Superior Photodegradation of Bentazon and Nile Blue and Their Binary Mixture Using Sol–Gel Synthesized TiO2 Nanoparticles Under UV and Sunlight Sources
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of TiO2 Nanoparticles
2.3. Photocatalytic Activity Experiment
2.4. Characterizations
3. Results and Discussions
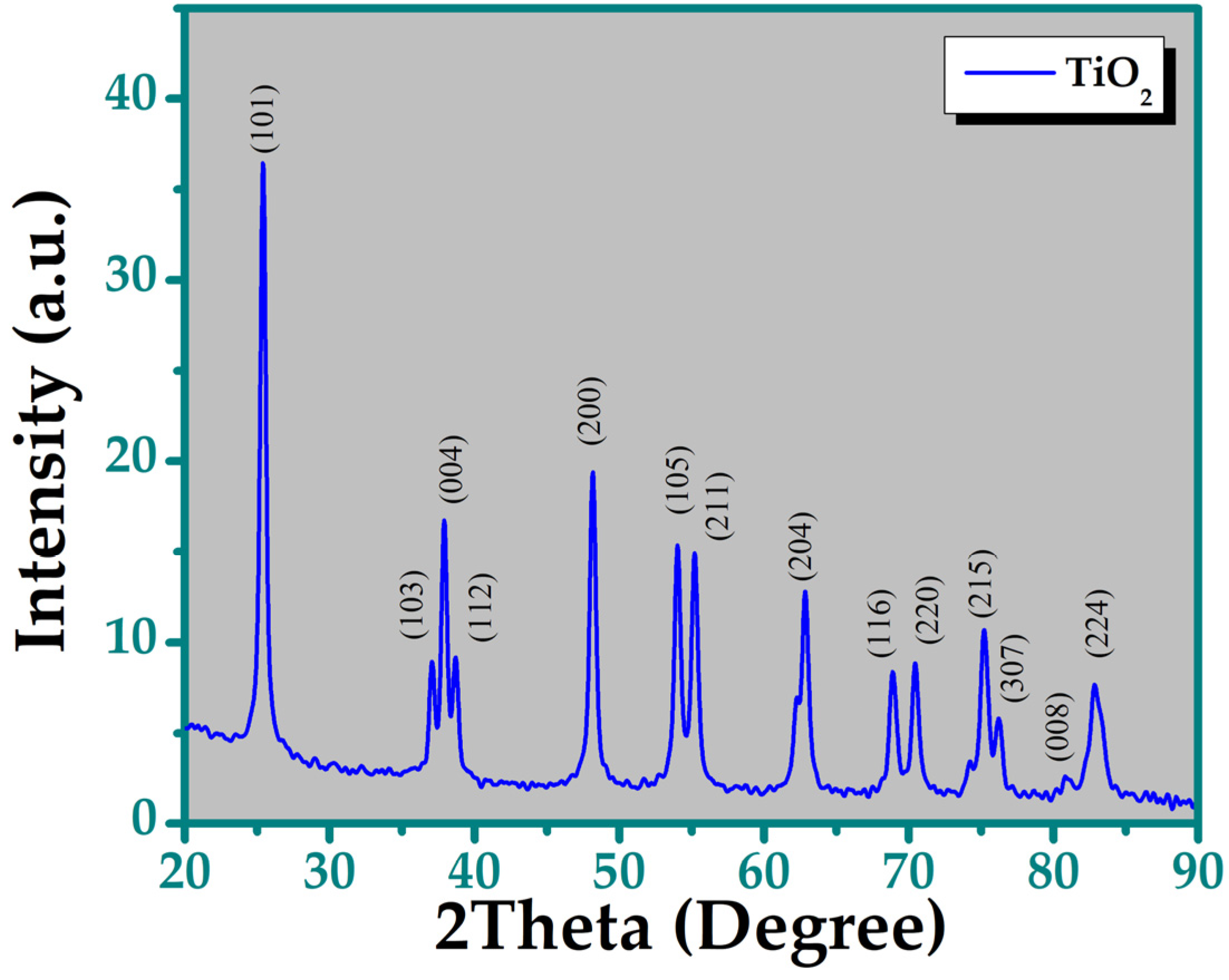
3.1. X-Ray Diffraction
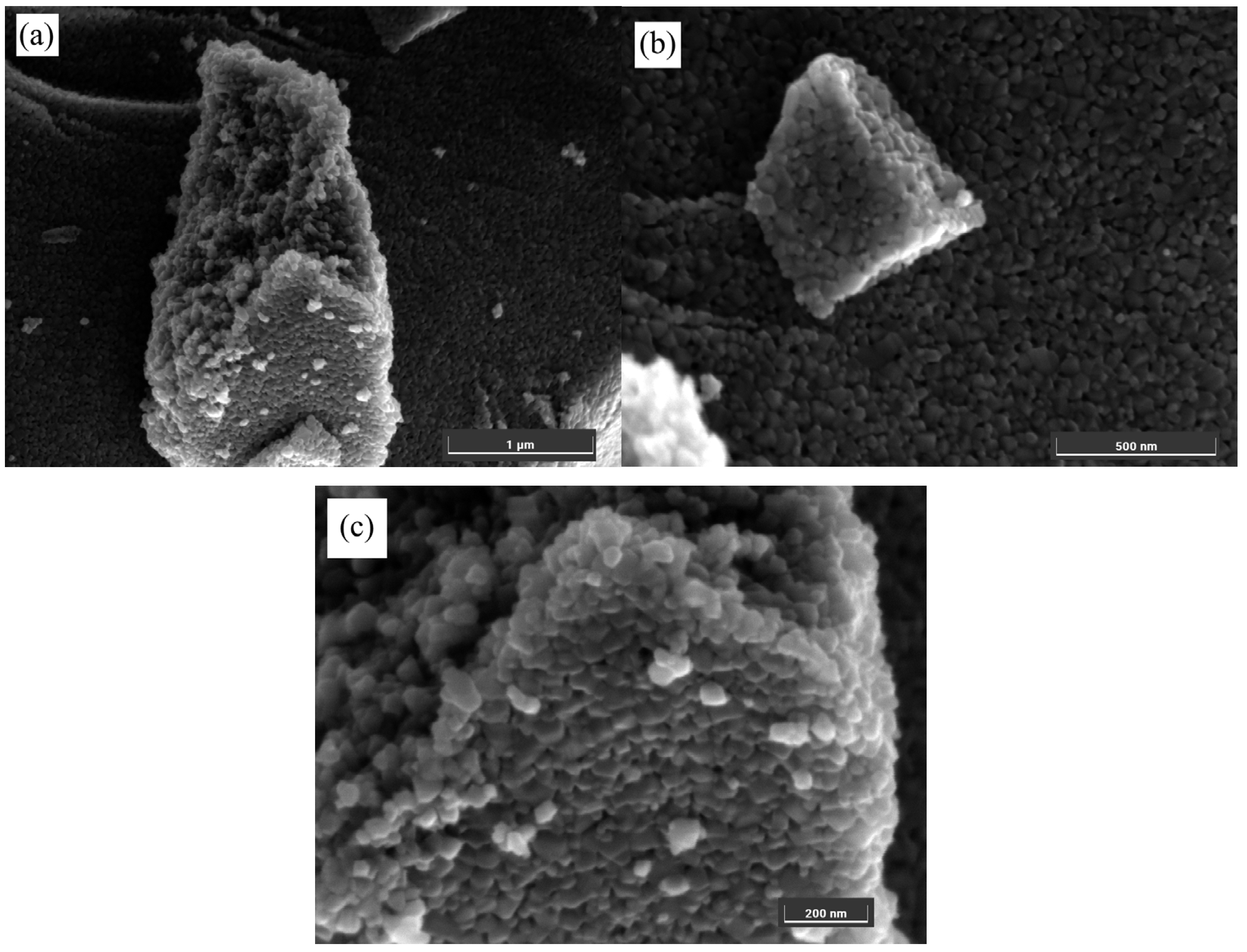
3.2. SEM Analysis
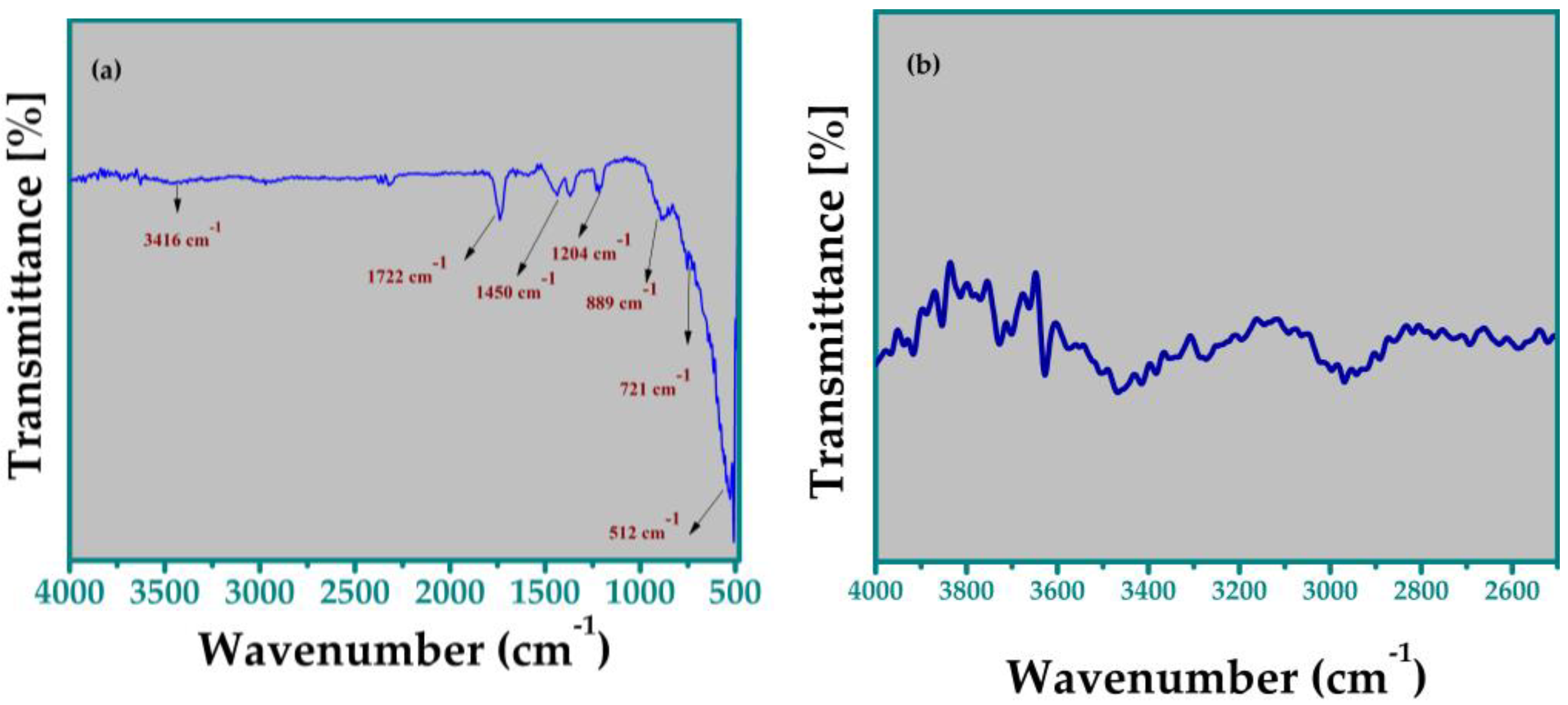
3.3. FTIR
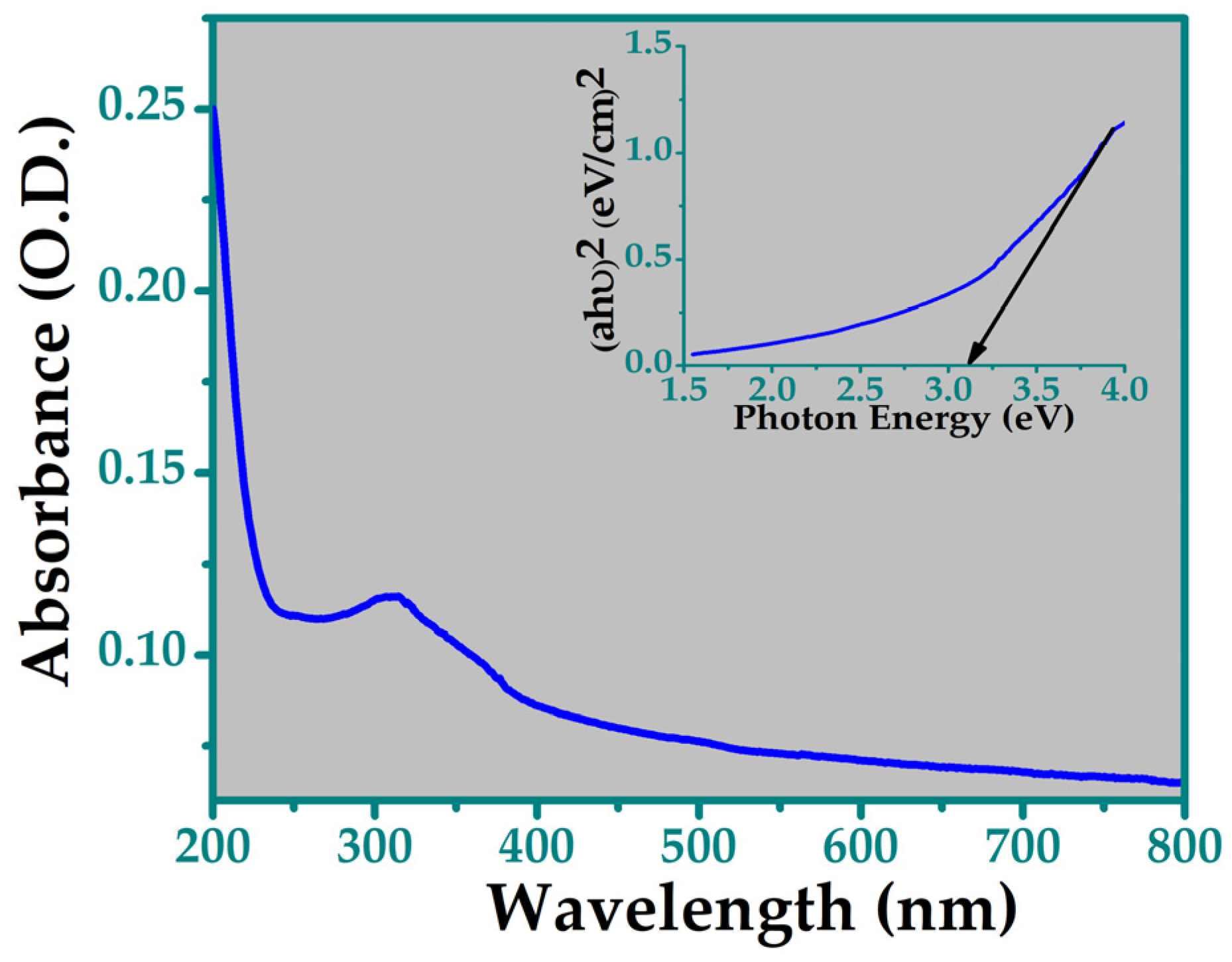
3.4. UV-Visible Spectroscopy
3.5. Photocatalytic Activity
3.5.1. Degradation of Bentazon Herbicide
3.5.2. Degradation of Nile Blue Dye
3.5.3. Simultaneous Degradation of Bentazon and Nile Blue
3.5.4. Scavenger Test
3.5.5. Stability and Reusability of TiO2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tölgyesi, Á.; Korozs, G.; Tóth, E.; Bálint, M.; Ma, X.; Sharma, V.K. Automation in Quantifying Phenoxy Herbicides and Bentazon in Surface Water and Groundwater Using Novel Solid Phase Extraction and Liquid Chromatography Tandem Mass Spectrometry. Chemosphere 2022, 286, 131927. [Google Scholar] [CrossRef] [PubMed]
- Guelfi, D.R.V.; Brillas, E.; Gozzi, F.; Machulek, A.; de Oliveira, S.C.; Sirés, I. Influence of Electrolysis Conditions on the Treatment of Herbicide Bentazon Using Artificial UVA Radiation and Sunlight. Identification of Oxidation Products. J. Environ. Manag. 2019, 231, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Negash, A.; Mohammed, S.; Weldekirstos, H.D.; Ambaye, A.D.; Gashu, M. Enhanced Photocatalytic Degradation of Methylene Blue Dye Using Eco-Friendly Synthesized RGO@ZnO Nanocomposites. Sci. Rep. 2023, 13, 22234. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Białecki, J.; Urbańska, K.; Bryś, M. The Effects of Natural and Synthetic Blue Dyes on Human Health: A Review of Current Knowledge and Therapeutic Perspectives. Adv. Nutr. 2021, 12, 2301–2311. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Hitam, C.N.C.; Jalil, A.A. A Review on Exploration of Fe2O3 Photocatalyst towards Degradation of Dyes and Organic Contaminants. J. Environ. Manag. 2020, 258, 110050. [Google Scholar] [CrossRef]
- Ferreira, S.H.; Morais, M.; Nunes, D.; Oliveira, M.J.; Rovisco, A.; Pimentel, A.; Águas, H.; Fortunato, E.; Martins, R. High UV and Sunlight Photocatalytic Performance of Porous ZnO Nanostructures Synthesized by a Facile and Fast Microwave Hydrothermal Method. Materials 2021, 14, 2385. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Hidalgo, M.C.; Bahnemann, D.W.; Geissen, S.U.; Murugesan, V.; Vogelpohl, A. A Fine Route to Tune the Photocatalytic Activity of TiO2. Appl. Catal. B Environ. 2006, 63, 31–40. [Google Scholar] [CrossRef]
- Zarubica, A.; Ljupković, R.; Papan, J.; Vukoje, I.; Porobić, S.; Ahrenkiel, S.P.; Nedeljković, J.M. Visible-Light-Responsive Al2O3 Powder: Photocatalytic Study. Opt. Mater. 2020, 106, 110013. [Google Scholar] [CrossRef]
- Krishnan, S.; Shriwastav, A. Application of TiO2 Nanoparticles Sensitized with Natural Chlorophyll Pigments as Catalyst for Visible Light Photocatalytic Degradation of Methylene Blue. J. Environ. Chem. Eng. 2021, 9, 104699. [Google Scholar] [CrossRef]
- Verma, V.; Al-Dossari, M.; Singh, J.; Rawat, M.; Kordy, M.G.M.; Shaban, M. A Review on Green Synthesis of TiO2 NPs: Synthesis and Applications in Photocatalysis and Antimicrobial. Polymers 2022, 14, 1444. [Google Scholar] [CrossRef]
- Banerjee, A.N.; Hamnabard, N.; Joo, S.W. A Comparative Study of the Effect of Pd-Doping on the Structural, Optical, and Photocatalytic Properties of Sol–Gel Derived Anatase TiO2 Nanoparticles. Ceram. Int. 2016, 42, 12010–12026. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A Critical Review on Application of Photocatalysis for Toxicity Reduction of Real Wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Racovita, A.D. Titanium Dioxide: Structure, Impact, and Toxicity. Int. J. Environ. Res. Public Health 2022, 19, 5681. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zeng, T. Preparation of TiO2 Anatase Nanocrystals by TiCl4 Hydrolysis with Additive H2SO4. PLoS ONE 2011, 6, e21082. [Google Scholar] [CrossRef]
- Lee, D.S.; Liu, T.K. Preparation of TiO2 Sol Using TiCl4 as a Precursor. J. Sol-Gel Sci. Technol. 2002, 25, 121–136. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Cao, X.; Wu, S.; Liu, C.; Li, G.; Jiang, W.; Wang, H.; Wang, N.; Ding, W. Preparation and Characterization of TiO2 Nanoparticles by Two Different Precipitation Methods. Ceram. Int. 2020, 46, 15333–15341. [Google Scholar] [CrossRef]
- Elsellami, L.; Dappozze, F.; Fessi, N.; Houas, A.; Guillard, C. Highly Photocatalytic Activity of Nanocrystalline TiO2 (Anatase, Rutile) Powders Prepared from TiCl4 by Sol–Gel Method in Aqueous Solutions. Process Saf. Environ. Prot. 2018, 113, 109–121. [Google Scholar] [CrossRef]
- Subhan, M.A.; Uddin, N.; Sarker, P.; Azad, A.K.; Begum, K. Photoluminescence, Photocatalytic and Antibacterial Activities of CeO2·CuO·ZnO Nanocomposite Fabricated by Co-Precipitation Method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 149, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, Y.N.; Magdalane, C.M.; Ramalingam, G.; Kumar, L.B.; Alwadai, N.; Al-Buriahi, M.S. Photocatalytic Activity of Hierarchical CTAB-Assisted TiO2 Nanoparticles for Polluted Water Treatment Using Solar Light Illumination. Appl. Phys. A 2022, 128, 299. [Google Scholar] [CrossRef]
- Suresh, R.; Ponnuswamy, V.; Mariappan, R. Effect of Annealing Temperature on the Microstructural, Optical and Electrical Properties of CeO2 Nanoparticles by Chemical Precipitation Method. Appl. Surf. Sci. 2013, 273, 457–464. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Mushtaq, S.; Al Qahtani, H.S.; Sedky, A.; Alam, M.W. Investigation of Tio2 Nanoparticles Synthesized by Sol-Gel Method for Effectual Photodegradation, Oxidation and Reduction Reaction. Crystals 2021, 11, 1456. [Google Scholar] [CrossRef]
- Gholami, M.; Jonidi-Jafari, A.; Farzadkia, M.; Esrafili, A.; Godini, K.; Shirzad-Siboni, M. Photocatalytic Removal of Bentazon by Copper Doped Zinc Oxide Nanorods: Reaction Pathways and Toxicity Studies. J. Environ. Manag. 2021, 294, 112962. [Google Scholar] [CrossRef]
- Haque, F.Z.; Nandanwar, R.; Singh, P. Evaluating Photodegradation Properties of Anatase and Rutile TiO2 Nanoparticles for Organic Compounds. Optik 2017, 128, 191–200. [Google Scholar] [CrossRef]
- John, A.K.; Palaty, S.; Sharma, S.S. Greener Approach towards the Synthesis of Titanium Dioxide Nanostructures with Exposed {001} Facets for Enhanced Visible Light Photodegradation of Organic Pollutants. J. Mater. Sci. Mater. Electron. 2020, 31, 20868–20882. [Google Scholar] [CrossRef]
- Zainab, S.; Azeem, M.; Awan, S.U.; Rizwan, S.; Iqbal, N.; Rashid, J. Optimization of Bandgap Reduction in 2-Dimensional GO Nanosheets and Nanocomposites of GO/Iron-Oxide for Electronic Device Applications. Sci. Rep. 2023, 13, 1–19. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY Hollow Mushrooms-like Nanocomposite Photoanode for Hydrogen Electrogeneration. J. Porous Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Yasmeen, S.; Burratti, L.; Duranti, L.; Agresti, A. Study of Photodegradation of Bentazon Herbicide by Using ZnO-Sm2O3 Nanocomposite Under UV Light. Int. J. Mol.Sci. 2024, 25, 13319. [Google Scholar] [CrossRef]
- Seck, E.I.; Doña-Rodríguez, J.M.; Fernández-Rodríguez, C.; Portillo-Carrizo, D.; Hernández-Rodríguez, M.J.; González-Díaz, O.M.; Pérez-Peña, J. Solar Photocatalytic Removal of Herbicides from Real Water by Using Sol-Gel Synthesized Nanocrystalline TiO2: Operational Parameters Optimization and Toxicity Studies. Sol. Energy 2013, 87, 150–157. [Google Scholar] [CrossRef]
- Kinkennon, A.E.; Green, D.B.; Hutchinson, B. The Use of Simulated or Concentrated Natural Solar Radiation for the TiO2-Mediated Photodecomposition of Basagran, Diquat, and Diuron. Chemosphere 1995, 31, 8663–8671. [Google Scholar] [CrossRef]
- Ahmed, S. Preparation and Characterization of ZnO & TiO2 Nanocatalysts for Photo Degradation of Bentazon Existing in Polluted Water. J. Phys. Conf. Ser. 2019, 1310, 012015. [Google Scholar] [CrossRef]
- Mungondori, H.H.; Tichagwa, L.; Katwire, D.M.; Aoyi, O. Preparation of Photo-Catalytic Copolymer Grafted Asymmetric Membranes (N-TiO2-PMAA-g-PVDF/PAN) and Their Application on the Degradation of Bentazon in Water. Iran. Polym. J. 2016, 25, 135–144. [Google Scholar] [CrossRef]
- Yaseen, M.; Humayun, M.; Khan, A.; Idrees, M.; Shah, N.; Bibi, S. Photo-Assisted Removal of Rhodamine B and Nile Blue Dyes from Water Using CuO–SiO2 Composite. Molecules 2022, 27, 5343. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.H.; Mosavi, V. Wet Coprecipitation Preparation of Perovskite-Type Iron Manganite Nano Powder Pure Phase Using Nitrate Precursors: Structural, Opto-Electronic, Morphological and Photocatalytic Activity for Degradation of Nile Blue Dye. J. Mater. Sci. Mater. Electron. 2017, 28, 10270–10276. [Google Scholar] [CrossRef]
- Mirzaei, M.; Habibi, M.H.; Sabzyan, H. Synthesis, Characterization, and Dye Degradation Photocatalytic Activity of the Nano-Size Copper Iron Binary Oxide. Environ. Sci. Pollut. Res. 2022, 29, 9173–9192. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, S.; Burratti, L.; Duranti, L.; Sgreccia, E.; Prosposito, P. Photocatalytic Degradation of Organic Pollutants—Nile Blue, Methylene Blue, and Bentazon Herbicide—Using NiO-ZnO Nanocomposite. Nanomaterials 2024, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Prabhudesai, V.S.; Meshram, A.A.; Vinu, R.; Sontakke, S.M. Superior Photocatalytic Removal of Metamitron and Its Mixture with Rhodamine B Dye Using Combustion Synthesized TiO2 Nanomaterial. Chem. Eng. J. Adv. 2021, 5, 100084. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Karimi, H.; Ghaedi, M. Degradation of Binary Mixtures of Dyes by Step-Scheme Quaternary Photocatalyst in Continuous Flow-Loop Ultrasound Assisted Micro-Photoreactor. J. Mol. Liq. 2023, 388, 122830. [Google Scholar] [CrossRef]

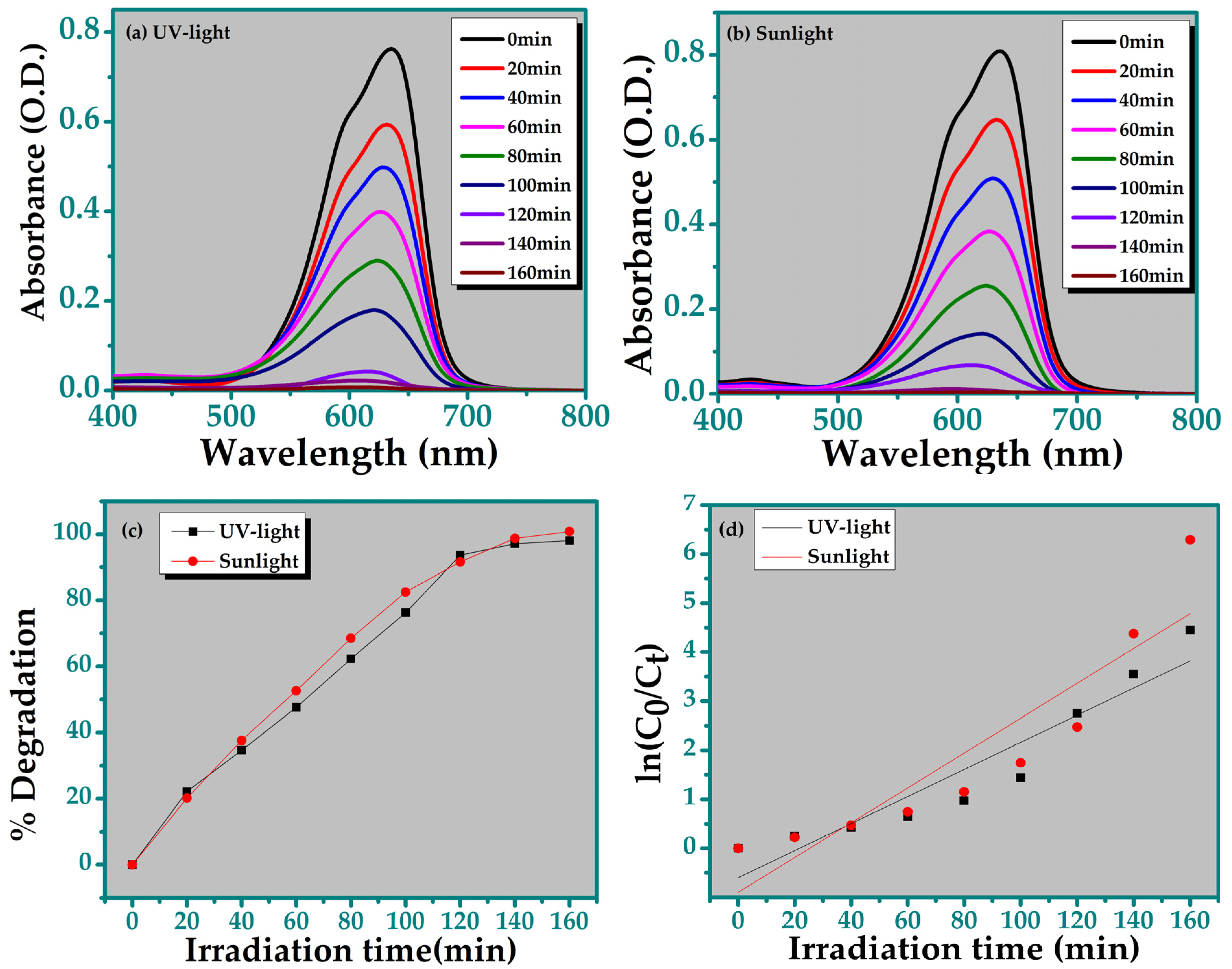
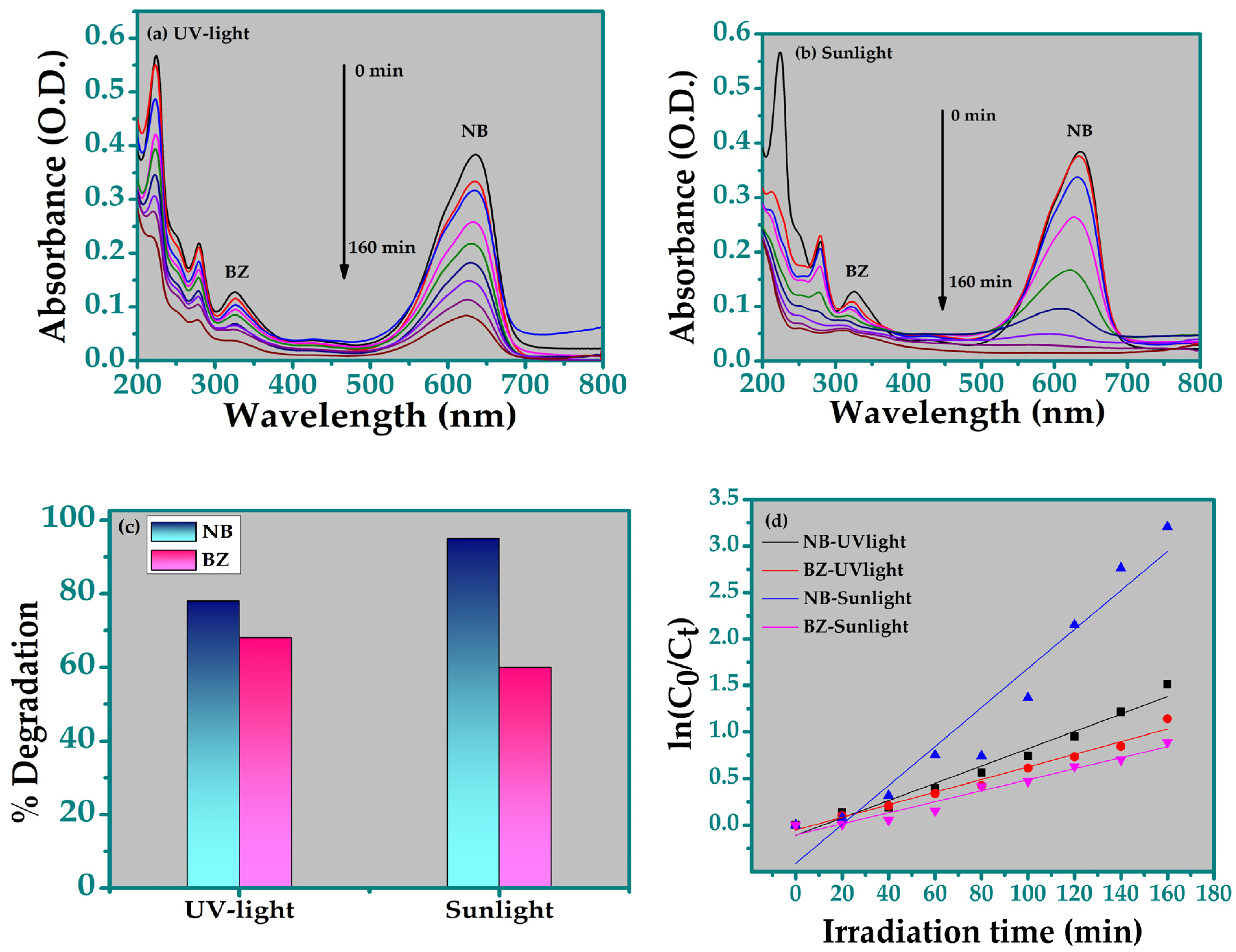
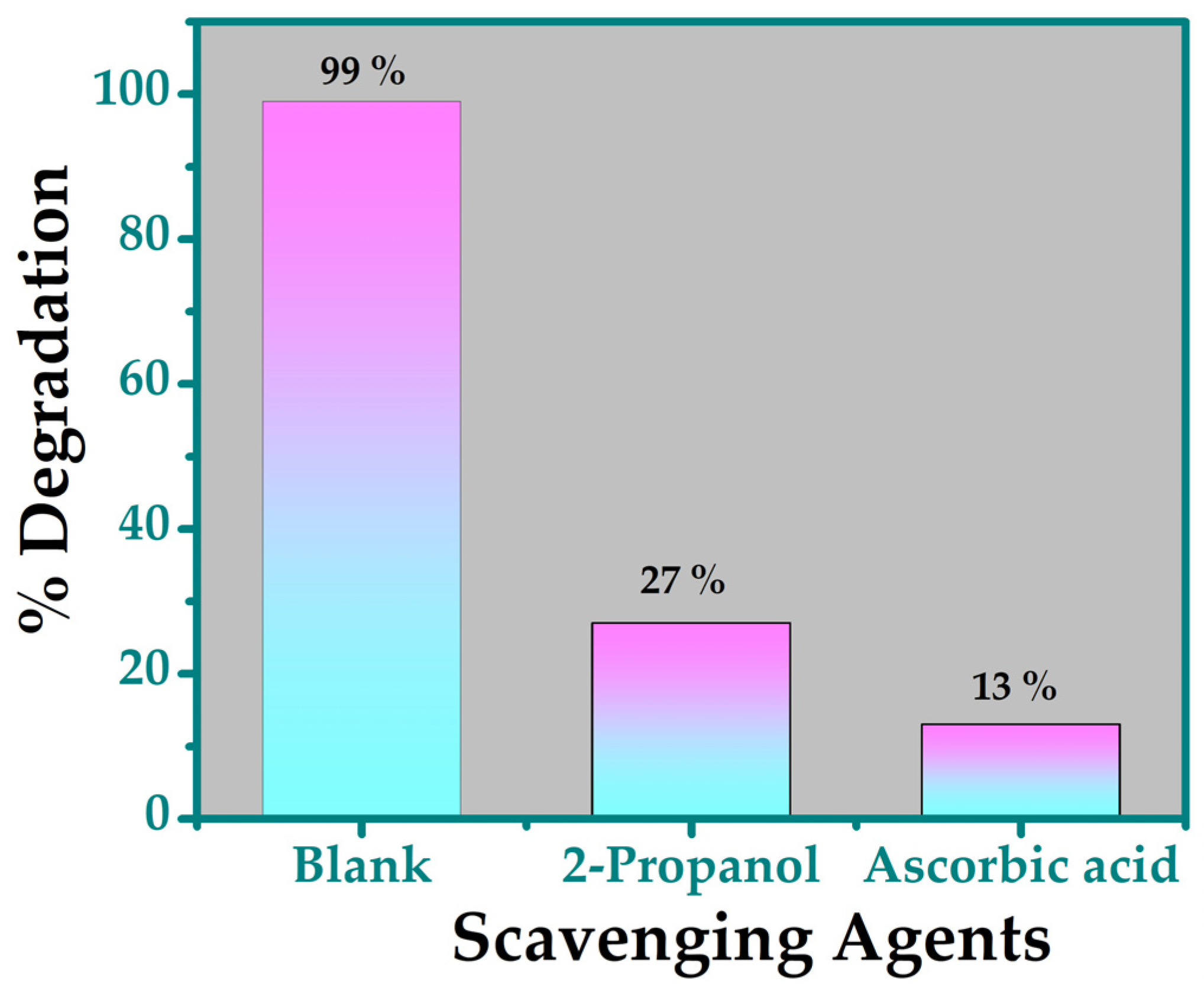

| Parameters | TiO2 |
|---|---|
| Symmetry | Tetragonal Anatase |
| Lattice constants | a = 3.775 Å b = 9.581 Å |
| Cell volume | 136.54 Å3 |
| Crystalline size D | 20 nm |
| Catalyst | Catalyst Concentration (gL−1) | Irradiation Source | Bentazon Concentration (mgL−1) | % Degradation | Ref. |
|---|---|---|---|---|---|
| TiO2 P25 | 1.0 | Philips HB 175 60 W | 20 | 85% in 120 min | [30] |
| TiO2 nanocrystal | 1.0 | Philips HB 175 60 W | 20 | 63% in 120 min | [30] |
| TiO2 suspension | 1.0 | solar simulator 1000 W | 10 | 95% in 60 min | [31] |
| TiO2 suspension | 1.0 | sunlight | 10 | 95% in 60 in | [31] |
| ZnO/TiO2 | 0.5 | UV lamp | 20 | 80% in 120 min | [32] |
| TiO2/PMAA | 0.5 | sunlight | 10 | 100% in 200 min | [33] |
| TiO2 nanoparticles | 0.3 | Hg lamp 300 W | 5 | 99% in 40 min | Present work |
| - | - | sunlight | - | 75% in 40 min | Present work |
| Pollutants | % Degradation | Radiation Source | K (min−1) | R2 | Y = a + bx |
|---|---|---|---|---|---|
| BZ | 99% | UV | 0.1110 | 0.9769 | −0.2805 + 0.0110x |
| BZ | 75% | sunlight | 0.03491 | 0.9987 | −0.0100 + 0.3491x |
| NB | 98% | UV | 0.0762 | 0.8859 | −0.5997 + 0.0762x |
| NB | 100% | sunlight | 0.0355 | 0.8157 | −0.8987 + 0.0355x |
| BZ (in BZ + NB mixture) | 68% | UV | 0.00679 | 0.9757 | −0.0531 + 0.00679x |
| BZ (in BZ + NB mixture) | 60% | Sunlight | 0.0059 | 0.9575 | −0.10543 + 0.0059x |
| NB (in BZ + NB mixture) | 78% | UV | 0.0093 | 0.9669 | −0.1092 + 0.0093x |
| NB (in BZ + NB mixture) | 95% | sunlight | 0.0209 | 0.9289 | −0.4136 + 0.0209x |
| Catalyst | Catalyst Concentration (gL−1) | Irradiation Source | Nile Blue Concentration (mgL−1) | Degradation Percentage | Ref. |
|---|---|---|---|---|---|
| CuO-SiO2 | 0.1 | UV lamp | 20 | 90% | [34] |
| FeMnO3 | / | 250 W mercury lamps | 10 | 95% in 80 min | [35] |
| CuFe2O4 | 0.03 | Hg lamp | 10 | 93% in 120 min | [36] |
| NiO-ZnO | 0.03 | sunlight | 5 | 97% in 140 min | [37] |
| TiO2 | 0.3 | UV light | 5 | 98% in 160 min | Present work |
| TiO2 | 0.3 | Sunlight | 5 | 100% in 160 min | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yasmeen, S.; Burratti, L.; Duranti, L.; Sgreccia, E.; Agresti, A.; Prosposito, P. Superior Photodegradation of Bentazon and Nile Blue and Their Binary Mixture Using Sol–Gel Synthesized TiO2 Nanoparticles Under UV and Sunlight Sources. Appl. Sci. 2025, 15, 1899. https://doi.org/10.3390/app15041899
Yasmeen S, Burratti L, Duranti L, Sgreccia E, Agresti A, Prosposito P. Superior Photodegradation of Bentazon and Nile Blue and Their Binary Mixture Using Sol–Gel Synthesized TiO2 Nanoparticles Under UV and Sunlight Sources. Applied Sciences. 2025; 15(4):1899. https://doi.org/10.3390/app15041899
Chicago/Turabian StyleYasmeen, Sadaf, Luca Burratti, Leonardo Duranti, Emanuela Sgreccia, Antonio Agresti, and Paolo Prosposito. 2025. "Superior Photodegradation of Bentazon and Nile Blue and Their Binary Mixture Using Sol–Gel Synthesized TiO2 Nanoparticles Under UV and Sunlight Sources" Applied Sciences 15, no. 4: 1899. https://doi.org/10.3390/app15041899
APA StyleYasmeen, S., Burratti, L., Duranti, L., Sgreccia, E., Agresti, A., & Prosposito, P. (2025). Superior Photodegradation of Bentazon and Nile Blue and Their Binary Mixture Using Sol–Gel Synthesized TiO2 Nanoparticles Under UV and Sunlight Sources. Applied Sciences, 15(4), 1899. https://doi.org/10.3390/app15041899










