Preliminary Investigation of a Cd0.9Zn0.1Te Detector for Small-Field Dosimetry Applications Using Therapeutic MV Beams
Abstract
1. Introduction
2. Materials and Methods
2.1. Growth of CZT
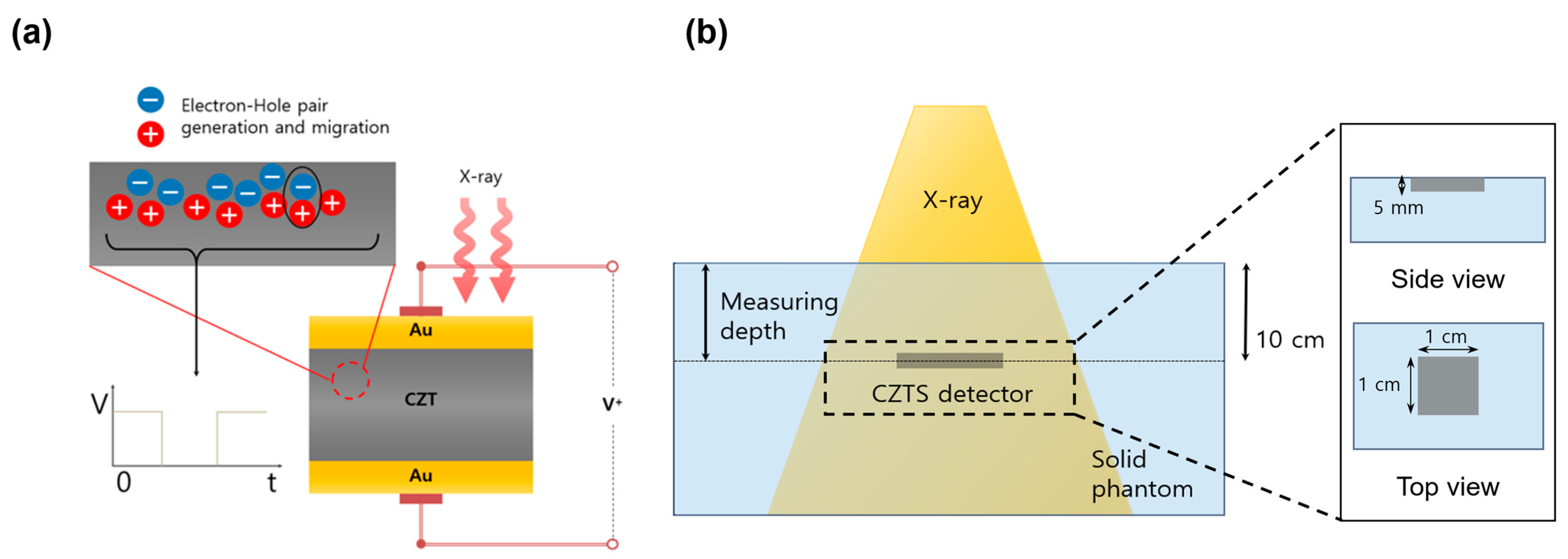
2.2. Measurement Configuration
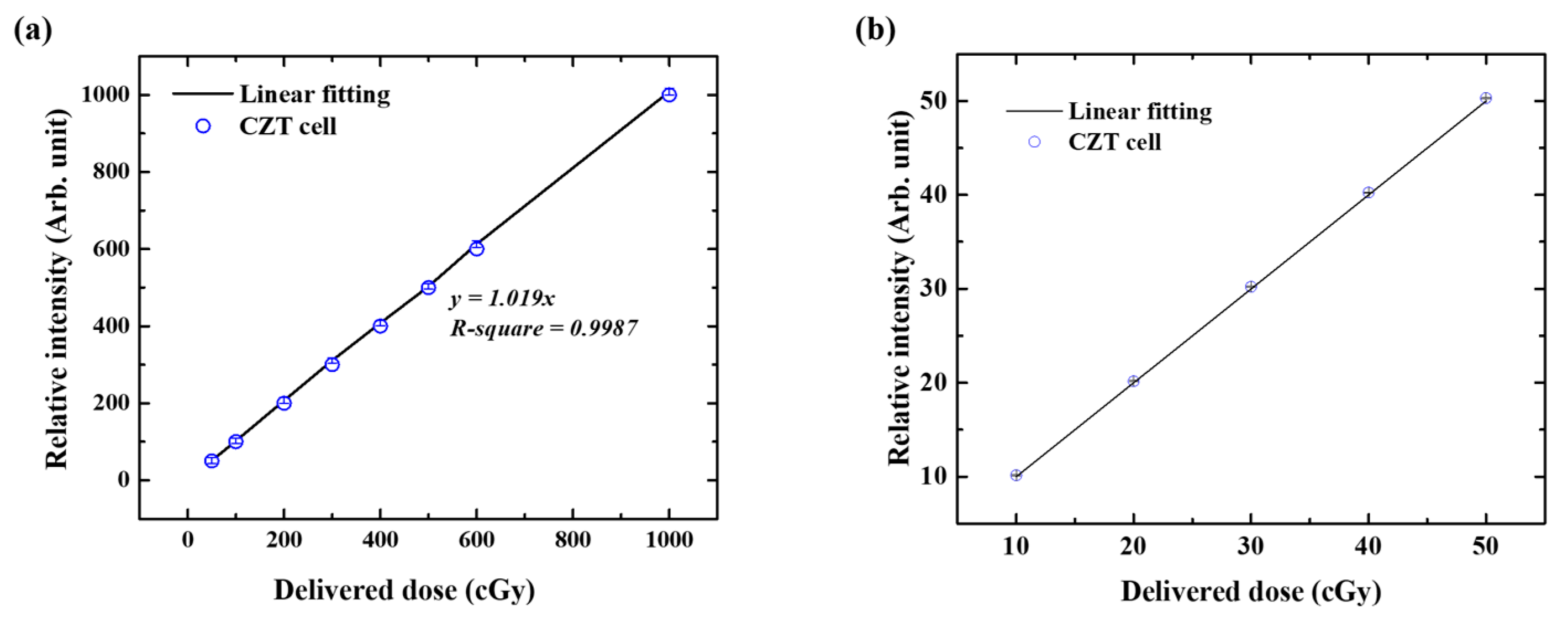
2.3. Dose Linearity
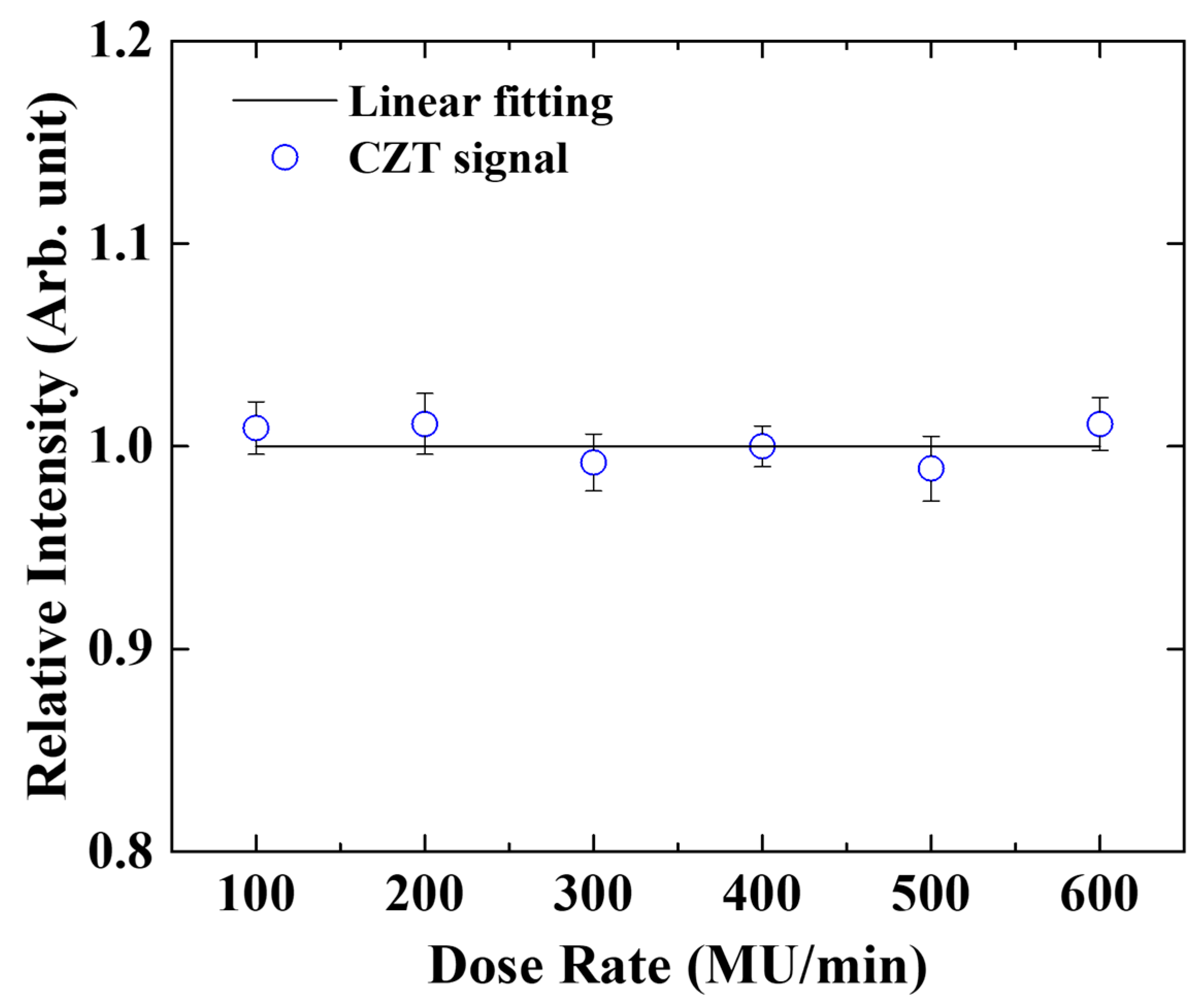
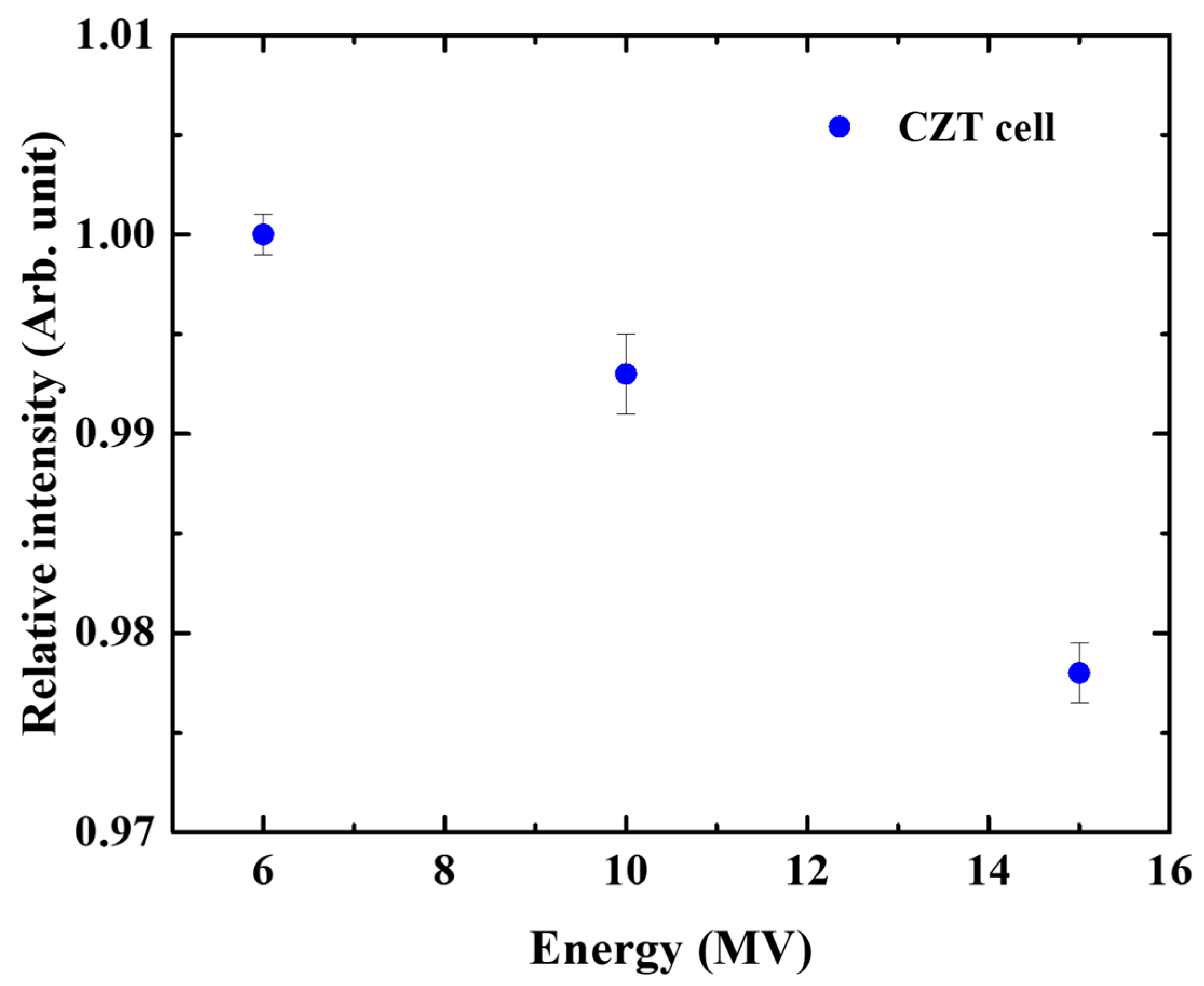
2.4. Dose Rate and Energy Dependence
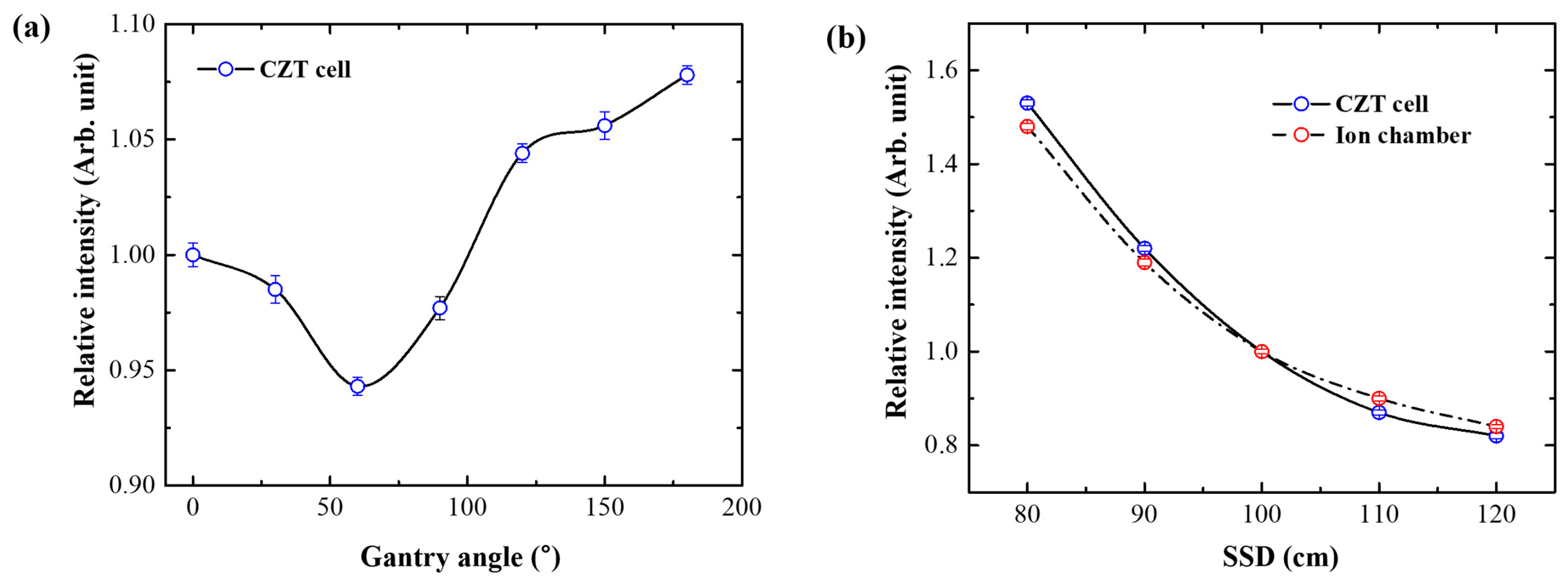
2.5. Angular and SSD Dependence
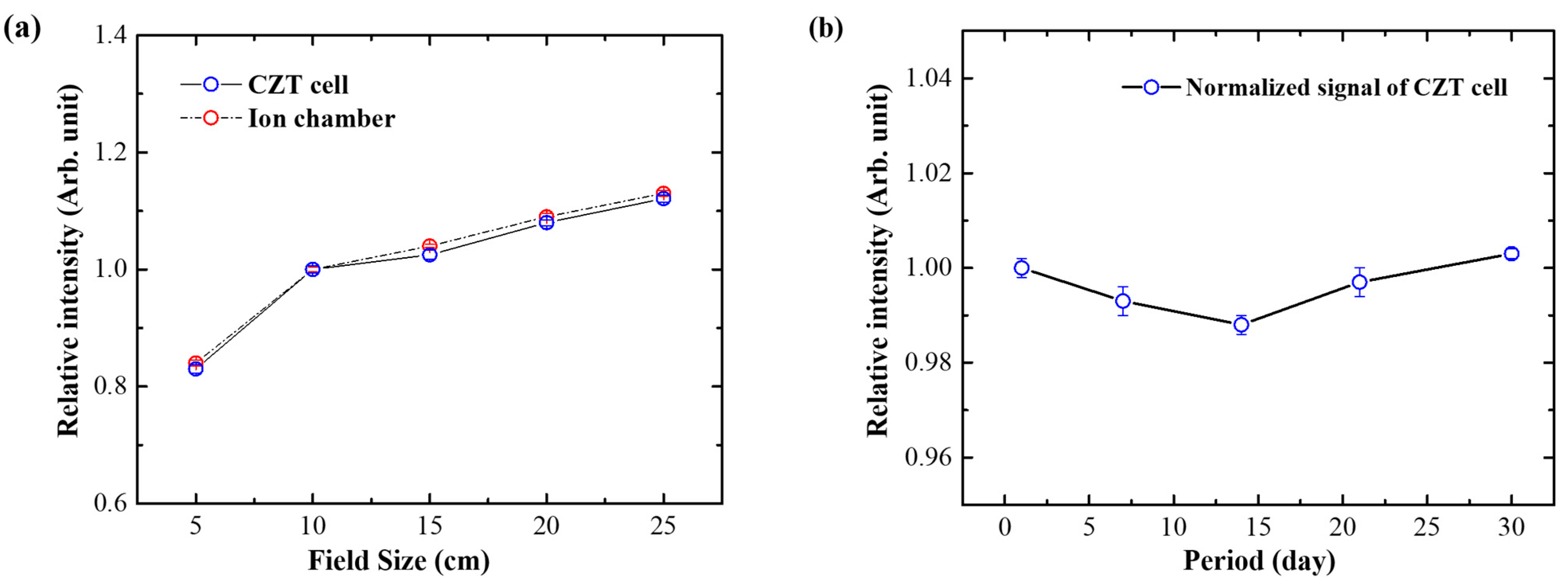
2.6. Field Size Dependence and Reproducibility
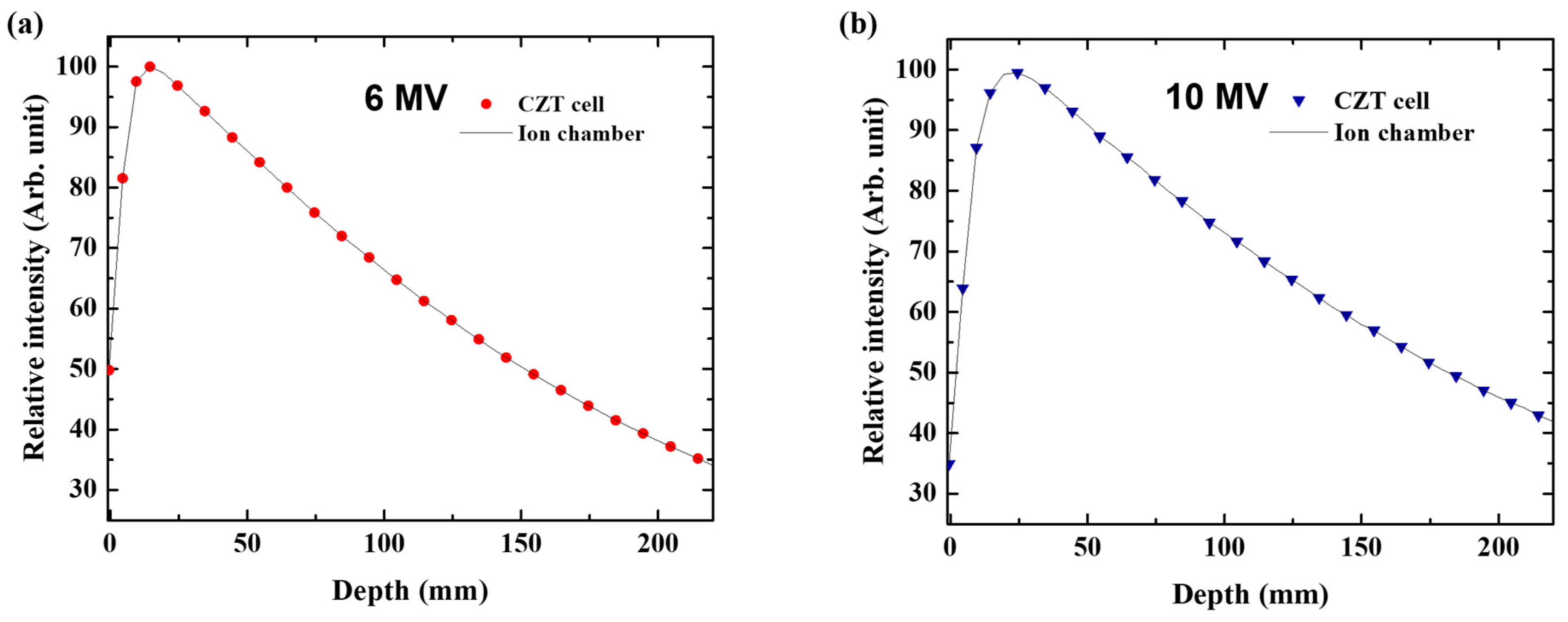
2.7. Percentage Depth Dose
3. Results
3.1. Dose Linearity
3.2. Dose Rate Dependence
3.3. Energy Dependence
3.4. Angular and SSD Dependence
3.5. Field Size Dependence and Reproducibility
3.6. Percentage Depth Dose
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lo, S.S.; Fakiris, A.J.; Chang, E.L.; Mayr, N.A.; Wang, J.Z.; Papiez, L.; Teh, B.S.; McGarry, R.C.; Cardenes, H.R.; Timmerman, R.D. Stereotactic body radiation therapy: A novel treatment modality. Nat. Rev. Clin. Oncol. 2010, 7, 44–54. [Google Scholar] [CrossRef]
- Kann, B.H.; Park, H.S.; Johnson, S.B.; Chiang, V.L.; Yu, J.B. Radiosurgery for brain metastases: Changing practice patterns and disparities in the United States. J. Natl. Compr. Canc. Netw. 2017, 15, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; Guckenberger, M.; Hurkmans, C.; Nestle, U.; Belderbos, J.; De Ruysscher, D.; Faivre-Finn, C.; Le Péchoux, C. SBRT dose and survival in non-small cell lung cancer: In regard to Koshy et al. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 945–946. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D.; Dragojević, I.; Hoisak, J.; Hoopes, D.; Manger, R. Lung stereotactic body radiation therapy (SBRT) dose gradient and PTV volume: A retrospective multicenter analysis. Radiat. Oncol. 2019, 14, 162. [Google Scholar] [CrossRef] [PubMed]
- Narayanasamy, G.; Desai, D.; Maraboyina, S.; Penagaricano, J.; Zwicker, R.; Johnson, E.L. A dose falloff gradient study in RapidArc planning of lung stereotactic body radiation therapy. J. Med. Phys. 2018, 43, 147–154. [Google Scholar] [CrossRef]
- Shaw, M.; Lye, J.; Alves, A.; Keehan, S.; Lehmann, J.; Hanlon, M.; Kenny, J.; Baines, J.; Porumb, C.; Geso, M.; et al. Characterisation of a synthetic diamond detector for end-to-end dosimetry in stereotactic body radiotherapy and radiosurgery. Phys. Imaging Radiat. Oncol. 2021, 20, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, P.H.; Cirino, E.; Das, I.J.; Garrett, J.A.; Yang, J.; Yin, F.-F.; Fairobent, L.A. AAPM-RSS medical physics practice Guideline 9.a. for SRS-SBRT. J. Appl. Clin. Med. Phys. 2017, 18, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
- Agency, I.A.E. Dosimetry of Small Static Fields Used in External Beam Radiotherapy: An International Code of Practice for Reference and Relative Dose Determination; Technical Reports Series; International Atomic Energy Agency (IAEA): Vienna, Austria, 2017; Volume 483. [Google Scholar]
- Foote, M.; Bailey, M.; Smith, L.; Siva, S.; Hegi-Johnson, F.; Seeley, A.; Barry, T.; Booth, J.; Ball, D.; Thwaites, D. Guidelines for safe practice of stereotactic body (ablative) radiation therapy. J. Med. Imaging Radiat. Oncol. 2015, 59, 646–653. [Google Scholar] [CrossRef]
- Sahgal, A.; Roberge, D.; Schellenberg, D.; Purdie, T.G.; Swaminath, A.; Pantarotto, J.; Filion, E.; Gabos, Z.; Butler, J.; Letourneau, D.; et al. The Canadian association of radiation oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin. Oncol. (R Coll Radiol) 2012, 24, 629–639. [Google Scholar] [CrossRef]
- Matuszak, M.M.; Hadley, S.W.; Feng, M.; Hayman, J.A.; Brock, K.K.; Burger, P.; Owen, D.; Suresh, K.; Schipper, M.; Lawrence, T.S.; et al. Enhancing safety and quality through preplanning peer review for patients undergoing stereotactic body radiation therapy. Pract. Radiat. Oncol. 2016, 6, e39–e46. [Google Scholar] [CrossRef] [PubMed]
- Van Elmpt, W.; McDermott, L.; Nijsten, S.; Wendling, M.; Lambin, P.; Mijnheer, B. A literature review of electronic portal imaging for radiotherapy dosimetry. Radiother. Oncol. 2008, 88, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Bjärngard, B.E.; Tsai, J.S.; Rice, R.K. Doses on the central axes of narrow 6-MV x-ray beams. Med. Phys. 1990, 17, 794–799. [Google Scholar] [CrossRef]
- Niroomand-Rad, A.; Blackwell, C.R.; Coursey, B.M.; Gall, K.P.; Galvin, J.M.; McLaughlin, W.L.; Meigooni, A.S.; Nath, R.; Rodgers, J.E.; Soares, C.G. Radiochromic film dosimetry: Recommendations of AAPM radiation therapy committee task group 55. American Association of Physicists in Medicine. Med. Phys. 1998, 25, 2093–2115. [Google Scholar] [CrossRef]
- Butson, M.J. Scanning orientation effects on Gagchromic EBT film dosimetry. Australas. Phys. Eng. Sci. Med. 2006, 293, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Paelinck, L.; Neve, W.D.; Wagter, C.D. Precautions and strategies in using a commercial flatbed scanner for radiochromic film dosimetry. Phys. Med. Biol. 2007, 521, 231–242. [Google Scholar] [CrossRef]
- Tyler, M.; Liu, P.Z.Y.; Chan, K.W.; Ralston, A.; McKenzie, D.R.; Downes, S.; Suchowerska, N. Characterization of small-field stereotactic radiosurgery beams with modern detectors. Phys. Med. Biol. 2013, 58, 7595–7608. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, A.A.A.; Wieker, S.; Harder, D.; Poppe, B. The origin of the flatbed scanner artifacts in radiochromic film dosimetry—Key experiments and theoretical descriptions. Phys. Med. Biol. 2016, 61, 7704–7724. [Google Scholar] [CrossRef]
- Darafsheh, A.; Zhao, T.; Khan, R. Spectroscopic analysis of irradiated radiochromic EBT-XD films in proton and photon beams. Phys. Med. Biol. 2020, 65, 205002. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Davis, S.; Seuntjens, J. Experimental analysis of general ion recombination in a liquid-filled ionization chamber in high energy photon beams. Med. Phys. 2013, 40, 062104. [Google Scholar] [CrossRef]
- Markovic, M.; Stathakis, S.; Mavroidis, P.; Jurkovic, I.A.; Papanikolaou, N. Characterization of a two-dimensional liquid-filled ion chamber detector array used for verification of the treatments in radiotherapy. Med. Phys. 2014, 41, 051704. [Google Scholar] [CrossRef]
- Lechner, W.; Palmans, H.; Sölkner, L.; Grochowska, P.; Georg, D. Detector comparison for small field output factor measurements in flattening filter free photon beams. Radiother. Oncol. 2013, 109, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Yarahmadi, M.; Wegener, S.; Sauer, O.A. Energy and field size dependence of a silicon diode designed for small-field dosimetry. Med. Phys. 2017, 44, 1958–1964. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Kuo, L.; Li, T.; Li, X.; Ballangrud, A.M.; Lovelock, M.; Chan, M.F. Comparative study of SRS end-to-end QA processes of a diode array device and an anthropomorphic phantom loaded with GafChromic XD film. J. Appl. Clin. Med. Phys. 2022, 23, e13747. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Mazurier, J.; Franck, D.; Dudouet, P.; Latorzeff, I.; Franceries, X. 2D EPID dose calibration for pretreatment quality control of conformal and IMRT fields: A simple and fast convolution approach. Phys. Med. 2016, 32, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Novais, J.C.; Molina López, M.Y.; Ruiz Maqueda, S. On flattening filter-free portal dosimetry. J. Appl. Clin. Med. Phys. 2016, 17, 132–145. [Google Scholar] [CrossRef]
- Podesta, M.; Nijsten, S.M.J.J.G.; Persoon, L.C.G.G.; Scheib, S.G.; Baltes, C.; Verhaegen, F. Time dependent pre-treatment EPID dosimetry for standard and FFF VMAT. Phys. Med. Biol. 2014, 59, 4749–4768. [Google Scholar] [CrossRef]
- Agarwal, A.; Rastogi, N.; Maria Das, K.J.; Yoganathan, S.A.; Udayakumar, D.; Kumar, S. Investigating the electronic portal imaging device for small radiation field measurements. J. Med. Phys. 2017, 42, 59–64. [Google Scholar] [CrossRef]
- Arlt, R.; Brutscher, J.; Gunnink, R.; Ivanov, V.; Parnham, K.; Soldner, S.; Stein, J. Use of CdZnTe detectors in hand-held and portable isotope identifiers to detect illicit trafficking of nuclear material and radioactive sources. In Proceedings of the 2000 IEEE Nuclear Science Symposium Conference Record (cat. No. 00CH37149), Lyon, France, 15–20 October 2000; Volume 11, pp. 4–14. [Google Scholar] [CrossRef]
- Sordo, S.D.; Abbene, L.; Caroli, E.; Mancini, A.M.; Zappettini, A.; Ubertini, P. Progress in the development of CdTe and CdZnTe semiconductor radiation detectors for astrophysical and medical applications. Sensors 2009, 9, 3491–3526. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chu, J.H.; Li, L.X.; Dai, N.; Zhang, F.J. Investigation of room temperature nuclear radiation CdZnTe pixel array detector. J. Optoelectron. Laser 2008, 19, 751–753. [Google Scholar]
- Haijun, X. Data Acquisition System of CZT Nuclear Detector Based on FPGA. Master’s Thesis, Chongqing University, Chongqing, China, 2013. [Google Scholar]
- Roy, U.N.; Bolotnikov, A.E.; Camarda, G.S.; Cui, Y.; Hossain, A.; Lee, K.; Marshall, M.; Yang, G.; James, R.B. Growth of CdTexSe1-x from a Te-rich solution for applications in radiation detection. J. Cryst. Growth 2014, 386, 43–46. [Google Scholar] [CrossRef]
- Veale, M.C.; Bell, S.J.; Jones, L.L.; Seller, P.; Wilson, M.D.; Allwork, C.; Kitou, D.; Sellin, P.J.; Veeramani, P.; Cernik, R.C. An ASIC for the study of charge sharing effects in small pixel CdZnTe X-ray detectors. IEEE Trans. Nucl. Sci. 2011, 58, 2357–2362. [Google Scholar] [CrossRef]
- Alashrah, S.; El-Taher, A. Intensity modulated radiation therapy plans verification using a Gaussian convolution kernel to correct the single chamber response Function of the I’mRT MatriXX Array. J. Appl. Sci. 2015, 15, 483–491. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, J.; Tang, X.; Zhao, R.; Lu, P.; Qv, B.; Li, M.; Qin, F. Simulation of energy spectrum characteristics of planar CdZnTe detector. Nucl. Technol. 2023, 46, 62–68. [Google Scholar]
- Luke, P.N.; Amman, M.; Lee, J.S.; Ludewigt, B.A.; Yaver, H. A CdZnTe coplanar-grid detector array for environmental remediation. Nucl. Instrum. Methods Phys. Res. Sect. A 2001, 458, 319–324. [Google Scholar] [CrossRef]
- Bennett, P.R.; Shah, K.S.; Klugerman, M.; Squillante, M.R. High efficiency pixellated CdTe detector. Nucl. Instrum. Methods Phys. Res. 1997, 392, 260–263. [Google Scholar] [CrossRef]
| Energy (MV) | Field Size (cm2) | SSD (cm) | Dose (cGy) | Dose Rate (MU/min) | Measuring Depth (cm) | |
|---|---|---|---|---|---|---|
| Dose linearity | 6 | 10 × 10 | 100 | 0–1000 | 400 | 10 |
| Dose rate dependence | 6 | 10 × 10 | 100 | 200 | 100–600 | 10 |
| Energy dependence | 6, 10, and 15 | 10 × 10 | 100 | 200 | 400 | 10 |
| Angular dependence | 6 | 10 × 10 | 100 | 200 | 400 | - |
| SSD dependence | 6 | 10 × 10 | 80–120 | 200 | 400 | 10 |
| Field size dependence | 6 | 5 × 5 to 25 × 25 | 100 | 200 | 400 | 10 |
| Reproducibility | 6 | 10 × 10 | 100 | 200 | 400 | 10 |
| PDD | 6, 10 | 10 × 10 | 100 | 500 | 400 | 0–25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Song, J.-Y.; Kim, Y.-H.; Jeong, J.-U.; Yoon, M.S.; Nam, T.-K.; Ahn, S.-J.; Cho, S. Preliminary Investigation of a Cd0.9Zn0.1Te Detector for Small-Field Dosimetry Applications Using Therapeutic MV Beams. Appl. Sci. 2025, 15, 1693. https://doi.org/10.3390/app15041693
Kim S, Song J-Y, Kim Y-H, Jeong J-U, Yoon MS, Nam T-K, Ahn S-J, Cho S. Preliminary Investigation of a Cd0.9Zn0.1Te Detector for Small-Field Dosimetry Applications Using Therapeutic MV Beams. Applied Sciences. 2025; 15(4):1693. https://doi.org/10.3390/app15041693
Chicago/Turabian StyleKim, Sangsu, Ju-Young Song, Yong-Hyub Kim, Jae-Uk Jeong, Mee Sun Yoon, Taek-Keun Nam, Sung-Ja Ahn, and Shinhaeng Cho. 2025. "Preliminary Investigation of a Cd0.9Zn0.1Te Detector for Small-Field Dosimetry Applications Using Therapeutic MV Beams" Applied Sciences 15, no. 4: 1693. https://doi.org/10.3390/app15041693
APA StyleKim, S., Song, J.-Y., Kim, Y.-H., Jeong, J.-U., Yoon, M. S., Nam, T.-K., Ahn, S.-J., & Cho, S. (2025). Preliminary Investigation of a Cd0.9Zn0.1Te Detector for Small-Field Dosimetry Applications Using Therapeutic MV Beams. Applied Sciences, 15(4), 1693. https://doi.org/10.3390/app15041693






