Growth and Rhamnolipid Production Performance of Pseudomonas aeruginosa on Crude Biomass Carbohydrates and Bioenhancer-Based Growth Media
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Media Preparation
2.3. Culture Conditions and Sampling Details
2.4. Design of Response Surface Experiment and Statistical Analyses for Bioscreen Study
2.5. Experimental Design and Determination of Rhamnolipid Concentration in 100 mL Shake-Flask Experiments
3. Results and Discussion
3.1. Results
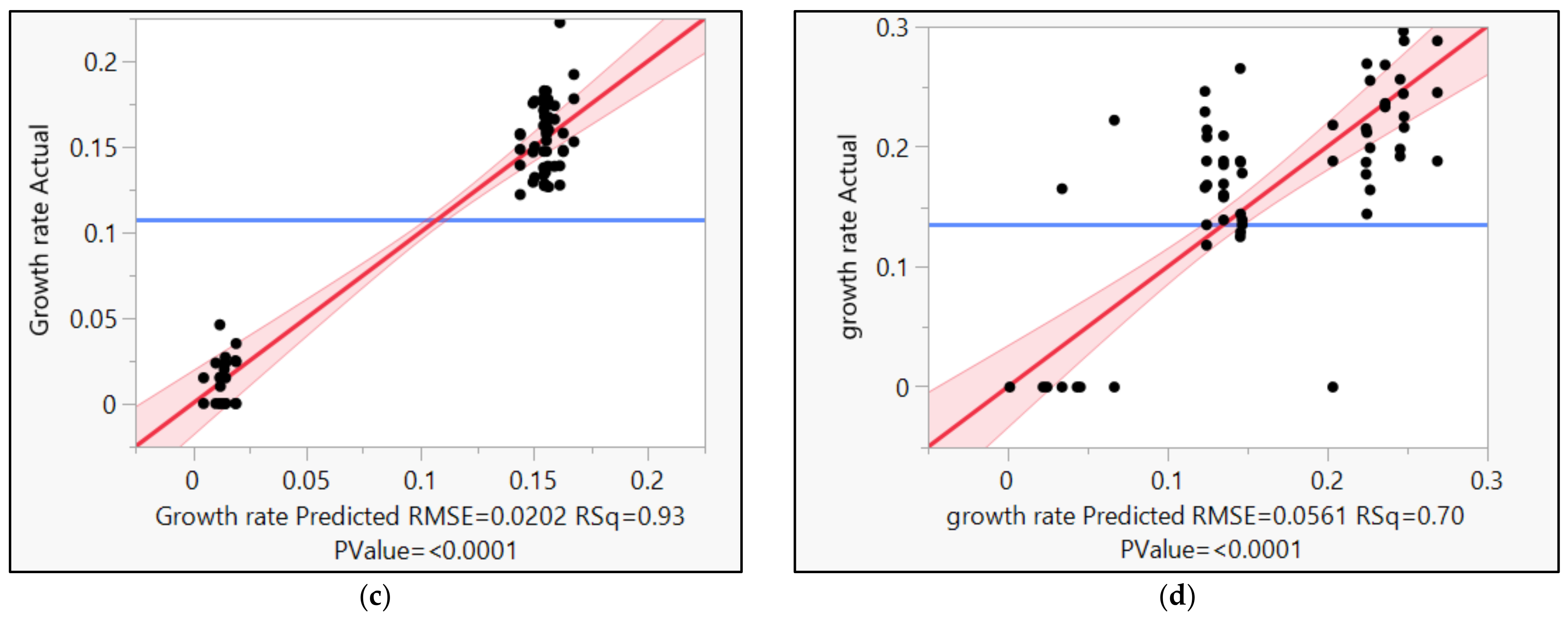
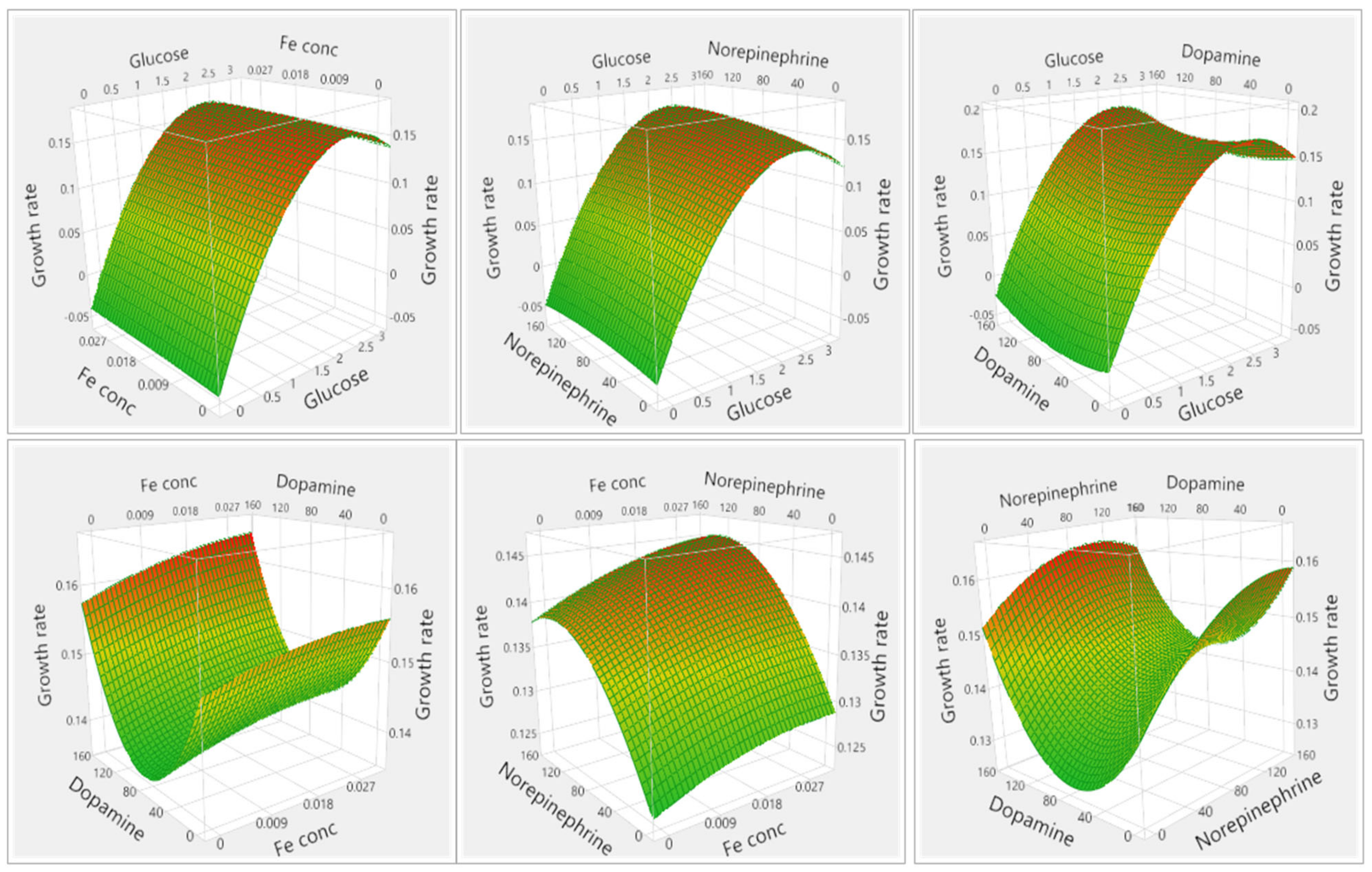
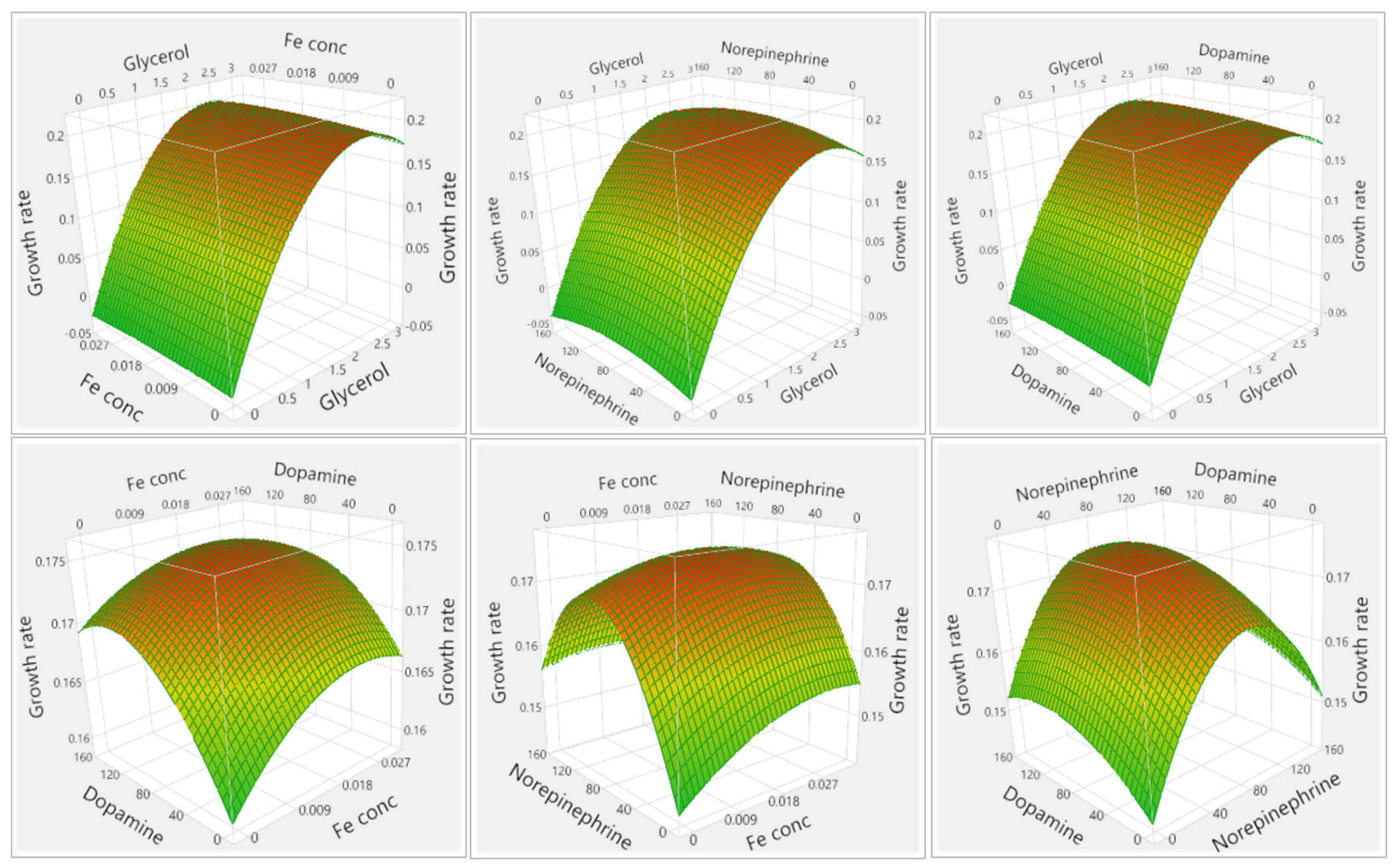
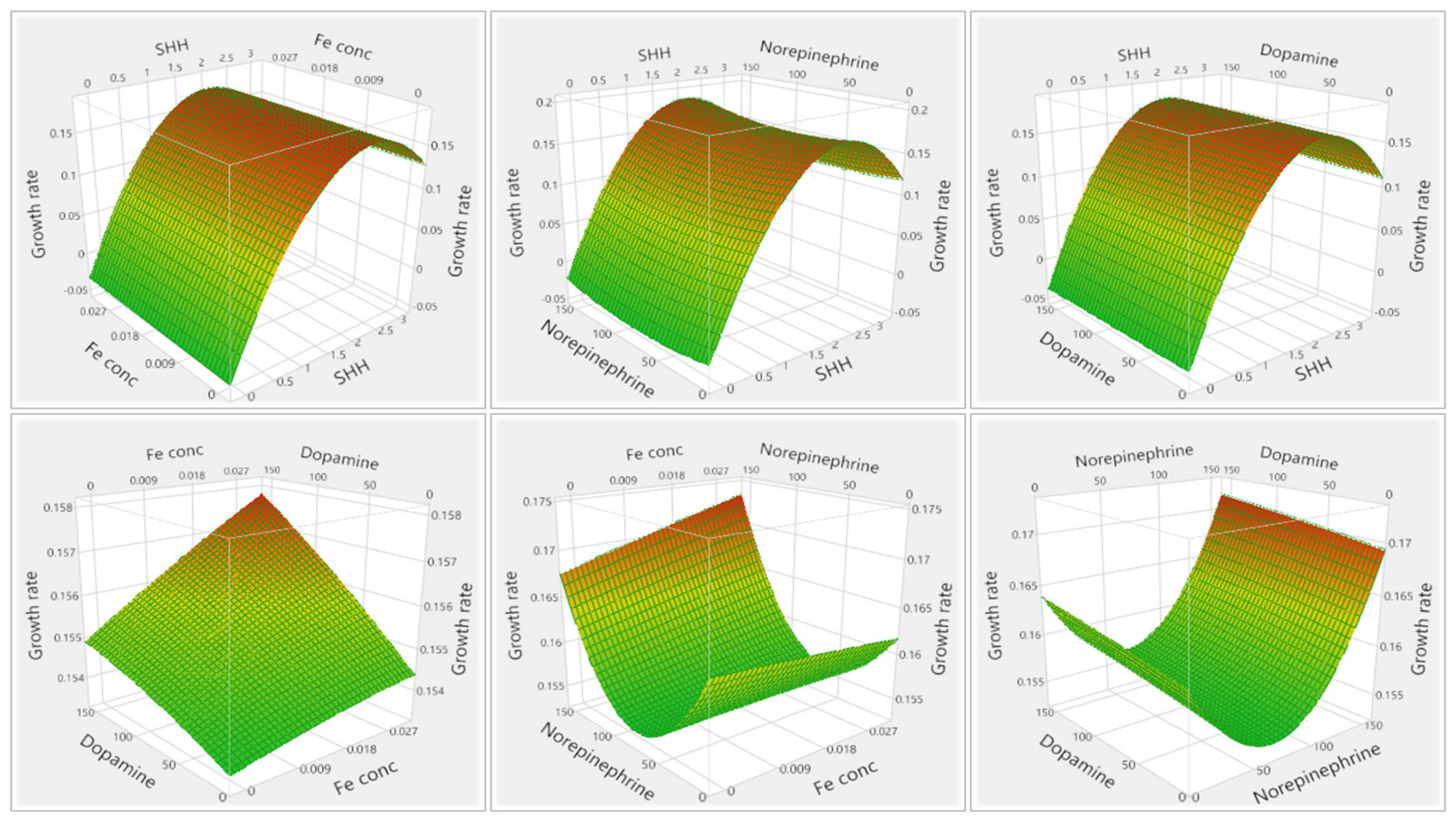
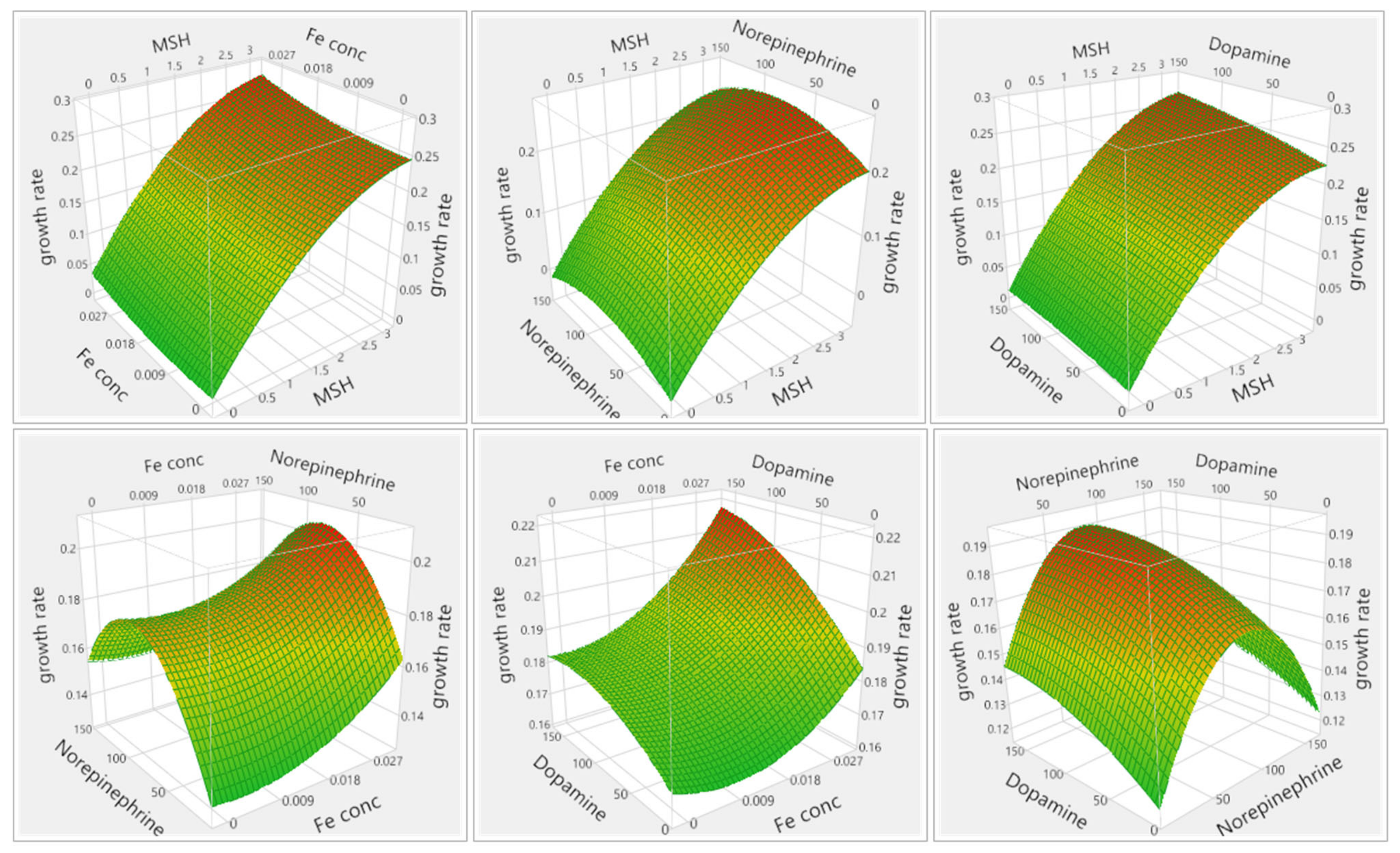
3.1.1. Results of Media Optimization with High-Throughput Bioscreen Study
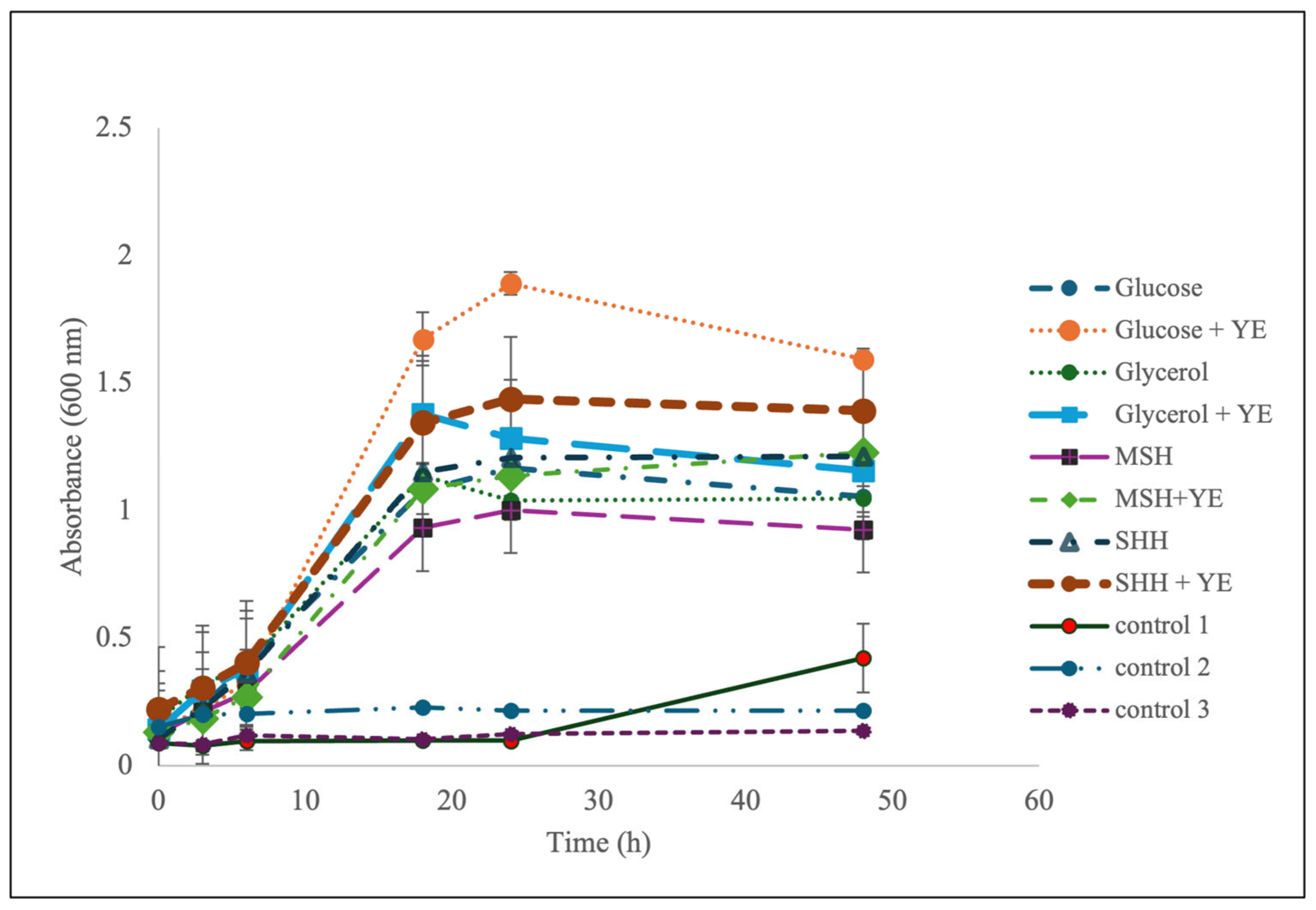
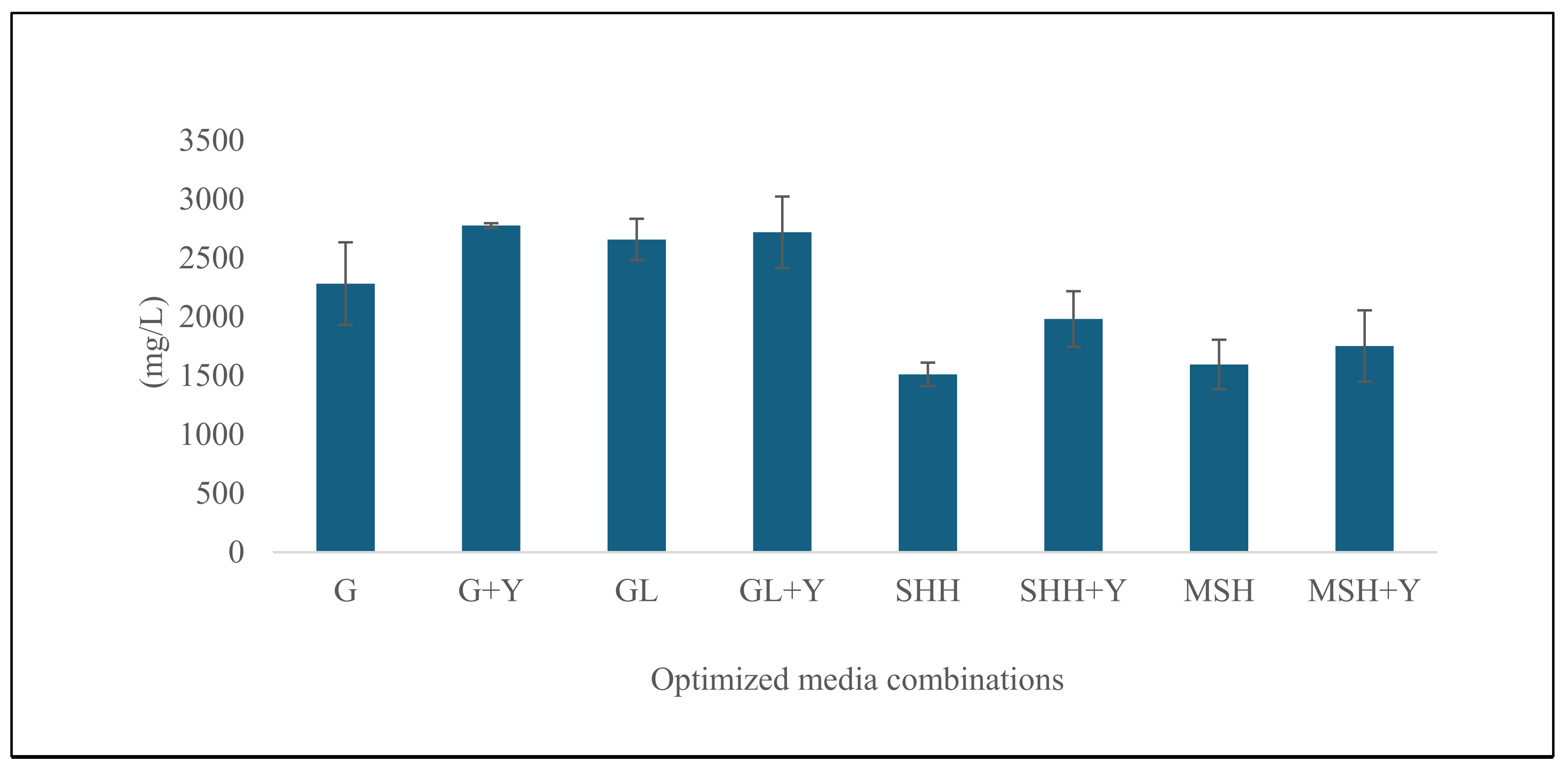
3.1.2. Results of Verification of Optimized Conditions in 100 mL Shake-Flask Study for Growth Performance and Rhamnolipid Titers
3.2. Discussion
3.2.1. Analysis of Media Optimization with High-Throughput Bioscreen Study
3.2.2. Analysis of Verification of Optimized Conditions in 100 mL Shake-Flask Study for Growth Performance and Rhamnolipid Titers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allied Market Research. Available online: https://www.alliedmarketresearch.com/surfactant-market (accessed on 2 December 2023).
- Jimoh, A.A. Biosurfactant: A new frontier for greener technology and environmental sustainability. Ecotox Environ. Safe 2019, 184, 109607. [Google Scholar] [CrossRef] [PubMed]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications, and current developments. Bioresource Technol. 2021, 330, 124963. [Google Scholar] [CrossRef] [PubMed]
- Paulino, B.N.; Pessoa, M.G.; Mano, M.C.R.; Molina, G.; Neri-Numa, I.A.; Pastore, G.M. Current status in biotechnological production and applications of glycolipid biosurfactants. Appl. Microbiol. Biotechnol. 2016, 100, 10265–10293. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Lépine, F.; Déziel, E. Rhamnolipids: Diversity of structures, microbial origins and roles. Appl. Microbiol. and Biotechnol. 2010, 86, 1323–1336. [Google Scholar] [CrossRef]
- Cortés-Sánchez, A.J.; Hernández-Sánchez, H.; Jaramillo-Flores, M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013, 168, 22–32. [Google Scholar] [CrossRef]
- Maier, R.M.; Soberón-Chávez, G. Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl. Microbiol. Biotechnol. 2000, 54, 625–633. [Google Scholar] [CrossRef]
- Fu, H.; Chai, T.; Huang, G.; Gao, P.; Liu, Z. Effects of rhamnolipid on the adsorption of Pb2+ onto compost humic acid. Desalin. Water Treat. 2015, 54, 3177–3183. [Google Scholar] [CrossRef]
- DeSanto, K. Rhamnolipid-Based Formulations. U.S. Patent 8,183,198 B2, 22 May 2012. [Google Scholar]
- Lourith, N.; Kanlayavattanakul, M. Natural surfactants used in cosmetics: Glycolipids. Int. J. Cosmet. 2009, 31, 255–261. [Google Scholar] [CrossRef]
- Henkel, M.; Muller, M.M.; Kugler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausman, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost-effective technologies and renewable substrates for biosurfactants’ production. Front. Microbiol. 2012, 5, 697. [Google Scholar]
- Sharma, R.; Lamsal, B.P.; Colonna, W.J. Pretreatment of fibrous biomass and growth of biosurfactant-producing Bacillus subtilis on biomass-derived fermentable sugars. Bioproc. Biosyst. Eng. 2016, 15, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Lamsal, B.P.; Mba-Wright, M. Performance of Bacillus subtilis on fibrous biomass sugar hydrolysates in producing biosurfactants and techno-economic comparison. Bioproc. Biosyst. Eng. 2018, 41, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.; Wood, T.K.; Jayaraman, A. The neuroendocrine hormone norepinephrine increases Pseudomonas aeruginosa PA14 virulence through the las quorum-sensing pathway. Appl. Microbiol. Biotechnol. 2009, 84, 763–776. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lyte, M.; Freestone, P.P.; Ajmal, A.; Colmer-Hamood, J.A.; Hamood, A.N. Norepinephrine represses the expression of toxA and the siderophore genes in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2009, 299, 100–109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lyte, M.; Ernst, S. Catecholamine induced growth of gram-negative bacteria. Life Sci. 1992, 50, 203–212. [Google Scholar] [CrossRef]
- Gunther, N.W.; Nunez, A.; Fett, W.; Solaiman, D.K. Production of rhamnolipids by Pseudomonas chlororaphis, a nonpathogenic bacterium. Appl. Environ. Microbiol. 2016, 71, 2288–2293. [Google Scholar] [CrossRef]
- Zhao, F.; Liang, X.; Ban, Y.; Han, S.; Zhang, J.; Zhang, Y.; Ma, F. Comparison of Methods to Quantify Rhamnolipid and Optimization of Oil Spreading Method. Tenside Surfact. Det. 2016, 53, 243–248. [Google Scholar] [CrossRef]
- Kim, S.K.; Lee, J.H. Biofilm dispersion in Pseudomonas aeruginosa. J. Microbiol. 2016, 54, 71–85. [Google Scholar] [CrossRef]
- Dasgupta, S.; Yang, C.; Castro, L.M.; Tashima, A.K.; Ferro, E.S.; Moir, R.; Willis, I.M.; Fricker, L.D. Analysis of the Yeast Peptidome and Comparison with the Human Peptidome. PLoS ONE 2016, 11, e163312. [Google Scholar] [CrossRef]
- Santa-Anna, L.; Sebastian, G.; Menezes, E.; Alves, T.; Santos, A.; Pereira, N., Jr.; Freire, D.M.G. Production of biosurfactants from Pseudomonas aeruginosa PA1 isolated in oil environments. Braz. J. Chem. Eng. 2002, 19, 159–166. [Google Scholar] [CrossRef]
- Chen, S.Y.; Lu, W.B.; Wie, Y.H.; Chen, W.M.; Chang, J.S. Improved production of biosurfactant with newly isolated Pseudomonas aeruginosa S2. Biotechnol. Prog. 2007, 23, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Syldatk, C.; Lang, S.; Wagner, F.; Wray, V.; Witte, L. Chemical and physical characterization of four interfacial-active rhamnolipids from Pseudomonas spec. DSM 2874 grown on n-alkanes. Z. Naturforsch. 1985, 40, 51–60. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, R.; Lamsal, B.P. Growth and Rhamnolipid Production Performance of Pseudomonas aeruginosa on Crude Biomass Carbohydrates and Bioenhancer-Based Growth Media. Appl. Sci. 2025, 15, 2531. https://doi.org/10.3390/app15052531
Sharma R, Lamsal BP. Growth and Rhamnolipid Production Performance of Pseudomonas aeruginosa on Crude Biomass Carbohydrates and Bioenhancer-Based Growth Media. Applied Sciences. 2025; 15(5):2531. https://doi.org/10.3390/app15052531
Chicago/Turabian StyleSharma, Rajat, and Buddhi P. Lamsal. 2025. "Growth and Rhamnolipid Production Performance of Pseudomonas aeruginosa on Crude Biomass Carbohydrates and Bioenhancer-Based Growth Media" Applied Sciences 15, no. 5: 2531. https://doi.org/10.3390/app15052531
APA StyleSharma, R., & Lamsal, B. P. (2025). Growth and Rhamnolipid Production Performance of Pseudomonas aeruginosa on Crude Biomass Carbohydrates and Bioenhancer-Based Growth Media. Applied Sciences, 15(5), 2531. https://doi.org/10.3390/app15052531





