 | 5.50 |
| 11H-Benzo[b]fluorene | ||||
| Pyrene, 2-methyl- | ||||
| 18 | Fluoranthene, 2-methyl- | C17H12 | 216.28 | 2.08 |
| Pyrene, 2-methyl- | ||||
| Fluoranthene, 2-methyl- | ||||
| 19 | Pyrene, 1-methyl- | C17H12 | 216.28 | 4.67 |
| Pyrene, 1-methyl- | ||||
| Fluoranthene, 2-methyl- | ||||
| 23 | Pyrene, 1,3-dimethyl- | C18H14 | 230.30 | 3.82 |
| Pyrene, 1,3-dimethyl- | C18H14 | 230.30 | ||
| Succinic acid, 2,2,3,3-tetrafluoropropyl heptadecyl ester | C24H42F4O4 | 470.60 | ||
| 27 | Benz[a]anthracene | C18H12 | 228.30 | 1.17 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Tetradecanoic acid, hexadecyl ester | C30H60O2 | 452.80 | ||
| 28 | Triphenylene | C18H12 | 228.30 | 6.60 |
| 2-Aminopent-4-enoic acid, N-(2-ethylhexyloxycarbonyl)-, undecyl ester | C25H47NO4 | 425.60 | ||
| 2-Aminopent-4-enoic acid, N-octyloxycarbonyl-, undecyl ester | C25H47NO4 | 425.60 | ||
| 30 | 1,3,9-Trimethylpyrene | C19H16 | 244.33 | 0.95 |
| 5-Methyl-Z-5-docosene | C23H46 | 322.60 | ||
| Tetradecanoic acid, hexadecyl ester | C30H60O2 | 452.80 | ||
| 37 | Bicyclo[4.1.0]hepta-1,3,5-triene, 2,5-diphenyl- | C19H14 | 242.30 | 2.15 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Carbonic acid, decyl heptadecyl ester | C28H56O3 | 440.70 | ||
| 38 | Benz[a]anthracene, 8-methyl- | C19H14 | 242.30 | 1.97 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Benz[a]anthracene, 8-methyl- | C19H14 | 242.30 | ||
| Pentacyclic aromatic hydrocarbons | ||||
| 43 | Chrysene, 5-ethyl- | C20H16 | 256.30 | 0.57 |
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| 47 | Perylene | C20H12 | 252.30 | 3.60 |
| E-10,13,13-Trimethyl-11-tetradecen-1-ol acetate | C19H36O2 | 296.50 | ||
| Pentafluoropropionic acid, octadecyl ester | C21H37F5O2 | 416.51 | ||
| 50 | Benzo[a]pyrene | C20H12 | 252.30 | 1.77 |
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| Alcohols | ||||
| 42 | 1-Decanol, 2-hexyl- | C16H34O | 242.30 | 1.01 |
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| Heptacosyl trifluoroacetate | C29H55F3O2 | 492.70 | ||
| 58 | 4a,7,7,10a-Tetramethyldodecahydrobenzo[f]chromen-3-ol | C17H30O2 | 266.40 | 0.14 |
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| 2-Dodecen-1-yl(-)succinic anhydride | C16H26O3 | 266.38 | ||
| Esters | ||||
| 31 | Succinic acid, 4-methylhept-3-yl nonyl ester | C21H40O4 | 356.50 | 0.28 |
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 36 | Palmitic acid vinyl ester | C18H34O2 | 282.50 | 0.27 |
| 17-Pentatriacontene | C35H70 | 490.90 | ||
| E-10,13,13-Trimethyl-11-tetradecen-1-ol acetate | C19H36O2 | 296.50 | ||
| 45 | Palmitic acid 2-nonanol ester | C27H54O2 | 410.70 | 0.25 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| 46 | Carbonic acid, decyl heptadecyl ester | C28H56O3 | 440.70 | 0.69 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| 54 | Phytyl, 2-methylbutanoate | C25H48O2 | 380.60 | 0.39 |
| E-10,13,13-Trimethyl-11-tetradecen-1-ol acetate | C19H36O2 | 296.50 | ||
| Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | ||
| Ketones | ||||
| 26 | 2-(2-Indanylidene)-1-indanone | C18H14O | 246.30 | 1.46 |
| 2-(2-Indanylidene)-1-indanone | C18H14O | 246.30 | ||
| Undec-10-ynoic acid, tetradecyl ester | C25H46O2 | 378.60 | ||
| Phenols | ||||
| 13 | 1-Hydroxypyrene | C16H10O | 218.25 | 1.87 |
| 1-Hydroxypyrene | C16H10O | 218.25 | ||
| Olean-12-ene | C30H50 | 410.70 | ||
| Ethers | ||||
| 33 | Nonyl tetracosyl ether | C33H68O | 480.90 | 0.48 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 5-Methyl-Z-5-docosene | C23H46 | 322.60 | ||
| Nitrogen compounds | ||||
| 20 | Naphthalen-2-ol, 1-(4-morpholyl)(phenyl)methyl- | C21H21NO2 | 319.40 | 1.52 |
| 2-[1-(2-[1,3]Dithian-2-yl-ethyl)-pent-4-enyloxy]-tetrahydropyran | C16H28O2S2 | 316.50 | ||
| Fumaric acid, dodecyl 2,3,4,6-tetrachlorophenyl ester | C22H28Cl4O4 | 498.30 | ||
| 25 | Ellipticine | C17H14N2 | 246.31 | 1.70 |
| Fumaric acid, heptadecyl octyl ester | C29H5O2 | 466.70 | ||
| Tetradecanoic acid, hexadecyl ester | C30H60O2 | 452.80 | ||
| Sulfurous compounds | ||||
| 1 | Sulfurous acid, 2-ethylhexyl isohexyl ester | C17H30O3S | 314.50 | 0.27 |
| Tridecane, 1-iodo- | C13H27I | 310.26 | ||
| Sulfurous acid, butyl nonyl ester | C13H28O3S | 264.43 | ||
| Other compounds | ||||
| 9 | Silane, trichlorooctadecyl- | C18H37Cl3Si | 387.90 | 0.90 |
| Di-n-decylsulfone | C20H42O2S | 346.60 | ||
| Diglycolic acid, 2-bromo-4-fluorophenyl decyl ester | C20H28BrFO5 | 447.30 | ||
| 44 | Hexacosane, 1-iodo- | C26H53I | 492.60 | 2.08 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 1-Decanol, 2-octyl- | C18H38O | 270.50 |
| No. | Compound Names | Proposed Formula | Molecular Weight | Relative Content, % |
|---|---|---|---|---|
| Aliphatic hydrocarbons | ||||
| 3 | Cyclohexane, tricosyl- | C29H58 | 406.80 | 2.73 |
| Dodecane, 5-cyclohexyl- | C18H36 | 252.50 | ||
| Undecane, 5-cyclohexyl- | C17H34 | 238.50 | ||
| 5 | 1-(1,5-dimethylhexyl) -4-(4-methylpentyl)-Cyclohexane | C20H40 | 280.50 | 2.23 |
| Octadecanoic acid, 2-oxo-, methyl ester | C19H36O3 | 312.50 | ||
| Heneicosane, 11-cyclopentyl- | C26H52 | 364.70 | ||
| 6 | pentacosyl-Cyclohexane | C31H62 | 434.80 | 3.44 |
| Dodecane, 5-cyclohexyl- | C18H36 | 252.50 | ||
| Undecane, 5-cyclohexyl- | C17H34 | 238.50 | ||
| 8 | 2-methyl-Octacosane | C29H60 | 408.80 | 7.13 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Hexacosyl trifluoroacetate | C28H53F3O2 | 478.70 | ||
| 9 | 2,6,10,15-tetramethyl-Heptadecane | C21H44 | 296.60 | 6.11 |
| Hexacosyl trifluoroacetate | C28H53F3O2 | 478.70 | ||
| Sulfurous acid, octadecyl 2-propyl ester | C21H44O3S | 376.60 | ||
| 13 | 2-Methyltetracosane | C25H52 | 352.70 | 8.38 |
| Hexacosyl trifluoroacetate | C28H53F3O2 | 478.70 | ||
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 16 | 17.alpha.(H),21.beta.(H)-Homohopane | C31H54 | 426.80 | 7.09 |
| 17.alpha.(H),21.beta.(H)-Hopane | C30H52 | 412.70 | ||
| 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | ||
| Carboxylic acids | ||||
| 12 | Tetracosyl benzoate | C31H54O2 | 458.80 | 1.96 |
| Hexacosyl trifluoroacetate | C28H53F3O2 | 478.70 | ||
| Dotriacontanal $$ n-Dotriacontanal | C32H66 | 450.90 | ||
| Alcohols | ||||
| 15 | 5-(7a-Isopropenyl-4,5-dimethyl-octahydroinden-4-yl)-3-methyl-pent-2-en-1-ol | C20H34O | 290.50 | 3.55 |
| 2,4a,8,8-Tetramethyldecahydrocyclopropa[d]naphthalene | C15H26 | 206.37 | ||
| 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | ||
| Aldehydes | ||||
| 1 | 2-Butyn-1-al diethyl acetal | C8H14O2 | 142.20 | 1.36 |
| 2-Propenamide | C3H5NO | 71.08 | ||
| Stearic acid hydrazide | C18H38N2O | 298.50 | ||
| Ethers | ||||
| 2 | Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | 6.40 |
| Octadecanoic acid, 2-oxo-, methyl ester | C19H36O3 | 312.50 | ||
| 10-Methylnonadecane | C20H42 | 282.50 | ||
| 10 | Fumaric acid, trans-hex-3-enyl tridecyl ester | C23H40O4 | 380.60 | 7.65 |
| Tricosyl pentafluoropropionate | C26H47F5O2 | 486.60 | ||
| Docosyl pentafluoropropionate | C25H45F5O2 | 472.60 | ||
| 11 | Fumaric acid, octadecyl trans-hex-3-enyl ester | C28H50O4 | 450.70 | 4.45 |
| Hexacosyl trifluoroacetate | C28H53F3O2 | 478.70 | ||
| Docosyl pentafluoropropionate | C25H45F5O2 | 472.60 | ||
| Sulfurous compounds | ||||
| 7 | Sulfurous acid, octadecyl 2-propyl ester | C21H44O3S | 376.60 | 11.26 |
| Sulfurous acid, octadecyl 2-propyl ester | C21H44O3S | 376.60 | ||
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| Other compounds | ||||
| 4 | 2-Bromo dodecane | C12H25Br | 249.23 | 5.68 |
| 1-Dodecanol, 2-octyl- | C20H42O | 298.50 | ||
| 1-Decanol, 2-octyl- | C18H38O | 270.50 | ||
| 14 | Phosphonofluoridic acid, ethyl-, decyl ester | C12H26FO2P | 252.31 | 1.56 |
| Heptyl ethylphosphonofluoridate | C9H20FO2P | 210.23 | ||
| 1,1-Dimethylpropyl ethylphosphonofluoridate | C7H16FO2P | 182.17 |
| No. | Compound Names | Proposed Formula | Molecular Weight | Relative Content, % |
|---|---|---|---|---|
| Aliphatic hydrocarbons | ||||
| 1 | Octacosane,2-methyl- | C29H60 | 408.80 | 1.19 |
| Heneicosane | C21H44 | 296.60 | ||
| Pentadecane, 8-hexyl- | C21H44 | 296.60 | ||
| 2 | Heneicosane | C21H44 | 296.60 | 1.08 |
| Tetracosane, 1-iodo- | C24H49I | 464.50 | ||
| Pentacosane | C25H52 | 352.70 | ||
| 3 | Eicosane | C20H42 | 282.50 | 1.97 |
| 2-Methylhexacosane | C27H56 | 380.70 | ||
| Octacosane, 2-methyl- | C29H60 | 408.80 | ||
| 5 | 3,8-dimethyl-Decane | C12H26 | 170.33 | 0.43 |
| Decane, 2,3,5-trimethyl- | C13H28 | 184.36 | ||
| Hexadecane, 1-iodo- | C16H33I | 352.34 | ||
| 6 | Tridecane, 5-cyclohexyl- | C19H38 | 266.50 | 0.44 |
| Cyclohexane, (1-butylhexadecyl)- | C26H52 | 364.70 | ||
| Tridecane, 4-cyclohexyl | C19H38 | 266.50 | ||
| 7 | Pentacosane | C25H52 | 352.70 | 1.19 |
| Octacosane,2-methyl- | C29H60 | 408.80 | ||
| Octacosane,2-methyl- | C29H60 | 408.80 | ||
| 9 | Cyclohexane, 1-(cyclohexylmethyl)-2-methyl-, cis- | C14H26 | 194.36 | 0.45 |
| Cyclohexane, 1-bromo-2-methyl- | C7H13Br | 177.08 | ||
| Cyclohexane, 1-isopropyl-1-methyl- | C10H20 | 140.27 | ||
| 10 | Eicosane | C20H42 | 282.50 | 1.46 |
| 11-Methyltricosane | C24H50 | 338.70 | ||
| Carbonic acid, octadecyl vinyl ester | C21H40O3 | 340.50 | ||
| 11 | 2-Methylhexacosane | C27H56 | 380.70 | 2.04 |
| Dotriacontane | C32H66 | 450.90 | ||
| Heneicosane | C21H44 | 296.60 | ||
| 13 | Cyclohexane, octadecyl- | C24H48 | 336.60 | 0.76 |
| Cyclohexane, 1,1′-(1,3-propanediyl)bis- | C15H28 | 208.38 | ||
| n-Heptadecylcyclohexane | C23H46 | 322.60 | ||
| 15 | 11-Methyltricosane | C24H50 | 338.70 | 0.29 |
| 9-Methylheneicosane | C22H46 | 310.60 | ||
| 9-methylheptadecane | C18H38 | 254.50 | ||
| 16 | Heneicosane | C21H44 | 296.60 | 3.89 |
| Pentacosane | C25H52 | 352.70 | ||
| Tetracontane | C40H82 | 563.10 | ||
| 17 | 1,1-dicyclohexyl-Heptane | C19H36 | 264.50 | 0.19 |
| Cyclohexane, 1-(cyclohexylmethyl)-2-methyl-, cis- | C14H26 | 194.36 | ||
| Cyclohexane, 1-(cyclohexylmethyl)-4-methyl- | C14H26 | 194.36 | ||
| 19 | Tetrapentacontane | C54H110 | 759.40 | 0.78 |
| Eicosane | C20H42 | 282.50 | ||
| Hexadecane, 2,6,10,14-tetramethyl- | C20H42 | 282.50 | ||
| 20 | Pentatriacontane | C35H72 | 492.90 | 10.47 |
| 2-Methylhexacosane | C27H56 | 380.70 | ||
| Octacosane, 2-methyl- | C29H60 | 408.80 | ||
| 21 | 7,7-Diethylheptadecane | C21H44 | 296.60 | 2.42 |
| Pentadecane, 6-methyl- | C16H34 | 226.44 | ||
| Hentriacontane | C31H64 | 436.80 | ||
| 23 | Octacosane, 2-methyl- | C29H60 | 408.80 | 0.86 |
| Heptadecane, 2,6,10,15-tetramethyl- | C21H44 | 296.60 | ||
| Hexadecane, 2,6,10,14-tetramethyl- | C20H42 | 282.50 | ||
| 25 | Cyclohexane, octadecyl- | C24H48 | 336.60 | 0.85 |
| Cyclohexane, nonadecyl- | C25H50 | 350.70 | ||
| 2-Cyclohexylnonadecane | C25H50 | 350.70 | ||
| 27 | 11-Methyltricosane | C24H50 | 338.70 | 1.14 |
| Carbonic acid, octadecyl vinyl ester | C21H40O3 | 340.50 | ||
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| 30 | Tetracontane | C40H82 | 563.10 | 16.35 |
| Dotriacontane | C32H66 | 450.90 | ||
| Pentacosane | C25H52 | 352.70 | ||
| 32 | Pentadecane, 2,6,10,14-tetramethyl- | C19H40 | 268.50 | 1.90 |
| 11-Methylpentacosane | C26H54 | 366.70 | ||
| 11-Methyltricosane | C24H50 | 338.70 | ||
| 33 | 3,3-Diethylpentadecane | C19H40 | 268.50 | 0.87 |
| 3,3,13,13-Tetraethylpentadecane | C23H48 | 324.60 | ||
| Hentriacontane | C31H64 | 436.80 | ||
| 34 | Dotriacontane | C32H66 | 450.90 | 4.52 |
| Tetracontane | C40H82 | 563.10 | ||
| Pentacosane | C25H52 | 352.70 | ||
| 35 | Pentacosane | C25H52 | 352.70 | 1.22 |
| Pentacosane | C25H52 | 352.70 | ||
| Dotriacontane | C32H66 | 450.90 | ||
| 37 | 2-Methylheptacosane | C28H58 | 394.80 | 4.41 |
| Dotriacontane | C32H66 | 450.90 | ||
| Tetrapentacontane | C54H110 | 759.40 | ||
| 40 | Octacosane, 2-methyl- | C29H60 | 408.80 | 1.71 |
| Pentatriacontane | C35H72 | 492.90 | ||
| Carbonic acid, octadecyl vinyl ester | C21H40O3 | 340.50 | ||
| 41 | 11-Methyltricosane | C24H50 | 338.70 | 5.33 |
| Pentatriacontane | C35H72 | 492.90 | ||
| Carbonic acid, octadecyl vinyl ester | C21H40O3 | 340.50 | ||
| 42 | 2-Methylhexacosane | C27H56 | 380.70 | 4.16 |
| Pentatriacontane | C35H72 | 492.90 | ||
| Hexacosane, 1-iodo- | C26H53I | 492.60 | ||
| 44 | 17.alpha.(H),21.beta.(H)-Hopane | C30H52 | 412.70 | 0.40 |
| 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | ||
| A’-Neogammacer-22(29)-ene | C30H50 | 410.70 | ||
| 45 | Tetrapentacontane | C54H110 | 759.40 | 2.91 |
| Eicosane | C20H42 | 282.50 | ||
| Dotriacontane | C32H66 | 450.90 | ||
| 46 | 17.alpha.(H),21.beta.(H)-Homohopane | C30H52 | 412.70 | 0.64 |
| 17.alpha.(H),21.beta.(H)-Homohopane | C30H52 | 412.70 | ||
| 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | ||
| 49 | Trispiro[4.2.4.2.4.2.]heneicosane | C21H36 | 288.50 | 0.44 |
| 4a,7,7,10a-Tetramethyldodecahydrobenzo[f]chromen-3-ol | C17H30O2 | 266.40 | ||
| 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | ||
| 53 | 1-(2,2,3,5,6-Pentamethylcyclohex-4-enyl)- 9-(3,3,4-trimethylcyclohex-1-enyl)- 3,6-dimethyl-6-ethenyl-dec-4-ene | C34H58 | 466.80 | 0.12 |
| 1,1,6-trimethyl-3-methylene-2-(3,6,9,13-tetramethyl-6-ethenye-10,14-dimethylene-pentadec-4-enyl)cyclohexane | C33H56 | 452.80 | ||
| Cholestane, (5.alpha.,14.beta.)- | C27H48 | 372.70 | ||
| 54 | 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | 1.72 |
| 17.alpha.(H),21.beta.(H)-Homohopane | C31H54 | 426.80 | ||
| 17.alpha.(H),21.beta.(H)-Homohopane | C31H54 | 426.80 | ||
| 56 | 17.alpha.(H),21.beta.(H)-Hopane | C30H52 | 412.70 | 1.95 |
| 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | ||
| 28-Nor-17.alpha.(H)-hopane | C29H50 | 398.70 | ||
| 58 | 17.alpha.(H),21.beta.(H)-Homohopane | C31H54 | 426.80 | 1.23 |
| 17.alpha.(H),21.beta.(H)-Hopane | C30H52 | 412.70 | ||
| 17.alpha.(H),21.beta.(H)-Hopane | C30H52 | 412.70 | ||
| Alcohols | ||||
| 8 | 1-Decanol, 2-hexyl- | C16H34O | 242.44 | 2.02 |
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| 2-Methylhexacosane | C27H56 | 380.70 | ||
| 22 | Octacosanol | C28H58O | 410.80 | 0.43 |
| Tetracosyl trifluoroacetate | C26H49F3O2 | 450.70 | ||
| Tricosyl trifluoroacetate | C25H47F3O2 | 436.60 | ||
| 28 | 1-Dodecanol, 2-octyl- | C20H42O | 298.50 | 0.43 |
| Nonyl tetracosyl ether | C33H68O | 480.90 | ||
| Carbonic acid, octadecyl vinyl ester | C21H40O3 | 340.50 | ||
| 38 | 1-Decanol, 2-hexyl- | C16H34O | 242.44 | 2.18 |
| Docosyl octyl ether | C30H62O | 438.80 | ||
| Octyl tetracosyl ether | C32H66O | 466.90 | ||
| Ethers | ||||
| 12 | Docosyl octyl ether | C30H62O | 438.80 | 0.40 |
| 2-Methylhexacosane | C27H56 | 380.70 | ||
| 5-Methylnonacosane | C30H62 | 422.80 | ||
| 26 | Nonyl tetracosyl ether | C33H68O | 480.90 | 0.19 |
| Dotriacontane | C32H66 | 450.90 | ||
| Eicosane | C20H42 | 282.50 | ||
| 50 | Isophthalic acid, 3,7-dimethyloct-6-enyl tridecyl ester | C31H50O4 | 486.70 | 0.26 |
| Citronellyl palmitoleate | C26H48O2 | 392.70 | ||
| Citronellyl oleate | C28H52O2 | 420.70 | ||
| Esters | ||||
| 14 | Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | 1.04 |
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| Pentatriacontane | C35H72 | 492.90 | ||
| 24 | Carbonic acid, octadecyl vinyl ester | C21H40O3 | 340.50 | 0.65 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Docosyl octyl ether | C30H62O | 438.80 | ||
| 36 | Carbonic acid, decyl hexadecyl ester | C27H54O3 | 426.70 | 1.46 |
| Heptacosyl trifluoroacetate | C29H55F3O2 | 492.70 | ||
| Carbonic acid, decyl heptadecyl ester | C28H56O3 | 440.70 | ||
| 39 | Carbonic acid, decyl octadecyl ester | C27H54O3 | 426.70 | 0.79 |
| 2-Methylhexacosane | C27H56 | 380.70 | ||
| 2-Methyltetracosane | C25H52 | 352.70 | ||
| 43 | Carbonic acid, decyl hexadecyl ester | C27H54O3 | 426.70 | 2.39 |
| Dotriacontane | C32H66 | 450.90 | ||
| Hentriacontane | C31H64 | 436.80 | ||
| 51 | Phytyl decanoate | C30H58O2 | 450.80 | 0.12 |
| 1-(2,2,3,5,6-Pentamethylcyclohex-4-enyl)-9-(3,3,4-trimethylcyclohex-1-enyl)-3,6-dimethyl-6-ethenyl-dec-4-ene | C34H58 | 466.80 | ||
| Allopregnane | C21H36 | 288.50 | ||
| 52 | Valtrate | C22H30O8 | 422.50 | 0.84 |
| Squalane $$ Tetracosane, 2,6,10,15,19,23-hexamethyl- | C30H62 | 422.80 | ||
| 11-Methyltricosane | C24H50 | 338.70 | ||
| 55 | Isophthalic acid, 3,7-dimethyloct-6-enyl tridecyl ester | C31H50O4 | 486.70 | 0.26 |
| Heptacosyl trifluoroacetate | C29H55F3O2 | 492.70 | ||
| Hexacosyl trifluoroacetate | C28H53F3O2 | 478.70 | ||
| Ketones | ||||
| 47 | 21-Nor-5.alpha.-cholest-24-en-20-one | C26H42O | 370.60 | 0.14 |
| 2-Methyl-E-7-octadecene | C19H38 | 266.50 | ||
| 5-Methyl-Z-5-docosene | C23H46 | 322.60 | ||
| Nitrogen compounds | ||||
| 4 | Hexadecanedinitrile | C16H28N2 | 248.41 | 0.09 |
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| Carbonic acid, eicosyl vinyl ester | C23H44O3 | 368.60 | ||
| Sulfurous compounds | ||||
| 18 | Sulfurous acid, cyclohexylmethyl heptyl ester | C14H28O3S | 276.44 | 0.37 |
| Sulfurous acid, di(cyclohexylmethyl) ester | C14H26O3S | 274.42 | ||
| Sulfurous acid, cyclohexylmethyl pentadecyl ester | C22H44O3S | 388.60 | ||
| 31 | Sulfurous acid, cyclohexylmethyl pentadecyl ester | C22H44O3S | 388.60 | 1.06 |
| Sulfurous acid, di(cyclohexylmethyl) ester | C14H26O3S | 274.42 | ||
| 2-Pyrazoline, 5-ethyl-1,4-dimethyl- | C7H14N2 | 126.20 | ||
| Other compounds | ||||
| 29 | Tetracosyl pentafluoropropionate | C27H49F5O2 | 500.70 | 0.44 |
| Nonyl tetracosyl ether | C33H68O | 480.90 | ||
| 1-Decanol, 2-hexyl- | C16H34O | 242.44 | ||
| 48 | Triacontane, 1-bromo- | C30H61Br | 501.70 | 0.61 |
| Hexacosane, 1-iodo- | C26H53I | 492.60 | ||
| Dotriacontane | C32H66 | 450.90 | ||
| 57 | 2- Bromopropionic acid, octadecyl ester | C21H41BrO2 | 405.50 | 0.53 |
| Heptadecane, 9-(2-cyclohexylethyl)- | C25H50 | 350.70 | ||
| Cyclohexane, (2-decyldodecyl)- | C28H56 | 392.70 |
| No. | Compound Names | Proposed Formula | Molecular Weight | Relative Content, % |
|---|---|---|---|---|
| Aliphatic hydrocarbons | ||||
| 2 | Cyclododecane | C12H24 | 168.32 | 0.79 |
| 1-Tetradecanol | C14H30O | 214.39 | ||
| Cyclotetradecane | C14H28 | 196.37 | ||
| Aldehydes | ||||
| 1 | Benzaldehyde | C7H6O | 106.12 | 7.60 |
| Benzaldehyde | ||||
| Benzaldehyde | ||||
| Carboxylic acids | ||||
| 3 | n-Hexadecanoic acid | C16H32O2 | 256.42 | 37.56 |
| Butyraldehyde, semi carbazone | C3H7N3O | 101.11 | ||
| n-Hexadecanoic acid | C16H32O2 | 256.42 |
| No. | Compound Names | Proposed Formula | Molecular Weight | Relative Content, % |
|---|---|---|---|---|
| Aldehydes | ||||
| 1 | Benzaldehyde | C7H6O | 106.12 | 7.70 |
| Benzaldehyde | ||||
| Benzaldehyde | ||||
| Carboxylic acids | ||||
| 2 | n-Hexadecanoic acid | C16H32O2 | 256.42 | 37.55 |
| n-Hexadecanoic acid | ||||
| n-Hexadecanoic acid |
| No. | Compound Names | Proposed Formula | Molecular Weight | Relative Content, % |
|---|---|---|---|---|
| Aldehydes | ||||
| 1 | Benzaldehyde | C7H6O | 106.12 | 11.84 |
| Benzaldehyde | ||||
| Benzaldehyde |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhu, Z.; Zhao, J. Subcritical Extraction of Coal Tar Slag and Analysis of Extracts and Raffinates. Appl. Sci. 2025, 15, 2694. https://doi.org/10.3390/app15052694
Wang X, Zhu Z, Zhao J. Subcritical Extraction of Coal Tar Slag and Analysis of Extracts and Raffinates. Applied Sciences. 2025; 15(5):2694. https://doi.org/10.3390/app15052694
Chicago/Turabian StyleWang, Xiaohua, Zhongchao Zhu, and Jianyou Zhao. 2025. "Subcritical Extraction of Coal Tar Slag and Analysis of Extracts and Raffinates" Applied Sciences 15, no. 5: 2694. https://doi.org/10.3390/app15052694
APA StyleWang, X., Zhu, Z., & Zhao, J. (2025). Subcritical Extraction of Coal Tar Slag and Analysis of Extracts and Raffinates. Applied Sciences, 15(5), 2694. https://doi.org/10.3390/app15052694







