Pollutant Removal Efficiency of Pilot-Scale Horizontal Subsurface Flow Constructed Wetlands Treating Landfill Leachate
Abstract
1. Introduction
2. Materials and Methods
2.1. Leachate Qualitative Characteristics
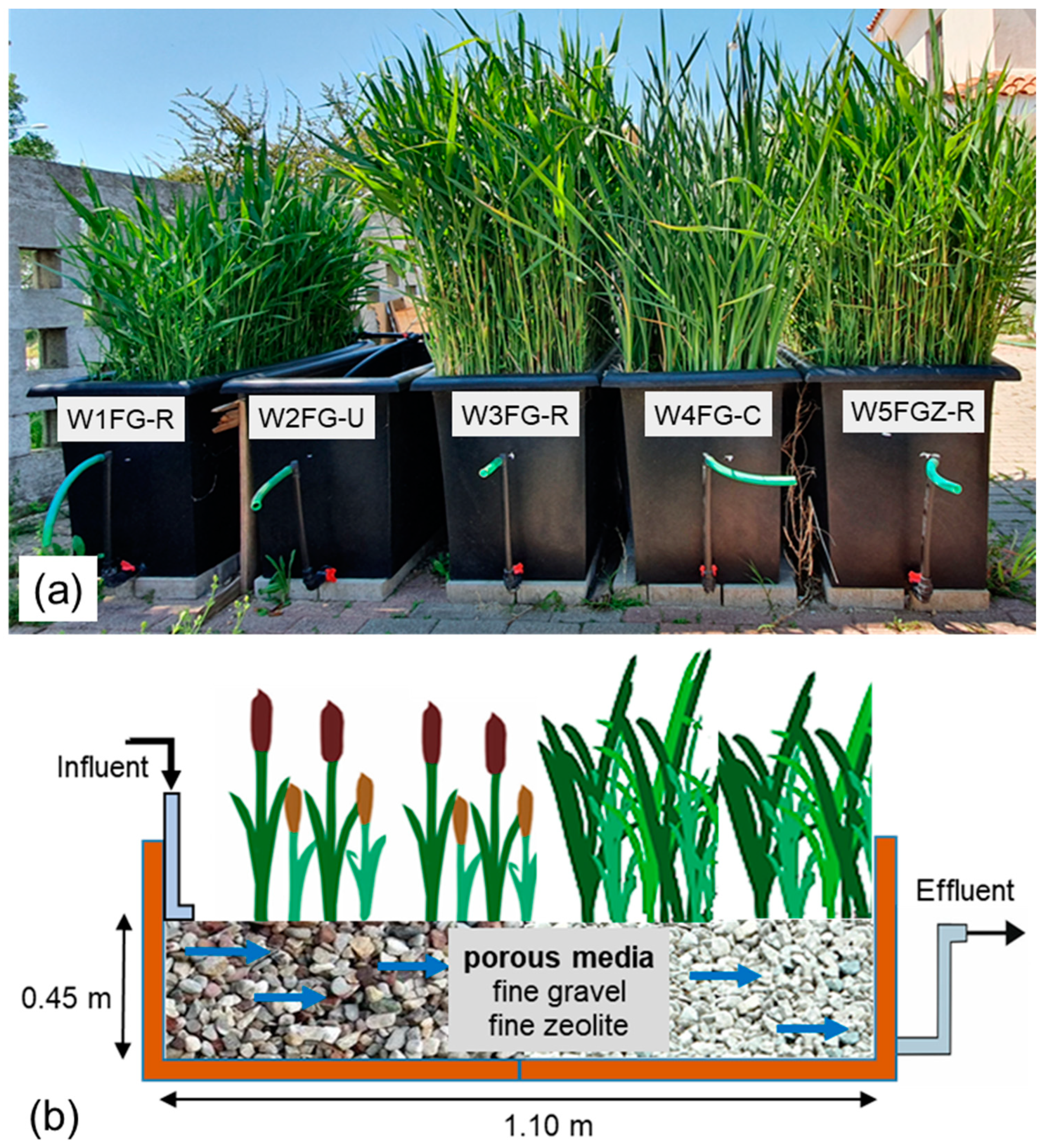
2.2. System Configuration and Operation
2.3. Sampling and Monitoring
2.4. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters of Leachate Landfill in Pilot-Scale CW Units
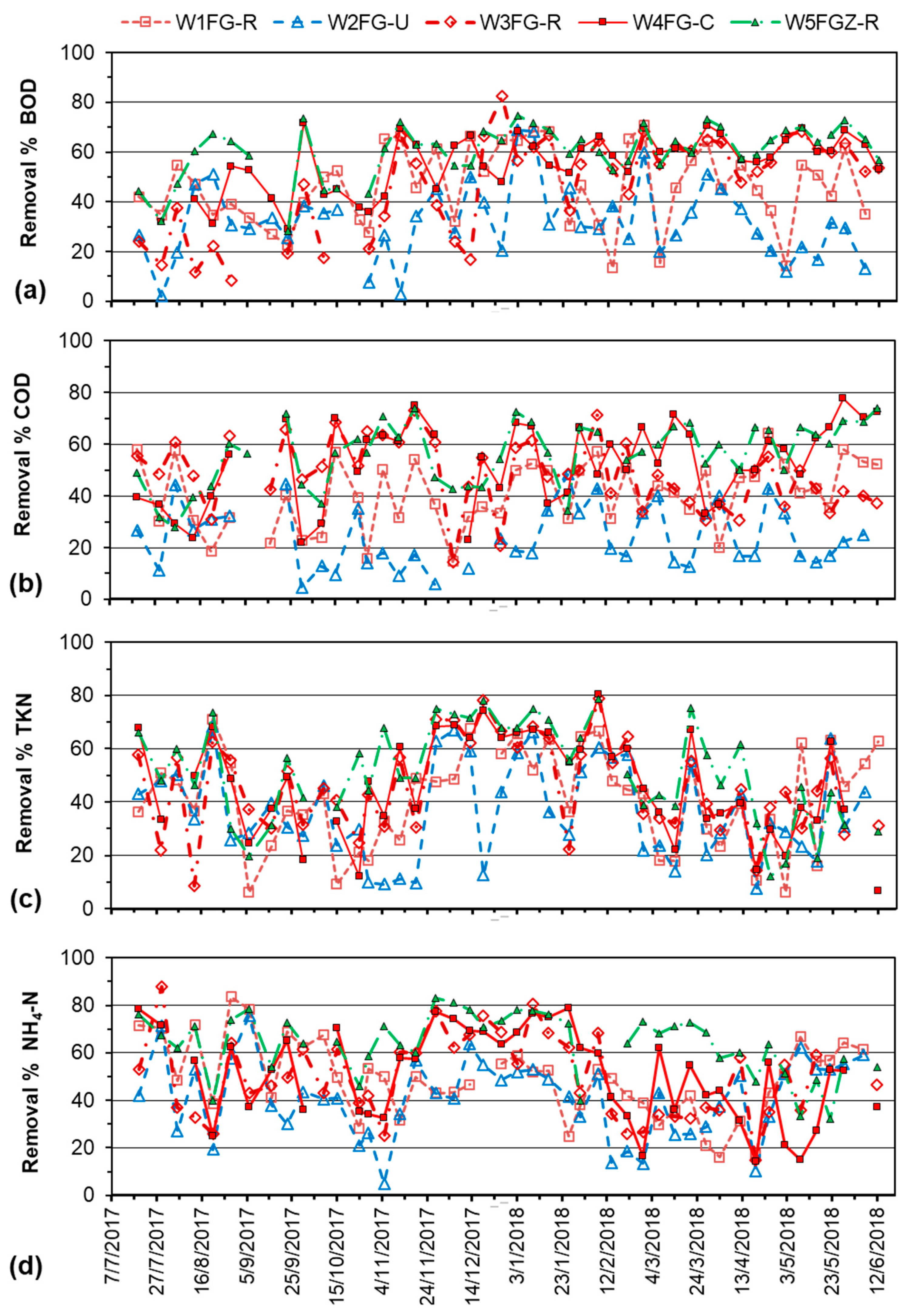
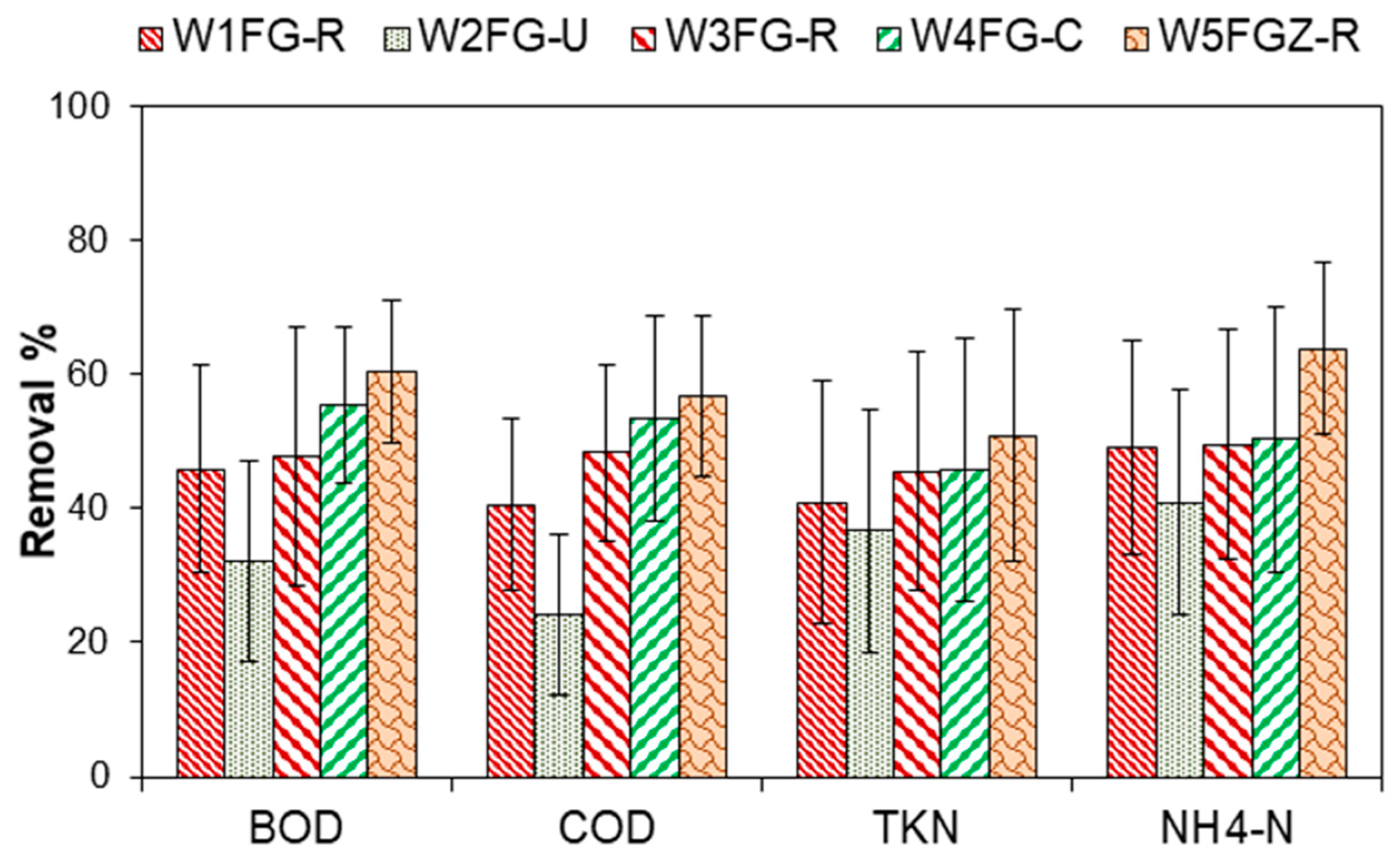
3.2. Overall Performance of Pilot-Scale CW Units
3.3. Effect of Vegetation
3.4. Effect of Hydraulic Residence Time and Porous Media
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdel-Shafy, H.; Ibrahim, A.; Al-Sulaiman, A.; Okasha, R. Landfill leachate: Sources, nature, organic composition, and treatment: An environmental overview. Ain Shams Eng. J. 2024, 15, 102293. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Z. A comprehensive review of landfill leachate treatment technologies. Front. Environ. Sci. 2024, 12, 1439128. [Google Scholar] [CrossRef]
- El-Saadony, Μ.; Saad, A.; El-Wafai, N.; Abou-Aly, H.; Salem, H.; Soliman, S.; Abd El-Mageed, T.; Elrys, A.; Selim, S.; Abd El-Hack, M.; et al. Hazardous wastes and management strategies of landfill leachates: A comprehensive review. Environ. Technol. Innov. 2023, 31, 103150. [Google Scholar] [CrossRef]
- Maddela, N.R.; Ramakrishnan, B.; Kakarla, D.; Venkateswarlu, K.; Megharaj, M. Major contaminants of emerging concern in soils: A perspective on potential health risks. RSC Adv. 2022, 12, 12396–12415. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and treatment of landfill leachate: A review. Water Res. 2021, 203, 117525. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Filho, F.J.C.M.; Cavalheri, P.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total Environ. 2020, 705, 135568. [Google Scholar] [CrossRef] [PubMed]
- Igwegbe, C.; López-Maldonado, E.; Landázuri, A.; Ovuoraye, P.; Ogbu, A.; Vela-García, N.; Białowiec, A. Sustainable municipal landfill leachate management: Current practices, challenges, and future directions. Desalination Water Treat. 2024, 320, 100709. [Google Scholar] [CrossRef]
- Koutsou, O.P.; Mandylas, C.; Fountoulakis, M.S.; Stasinakis, A. Leachate management in medium- and small-sized sanitary landfills: A Greek case study. Environ. Sci. Pollut. Res. 2023, 30, 120994–121006. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cui, H.; Li, Y.; Song, X.; Liu, W.; Wang, Y.; Hou, H.; Zhang, H.; Li, Y.; Wang, F.; et al. Challenges and engineering application of landfill leachate concentrate treatment. Environ. Res. 2023, 231, 116028. [Google Scholar] [CrossRef]
- Lebron, Y.A.R.; Moreira, V.R.; Brasil, Y.L.; Silva, A.F.R.; de Souza Santos, L.V.; Lange, L.C.; Amaral, M.C.S. A survey on experiences in leachate treatment: Common practices, differences worldwide and future perspectives. J. Environ. Manag. 2021, 288, 112475. [Google Scholar] [CrossRef]
- Chen, H.; Xu, H.; Zhong, C.; Liu, M.; Yang, L.; He, J.; Sun, Y.; Zhao, C.; Wang, D. Treatment of landfill leachate by coagulation: A review. Sci. Total Environ. 2024, 912, 169294. [Google Scholar] [CrossRef]
- Saxena, V.; Padhi, S.K.; Dikshit, P.K.; Pattanaik, L. Recent developments in landfill leachate treatment: Aerobic granular reactor and its future prospects. Environ. Nanotechn. Monit. Manag. 2022, 18, 100689. [Google Scholar] [CrossRef]
- Abujazar, M.S.S.; Karaağaç, S.U.; Abu Amr, S.S.; Alazaiza, M.Y.D.; Bashir, M.J.K. Recent advancement in the application of hybrid coagulants in coagulationflocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Gikas, G.D.; Tsihrintzis, V.A. Stabilization pond systems for wastewater treatment: Facility costs and environmental footprint assessment. Glob. Nest J. 2014, 16, 375–385. [Google Scholar] [CrossRef]
- Miao, L.; Yang, G.; Tao, T.; Peng, Y. Recent advances in nitrogen removal from landfill leachate using biological treatments–A review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, L.; Tan, F.; Wu, D. Treatment of landfill leachate using activated sludge technology: A review. Archaea 2018, 2018, 1039453. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Kutty, S.R.M.; Lawal, I.M.; Abubakar, S.; Hassan, I.; Zubairu, I.; Abdurrasheed, A.S.; Adam, A.A.; Ghaleb, A.A.S.; Almahbashi, N.M.Y.; et al. Sequencing batch reactor technology for landfill leachate treatment: A state-of-the-art review. J. Environ. Manag. 2021, 282, 111946. [Google Scholar] [CrossRef]
- Men, Y.; Li, Z.; Zhu, L.; Wang, X.; Cheng, S.; Lyu, Y. New insights into membrane fouling during direct membrane filtration of municipal wastewater and fouling control with mechanical strategies. Sci. Total Environ. 2023, 869, 161775. [Google Scholar] [CrossRef] [PubMed]
- Agabo-Garcia, C.; Repetto, G.; Albqmi, M.; Hodaifa, G. Evaluation of the olive mill wastewater treatment based on advanced oxidation processes (AOPs), flocculation, and filtration. J. Environ. Chem. Eng. 2023, 11, 109789. [Google Scholar] [CrossRef]
- Yang, L.; Hu, W.; Chang, Z.; Liu, T.; Fang, D.; Shao, P.; Shi, H.; Luo, X. Electrochemical recovery and high value-added reutilization of heavy metal ions from wastewater: Recent advances and future trends. Environ. Int. 2021, 152, 106512. [Google Scholar] [CrossRef]
- Jamrah, A.; Al-Zghoul, T.; Al-Qodah, Z. An extensive analysis of combined processes for landfill leachate treatment. Water Wastewater Wast. Manag. Sustain. Dev. 2024, 16, 1640. [Google Scholar] [CrossRef]
- Retta, B.; Coppola, E.; Ciniglia, C.; Grilli, E. Constructed wetlands for the wastewater treatment: A review of Italian case studies. Appl. Sci. 2023, 13, 6211. [Google Scholar] [CrossRef]
- Santos, J.; Rodrigues, S.; Magalhães, M.; Rodrigues, K.; Pereira, L.; Marinho, G. A state-of-the-art review (2019–2023) on constructed wetlands for greywater treatment and reuse. Environ. Chall. 2024, 16, 100973. [Google Scholar] [CrossRef]
- Vymazal, J. The Historical Development of Constructed Wetlands for Wastewater Treatment. Land 2022, 11, 174. [Google Scholar] [CrossRef]
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A. Effect of operational and design parameters on performance of pilot-scale vertical flow constructed wetlands treating university campus wastewater. Water Res. Manag. 2016, 30, 5875–5899. [Google Scholar] [CrossRef]
- Gikas, G.D.; Tsihrintzis, V.A. Municipal wastewater treatment using constructed wetlands. Water Util. J. 2014, 8, 57–65. [Google Scholar]
- Gkika, D.; Gikas, G.D.; Tsihrintzis, V.A. Construction and operation costs of constructed wetlands treating wastewater. Water Sci. Technol. 2014, 70, 803–810. [Google Scholar] [CrossRef]
- Akyürek, A.; Ağdağ, O. Comparison of constructed wetlands and package type sequencing batch biological treatment plants in rural areas in terms of efficiency and cost in a full-scale example. Ecol. Eng. 2024, 201, 107190. [Google Scholar] [CrossRef]
- Stefanakis, A. Constructed Wetlands: Description and Benefits of an Eco-Tech Water Treatment System. In Impact of Water Pollution on Human Health and Environmental Sustainability; IGI Global: Hershey, PA, USA, 2016; pp. 281–303. [Google Scholar] [CrossRef]
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A. Chromium removal from wastewater using HSF and VF pilot-scale constructed wetlands: Overall performance, and fate and distribution of this element within the wetland environment. Chemosphere 2017, 168, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.M.; Alfaia, R.G.D.S.M.; Campos, J.C. Landfill leachate treatment in Brazil—An overview. J. Environ. Manag. 2019, 232, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Dhamsaniya, M.; Sojitra, D.; Modi, H.; Shabiimam, M.A.; Kandya, A. A review of the techniques for treating the landfill leachate. Mater. Today Proc. 2023, 77, 358–364. [Google Scholar] [CrossRef]
- Guerrero, L.; Montalvo, S.; Huiliñir, C.; Barahona, A.; Borja, R.; Cortés, A. Simultaneous nitrification–denitrification of wastewater: Effect of zeolite as a support in sequential batch reactor with step-feed strategy. Int. J. Environ. Sci. Technol. 2016, 13, 2325–2338. [Google Scholar] [CrossRef]
- Akinbile, C.O.; Yusoff, M.S.; Zuki, A.A. Landfill leachate treatment using sub-surface flow constructed wetland by Cyperus haspan. Waste Manag. 2012, 32, 1387–1393. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Wallace, S.D. Treatment Wetlands, 2nd ed.; CRC Press/Taylor and Francis Group: Boca Raton, FL, USA, 2009; ISBN 978-1-56670-526-4. [Google Scholar]
- Białowiec, A.; Davies, L.; Albuquerque, A.; Randerson, P.F. Nitrogen removal from landfill leachate in constructed wetlands with reed and willow: Redox potential in the root zone. J. Environ. Manag. 2012, 97, 22–27. [Google Scholar] [CrossRef]
- Bakhshoodeh, R.; Alavi, N.; Majlesi, M.; Paydary, P. Compost leachate treatment by a pilot-scale subsurface horizontal flow constructed wetland. Ecol. Eng. 2017, 105, 7–14. [Google Scholar] [CrossRef]
- Papaevangelou, V.A.; Gikas, G.D.; Tsihrintzis, V.A. Effect of operational and design parameters on performance of pilot-scale horizontal subsurface flow constructed wetlands treating university campus wastewater. Environ. Sci. Pollut. Res. 2016, 23, 19504–19519. [Google Scholar] [CrossRef] [PubMed]
- Yalcuk, A.; Ugurlu, A. Comparison of horizontal and vertical constructed wetland systems for landfill leachate treatment. Bioresour. Technol. 2009, 100, 2521–2526. [Google Scholar] [CrossRef] [PubMed]
- Bakhshoodeh, R.; Alavi, N.; Oldham, C.; Santos, R.M.; Babaei, A.A.; Vymazal, J.; Paydary, P. Constructed wetlands for landfill leachate treatment: A review. Ecol. Eng. 2020, 146, 105725. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Waste Water, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Othman, E.; Yusoff, M.S.; Aziz, H.A.; Adlan, M.N.; Bashir, M.J.K.; Hung, Y.T. The effectiveness of silica sand in semi-aerobic stabilized landfill leachate treatment. Water 2010, 2, 904–915. [Google Scholar] [CrossRef]
- Parlakidis, P.; Mavropoulos, T.; Vryzas, Z.; Gikas, G.D. Fluopyram removal from agricultural equipment rinsing water using HSF pilot-scale constructed wetlands. Environ. Sci. Pollut. Res. 2022, 29, 29584–29596. [Google Scholar] [CrossRef]
- Crites, R.W.; Middlebrooks, E.J.; Reed, S.C. Natural Wastewater Treatment Systems, 1st ed.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: New York, NY, USA, 2006; p. 552. [Google Scholar]
- Lam, T.; Yang, X.; Ergas, S.J.; Arias, M.E. Feasibility of landfill leachate reuse through adsorbent-enhanced constructed wetlands and ultrafiltration-reverse osmosis. Desalination 2023, 545, 116163. [Google Scholar] [CrossRef]
- Dai, S.; Wang, R.; Lin, J.; Zhang, G.; Chen, Z.; Li, L.; Shi, Q. Study on physical clogging process and practical application of horizontal subsurface flow constructed wetland. Sci. Rep. 2025, 15, 523. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Feng, X.; Pyo, S. Current problems and countermeasures of constructed wetland for wastewater treatment: A review. J. Water Process Eng. 2024, 57, 104569. [Google Scholar] [CrossRef]
- Weerakoon, G.M.P.R.; Jinadasa, K.B.S.N.; Herath, G.B.B.; Mowjood, M.I.M.; van Bruggen, J.J. Impact of the hydraulic loading rate on pollutants removal in tropical horizontal subsurface flow constructed wetlands. Ecol. Eng. 2013, 61, 154–160. [Google Scholar] [CrossRef]
- Ye, F.X.; Li, Y. Enhancement of nitrogen removal in towery hybrid constructed wetland to treat domestic wastewater for small rural communities. Ecol. Eng. 2009, 35, 1043–1050. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, Q.; Bai, G.; Guo, D.; Chi, Y.; Li, B.; Yang, L.; Ren, Y. Inducing root redundant development to release oxygen: An efficient natural oxygenation approach for subsurface flow constructed wetland. Environ. Res. 2023, 239, 117377. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Sun, G. A review on nitrogen and organics removal mechanisms in subsurface flow constructed wetlands: Dependency on environmental parameters, operating conditions and supporting media. J. Environ. Manag. 2012, 112, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Tsihrintzis, V.A.; Gikas, G.D. Constructed wetlands for wastewater and activated sludge treatment in North Greece: A review. Water Sci. Technol. 2010, 61, 2653–2672. [Google Scholar] [CrossRef] [PubMed]
- Chiemchaisri, C.; Chiemchaisri, W.; Junsod, J.; Threedeach, S.; Wicranarachchi, P.N. Leachate treatment and greenhouse gas emission in subsurface horizontal flow constructed wetland. Bioresour. Technol. 2009, 100, 3808–3814. [Google Scholar] [CrossRef] [PubMed]
- Bulc, T.G. Long term performance of a constructed wetland for landfill leachate treatment. Ecol. Eng. 2006, 26, 365–374. [Google Scholar] [CrossRef]
- Madera-Parra, C.; Peña, M.; Peña, E.; Lens, P. Cr (VI) and COD removal from landfill leachate by polyculture constructed wetland at a pilot scale. Environ. Sci. Pollut. Res. 2015, 22, 12804–12815. [Google Scholar] [CrossRef]
- Minakshi, D.; Sharma, P.K.; Rani, A.; Malaviya, P.; Srivastava, V.; Kumar, M. Performance evaluation of vertical constructed wetland units with hydraulic retention time as a variable operating factor. Groundw. Sustain. Dev. 2022, 19, 100834. [Google Scholar] [CrossRef]
- Martins, T.H.; Souza, T.S.O.; Foresti, E. Ammonium removal from landfill leachate by Clinoptilolite adsorption followed by bioregeneration. J. Environ. Chem. Eng. 2017, 5, 63–68. [Google Scholar] [CrossRef]
- Saeed, T.; Miah, M.J.; Majed, N.; Hasan, M.; Khan, T. Pollutant removal from landfill leachate employing two-stage constructed wetland mesocosms: Co-treatment with municipal sewage. Environ. Sci. Pollut. Res. 2020, 27, 28316–28332. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Landfill Age | Present Study | ||
|---|---|---|---|---|
| Young | Middle-Aged | Old | ||
| Age (years) | <10 years | 10–20 years | >20 years | |
| pH | 3–6 | 6–7 | >7.5 | 8.24 3 (±0.67) |
| 1 EC (mS/cm) | 15–41.5 | 6–14 | 28.5 (±5.2) | |
| BOD (mg/L) | 10,000–25,000 | 1000–4000 | 50–1000 | 685 (±102) |
| COD (mg/L) | 15,000–40,000 | 10,000–20,000 | 1000–5000 | 9344 (±1956) |
| BOD/COD | 0.6–0.7 | 0.1–0.2 | <0.1 | 0.07 (±0.005) |
| 2 TKN (mg/L) | 1500–4500 | 400–800 | 75–300 | 762 (±151) |
| NH4-N (mg/L) | 1500–4250 | 250–700 | 50–200 | 579 (±97) |
| CW Unit | Porous Media | Plant Species | HRT (Days) | Qin (L/Day) | HLR (mm/Day) |
|---|---|---|---|---|---|
| W1FG-R | FG | Phragmites australis | 8 | 9.5 | 19.4 |
| W2FG-U | FG | None | 10 | 7.6 | 15.3 |
| W3FG-R | FG | Phragmites australis | 10 | 7.6 | 15.3 |
| W4FG-C | FG | Typha latifolia | 10 | 7.6 | 15.3 |
| W5FGZ-R | 75% FG and 25% FZ | Phragmites australis | 10 | 7.6 | 15.3 |
| Parameters | Influent | Effluent | |||||
|---|---|---|---|---|---|---|---|
| W1FG-R | W2FG-U | W3FG-R | W4FG-C | W5FGZ-R | |||
| Τ (°C) | mean | 21.5 | 21.0 | 20.8 | 20.7 | 21.1 | 21.1 |
| SD (n) 1 | 6.4 (106) | 6.4 (106) | 6.6 (106) | 6.6 (106) | 6.8 (106) | 6.7 (106) | |
| min | 7.0 | 7.0 | 7.1 | 7.0 | 7.2 | 7.7 | |
| max | 36.2 | 35.8 | 36.1 | 33.5 | 34.8 | 34.3 | |
| DO (mg/L) | mean | 3.24 | 4.49 | 4.27 | 4.50 | 4.41 | 5.14 |
| SD (n) | 1.18 (106) | 1.40 (106) | 1.10 (106) | 1.39 (106) | 1.49 (106) | 1.19 (106) | |
| min | 0.33 | 1.66 | 1.78 | 0.57 | 1.37 | 2.13 | |
| max | 5.01 | 8.58 | 6.91 | 7.65 | 7.74 | 8.14 | |
| pH | mean | 8.11 | 7.73 | 7.91 | 7.70 | 7.65 | 7.53 |
| SD (n) | 0.87 (106) | 0.71 (106) | 0.81 (106) | 0.71 (106) | 0.61 | 0.66 (106) | |
| min | 6.11 | 6.11 | 6.11 | 6.11 | 6.23 | 5.98 | |
| max | 9.47 | 8.68 | 8.77 | 8.57 | 8.47 | 8.45 | |
| EC (mS/cm) | mean | 7.37 | 9.80 | 6.04 | 10.45 | 9.61 | 10.38 |
| SD (n) | 0.88 (106) | 4.71 (106) | 1.05 (106) | 5.55 (106) | 4.50 (106) | 6.31 (106) | |
| min | 4.05 | 4.04 | 3.69 | 3.54 | 3.56 | 3.12 | |
| max | 9.50 | 27.70 | 9.48 | 25.80 | 24.50 | 27.00 | |
| Parameters | Influent | Effluent | |||||
|---|---|---|---|---|---|---|---|
| W1FG-R | W2FG-U | W3FG-R | W4FG-C | W5FGZ-R | |||
| BOD (mg/L) | mean | 195.9 | 105.4 | 133.5 | 102.0 | 85.7 | 77.0 |
| SD (n) 1 | 29.8 (46) | 31.2 (46) | 32.8 (44) | 36.5 (42) | 19.5 (44) | 20.0 (44) | |
| min | 146.0 | 50.7 | 56.3 | 28.0 | 51.5 | 47.5 | |
| max | 300.0 | 180.0 | 200.0 | 197.0 | 130.5 | 131.0 | |
| COD (mg/L) | mean | 3115 | 1875 | 2446 | 1516 | 1403 | 1354 |
| SD (n) | 652 (46) | 418 (45) | 706 (41) | 422 (45) | 318 (43) | 438 (44) | |
| min | 2065 | 1065 | 1065 | 710 | 898 | 900 | |
| max | 5207 | 2795 | 4312 | 2842 | 2085 | 3751 | |
| TKN (mg/L) | mean | 345.3 | 204.1 | 220.1 | 202.3 | 201.9 | 172.8 |
| SD (n) | 60.7 (46) | 75.2 (45) | 74.1 (45) | 72.3 (45) | 87.6 (43) | 65.8 (43) | |
| min | 210.0 | 101.0 | 119.0 | 84.0 | 91.0 | 84.0 | |
| max | 483.0 | 350.0 | 385.0 | 317.3 | 396.7 | 308.0 | |
| NH4-N (mg/L) | mean | 231.6 | 117.3 | 134.9 | 115.4 | 114.0 | 81.4 |
| SD (n) | 38.9 (46) | 42.8 (44) | 39.8 (45) | 40.4 (43) | 45.2 (43) | 25.8 (42) | |
| min | 140.0 | 35.0 | 49.0 | 21.0 | 41.0 | 42.0 | |
| max | 308.0 | 219.3 | 266.0 | 210.0 | 196.0 | 137.0 | |
| One-Way ANOVA | Post Hoc (Tukey HSD) Test | ||||
|---|---|---|---|---|---|
| Parameter | F | p | CW (I)–CW (J) | Mean Difference (I–J) | p |
| BOD | 25.106 | 0.001 | W2FG-U–W3FG-R | −15.51 | 0.001 * |
| W2FG-U–W4FG-C | −23.26 | 0.001 * | |||
| W3FG-R–W4FG-C | −7.74 | 0.061 | |||
| COD | 54.412 | 0.001 | W2FG-U–W3FG-R | −24.23 | 0.001 * |
| W2FG-U–W4FG-C | −29.20 | 0.001 * | |||
| W3FG-R–W4FG-C | −4.96 | 0.209 | |||
| TKN | 3.548 | 0.032 | W2FG-U–W3FG-R | −8.93 | 0.063 |
| W2FG-U–W4FG-C | −9.26 | 0.045 * | |||
| W3FG-R–W4FG-C | −0.32 | 0.996 | |||
| NH4-N | 3.722 | 0.027 | W2FG-U–W3FG-R | −8.59 | 0.071 |
| W2FG-U–W4FG-C | −9.52 | 0.040 * | |||
| W3FG-R–W4FG-C | −0.93 | 0.969 | |||
| Parameter | Mean Values of Pollutant Removal (%) | t-Test for Equality of Means | |||
|---|---|---|---|---|---|
| W1FG-R | W3FG-R | t | df | p (2-Tailed) | |
| BOD | 45.9 | 47.7 | −0.486 | 86 | 0.628 |
| COD | 40.6 | 48.3 | −2.802 | 88 | 0.006 * |
| TKN | 40.9 | 45.5 | −1.214 | 88 | 0.228 |
| NH4-Ν | 49.0 | 49.4 | −0.119 | 85 | 0.905 |
| W3FG-R | W5FGZ-R | ||||
| BOD | 47.7 | 60.4 | −3.740 | 63 | 0.001 * |
| COD | 48.3 | 56.7 | −3.091 | 87 | 0.003 * |
| TKN | 45.5 | 50.9 | −1.356 | 86 | 0.179 |
| NH4-Ν | 49.4 | 63.8 | −4.334 | 78 | 0.001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntountounakis, I.; Margaritou, I.-E.; Pervelis, I.; Kyrou, P.; Parlakidis, P.; Gikas, G.D. Pollutant Removal Efficiency of Pilot-Scale Horizontal Subsurface Flow Constructed Wetlands Treating Landfill Leachate. Appl. Sci. 2025, 15, 2595. https://doi.org/10.3390/app15052595
Ntountounakis I, Margaritou I-E, Pervelis I, Kyrou P, Parlakidis P, Gikas GD. Pollutant Removal Efficiency of Pilot-Scale Horizontal Subsurface Flow Constructed Wetlands Treating Landfill Leachate. Applied Sciences. 2025; 15(5):2595. https://doi.org/10.3390/app15052595
Chicago/Turabian StyleNtountounakis, Ioannis, Ioanna-Eirini Margaritou, Ioannis Pervelis, Pavlos Kyrou, Paraskevas Parlakidis, and Georgios D. Gikas. 2025. "Pollutant Removal Efficiency of Pilot-Scale Horizontal Subsurface Flow Constructed Wetlands Treating Landfill Leachate" Applied Sciences 15, no. 5: 2595. https://doi.org/10.3390/app15052595
APA StyleNtountounakis, I., Margaritou, I.-E., Pervelis, I., Kyrou, P., Parlakidis, P., & Gikas, G. D. (2025). Pollutant Removal Efficiency of Pilot-Scale Horizontal Subsurface Flow Constructed Wetlands Treating Landfill Leachate. Applied Sciences, 15(5), 2595. https://doi.org/10.3390/app15052595








