Zirconium-Containing Metal–Organic Frameworks (MOFs) as Catalysts for Biomass Conversion
Abstract
1. Introduction
2. Metal–Organic Frameworks (MOFs) and Their Use in Catalysis
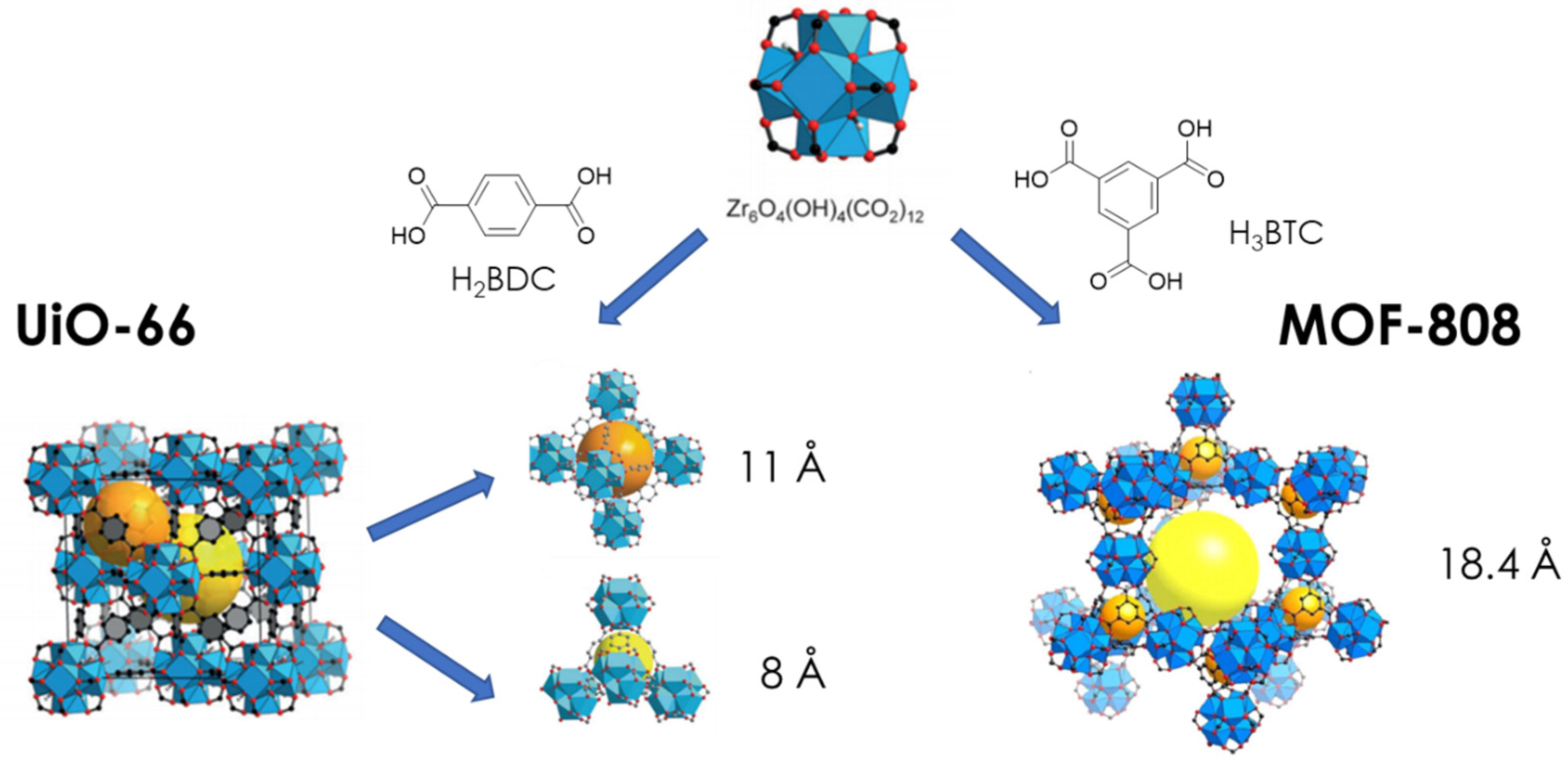
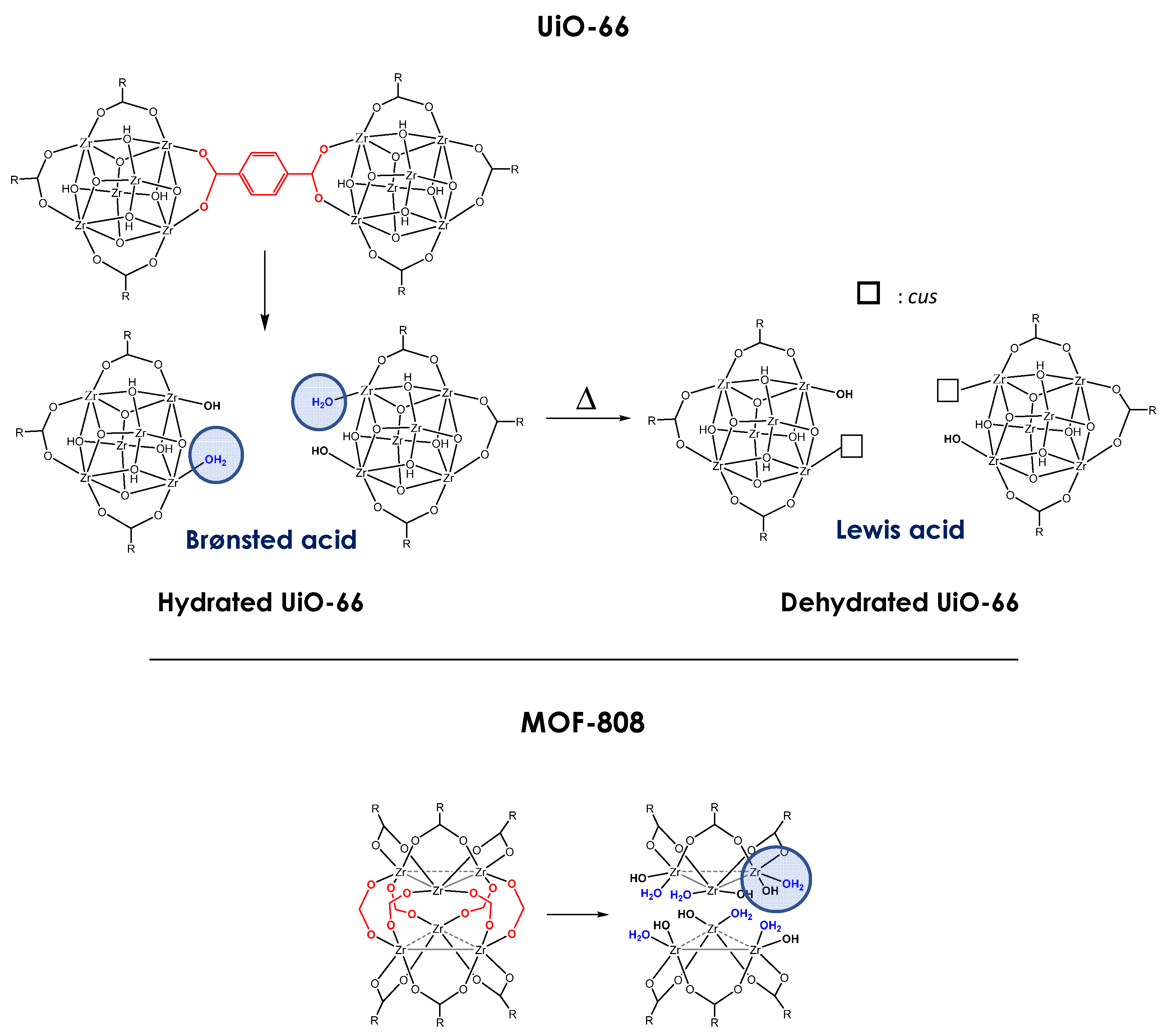
3. Structure and Properties of UiO-66 and MOF-808: Origin of Lewis and Brønsted-Induced Acidity
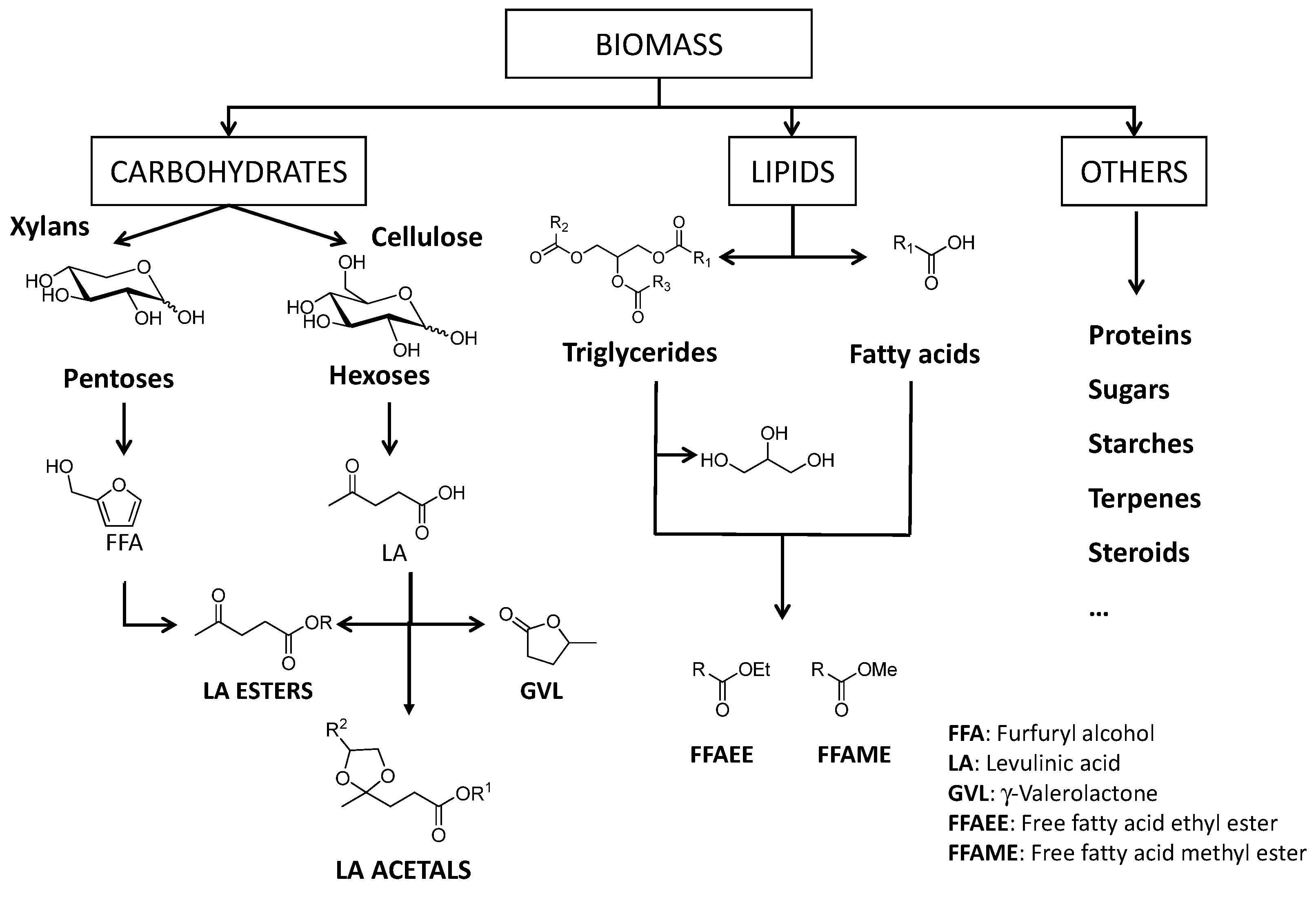
4. Conversion of Biomass into Chemicals over UiO-66 and MOF-808
- -
- Conversion of carbohydrates;
- -
- Conversion of lipids (fatty acids and glycerol);
- -
- Conversion of other biomass-derived compounds.
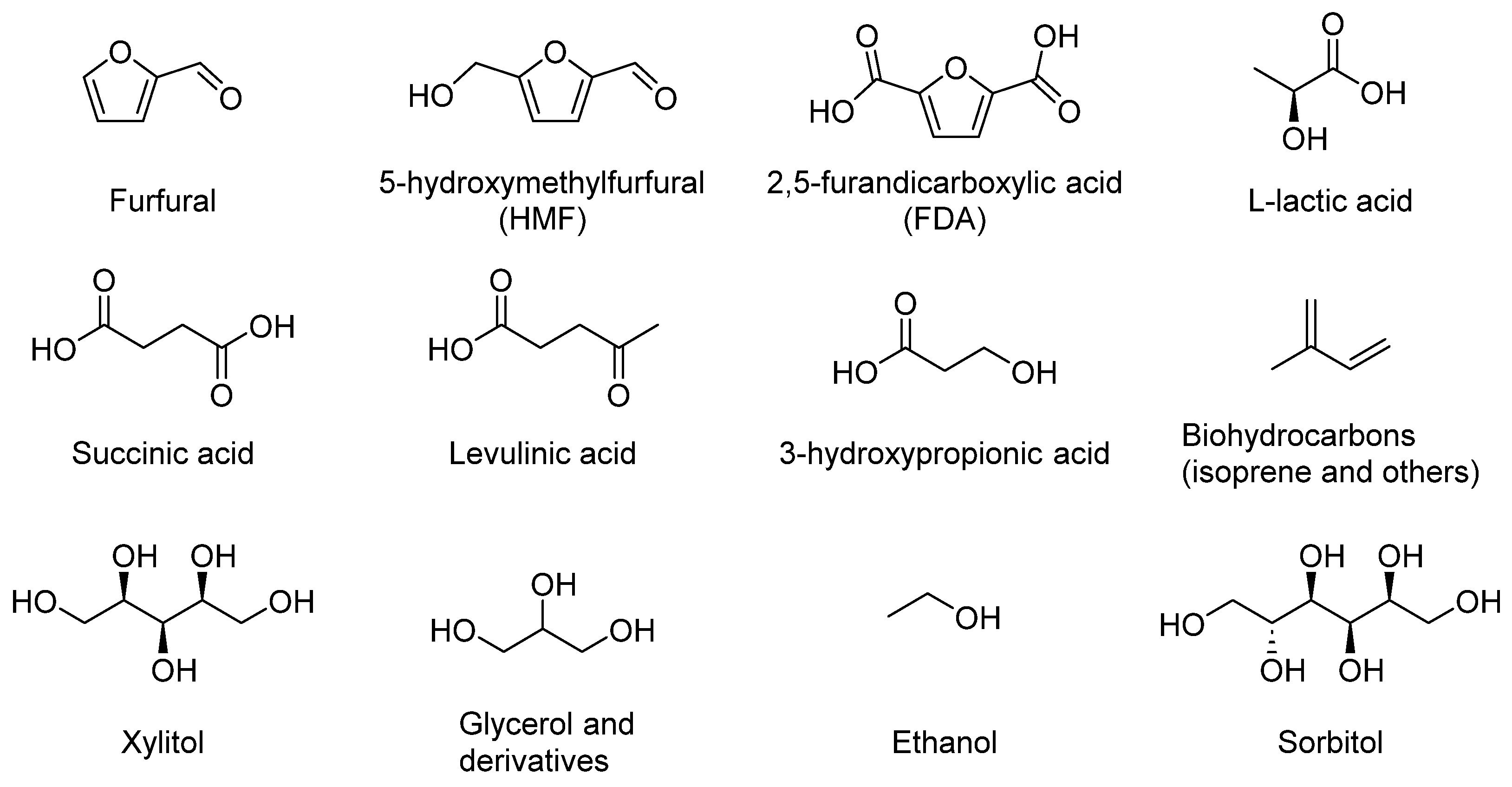
5. Conversion of Carbohydrates
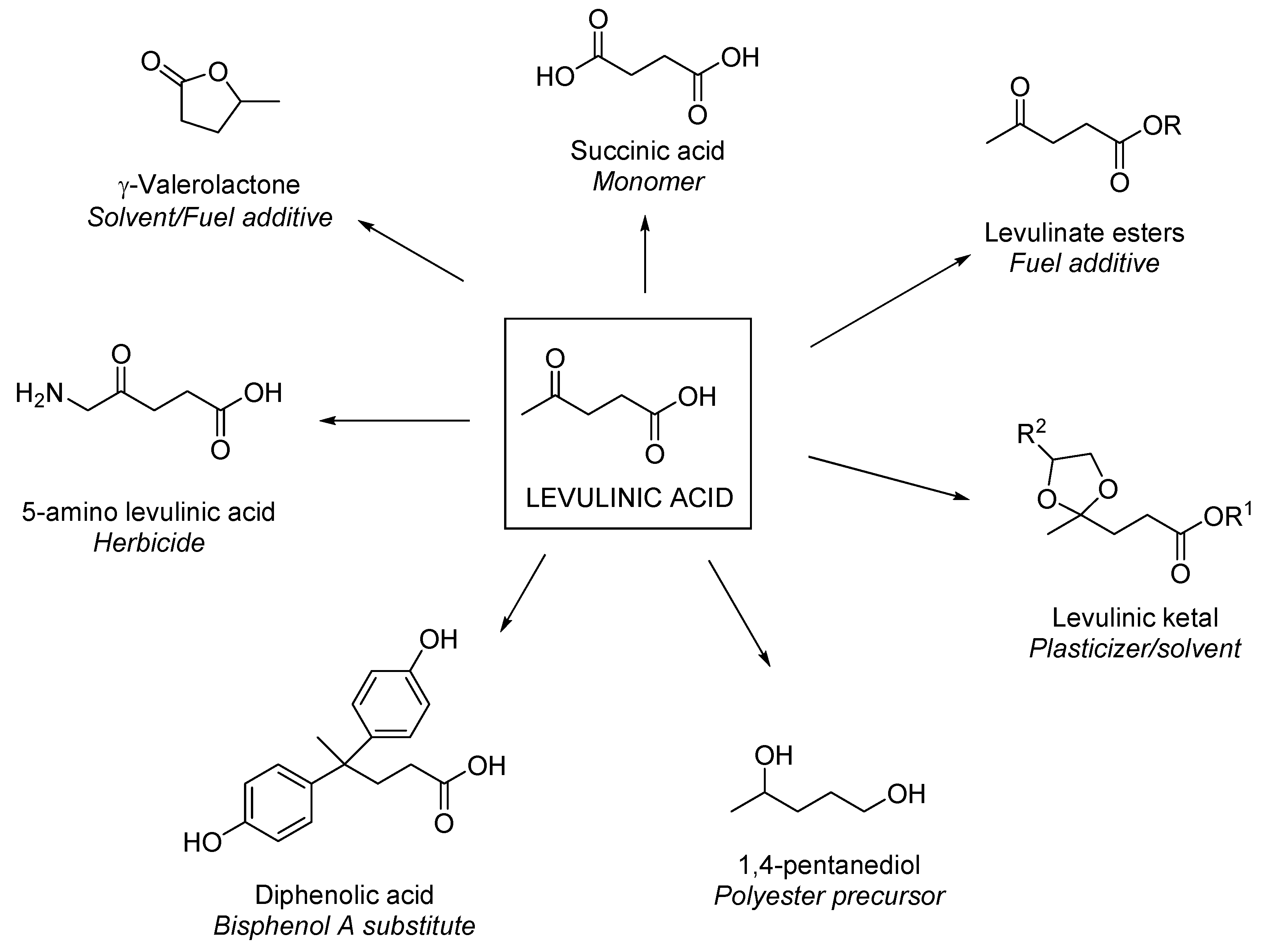
5.1. Levulinic Acid into Chemicals
5.1.1. Levulinic Acid Derivates as Value-Added Chemicals
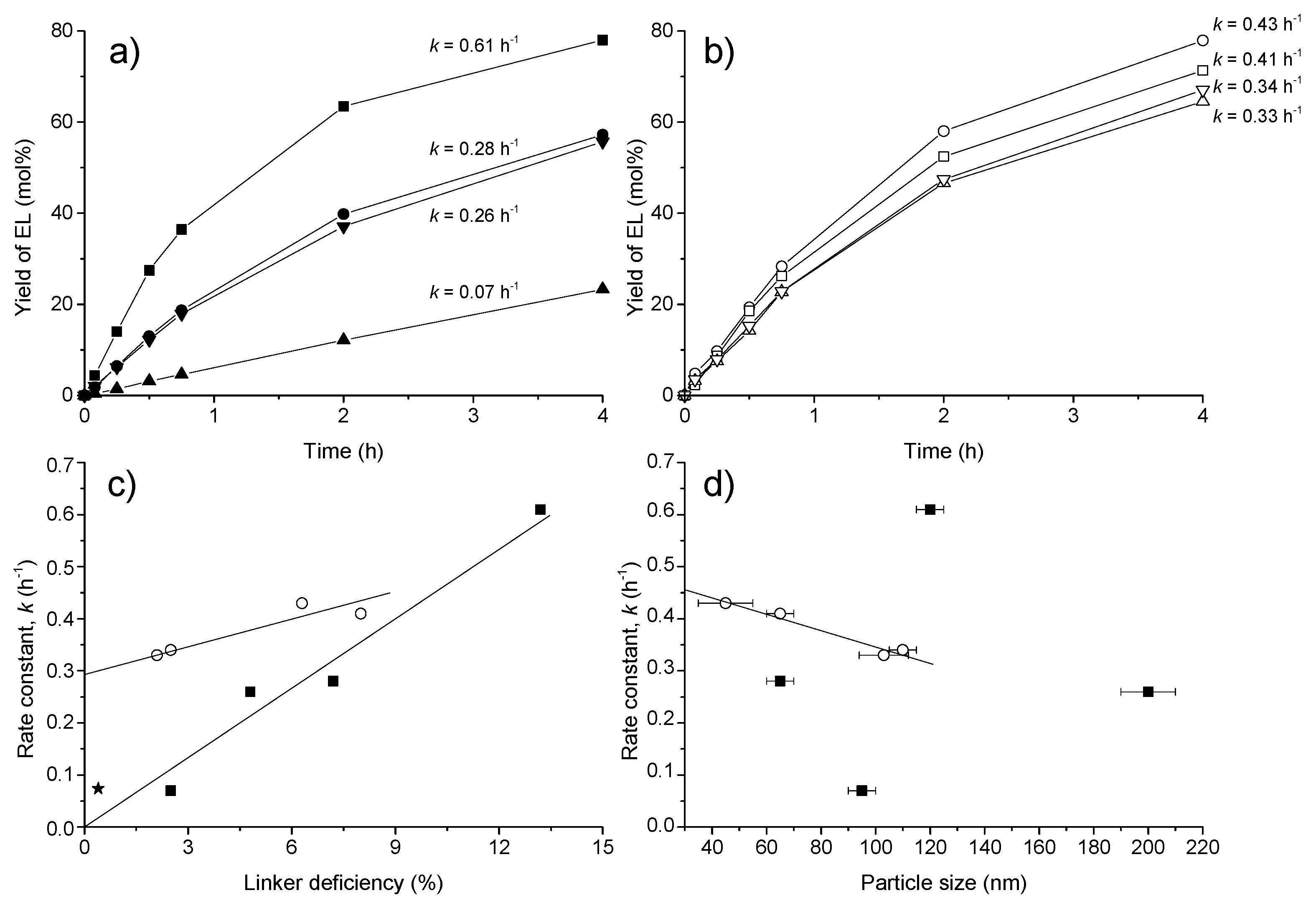
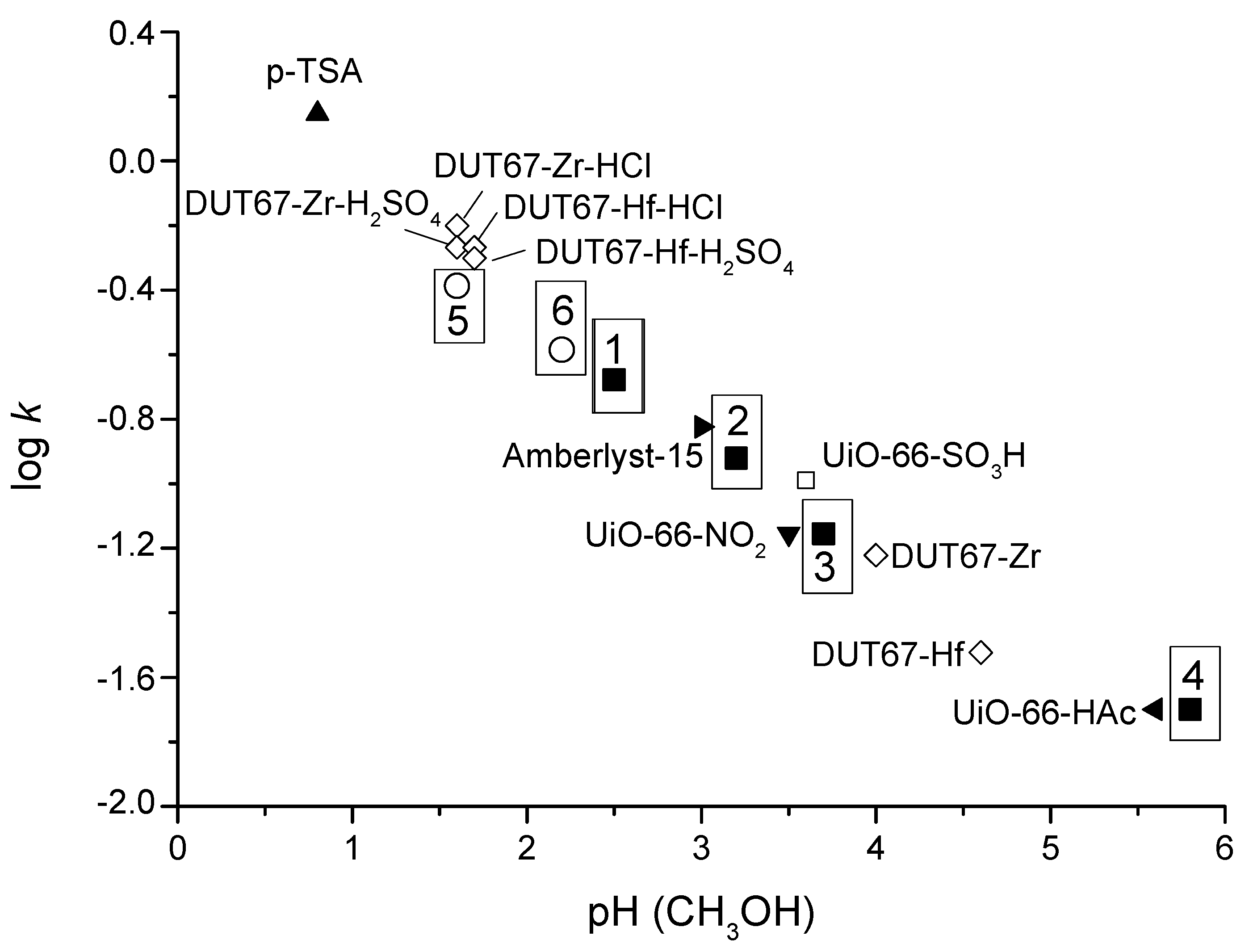
5.1.2. Synthesis of Levulinate Esters
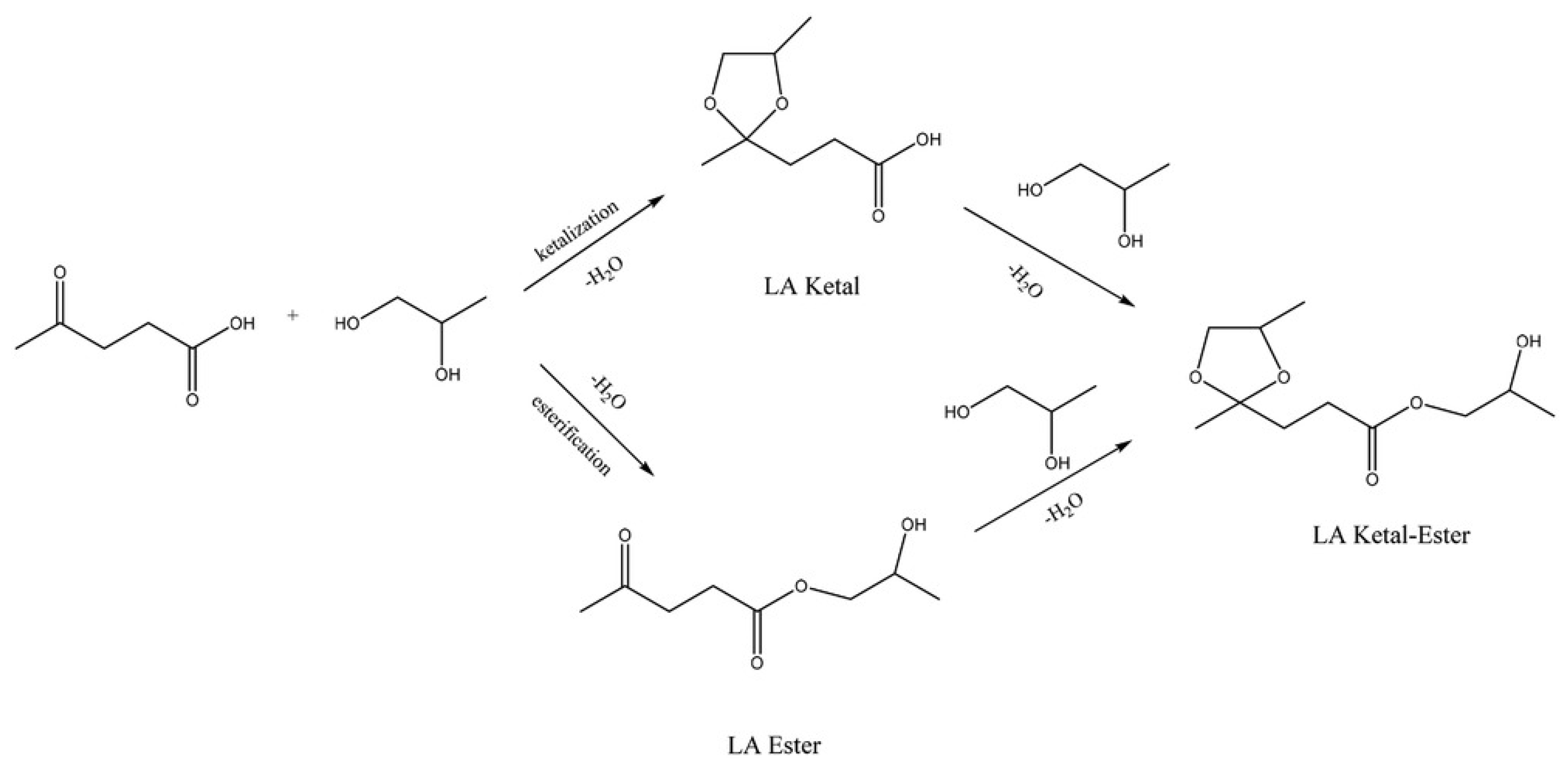
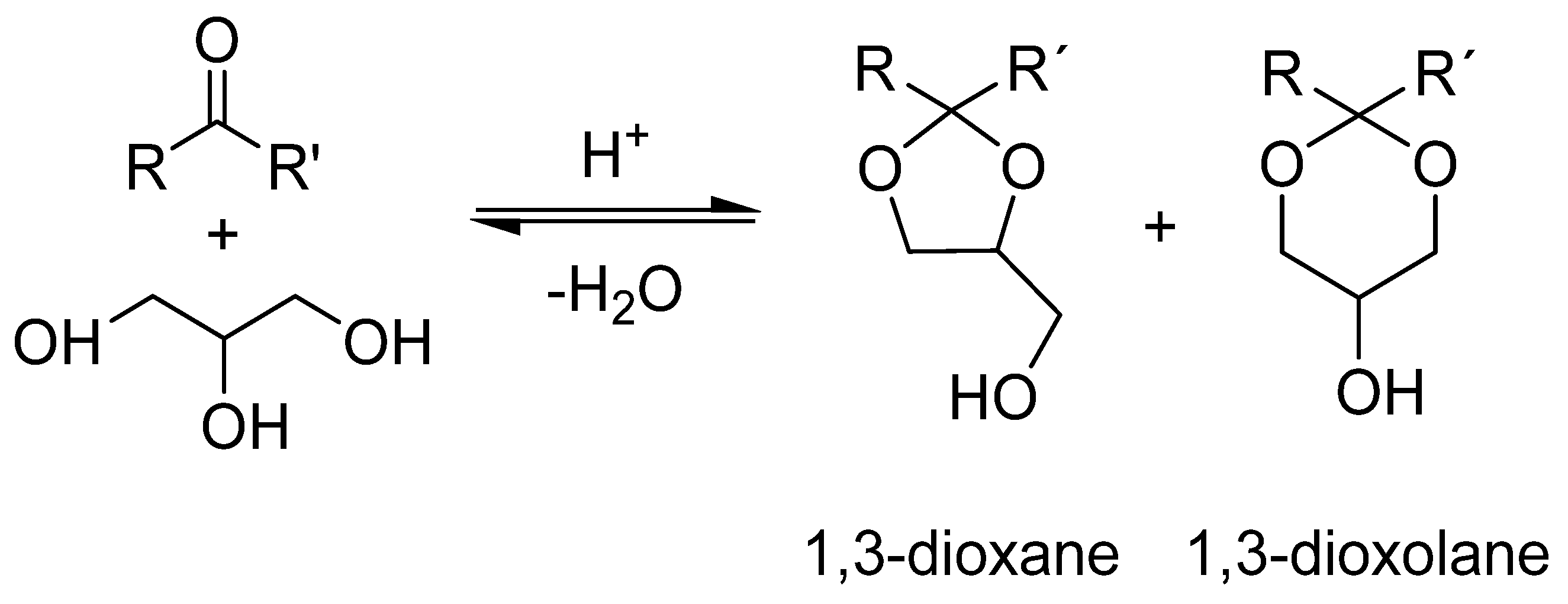
5.1.3. Direct Ketalization of LA
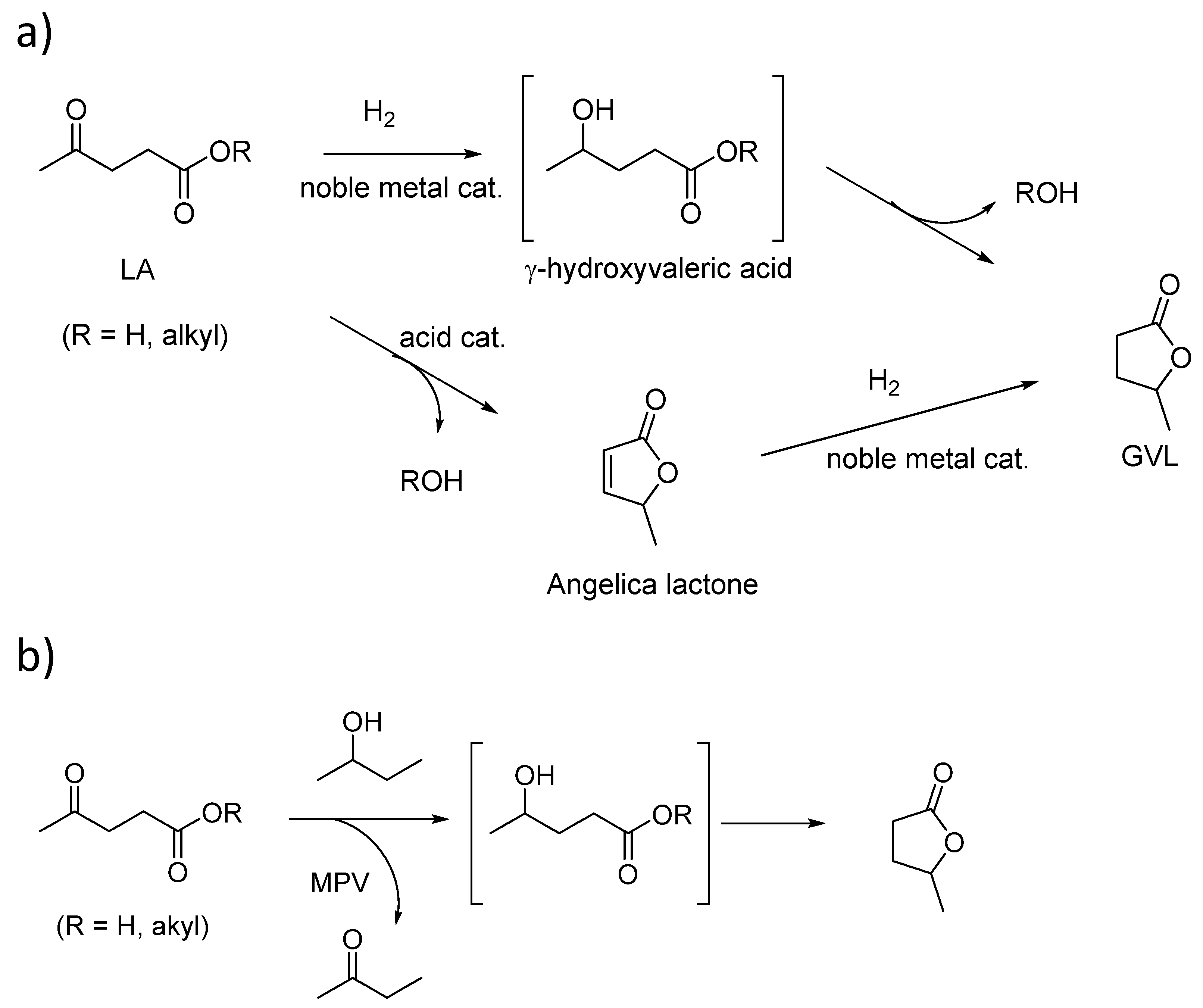
5.1.4. Synthesis of γ-Valerolactone (GVL)
6. Conversion of Lipids
6.1. Esterification of Free Fatty Acids (FFAs)
6.2. Glycerol Valorization: Formation of Glyceryl Acetates
7. Conversion of Other Biomass-Derived Compounds
7.1. Isomerization of Terpenoids: Citronellal to Isopulegol Conversion
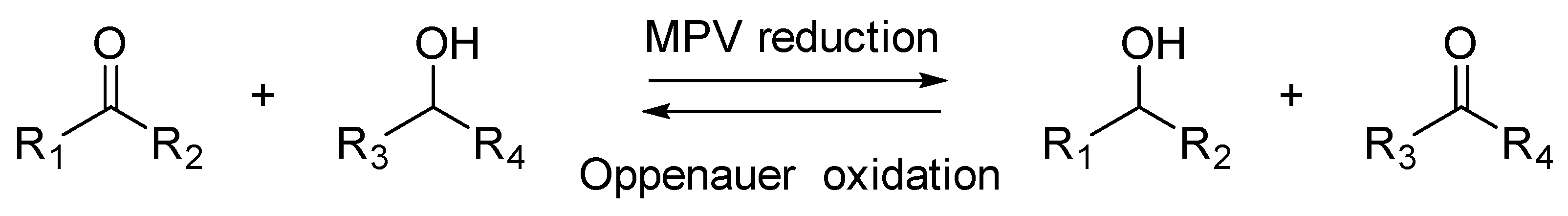
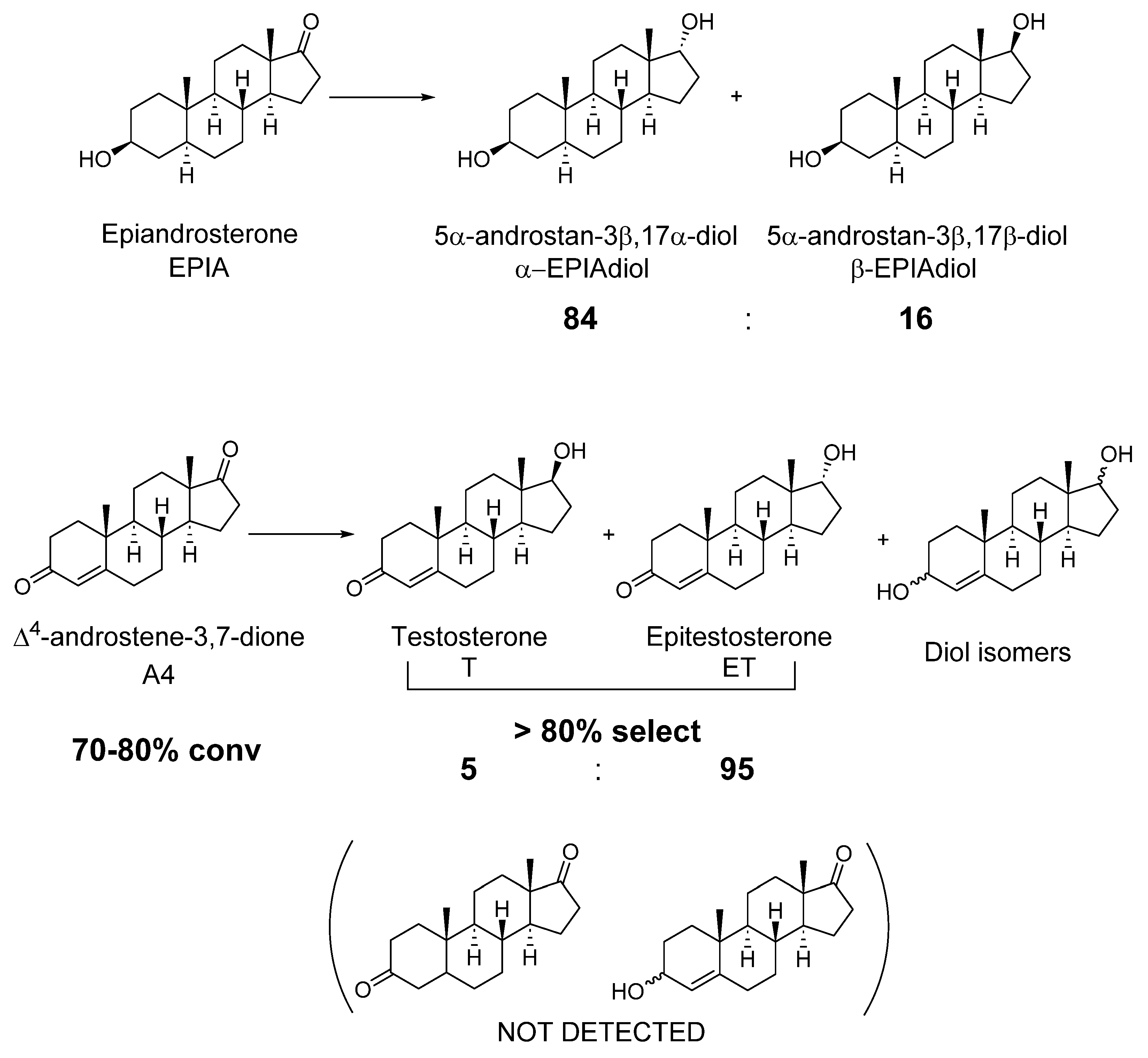
7.2. Reduction of Carbonyl Compounds: Synthesis of Hydroxysteroids
8. Conclusions
Funding
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Corma Canos, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-Organic Frameworks: A New Class of Porous Materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eddaoudi, M.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Advances in the Chemistry of Metal–Organic Frameworks. CrystEngComm 2002, 4, 401–404. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary Building Units, Nets and Bonding in the Chemistry of Metal-Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydin, A.O.; Hupp, J.T. Metal–Organic Framework Materials with Ultrahigh Surface Areas: Is the Sky the Limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef]
- Hönicke, I.M.; Senkovska, I.; Bon, V.; Baburin, I.A.; Bönisch, N.; Raschke, S.; Evans, J.D.; Kaskel, S. Balancing Mechanical Stability and Ultrahigh Porosity in Crystalline Framework Materials. Angew. Chem. Int. Ed. 2018, 57, 13780–13783. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H.; Llabrés i Xamena, F.X. Engineering Metal Organic Frameworks for Catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef]
- Gascon, J.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X. Metal Organic Framework Catalysis: Quo Vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepúlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal–Organic and Covalent Organic Frameworks as Single-Site Catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Gándara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water Adsorption in Porous Metal-Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Wu, H.; Yildirim, T.; Zhou, W. Exceptional Mechanical Stability of Highly Porous Zirconium Metal–Organic Framework UiO-66 and Its Important Implications. J. Phys. Chem. Lett. 2013, 4, 925–930. [Google Scholar] [CrossRef]
- Jakobsen, S.; Gianolio, D.; Wragg, D.S.; Nilsen, M.H.; Emerich, H.; Bordiga, S.; Lamberti, C.; Olsbye, U.; Tilset, M.; Lillerud, K.P. Structural Determination of a Highly Stable Metal-Organic Framework with Possible Application to Interim Radioactive Waste Scavenging: Hf-UiO-66. Phys. Rev. B Condens. Matter Mater. Phys. 2012, 86, 125429. [Google Scholar] [CrossRef]
- Lammert, M.; Wharmby, M.T.; Smolders, S.; Bueken, B.; Lieb, A.; Lomachenko, K.A.; De Vos, D.; Stock, N. Cerium-Based Metal Organic Frameworks with UiO-66 Architecture: Synthesis, Properties and Redox Catalytic Activity. Chem. Commun. 2015, 51, 12578–12581. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Ladewig, B.P.; Hill, A.J.; Lau, C.H.; Hill, M.R. Post-Synthetic Ti Exchanged UiO-66 Metal-Organic Frameworks That Deliver Exceptional Gas Permeability in Mixed Matrix Membranes. Sci. Rep. 2015, 5, 7823. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-Based Metal–Organic Frameworks: Design, Synthesis, Structure, and Applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Schaate, A.; Roy, P.; Preuße, T.; Lohmeier, S.J.; Godt, A.; Behrens, P. Porous Interpenetrated Zirconium–Organic Frameworks (PIZOFs): A Chemically Versatile Family of Metal–Organic Frameworks. Chem.-A Eur. J. 2011, 17, 9320–9325. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; Llabrés i Xamena, F.X. Conversion of Levulinic Acid into Chemicals: Synthesis of Biomass Derived Levulinate Esters over Zr-Containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. [Google Scholar] [CrossRef]
- Shearer, G.C.; Chavan, S.; Bordiga, S.; Svelle, S.; Olsbye, U.; Petter Lillerud, K. Defect Engineering: Tuning the Porosity and Composition of the Metal–Organic Framework UiO-66 via Modulated Synthesis. Chem. Mater. 2016, 28, 3749–3761. [Google Scholar] [CrossRef]
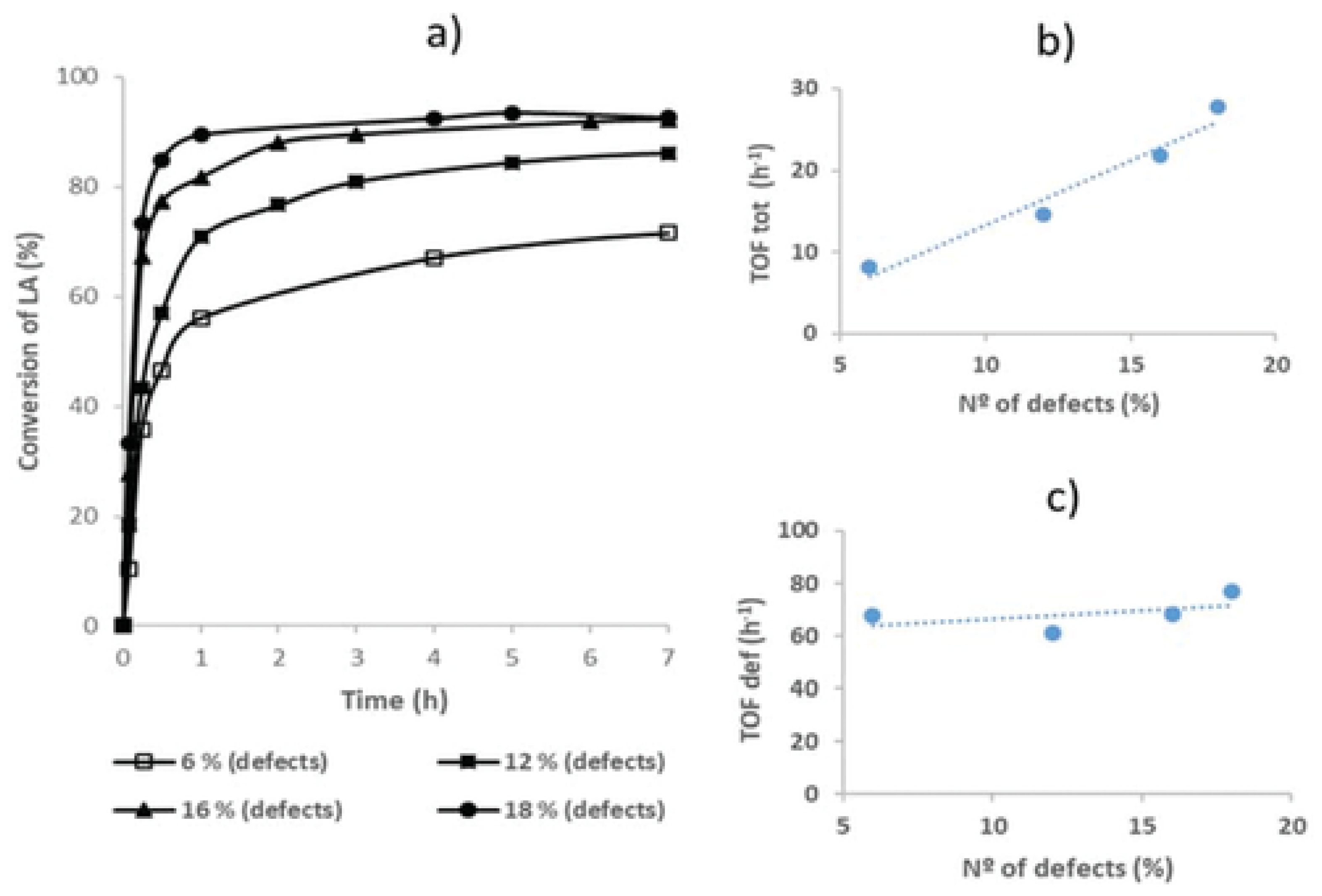
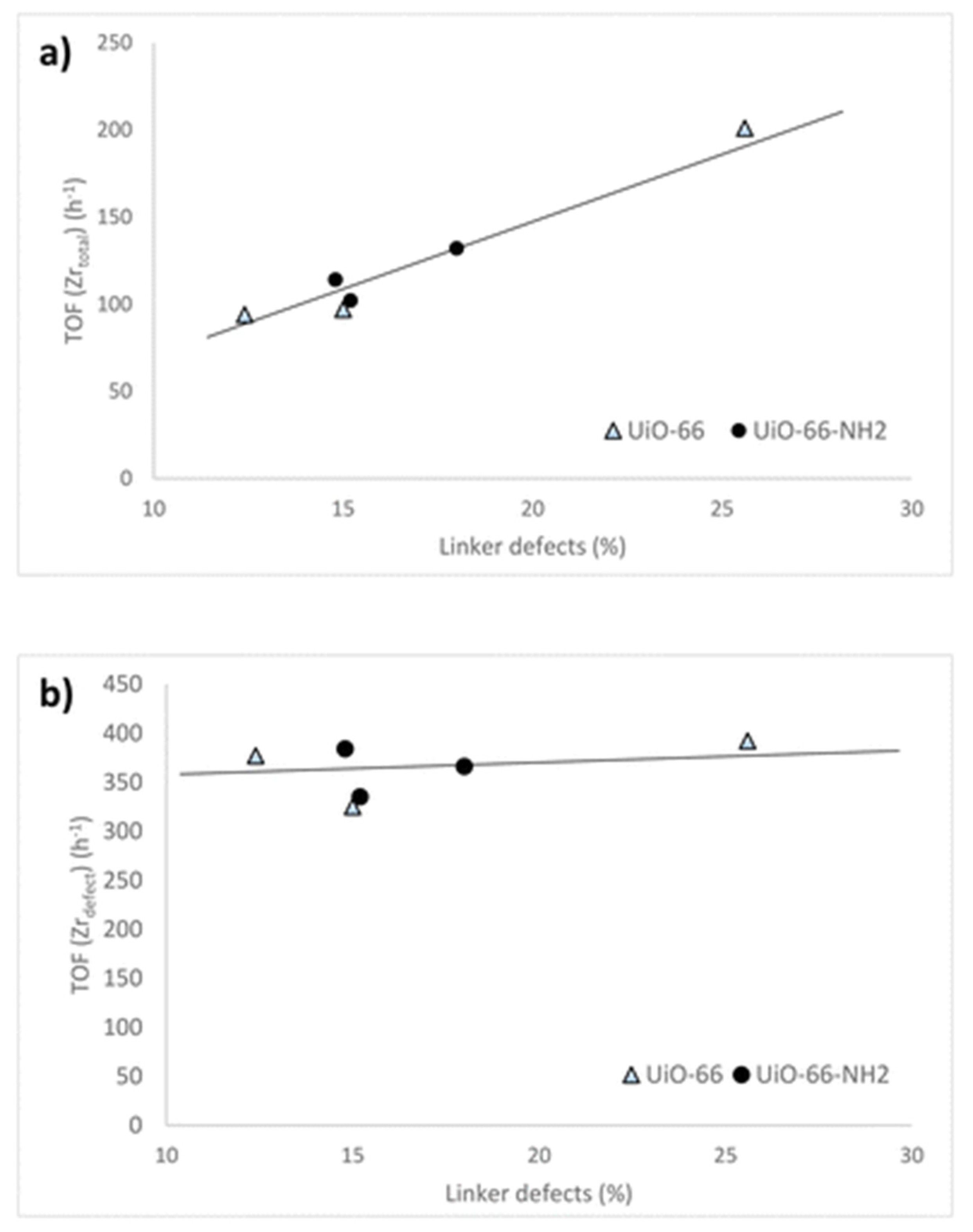
- Cirujano, F.G.; Llabrés i Xamena, F.X. Tuning the Catalytic Properties of UiO-66 Metal-Organic Frameworks: From Lewis to Defect-Induced Brønsted Acidity. J. Phys. Chem. Lett. 2020, 11, 4879–4890. [Google Scholar] [CrossRef] [PubMed]
- Mautschke, H.-H.; Drache, F.; Senkovska, I.; Kaskel, S.; Llabrés i Xamena, F.X. Catalytic Properties of Pristine and Defect-Engineered Zr-MOF-808 Metal Organic Frameworks. Catal. Sci. Technol. 2018, 8, 3610–3616. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Li, B.; Zhang, H. Advances in the Catalytic Production of Valuable Levulinic Acid Derivatives. ChemCatChem 2012, 4, 1230–1237. [Google Scholar] [CrossRef]
- Hayes, D.J. An Examination of Biorefining Processes, Catalysts and Challenges. Catal. Today 2009, 145, 138–151. [Google Scholar] [CrossRef]
- Allaoua, I.; Goi, B.E.; Obadia, M.M.; Debuigne, A.; Detrembleur, C.; Drockenmuller, E. (Co)Polymerization of Vinyl Levulinate by Cobalt-Mediated Radical Polymerization and Functionalization by Ketoxime Click Chemistry. Polym. Chem. 2014, 5, 2973–2979. [Google Scholar] [CrossRef]
- Sinisi, A.; Degli Esposti, M.; Toselli, M.; Morselli, D.; Fabbri, P. Biobased Ketal-Diester Additives Derived from Levulinic Acid: Synthesis and Effect on the Thermal Stability and Thermo-Mechanical Properties of Poly(Vinyl Chloride). ACS Sustain. Chem. Eng. 2019, 7, 13920–13931. [Google Scholar] [CrossRef]
- Mallesham, B.; Govinda Rao, B.; Reddy, B.M. Production of Biofuel Additives by Esterification and Acetalization of Bioglycerol. Comptes Rendus Chim. 2016, 19, 1194–1202. [Google Scholar] [CrossRef]
- Xuan, W.; Hakkarainen, M.; Odelius, K. Levulinic Acid as a Versatile Building Block for Plasticizer Design. ACS Sustain. Chem. Eng. 2019, 7, 12552–12562. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of Biomass Platform Molecules into Fuel Additives and Liquid Hydrocarbon Fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Fegyverneki, D.; Orha, L.; Láng, G.; Horváth, I.T. Gamma-Valerolactone-Based Solvents. Tetrahedron 2010, 66, 1078–1081. [Google Scholar] [CrossRef]
- Palkovits, R. Pentenoic Acid Pathways for Cellulosic Biofuels. Angew. Chem.—Int. Ed. 2010, 49, 4336–4338. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.P.; Price, R.; Ayoub, P.M.; Louis, J.; Petrus, L.; Clarke, L.; Gosselink, H. Valeric Biofuels: A Platform of Cellulosic Transportation Fuels. Angew. Chem.—Int. Ed. 2010, 49, 4479–4483. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic Upgrading of Levulinic Acid to 5-Nonanone. Green Chem. 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Caratelli, C.; Hajek, J.; Cirujano, F.G.; Waroquier, M.; Llabrés i Xamena, F.X.; Van Speybroeck, V. Nature of Active Sites on UiO-66 and Beneficial Influence of Water in the Catalysis of Fischer Esterification. J. Catal. 2017, 352, 401–414. [Google Scholar] [CrossRef]
- Valenzano, L.; Civalleri, B.; Chavan, S.; Bordiga, S.; Nilsen, M.H.; Jakobsen, S.; Lillerud, K.P.; Lamberti, C. Disclosing the Complex Structure of UiO-66 Metal Organic Framework: A Synergic Combination of Experiment and Theory. Chem. Mater. 2011, 23, 1700–1718. [Google Scholar] [CrossRef]
- Freitas, F.A.; Licursi, D.; Lachter, E.R.; Galletti, A.M.R.; Antonetti, C.; Brito, T.C.; Nascimento, R.S.V. Heterogeneous Catalysis for the Ketalisation of Ethyl Levulinate with 1,2-Dodecanediol: Opening the Way to a New Class of Bio-Degradable Surfactants. Catal. Commun. 2016, 73, 84–87. [Google Scholar] [CrossRef]
- Selifonov, S.; Rothstein, S.D.; Mullen, B.D. Method of Making Ketals and Acetals. U.S. Patent US8604223B22013, 10 December 2013. [Google Scholar]
- Mullen, B.D.; Badarinarayana, V.; Santos-Martinez, M.; Selifonov, S. Catalytic Selectivity of Ketalization versus Transesterification. Top. Catal. 2010, 53, 1235–1240. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Animashaun, M.A. Acid Catalyzed Competitive Esterification and Ketalization of Levulinic Acid with 1,2 and 1,3-Diols: The Effect of Heterogeneous and Homogeneous Catalysts. Catal. Lett. 2016, 146, 1819–1824. [Google Scholar] [CrossRef]
- Rapeyko, A.; Rodenas, M.; Llabrés i Xamena, F.X. Zr-Containing UiO-66 Metal-Organic Frameworks as Highly Selective Heterogeneous Acid Catalysts for the Direct Ketalization of Levulinic Acid. Adv. Sustain. Syst. 2022, 6, 2100451. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; West, R.M.; Dumesic, J.A. Catalytic Conversion of Renewable Biomass Resources to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 79–100. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.R.H.; Palkovits, R. Development of Heterogeneous Catalysts for the Conversion of Levulinic Acid to γ-Valerolactone. ChemSusChem 2012, 5, 1657–1667. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.M.; Silva, A.F.; Fernandes, A.; Valente, A.A. γ-Valerolactone Synthesis from α-Angelica Lactone and Levulinic Acid over Biobased Multifunctional Nanohybrid Catalysts. Catal. Today 2021, in press. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodrí Guez-Padró, D.; Luque, R.; Mauriello, F. Recent Catalytic Routes for the Preparation and the Upgrading of Biomass Derived Furfural and 5-Hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273. [Google Scholar] [CrossRef]
- Chia, M.; Dumesic, J.A. Liquid-Phase Catalytic Transfer Hydrogenation and Cyclization of Levulinic Acid and Its Esters to γ-Valerolactone over Metal Oxide Catalysts. Chem. Commun. 2011, 47, 12233–12235. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.B.; Guo, Q.X.; Fu, Y. Raney® Ni Catalyzed Transfer Hydrogenation of Levulinate Esters to γ-Valerolactone at Room Temperature. Chem. Commun. 2013, 49, 5328–5330. [Google Scholar] [CrossRef]
- Luo, H.Y.; Consoli, D.F.; Gunther, W.R.; Román-Leshkov, Y. Investigation of the Reaction Kinetics of Isolated Lewis Acid Sites in Beta Zeolites for the Meerwein–Ponndorf–Verley Reduction of Methyl Levulinate to g-Valerolactone. J. Catal. 2014, 32, 198–207. [Google Scholar] [CrossRef]
- Bui, L.; Luo, H.; Gunther, W.R.; Román-Leshkov, Y. Domino Reaction Catalyzed by Zeolites with Brønsted and Lewis Acid Sites for the Production of G-Valerolactone from Furfural. Angew. Chem.—Int. Ed. 2013, 52, 8180–8183. [Google Scholar] [CrossRef]
- Wang, J.; Jaenicke, S.; Chuah, G.K. Zirconium–Beta Zeolite as a Robust Catalyst for the Transformation of Levulinic Acid to g-Valerolactone via Meerwein–Ponndorf–Verley Reduction. RSC Adv. 2014, 4, 13481–13489. [Google Scholar] [CrossRef]
- Vermoortele, F.; Vandichel, M.; Van de Voorde, B.; Ameloot, R.; Waroquier, M.; Van Speybroeck, V.; de Vos, D.E. Electronic Effects of Linker Substitution on Lewis Acid Catalysis with Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2012, 51, 4887–4890. [Google Scholar] [CrossRef]
- Plessers, E.; Fu, G.; Tan, C.Y.X.; de Vos, D.E.; Roeffaers, M.B.J. Zr-Based MOF-808 as Meerwein–Ponndorf–Verley Reduction Catalyst for Challenging Carbonyl Compounds. Catalysts 2016, 6, 104. [Google Scholar] [CrossRef]
- Valekar, A.H.; Lee, M.; Yoon, J.W.; Kwak, J.; Hong, D.Y.; Oh, K.R.; Cha, G.Y.; Kwon, Y.U.; Jung, J.; Chang, J.S.; et al. Catalytic Transfer Hydrogenation of Furfural to Furfuryl Alcohol under Mild Conditions over Zr-MOFs: Exploring the Role of Metal Node Coordination and Modification. ACS Catal. 2020, 10, 3720–3732. [Google Scholar] [CrossRef]
- Rojas-Buzo, S.; Garcia-Garcia, P.; Corma, A. Catalytic Transfer Hydrogenation of Biomass-Derived Carbonyls over Hafnium-Based Metal–Organic Frameworks. ChemSusChem 2018, 11, 432–438. [Google Scholar] [CrossRef]
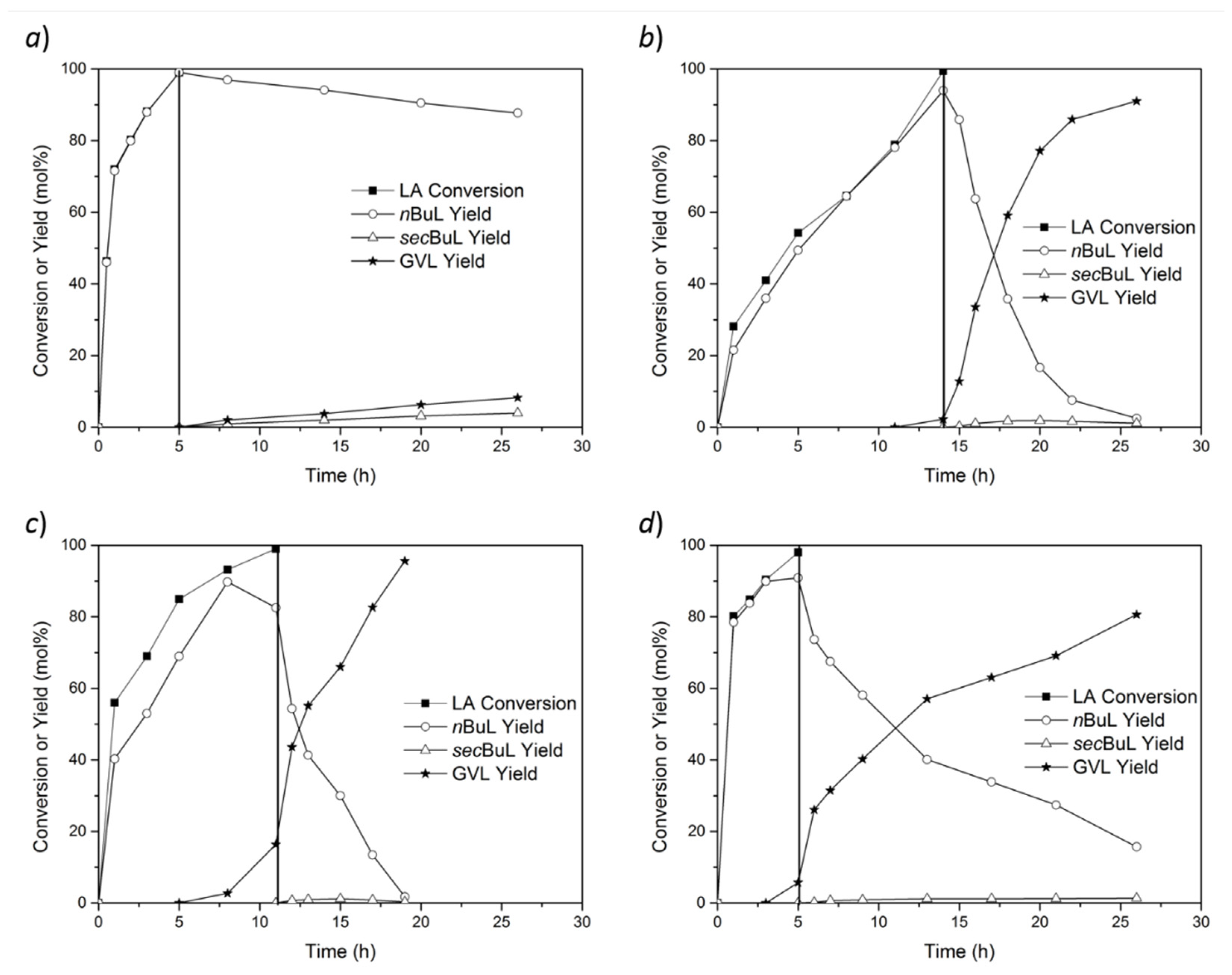
- Valekar, A.H.; Cho, K.-H.; Chitale, S.K.; Hong, D.-Y.; Cha, G.-Y.; Lee, U.-H.; Hwang, D.W.; Serre, C.; Chang, J.-S.; Hwang, Y.K. Catalytic Transfer Hydrogenation of Ethyl Levulinate to γ-Valerolactone over Zirconium-Based Metal–Organic Frameworks. Green Chem. 2016, 18, 4542–4552. [Google Scholar] [CrossRef]
- Guarinos, J.M.; Cirujano, F.G.; Rapeyko, A.; Llabrés i Xamena, F.X. Conversion of Levulinic Acid to γ-Valerolactone over Zr-Containing Metal-Organic Frameworks: Evidencing the Role of Lewis and Brønsted Acid Sites. Mol. Catal. 2021, 515, 111925. [Google Scholar] [CrossRef]
- Jiang, J.; Gándara, F.; Zhang, Y.-B.; Na, K.; Yaghi, O.M.; Klemperer, W.G. Superacidity in Sulfated Metal−Organic Framework-808. J. Am. Chem. Soc. 2014, 136, 12844–12847. [Google Scholar] [CrossRef]
- Trickett, C.A.; Popp, T.M.O.; Su, J.; Yan, C.; Weisberg, J.; Huq, A.; Urban, P.; Jiang, J.; Kalmutzki, M.J.; Liu, Q.; et al. Identification of the Strong Brønsted Acid Site in a Metal–Organic Framework Solid Acid Catalyst. Nat. Chem. 2019, 11, 170–176. [Google Scholar] [CrossRef]
- Vieira, S.S.; Magriotis, Z.M.; Santos, N.A.V.; Saczk, A.A.; Hori, C.E.; Arroyo, P.A. Biodiesel Production by Free Fatty Acid Esterification Using Lanthanum (La3+) and HZSM-5 Based Catalysts. Bioresour. Technol. 2013, 133, 248–255. [Google Scholar] [CrossRef]
- Ma, F.; Hanna, M.A. Biodiesel Production: A Review. Bioresour. Technol. 1999, 70, 1–15. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.A.; Bats, N. Catalysis of Transesterification by a Nonfunctionalized Metal-Organic Framework: Acido-Basicity at the External Surface of ZIF-8 Probed by FTIR and Ab Initio Calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef]
- Savonnet, M.; Camarata, A.; Canivet, J.; Bazer-Bachi, D.; Bats, N.; Lecocq, V.; Pinel, C.; Farrusseng, D. Tailoring Metal–Organic Framework Catalysts by Click Chemistry. Dalton Trans. 2012, 41, 3945–3948. [Google Scholar] [CrossRef] [PubMed]
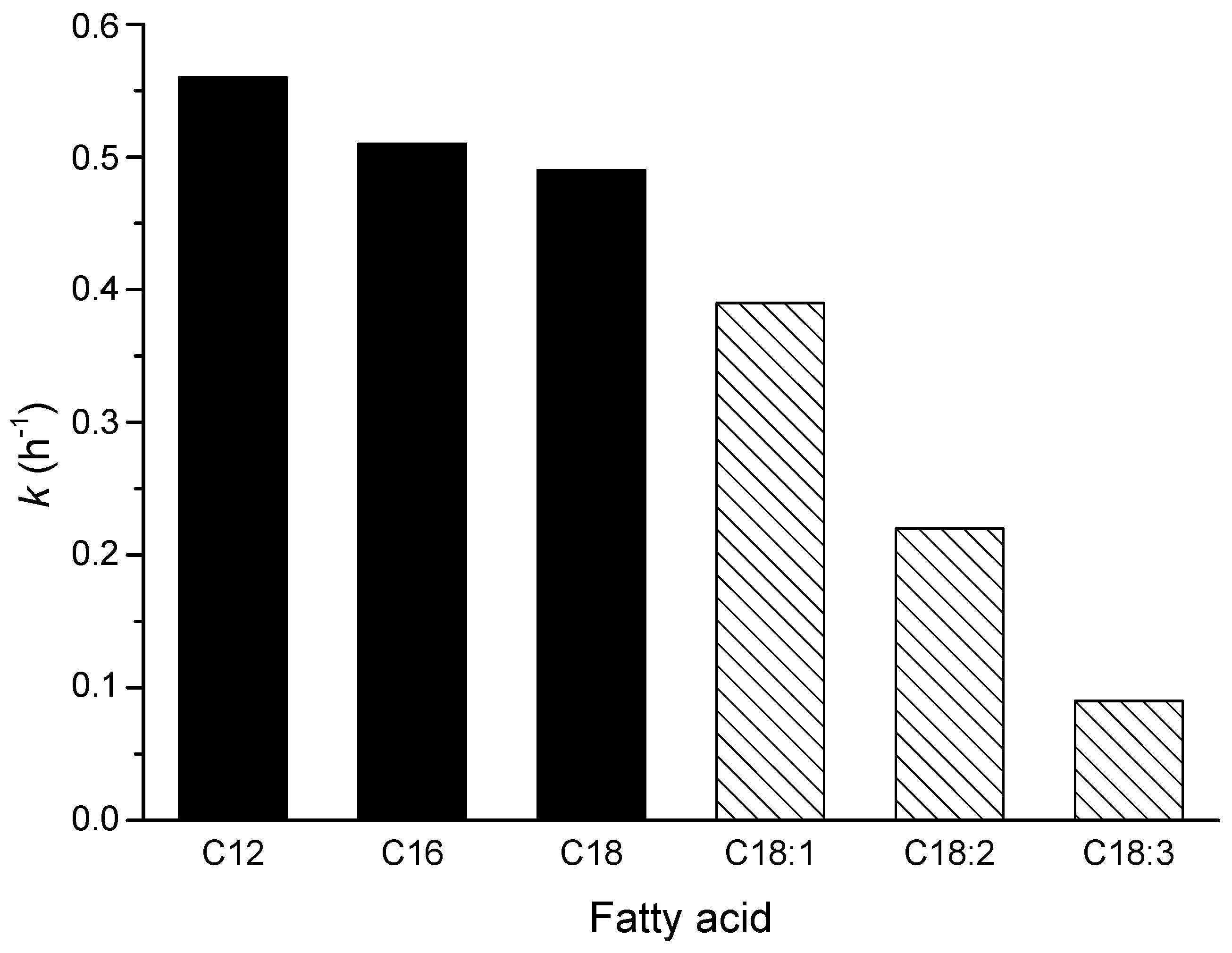
- Cirujano, F.G.G.; Corma, A.; Llabrés i Xamena, F.X. Zirconium-Containing Metal Organic Frameworks as Solid Acid Catalysts for the Esterification of Free Fatty Acids: Synthesis of Biodiesel and Other Compounds of Interest. Catal. Today 2015, 257, 213–220. [Google Scholar] [CrossRef]
- Mota, C.J.A.; Da Silva, C.X.A.; Rosenbach, N.; Costa, J.; Da Silva, F. Glycerin Derivatives as Fuel Additives: The Addition of Glycerol/Acetone Ketal (Solketal) in Gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Silva, P.H.R.; Gonçalves, V.L.C.; Mota, C.J.A. Glycerol Acetals as Anti-Freezing Additives for Biodiesel. Bioresour. Technol. 2010, 101, 6225–6229. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Fadillah, G.; Musawwa, M.M. Glycerol to Solketal for Fuel Additive: Recent. Energies 2019, 12, 2872. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Velty, A. Synthesis of Hyacinth, Vanilla, and Blossom Orange Fragrances: The Benefit of Using Zeolites and Delaminated Zeolites as Catalysts. Appl. Catal. A Gen. 2004, 263, 155–161. [Google Scholar] [CrossRef]
- Sari, P.; Razzak, M.; Tucker, I.G. Isotropic Systems of Medium-Chain Mono- and Diglycerides for Solubilization of Lipophilic and Hydrophilic Drugs. Pharm. Dev. Technol. 2004, 9, 97–106. [Google Scholar] [CrossRef]
- Piasecki, A.; Sokołowski, A.; Burczyk, B.; Kotlewska, U. Synthesis and Surface Properties of Chemodegradable Anionic Surfactants: Sodium (2-n-Alkyl-1,3-Dioxan-5-Yl)Sulfates. JAOCS J. Am. Oil Chem. Soc. 1997, 74, 33–37. [Google Scholar] [CrossRef]
- Rapeyko, A.; Díaz Infante, J.C.; Llabrés i Xamena, F.X. Zr-Containing UiO-66 Metal–Organic Frameworks as Efficient Heterogeneous Catalysts for Glycerol Valorization: Synthesis of Hyacinth and Other Glyceryl Acetal Fragrances. Mol. Syst. Des. Eng. 2023, 8, 775–785. [Google Scholar] [CrossRef]
- Otsuka, S.; Tani, K.; Yamagata, T.; Akutagawa, S.; Kumobayashi, H.; Yagi, M. Process for the Preparation of Enamines or Imines. EP Patent 68506, 3 January 1985. [Google Scholar]
- Alaerts, L.; Seguin, E.; Poelman, H.; Thibault-Starzyk, F.; Jacobs, P.A.; De Vos, D.E. Probing the Lewis Acidity and Catalytic Activity of the Metal-Organic Framework Cu3(Btc)2 (BTC = Benzene-1,3,5-Tricarboxylate). Chem.-A Eur. J. 2006, 12, 7353–7363. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Llabrés Xamena, F.X.; Corma, A. MOFs as Multifunctional Catalysts: One-Pot Synthesis of Menthol from Citronellal over a Bifunctional MIL-101 Catalyst. Dalton Trans. 2012, 41, 4249–4254. [Google Scholar] [CrossRef] [PubMed]
- Vandichel, M.; Vermoortele, F.; Cottenie, S.; de Vos, D.E.; Waroquier, M.; Van Speybroeck, V. Insight in the Activity and Diastereoselectivity of Various Lewis Acid Catalysts for the Citronellal Cyclization. J. Catal. 2013, 305, 118–129. [Google Scholar] [CrossRef][Green Version]
- Vermoortele, F.; Bueken, B.; Le Bars, G.; Van de Voorde, B.; Vandichel, M.; Houthoofd, K.; Vimont, A.; Daturi, M.; Waroquier, M.; Van Speybroeck, V.; et al. Synthesis Modulation as a Tool To Increase the Catalytic Activity of Metal-Organic Frameworks: The Unique Case of UiO-66(Zr). J. Am. Chem. Soc. 2013, 135, 11465–11468. [Google Scholar] [CrossRef] [PubMed]
- Meerwein, H.; Schmidt, R. Ein Neues Verfahren Zur Reduktion von Aldehyden Und Ketonen. Justus Liebigs Ann. Chem. 1925, 444, 221–238. [Google Scholar] [CrossRef]
- Ponndorf, W. Der Reversible Austausch Der Oxydationsstufen Zwischen Aldehyden Oder Ketonen Einerseits Und Primären Oder Sekundären Alkoholen Anderseits. Angew. Chem. 1926, 39, 138. [Google Scholar] [CrossRef]
- Verley, A. Exchange of Functional Groups between Two Molecules. Exchange of Alcohol and Aldehyde Groups. Bull. Soc. Chim. Fr 1925, 37, 537. [Google Scholar]
- Mautschke, H.H.; Llabrés i Xamena, F.X. MOF-808 as a Highly Active Catalyst for the Diastereoselective Reduction of Substituted Cyclohexanones. Molecules 2022, 27, 6315. [Google Scholar] [CrossRef]
- Mautschke, H.H.; Llabrés i Xamena, F.X. One-Step Chemo-, Regio- and Stereoselective Reduction of Ketosteroids to Hydroxysteroids over Zr-Containing MOF-808 Metal-Organic Frameworks. Chem.-A Eur. J. 2021, 27, 10766–10775. [Google Scholar] [CrossRef]
- Dodge, J.A.; Lugar III, C.W. Alcohol Inversion of 17b-Steroids. Bioorg. Med. Chem. Lett. 1996, 6, 1–2. [Google Scholar] [CrossRef]
- Han, G.-D.; Lin, Z.-Y.; Yan, C.-P. Synthesis of Musk Androgen 5a-Androstane-3b,17a-Diol. Zhongguo Yiyao Gongye Zazhi 1994, 25, 490–491. [Google Scholar]
- Ohta, T.; Zhang, H.; Torihara, Y.; Hida, T.; Furukawa, I. Practical Synthesis of Androgen: The Efficient Transformation of 17-Oxo Group to 17a-Hydroxy Group. Steroids 1998, 63, 630–632. [Google Scholar] [CrossRef] [PubMed]
- Göndös, G.; Orr, J.C. Reduction of Steroid 17-Ketones by Enantiomeric Chiral Reducing Agents. J. Chem. Soc. Chem. Commun. 1982, 1238–1239. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, Y.; Wang, Z.; Chen, M.; Sun, L.; Liu, X. Titanium Incorporated with UiO-66(Zr)-Type Metal–Organic Framework (MOF) for Photocatalytic Application. RSC Adv. 2016, 6, 3671–3679. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Churipard, S.R.; Maradur, S.P.; Kalidindi, S.B. Exploring the Brønsted Acidity of UiO-66 (Zr, Ce, Hf) Metal-Organic Frameworks for Efficient Solketal Synthesis from Glycerol Acetalization. Dalton Trans. 2019, 48, 843–847. [Google Scholar] [CrossRef]



| Lewis Acidity (Dehydrated Material) | Brønsted-Induced Acidity (Hydrated Material) | |
|---|---|---|
| UiO-66 |  Few acid sites (only at defect sites) Few acid sites (only at defect sites) | |
 Restricted pore space Restricted pore space(hindered Lewis sites) |  Strongly polarized H2O molecules Strongly polarized H2O molecules(strong Brønsted sites) | |
| MOF-808 |  All Zr4+ sites available All Zr4+ sites available | |
 Wide pores Wide pores (efficient Lewis sites) |  Weakly polarized H2O molecules due to geminal −OH groups Weakly polarized H2O molecules due to geminal −OH groups (weak Brønsted sites) | |
| Carbonyl | Time (h) | Conv. (%) | Select. Dioxane (%) | Select. Dioxolane (%) |
|---|---|---|---|---|
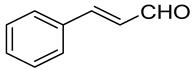 | 3 | 100 | 57.8 | 42.2 |
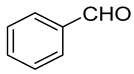 | 1 | 100 | 33.4 | 66.6 |
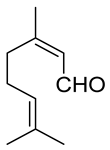 | 3 | 100 | 60.9 | 39.1 |
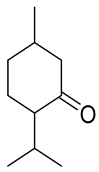 | 24 | 18.7 | 17.7 | 82.3 |
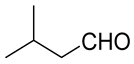 | 3 | 99 | 30.5 | 69.5 |
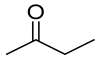 | 7 | 100 | 0 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rapeyko, A.; Llabrés i Xamena, F.X. Zirconium-Containing Metal–Organic Frameworks (MOFs) as Catalysts for Biomass Conversion. Appl. Sci. 2025, 15, 2609. https://doi.org/10.3390/app15052609
Rapeyko A, Llabrés i Xamena FX. Zirconium-Containing Metal–Organic Frameworks (MOFs) as Catalysts for Biomass Conversion. Applied Sciences. 2025; 15(5):2609. https://doi.org/10.3390/app15052609
Chicago/Turabian StyleRapeyko, Anastasia, and Francesc X. Llabrés i Xamena. 2025. "Zirconium-Containing Metal–Organic Frameworks (MOFs) as Catalysts for Biomass Conversion" Applied Sciences 15, no. 5: 2609. https://doi.org/10.3390/app15052609
APA StyleRapeyko, A., & Llabrés i Xamena, F. X. (2025). Zirconium-Containing Metal–Organic Frameworks (MOFs) as Catalysts for Biomass Conversion. Applied Sciences, 15(5), 2609. https://doi.org/10.3390/app15052609







