Preparation and Characterization of Poly(butylene succinate) Films Modified with Sea Buckthorn (Hippophae rhamnoides L.) Extract for Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Sea Buckthorn Chloroform Extracts
2.3. Preparation of PBS-Based Films
2.4. Determination of Moisture Content
2.5. Thickness and Mechanical Properties
2.6. Spectral Analysis of Films
2.7. Film Color Analysis
2.8. Antioxidant Potential of Films
2.9. Antimicrobial Activity
2.10. Microscopic Analysis
2.11. Statistical Analyses
3. Results and Discussion
3.1. Moisture Content
3.2. The Thickness and Mechanical Properties
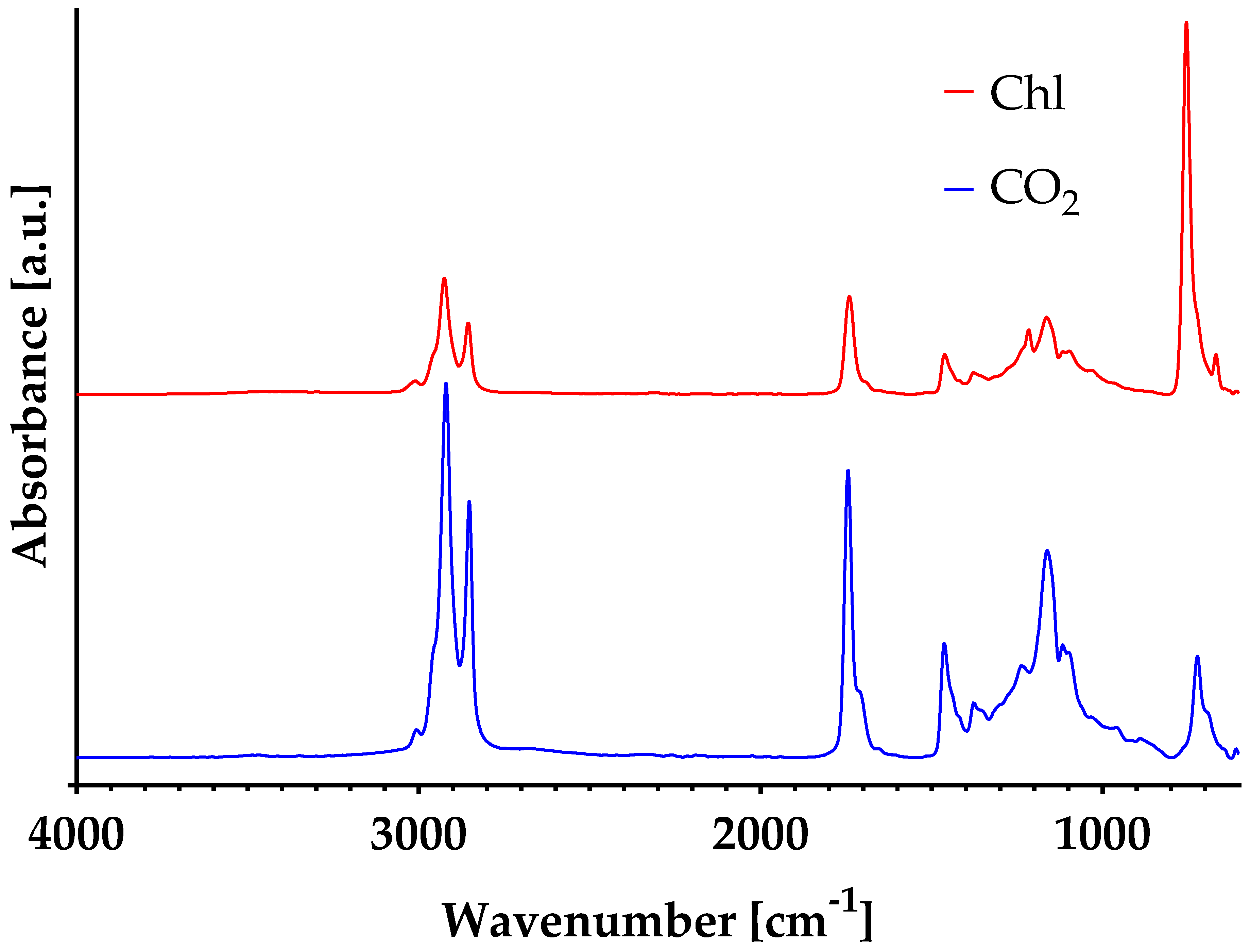
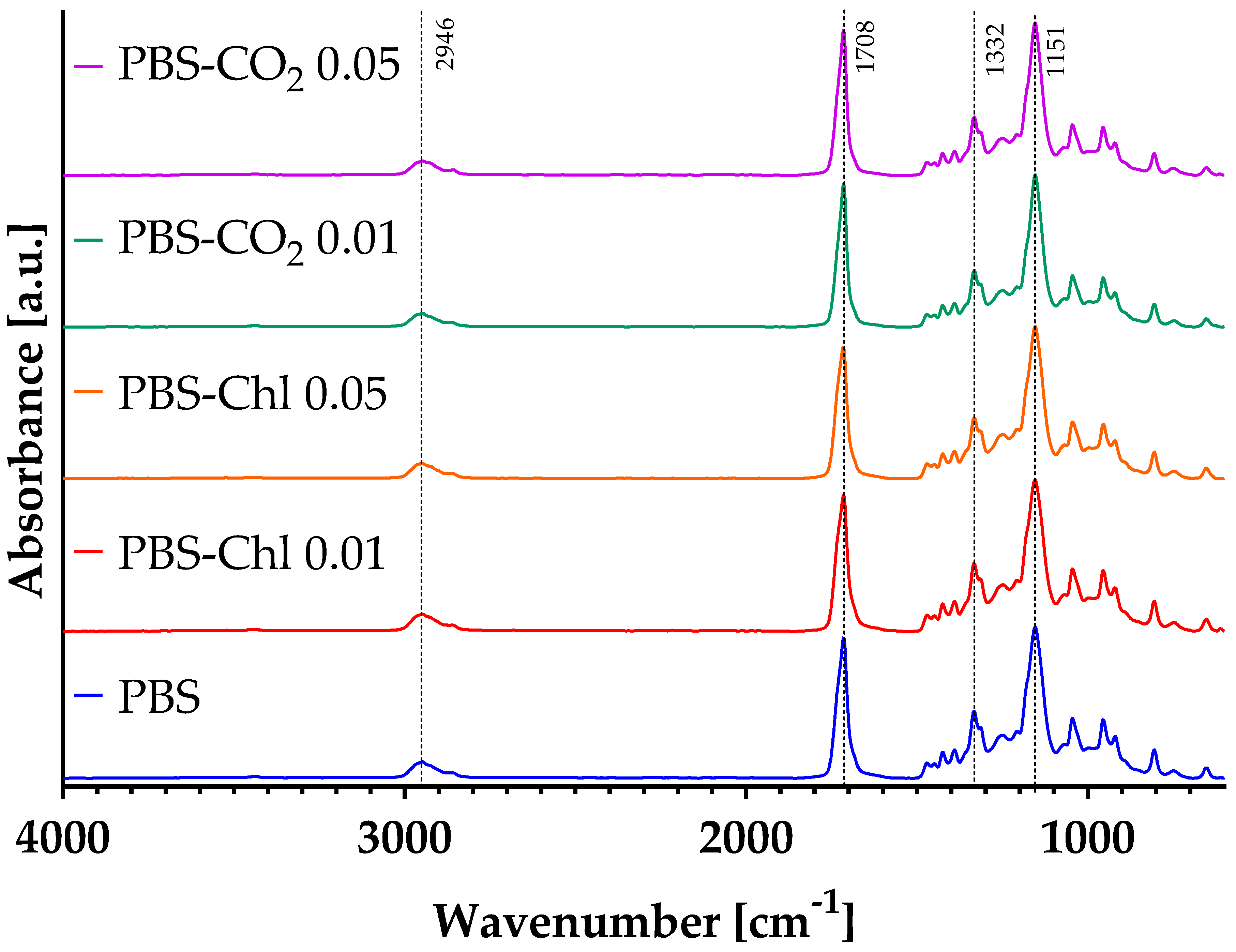
3.3. Spectral Analysis
3.4. Color Measurement
3.5. Antioxidant Characteristics
3.6. Antimicrobial Characteristics
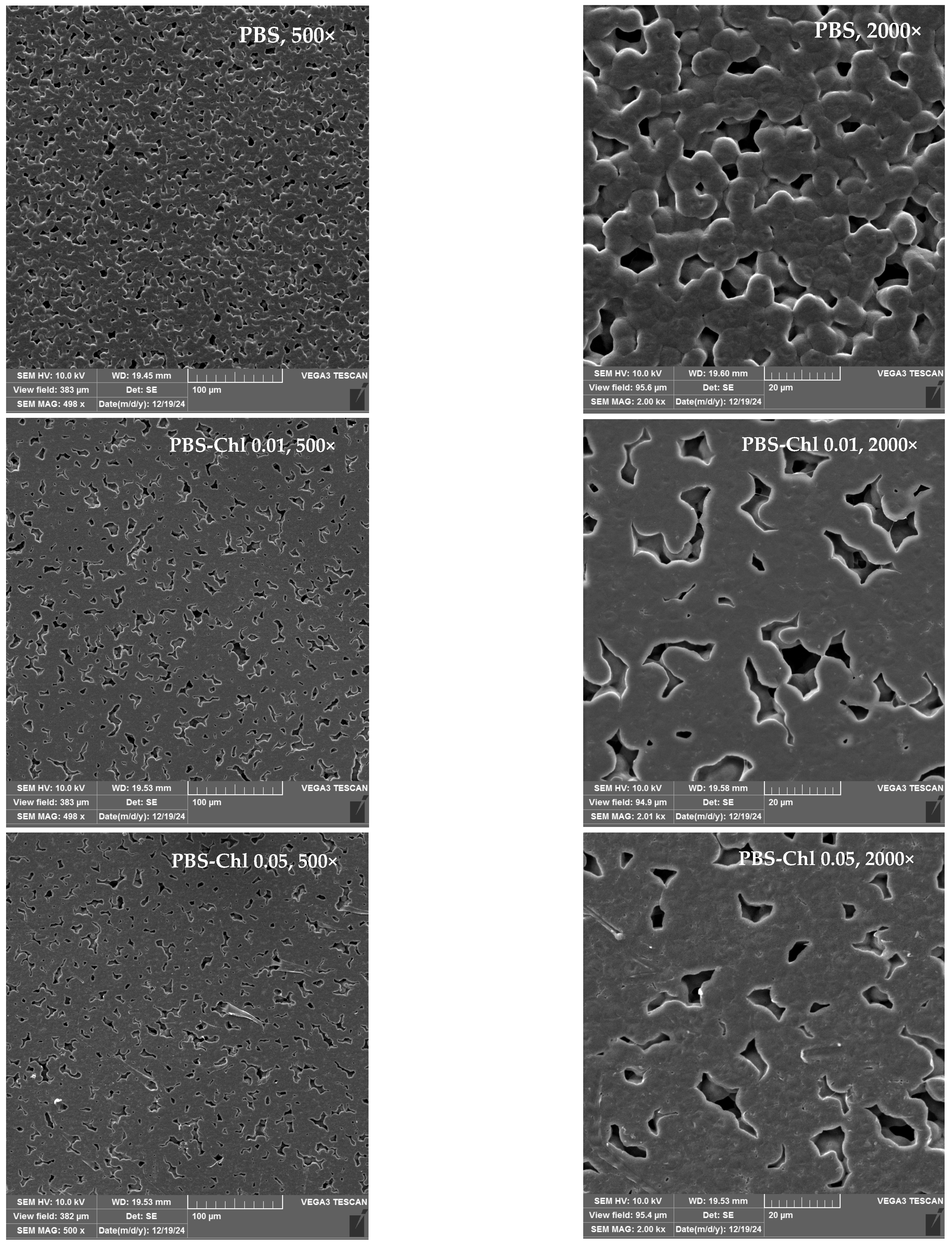
3.7. Microscopic Examination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, S.; Singh, E.; Mishra, R.; Kumar, A.; Caucci, S. Utilization of Plastic Wastes for Sustainable Environmental Management: A Review. ChemSusChem 2021, 14, 3985–4006. [Google Scholar] [CrossRef] [PubMed]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-Based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate)(PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. Food Packaging Materials with Special Reference to Biopolymers-Properties and Applications. Chem. Afr. 2022, 6, 117–144. [Google Scholar] [CrossRef]
- Kola, V.; Carvalho, I.S. Plant Extracts as Additives in Biodegradable Films and Coatings in Active Food Packaging. Food Biosci. 2023, 54, 102860. [Google Scholar] [CrossRef]
- Shavisi, N.; Khanjari, A.; Basti, A.A.; Misaghi, A.; Shahbazi, Y. Effect of PLA Films Containing Propolis Ethanolic Extract, Cellulose Nanoparticle and Ziziphora Clinopodioides Essential Oil on Chemical, Microbial and Sensory Properties of Minced Beef. Meat Sci. 2017, 124, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Sivakanthan, S.; Rajendran, S.; Gamage, A.; Madhujith, T.; Mani, S. Antioxidant and Antimicrobial Applications of Biopolymers: A Review. Food Res. Int. 2020, 136, 109327. [Google Scholar] [CrossRef] [PubMed]
- Petchwattana, N.; Naknaen, P. Utilization of Thymol as an Antimicrobial Agent for Biodegradable Poly(Butylene Succinate). Mater. Chem. Phys. 2015, 163, 369–375. [Google Scholar] [CrossRef]
- Wiburanawong, S.; Petchwattana, N.; Covavisaruch, S. Carvacrol as an Antimicrobial Agent for Poly(Butylene Succinate): Tensile Properties and Antimicrobial Activity Observations. Adv. Mat. Res. 2014, 931–932, 111–115. [Google Scholar] [CrossRef]
- Mohamad, N.; Mazlan, M.M.; Tawakkal, I.S.M.A.; Talib, R.A.; Kian, L.K.; Jawaid, M. Characterization of Active Polybutylene Succinate Films Filled Essential Oils for Food Packaging Application. J. Polym. Environ. 2022, 30, 585–596. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Zdanowicz, M.; Macieja, S.; Kowalczyk, K.; Bartkowiak, A. Development and Characterization of Bioactive Poly(Butylene-Succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers 2021, 13, 1798. [Google Scholar] [CrossRef]
- Pothinuch, P.; Promsorn, J.; Sablani, S.S.; Harnkarnsujarit, N. Antioxidant Release, Morphology and Packaging Properties of Gallic Acid Incorporated Biodegradable PBAT Blended PBS Active Packaging. Food Packag. Shelf Life 2024, 43, 101304. [Google Scholar] [CrossRef]
- Basbasan, A.J.; Hararak, B.; Winotapun, C.; Wanmolee, W.; Chinsirikul, W.; Leelaphiwat, P.; Chonhenchob, V.; Boonruang, K. Lignin Nanoparticles for Enhancing Physicochemical and Antimicrobial Properties of Polybutylene Succinate/Thymol Composite Film for Active Packaging. Polymers 2023, 15, 989. [Google Scholar] [CrossRef] [PubMed]
- Nansu, W.; Ross, S.; Waisarikit, A.; Ross, G.M.; Charoensit, P.; Suphrom, N.; Mahasaranon, S. Exploring the Potential of Roselle Calyx and Sappan Heartwood Extracts as Natural Colorants in Poly(Butylene Succinate) for Biodegradable Packaging Films. Polymers 2023, 15, 4193. [Google Scholar] [CrossRef] [PubMed]
- Łopusiewicz, Ł.; Macieja, S.; Bartkowiak, A.; El Fray, M. Antimicrobial, Antibiofilm, and Antioxidant Activity of Functional Poly(Butylene Succinate) Films Modified with Curcumin and Carvacrol. Materials 2021, 14, 7882. [Google Scholar] [CrossRef]
- Tereshchuk, L.V.; Starovoitova, K.V.; Vyushinsky, P.A.; Zagorodnikov, K.A. The Use of Sea Buckthorn Processing Products in the Creation of a Functional Biologically Active Food Emulsion. Foods 2022, 11, 2226. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhang, J.; Song, H.; Zhu, W.; Liu, Y. Antioxidant Activity and Phenolic Components of Sea Buckthorn (Hippophae rhamnoides) Seed Extracts. In Proceedings of the 2013 International Conference on Advanced Mechatronic Systems, Luoyang, China, 25–27 September 2013; pp. 96–101. [Google Scholar] [CrossRef]
- Rajkowska, K.; Rykała, E.; Czyżowska, A. Antibacterial Effect of Sea Buckthorn (Hippophae rhamnoides L.) Fruit Extract on Radish Seeds Prior to Sprouting. Pol. J. Food Nutr. Sci. 2024, 74, 120–129. [Google Scholar] [CrossRef]
- Feng, K.; Feng, X.; Tan, W.; Zheng, Q.; Zhong, W.; Liao, C.; Liu, Y.; Li, S.; Hu, W. Development of a Food Preservative from Sea Buckthorn Together with Chitosan: Application in and Characterization of Fresh-Cut Lettuce Storage. Front. Microbiol. 2023, 14, 1080365. [Google Scholar] [CrossRef] [PubMed]
- Rather, S.A.; Hussain, P.R.; Suradkar, P. Evaluating the Effects of Chitosan Incorporated with Seabuckthorn Leaf Extract Composite Edible Coatings on the Shelf Life of Peach (Prunus persica L.) Fruits. Food Humanit. 2024, 2, 100280. [Google Scholar] [CrossRef]
- Ali, A.; Banoo, M.; Banoo, H.; Ali, G. Fabrication and Characterization of Citric Acid Crosslinked, Sea Buckthorn Leaves Extract Incorporated PVA-Based Films with Improved Antioxidative and UV-Shielding Properties for Food Packaging Applications. J. Packag. Technol. Res. 2024, 8, 179–193. [Google Scholar] [CrossRef]
- Guo, Z.; Han, L.; Yu, Q.L.; Lin, L. Effect of a Sea Buckthorn Pomace Extract-Esterified Potato Starch Film on the Quality and Spoilage Bacteria of Beef Jerky Sold in Supermarket. Food Chem. 2020, 326, 127001. [Google Scholar] [CrossRef] [PubMed]
- Rather, S.A.; Mir, N.A. Effect of Carboxymethyl Cellulose Enriched with Seabuckthorn (Hippophaerhamnoides L.) Leaf Extract Edible Coatings on the Quality of Fresh Cut “Maharaji” Apple. Food Humanit. 2023, 1, 571–580. [Google Scholar] [CrossRef]
- Reverchon, E.; De Marco, I. Supercritical Fluid Extraction and Fractionation of Natural Matter. J. Supercrit. Fluids 2006, 38, 146–166. [Google Scholar] [CrossRef]
- ASTM D822-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM: West Conshohocken, PA, USA, 2018.
- Zdanowicz, M.; Mizielińska, M.; Kowalczyk, A. Cast Extruded Films Based on Polyhydroxyalkanoate/Poly(Lactic Acid) Blend with Herbal Extracts Hybridized with Zinc Oxide. Polymers 2024, 16, 1954. [Google Scholar] [CrossRef] [PubMed]
- Topală, C.M.; Mazilu, I.C.; Vulpe, M.; Vîjan, L.E. Quality study of fruits and extracts from six romanian sea buckthorn varieties. Curr. Trends Nat. Sci. 2020, 9, 273–283. [Google Scholar] [CrossRef]
- de Matos Costa, A.R.; Crocitti, A.; de Carvalho, L.H.; Carroccio, S.C.; Cerruti, P.; Santagata, G. Properties of Biodegradable Films Based on Poly(Butylene Succinate) (PBS) and Poly(Butylene Adipate-Co-Terephthalate) (PBAT) Blends. Polymers 2020, 12, 2317. [Google Scholar] [CrossRef] [PubMed]
- Sławińska, N.; Żuchowski, J.; Stochmal, A.; Olas, B. Extract from Sea Buckthorn Seeds—A Phytochemical, Antioxidant, and Hemostasis Study; Effect of Thermal Processing on Its Chemical Content and Biological Activity In Vitro. Nutrients 2023, 15, 686. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, Antioxidant and Phytochemical Investigations of Sea Buckthorn (Hippophaë rhamnoides L.) Leaf, Stem, Root and Seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Merijs-Meri, R.; Zicans, J.; Ivanova, T.; Mezule, L.; Ivanickins, A.; Bockovs, I.; Bitenieks, J.; Berzina, R.; Lebedeva, A. Melt-Processed Polybutylene-Succinate Biocomposites with Chitosan: Development and Characterization of Rheological, Thermal, Mechanical and Antimicrobial Properties. Polymers 2024, 16, 2808. [Google Scholar] [CrossRef]
- Brobbey, K.J.; Saarinen, J.J.; Alakomi, H.-L.; Yang, B.; Toivakka, M. Efficacy of Natural Plant Extracts in Antimicrobial Packaging Systems. J. Appl. Packag. Res. 2017, 9, 60–71. [Google Scholar]
- Mizielińska, M.; Zdanowicz, M.; Tarnowiecka-Kuca, A.; Bartkowiak, A. The Influence of Functional Composite Coatings on the Properties of Polyester Films before and after Accelerated UV Aging. Materials 2024, 17, 3048. [Google Scholar] [CrossRef] [PubMed]
- Tallawi, M.; Rai, R.; R-Gleixner, M.; Roerick, O.; Weyand, M.; Roether, J.A.; Schubert, D.W.; Kozlowska, A.; Fray, M.E.; Merle, B.; et al. Poly(Glycerol Sebacate)\Poly(Butylene Succinate-Dilinoleate) Blends as Candidate Materials for Cardiac Tissue Engineering. Macromol. Symp. 2013, 334, 57–67. [Google Scholar] [CrossRef]
- Ebrahimpour, M.; Safekordi, A.A.; Mousavi, S.M.; Heydarinasab, A. A Miscibility Study on Biodegradable Poly Butylene Succinate/Polydioxanone Blends. J. Polym. Res. 2018, 25, 1–8. [Google Scholar] [CrossRef]
- Karakehya, N. Comparison of the Effects of Various Reinforcements on the Mechanical, Morphological, Thermal and Surface Properties of Poly(Butylene Succinate). Int. J. Adhes. Adhes. 2021, 110, 102949. [Google Scholar] [CrossRef]



| Sample Name | Type of Extract Added | g of Extract/1 g of PBS |
|---|---|---|
| PBS | - | - |
| PBS-Chl 0.01 | Chloroform extract | 0.01 |
| PBS-Chl 0.05 | Chloroform extract | 0.05 |
| PBS-CO2 0.01 | scCO2 extract | 0.01 |
| PBS-CO2 0.05 | scCO2 extract | 0.05 |
| Sample | MC (%) |
|---|---|
| PBS | 1.47 ± 0.30 c |
| PBS-Chl 0.01 | 1.73 ± 0.61 a,c |
| PBS-Chl 0.05 | 2.55 ± 0.45 a,b |
| PBS-CO2 0.01 | 2.54 ± 0.47 a,b |
| PBS-CO2 0.05 | 3.34 ± 0.58 b |
| Sample | Thickness (mm) | TS (MPa) | EB (%) |
|---|---|---|---|
| PBS | 0.019 ± 0.001 a | 6.16 ± 1.70 b,c | 14.48 ± 2.54 a |
| PBS-Chl 0.01 | 0.018 ± 0.001 a | 6.86 ± 2.23 c | 13.95 ± 2.59 a |
| PBS-Chl 0.05 | 0.020 ± 0.001 a | 3.95 ± 1.26 a | 14.02 ± 2.99 a |
| PBS-CO2 0.01 | 0.021 ± 0.002 a | 5.93 ± 1.34 a,b,c | 13.17 ± 1.93 a |
| PBS-CO2 0.05 | 0.022 ± 0.002 a | 4.15 ± 0.61 a,b | 13.74 ± 2.64 a |
| Sample | L* | a* | b* | ∆E | YI | Opacity |
|---|---|---|---|---|---|---|
| PBS | 64.40 ± 0.33 c | 14.49 ± 0.14 b | 39.27 ± 0.47 b | 0.00 | 87.12 ± 0.96 a | 14.26 ± 0.73 b,c |
| PBS-Chl 0.01 | 63.82 ± 0.18 a | 14.65 ± 0.13 a | 40.16 ± 0.56 a | 1.12 ± 0.48 a | 89.89 ± 1.32 c | 12.04 ± 1.52 a |
| PBS-Chl 0.05 | 64.75 ± 0.08 d | 14.62 ± 0.06 a | 40.17 ± 0.47 a | 1.01 ± 0.39 a | 88.62 ± 1.11 b | 15.70 ± 2.32 c |
| PBS-CO2 0.01 | 63.90 ± 0.34 a | 14.87 ± 0.02 c | 41.22 ± 0.32 d | 2.05 ± 0.39 b | 92.15 ± 1.18 d | 13.73 ± 3.01 a,b |
| PBS-CO2 0.05 | 61.94 ± 0.10 b | 16.11 ± 0.03 d | 46.68 ± 0.26 d | 7.97 ± 0.25 c | 107.67 ± 0.65 e | 18.80 ± 1.56 d |
| Sample | DPPH (%) |
|---|---|
| PBS | 1.74 ± 1.92 b |
| PBS-Chl 0.01 | 15.60 ± 2.91 a |
| PBS-Chl 0.05 | 26.44 ± 3.01 c |
| PBS-CO2 0.01 | 19.55 ± 2.07 a |
| PBS-CO2 0.05 | 41.13 ± 1.31 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macieja, S.; Bartkowiak, A.; Mizielińska, M. Preparation and Characterization of Poly(butylene succinate) Films Modified with Sea Buckthorn (Hippophae rhamnoides L.) Extract for Packaging Applications. Appl. Sci. 2025, 15, 2099. https://doi.org/10.3390/app15042099
Macieja S, Bartkowiak A, Mizielińska M. Preparation and Characterization of Poly(butylene succinate) Films Modified with Sea Buckthorn (Hippophae rhamnoides L.) Extract for Packaging Applications. Applied Sciences. 2025; 15(4):2099. https://doi.org/10.3390/app15042099
Chicago/Turabian StyleMacieja, Szymon, Artur Bartkowiak, and Małgorzata Mizielińska. 2025. "Preparation and Characterization of Poly(butylene succinate) Films Modified with Sea Buckthorn (Hippophae rhamnoides L.) Extract for Packaging Applications" Applied Sciences 15, no. 4: 2099. https://doi.org/10.3390/app15042099
APA StyleMacieja, S., Bartkowiak, A., & Mizielińska, M. (2025). Preparation and Characterization of Poly(butylene succinate) Films Modified with Sea Buckthorn (Hippophae rhamnoides L.) Extract for Packaging Applications. Applied Sciences, 15(4), 2099. https://doi.org/10.3390/app15042099







